Abstract
We examined the influence of the gram-positive cell wall products peptidoglycan (PepG) and lipoteichoic acid (LTA), compared to lipopolysaccharide (LPS), on the monocyte expression of receptors involved in antigen presentation (HLA-DR, B7.1, and B7.2), cell adhesion (intercellular adhesion molecule-1 [ICAM-1] and lymphocyte function associated antigen-3 [LFA-3]), phagocytosis (FcγRI), and cell activation (CD14). We also evaluated possible influences of the immunosuppressive drugs cyclosporine A, tacrolimus, and sirolimus on the expression of these receptors. Pretreatment of whole blood for 4 h with the immunosuppressive drugs did not influence the expression of the surface receptors in normal or stimulated blood. Stimulation with both PepG and LTA caused significant up-regulation of the surface expression of ICAM-1 and HLA-DR on whole blood monocytes, similar to that obtained with LPS, whereas B7.1, B7.2, LFA-3, and FcγRI were not modulated. PepG and LTA also caused increased expression of CD14, whereas LPS down-regulated this molecule. In contrast, we did not detect any significant influence of any of the bacterial products on the plasma concentration of soluble CD14. We hypothesized that the increased expression of surface CD14 in blood stimulated with PepG would prime for cellular activation by LPS. Indeed, we show that PepG and the partial PepG structure muramyl dipeptide acted in synergy with LPS to cause the release of tumor necrosis factor-α. The results suggest that PepG and LPS provoke partly different responses on monocyte phenotype and that CD14 may play different roles in the innate response to gram-positive and gram-negative bacteria.
Cells of monocytic lineage play a critical role in innate immunity. Monocytes are involved in receptor-mediated pathogen recognition (48), chemotaxis, phagocytosis, antigen presentation, and mediator release (2). The conduct of these tasks is initiated through engagement of receptors expressed on the surface membrane. CD14 is a key receptor expressed on the surface of monocytes, and it is required for the induction of an inflammatory response triggered by low concentrations of lipopolysaccharide (LPS) (7, 26). The importance of membrane-bound CD14 in conferring sensitive responses to LPS has been well documented both in vitro and in vivo (21, 27, 48).
More recently, the CD14 molecule has also been recognized as a receptor for the gram-positive (G+) bacterial cell wall products peptidoglycan (PepG) and lipoteichoic acid (LTA) (6, 19, 20, 47). Accounting for up to 50% of severe sepsis or septic shock cases in modern intensive care units, G+ bacterial sepsis has been recognized as an important clinical condition (10, 20). Like LPS, PepG and LTA have been shown to initiate innate immune responses (6, 19, 20); however, the role of CD14 in G+ bacterial sepsis is unclear (22).
Recently, the family of Toll-like receptors (TLRs) has been described as important in mediating innate immune responses, and these receptors may have the capability to distinguish between different classes of pathogens (25, 41). TLR2 and TLR4 recognize different bacterial cell wall components, and TLR2 has been shown to play a major role in G+ bacterial recognition (34, 41, 49).
Despite clear differences in chemical structure, LPS, PepG, and LTA have striking similarities in biological activities (39). They signal partly through the same receptors and induce similar cytokine response. However, the potential influence of PepG and LTA on the expression of surface receptors involved in monocytic immune responses has not been well characterized.
Cell adhesion is a prerequisite for efficient host defense. Intercellular adhesion molecule-1 (ICAM-1 [CD54]) mediates leukocyte-leukocyte and leukocyte-endothelial-cell interactions by binding to the β2-integrins lymphocyte function associated antigen-1 (LFA-1) and macrophage antigen complex-1 (12). Furthermore, ICAM-1 serves as a costimulatory molecule for T-cell-receptor activation (9, 40).
It is now increasingly evident that T-cell activation is a complex multistep process involving several accessory molecules expressed on the surface of antigen-presenting cells. The B7 receptors B7.1 (CD80) and B7.2 (CD86), expressed on several monocyte-derived antigen-presenting cells (8, 14), interact with CD28 or cytotoxic lymphocyte-associated molecule-4 to deliver a key costimulatory signal in T-cell activation (35).
The interaction between LFA-3 (CD58) and its ligand CD2 has been shown to enhance the immune response, initiated by the presentation of antigens in complex with the major histocompatibility class molecules (31, 38).
The phagocytic capabilities of monocytes and macrophages are essential for the host clearance of pathogens as well as for effective uptake and presentation of antigens. FcγRI (CD64) is a high-affinity receptor for immunoglobulin G (IgG) which plays a central role in antibody-dependant cytotoxicity and clearance of immune complexes (42).
The capacity to enhance or suppress the expression of such receptors can be a powerful immunomodulatory tool. Mandatory for successful organ transplantation, immunosuppressive drugs have potentially serious side effects, including increased risk of infections. We have recently demonstrated that cyclosporine A (CsA) and tacrolimus inhibit the release of tumor necrosis factor-α (TNF-α) production induced by LPS in human whole blood, whereas sirolimus strongly attenuates the production of interleukin-10 induced by both LPS and PepG (24). Whether these immunosuppressive drugs influence the expression of surface receptors on human monocytes is unknown.
In the study reported here, we have investigated the influence of the G+ bacterial cell wall products PepG and LTA on monocyte expression of receptors involved in antigen presentation (HLA-DR, B7.1, and B7.2), cell adhesion (ICAM-1 and LFA-3), phagocytosis (FcγRI), and cell activation (CD14), in comparison with LPS. We also evaluated possible influences of the immunosuppressive drugs CsA, tacrolimus, and sirolimus on the expression of these surface receptors. Of particular importance we report a differential influence of PepG and LPS on the expression of CD14 on monocytes. In order to determine whether the PepG-induced rise in CD14 expression reported in the present study was due to reduced shedding of this receptor, we measured the levels of soluble CD14 in blood treated with PepG and/or LPS. Finally, we determined whether the ability of PepG to enhance the expression of CD14 would prime for cell activation by LPS, as measured by the release of tumor necrosis factor-α.
MATERIALS AND METHODS
Reagents.
Escherichia coli O26:B6 LPS (Difco Laboratories, Detroit, Mich.) was suspended in pyrogen-free sterile saline, and LPS was added directly to the blood samples in microcentrifuge tubes to a final concentration of 10 ng/ml. LTA from Staphylococcus aureus was purchased from Sigma Aldrich (L2515; St. Louis, Mo.). It was prepared using a phenol extraction protocol (15). According to the manufacturer, the protein content was less than 0.5%.
Purification of PepG.
PepG was isolated from S. aureus or Bacillus subtilis as previously described (16). Covalently attached proteins were removed by treatment with 2 mg of pronase per ml for 1 h at 60°C (3). Anionic polymers were removed from the PepG by the treatment of purified cell walls (10 mg [dry weight]/ml) with hydrofluoric acid (48% [vol/vol]) for 24 h at 4°C. The insoluble PepG was then washed by centrifugation (14,000 × g, 5 min) and resuspension, once in 100 ml of Tris-HCl (pH 8.0) and five times in distilled water, until the pH was neutral. The PepG was then recovered by centrifugation as described above and resuspended in saline (0.9% [wt/vol]) prior to sterilization by autoclaving and storage at −20°C. Extracts of PepG were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with no evidence of any protein whatsoever in the results. PepG was also enzymatically digested, and it gave the expected reversed-phase high-pressure liquid chromatography muropeptide profile with no spurious products. Moreover, LPS contamination of PepG could not be verified by the Limulus amoebocyte lysate test (detection limit, 10 ng/liter).
The PepG was dispersed by sonication (3,000 Hz, 3 × 10 s) on ice prior to the experiments. Nonsonicated PepG consisted of large visible aggregates of insoluble PepG material. Muramyl dipeptide (Sigma Aldrich) is a synthetic PepG subunit that consists of N-acetyl muramic acid linked to two amino acids (l-alanine and d-isoglutamine).
Drugs.
CsA (Sandimmune, 50 mg/ml; Novartis, Basel, Switzerland) used in this study was diluted in sterile 0.9% NaCl. Tacrolimus (Fujisawa GmbH, Munich, Germany) was dissolved in ethanol to 10 mg/ml, and then Tween 80 (Sigma Aldrich) was added to a 1:5 volume of ethanol solution. Further dilutions were obtained with 0.9% NaCl. Sirolimus powder (Wyeth-Ayerst Research, Princeton, N.J.) was dissolved in ethanol to a 2 mM stock solution which was stored at −70°C. Further dilutions were obtained with 0.9% NaCl.
Whole blood experiments.
A whole-blood model was used as previously described (45), with minor modifications. Briefly, venous blood from healthy volunteers was anticoagulated with Na-citrate. Whole blood was aliquoted into 1.6-ml microcentrifuge tubes (Sorenson BioScience Inc., Salt Lake City, Utah). Blood samples were stimulated for 6 h with 10 ng of LPS, 10 μg of sonicated PepG (from S. aureus or B. subtilis), 10 μg of nonsonicated PepG (from S. aureus), 1 μg of muramyl dipeptide (MDP) or 100 μg of LTA (from S. aureus) per ml of blood. The respective doses were chosen according to the optimal cytokine responses in human whole blood, as previously reported (44). In experiments aimed at studying the expression kinetics of receptors or soluble CD14, blood was incubated in Monovette syringes (Sarstedt) in the absence or presence of 10 ng of LPS or 10 μg of PepG per ml of blood, and samples were removed for analyses at 1, 3, 6, 12, and 24 h. In some experiments, whole blood was preincubated for 4 h with 250 ng of CsA, 10 ng of tacrolimus, or 10 ng of sirolimus per ml of blood prior to stimulation with 10 ng of LPS or 10 μg of PepG per ml of blood.
Antibody labeling and flow cytometry.
Subsequent to stimulation, whole-blood samples were immediately aliquoted at 100 μl into Falcon polystyrene tubes (Becton Dickinson, Lincoln Park, N.J.) and incubated with 10 μl of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies in darkness for 30 min at room temperature. Prior to washing and fixation, red blood cells were lysed using fluorescence-activated cell sorter lysing solution (Becton Dickinson, San Jose, Calif.). The samples were washed three times with CellWash (Becton Dickinson, Erembodegem, Belgium) before fixation with 1% formaldehyde (CellFIX; Becton Dickinson, Erembodegem). Expression of antigens was analyzed by flow cytometry using 10 μl of anti-CD14 (clone 18D11; Diatec AS, Oslo, Norway), anti-CD54 (clone HA58), anti-CD58 (clone 1C3), anti-CD64 (clone 10.1), anti-CD80 (clone L307.4), anti-CD86 (clone 2331 FUN-1), and anti-HLA-DR (clone G46-6) (all from Pharmingen, San Diego, Calif.), as well as isotype-negative control anti-IgG1 (clone 1B9) and anti-IgG2 (clone 5A7) (both Diatec AS). All samples were analyzed on the FACScan from Becton Dickinson (Immunocytometry Systems, San Jose, Calif.) using the CellQuest software.
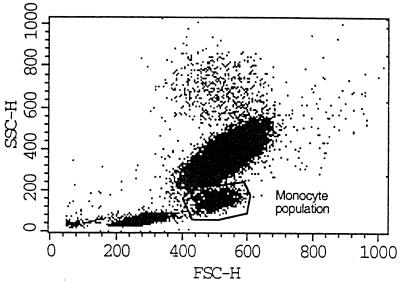
In each experiment the monocyte population was identified as CD14-positive cells with characteristic locations in the scatter diagram (Fig. 1). Fluorescence intensity (FI) was measured in the monocyte population after appropriate gating on the combination of forward scatter and sideways scatter, and mean (geometric) FI was recorded for each measurement. Thirty thousand cells were counted per sample.
FIG. 1.
Scatter characteristics of whole-blood leukocytes by flow cytometry. Subsequent to lysing of red blood cells, whole-blood leukocytes group into three main populations based on their scatter characteristics. Illustrated is a representative picture of leukocytes in unstimulated whole blood, where the monocyte population is encircled. This population stained heavily with anti-CD14 antibodies (data not shown). SSC-H, sideways scatter; FSC-H, forward scatter.
ELISA assays.
Plasma concentrations of TNF-α were determined with a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kit (PeliKine Compact; CLB Labs, Amsterdam, The Netherlands) according to the manufacturer's instructions. The detection limit of the TNF-α ELISA was 1 pg/ml. The plates were read at 450 nm in an ELISA reader (Thermomax microplate reader; Molecular Devices, Menlo Park, Calif.).
Measurements of sCD14 were done using a sandwich ELISA with CD14-specific monoclonal antibodies (clones 3C10 and 5C5) as previously described (28). The detection limit of the assay was 0.8 ng/ml, and the interassay and intra-assay variations were less than 10%.
Statistical evaluation.
Data are presented as means ± the standard error of the mean (SEM). Analysis of variance with Tukey post hoc assessment was used to evaluate the statistical significance of the results. Differences with P values of <0.05 were considered significant.
RESULTS
The scatter characteristics of whole-blood leukocytes are depicted in Fig. 1. The monocytes are identified as a distinct population between the lymphocytes and the granulocytes, staining strongly positive for CD14. Upon stimulation with LPS, PepG, or LTA, the scatter characteristics changed slightly, because a fraction of the monocytes became larger as indicated by an increase in the forward scatter.
In unstimulated blood incubated for 6 h, monocytes showed a basal expression of CD14, ICAM-1, LFA-3, FcγRI, and HLA-DR (Table 1) but not of B7.1 or B7.2.
TABLE 1.
Expression of surface inflammatory receptors on monocytes after stimulation of whole blood with LPS in the absence or presence of CsA, tacrolimus, or sirolimus
| Treatment | Mean specific fluorescence intensity (± SEM)a
|
||||
|---|---|---|---|---|---|
| CD14 | LFA-3 | ICAM-1 | FcγRI | HLA-DR | |
| Untreated | 54.8 ± 3.2 | 20.0 ± 4.3 | 32.5 ± 5.6 | 16.6 ± 3.6 | 53.5 ± 7.8 |
| LPS | 42.0 ± 2.0b | 21.6 ± 3.8 | 59.5 ±7.8b | 17.6 ± 4.1 | 83.3 ± 8.7b |
| LPS + CsA | 41.0 ± 2.3b | 20.0 ± 3.2 | 56.5 ± 6.5b | 19.0 ± 3.7 | 86.2 ± 11.3b |
| LPS + tacrolimus | 41.6 ± 2.3b | 19.3 ± 3.8 | 58.8 ± 8.0b | 18.6 ± 2.9 | 84.2 ± 9.6b |
| LPS + sirolimus | 42.5 ± 1.7b | 16.6 ± 5.6 | 54.8 ± 7.9b | 17.3 ±2.6 | 82.3 ± 11.7 |
Values are specific fluorescence intensity after appropriate gating for monocytes in whole blood treated with LPS (10 ng/ml), LPS and CsA (250 ng/ml), tacrolimus (10 ng/ml), or sirolimus (10 ng/ml).
Significantly different (P < 0.05) from the value for the untreated control group as calculated by analysis of variance with Tukey post hoc assessment.
Influence of LPS and immunosuppressive drugs on monocyte surface receptor expression.
Stimulation with LPS (10 ng/ml) for 6 h significantly increased the monocyte surface expression of ICAM-1 and HLA-DR (Table 1) (P < 0.05). The specific mean FI of ICAM-1 nearly doubled, while the FI of HLA-DR increased approximately 60%. In contrast, the surface expression of CD14 was decreased by 25% (P < 0.05) subsequent to LPS stimulation (Fig. 2 and Table 1). We could not observe any influence of LPS on the expression of LFA-3 and FcγRI (Table 1), and we did not find LPS to induce the expression of B7.1 or B7.2 (data not shown).
FIG. 2.
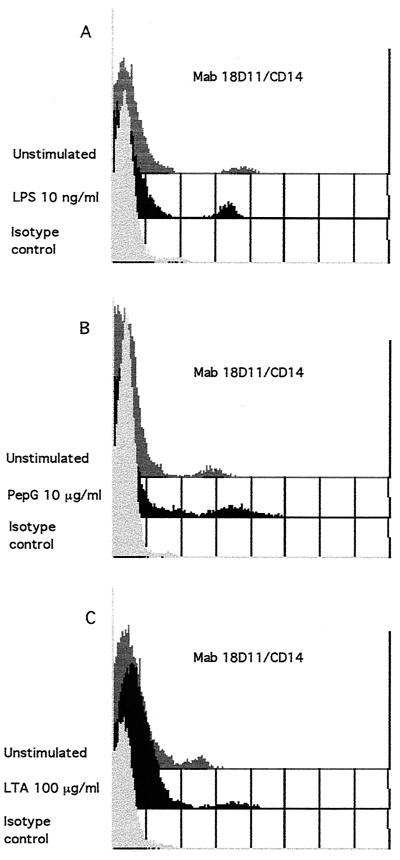
CD14 expression on monocytes 6 h after stimulation of whole blood with LPS (A), PepG (B), or LTA (C). Whole blood was stimulated with 10 ng of LPS, 10 μg of PepG, or 100 μg of LTA per ml of blood and incubated at 37°C for 6 h. One hundred microliters of blood was then spiked with FITC-conjugated anti-CD14 (clone 18D11) or isotype-negative control anti-IgG1 (clone 1B9) antibodies. Subsequent to lysing of red blood cells, washing, and fixation, the samples were analyzed by flow cytometry on the FACScan using CellQuest software. Results illustrated represent one of six experiments with each stimulant.
Pretreatment of whole blood with CsA (250 ng/ml), tacrolimus (10 ng/ml), or sirolimus (10 ng/ml) for 4 h influenced neither the basal expression of the surface receptors in question nor the expression characteristics subsequent to LPS stimulation (Table 1).
Influence of PepG and LTA on monocyte surface inflammatory receptor expression.
As seen in Table 2, PepG (10 μg/ml) from both S. aureus and B. subtilis significantly up-regulated the surface expression of ICAM-1 and HLA-DR, as efficiently as did LPS (P < 0.05). In contrast to LPS, PepG significantly up-regulated the surface expression of CD14 (Fig. 2 and Table 2). The FI of CD14 increased approximately 90% upon stimulation with PepG from both bacteria. However, neither the PepG from S. aureus nor that from B. subtilis had any influence on the expression of LFA-3 or FcγRI (Table 2), nor did either PepG induce the expression of B7.1 or B7.2 (data not shown).
TABLE 2.
Expression of surface inflammatory receptors on monocytes after stimulation of whole blood with PepG or LTA
| Treatment | Mean specific fluorescence intensity (± SEM)a
|
||||
|---|---|---|---|---|---|
| CD14 | ICAM-1 | LFA-3 | FcγRI | HLA-DR | |
| Untreated | 60.5 ± 12.2 | 27.3 ± 4.5 | 17.5 ± 1.1 | 18.4 ± 1.3 | 62.0 ± 11.7 |
| PepG (S. aureus) | 114.6 ± 20.2b | 59.3 ±13.3b | 15.8 ± 0.6 | 19.5 ± 1.7 | 105.7 ± 15.6b |
| PepG (B. subtilis) | 115.2 ± 21.3b | 69.7 ± 17.4b | 15.8 ± 1.0 | 18.8 ± 1.7 | 106.8 ± 16.3b |
| LTA | 103.2 ± 14.1b | 67.2 ± 16.7b | 16.2 ± 1.1 | 17.0 ± 1.8 | 127.3 ± 26.6b |
Values are for specific fluorescence intensity after appropriate gating for monocytes in whole blood treated with PepG (10 μg/ml) or LTA (100 μg/ml).
Significantly different (P < 0.05) from the value for the untreated control group as calculated by analysis of variance with Tukey post hoc assessment.
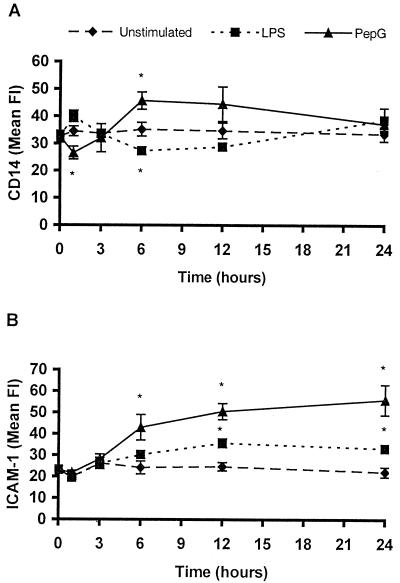
To study the regulation of CD14 and ICAM-1 over time, whole blood was stimulated with LPS (10 ng/ml) or PepG (10 μg/ml) for 1, 3, 6, 12, and 24 h. As presented in Fig. 3, the time-dependent expression of CD14 subsequent to stimulation with LPS or PepG showed an inverse biphasic pattern. LPS induced an early increase (16%) in CD14 expression at 1 h, followed by a decrease to subbasal levels at 6 and 12 h (24% [P < 0.05] and 18%, respectively) of incubation. After 24 h with LPS incubation, the CD14 expression again rose above basal levels. In contrast, stimulation with PepG induced a decrease (23% [P < 0.05]) in the CD14 expression at 1 h, followed by an increase to suprabasal levels after 3 h of incubation and throughout the experimental period (30% at 6 h [P < 0.05]).
FIG. 3.
Time-dependent expression of CD14 (A) and ICAM-1 (B) on monocytes during stimulation of whole blood with LPS or PepG. Whole blood was stimulated with 10 ng of LPS or 10 μg of PepG per ml of blood and incubated at 37°C for 24 h. After the indicated periods of time, 100 μl of blood was incubated with FITC-conjugated anti-CD14 (clone 18D11) or PE-conjugated anti-CD54 (clone HA58) as well as isotype control anti-IgG1 (clone 1B9) antibodies. Subsequent to lysing of red blood cells, washing, and fixation, the samples were analyzed by flow cytometry on the FACScan using CellQuest software. Data represent means ± SEMs of four donors. Asterisks indicates significant difference from unstimulated blood (P < 0.05).
In comparison, the expression of ICAM-1 increased after 3 h of incubation with both LPS and PepG. The mean FI increased by 78, 104, and 152% when stimulated with PepG for 6, 12, and 24 h, respectively (P < 0.05 at all timepoints) (Fig. 3), whereas the LPS-induced changes at the same time points were 25, 45, and 50%, respectively (P < 0.05 at 12 and 24 h) (Fig. 3). Thus, PepG seems to be more potent in inducing changes in ICAM-1 expression as compared to LPS.
Stimulation with LTA (100 μg/ml) for 6 h induced the same changes as those observed with PepG. The mean FI significantly increased by approximately 70% for CD14 (P < 0.05; Fig. 2 and Table 2) and more than 100% for CD54 and HLA-DR (P < 0.05) (Table 2).
As for LPS, pretreatment with the immunosuppressive drugs did not influence the expression characteristics subsequent to PepG or LTA stimulation (data not shown).
Influence of MDP and nonsonicated aggregated PepG from S. aureus on the expression of surface CD14 and ICAM-1.
We have previously shown that the synthetic PepG subunit MDP was unable to induce cytokine release in whole human blood, and the release of TNF-α was largely abrogated when the PepG was not dispersed (PepGn) (J. E. Wang and P. F. Jørgensen, unpublished data).
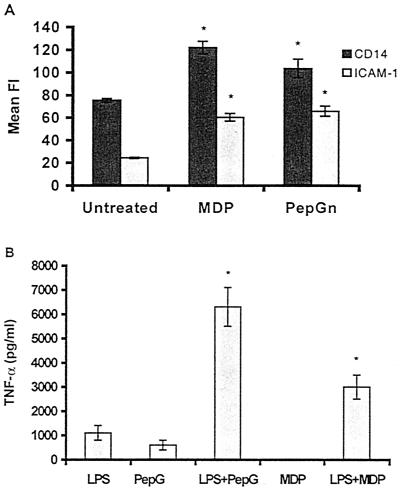
As reported here, we investigated whether MDP and PepGn are able to induce changes in the expression of CD14 and ICAM-1 in whole-blood monocytes. As illustrated in Fig. 4A, stimulation with both MDP (1 μg/ml) and PepGn (10 μg/ml) for 6 h significantly increased the expression of both CD14 (62 and 37%, respectively) and ICAM-1 (146 and 168%, respectively).
FIG. 4.
Effects of MDP and PepGn, without and with LPS, on expression by monocytes. (A) CD14 and ICAM-1 expression on monocytes in whole blood stiulated with MDP or PepGn. Whole blood was stimulated with 1 μg of MDP or 10 μg of PepGn per ml of blood and incubated at 37°C for 6 h. One hundred microliters of blood was subsequently incubated with FITC-conjugated anti-CD14 (clone 18D11) and PE-conjugated anti-CD54 (clone HA58) or isotype-negative control anti-IgG1 (clone 1B9) antibodies. Subsequent to lysing of red blood cells, washing, and fixation, the samples were analyzed by flow cytometry on the FACScan using CellQuest software. Data represent means ± SEMs of four donors. Asterisks indicate significant difference from unstimulated blood (P < 0.05). (B) Ability of PepG and MDP to cooperate with LPS to induce TNF-α. Whole blood from six donors was stimulated with 10 ng of LPS, 10 μg of PepG, or 1 μg of MDP per ml of blood or with a combination of LPS and PepG or LPS and MDP. Thereafter, the blood was incubated at 37°C for 6 h. Plasma was analyzed for the presence of TNF-α by ELISA. Data are means ± SEMs of six donors. Asterisks indicate significantly different values from those obtained with either stimulant alone (P < 0.05).
Thus, we hypothesized that the rise in the LPS receptor CD14 caused by PepG and MDP would prime monocytes for stimulation with LPS. Indeed, coadministration of LPS with PepG or MDP caused significantly increased values of TNF-α as compared with the sum of the values obtained by each stimulant alone (Fig. 4B). This phenomenon was seen with different doses of each stimulant in combination (data not shown).
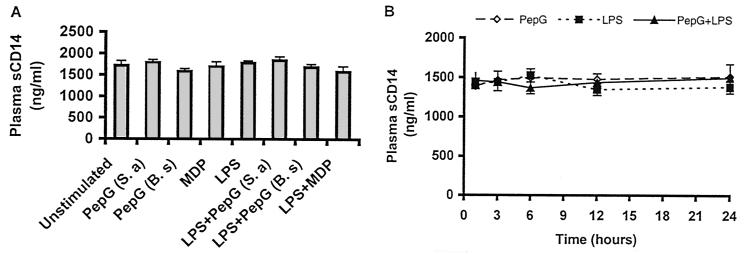
Influence of LPS, PepG, and LTA on sCD14.
sCD14 was found in plasma from unstimulated whole blood in all donors. Stimulation with PepG (10 μg/ml from both S. aureus and B. subtilis), MDP (1 μg/ml), or LPS (10 ng/ml) did not significantly alter the plasma levels of sCD14 (Fig. 5A). The plasma levels of sCD14 were found to be stable through a 24-h stimulation assay (Fig. 5B). Moreover, pretreatment of whole blood with sirolimus (10 ng/ml) for 4 h did not alter the plasma level of sCD14 in unstimulated or stimulated whole blood (data not shown).
FIG. 5.
Influence of PepG, MDP, and LPS on plasma levels of sCD14. (A) Whole blood was stimulated with 10 μg of PepG from S. aureus (S.a) or B. subtilis (B.s), 1 μg of MDP, and 10 ng of LPS per ml of blood, alone or in combination. Thereafter, the blood was incubated at 37°C for 6 h. Plasma was analyzed for sCD14 by ELISA. Data are means ± SEMs of six donors. (B) To examine time-dependent levels of sCD14 in plasma during stimulation of whole blood with PepG and LPS, Whole blood was incubated with 10 μg of PepG from S. aureus or 10 ng of LPS per ml of blood, alone or in combination. Thereafter, the blood was incubated at 37°C for 24 h. After the indicated periods of time, plasma was isolated and analyzed for sCD14 by ELISA. Data are means ± SEM of six donors.
DISCUSSION
In this paper we report that LPS, PepG, and LTA differentially influenced the expression of inflammatory surface receptors on human monocytes in whole blood. We have shown that PepG and LTA enhanced the expression of ICAM-1 and HLA-DR to a degree that was similar to that obtained by LPS. LPS up-regulates the expression of ICAM-1 and HLA-DR on human monocytes (29, 30, 33, 37). Our results confirm the findings of Heinzelmann et al. (23) that LPS and MDP increase the monocyte surface expression of HLA-DR and ICAM-1 in human whole blood. To the best of our knowledge, this is the first report to demonstrate that PepG and LTA up-regulate these surface molecules on human monocytes. Previous reports have documented up-regulation of ICAM-1 on endothelial cells stimulated with PepG (5, 18, 43). No reports are available demonstrating a stimulatory action of PepG or LTA on the expression of HLA-DR on human monocytes. Our results suggest that the G+ bacterial cell wall products induce important phenotypic changes on whole-blood monocytes. The strong up-regulation of both ICAM-1 and HLA-DR on monocytes may enhance essential interactions with other leukocytes, the effective presentation of antigenic peptides, and the induction of T-cell responses.
The up-regulation of ICAM-1 on macrophages by endotoxin was recently shown to involve activation of the transcription factor NF-κB (32). This ubiquitous transcription factor is also induced in macrophage-like cell lines when stimulated with both PepG and LTA (19, 34). The regulation of major histocompatibility complex class II genes involves several transcription factors (46); however, the relative importance of NF-κB is not clear. The immunosuppressive agents CsA and tacrolimus inhibit the activation of NF-κB (17). Sirolimus, a potent immunosuppressive and antiproliferative agent currently studied in clinical trials, has a primary effect on responses to cytokines rather than on the production of them (1, 36). Pretreatment of whole blood with either of these drugs did not interfere with the expression of surface receptors under normal or stimulated conditions. Thus, the up-regulation of ICAM-1 and HLA-DR in response to bacterial cell wall products possibly involves signaling pathways not influenced by these immunosuppressive drugs. Alternatively, the up-regulation is secondary to release of the molecules from preformed cytoplasmic vesicles. Experiments with the kinetics of ICAM-1 expression demonstrated an up-regulation starting at 3 h after stimulation with both PepG and LPS. The expression increased steadily up to 24 h with PepG but peaked at 12 h with LPS and leveled out thereafter. The time delay of 3 h before increased expression is most consistent with regulation at the RNA level (37).
Contrary to results with LPS, we found that PepG and LTA up-regulated the expression of membrane-bound CD14 (mCD14) on whole-blood monocytes. CD14, a key receptor on the surface of monocytes, is required for the induction of an inflammatory response triggered by low concentrations of endotoxin (7, 11, 26). There is now also clear evidence that mCD14 acts as a receptor for both PepG and LTA (6, 19, 20, 47). Furthermore, the ability to up-regulate mCD14 was demonstrated also for MDP. In our model, MDP was unable to induce the release of TNF-α when added alone; however, MDP significantly amplified the LPS-induced release of this proinflammatory cytokine when added concomitantly with LPS. We, therefore, speculate that the PepG-mediated up-regulation of mCD14 may contribute to the observed synergy with LPS. It is interesting that the time courses of mCD14 expression in response to LPS and PepG were very different. PepG induced an early decrease and later increase in this expression, while the contrary was observed with LPS. The role of CD14 in G+ bacterial sepsis has recently been questioned. Haziot et al. (22) demonstrated that the lethality of S. aureus in CD14-deficient mice was not different from that observed with control mice. Furthermore, their observation that S. aureus induced at least threefold higher serum concentrations of TNF-α in CD14-deficient mice as compared with normal control mice suggests important differences in the role of CD14 in the development of G+ versus gram-negative (G−) bacterial sepsis. The hypothesis that G+ bacteria signal through other receptors than CD14 is in line with our observations. Thus, the PepG-induced increase in CD14 expression may reflect a scavenging function of this receptor in G+ infections. This would explain the reported increased potency of G+ bacteria to induce TNF-α in CD14 knockout mice (22).
The difference in influence by LPS and PepG on mCD14 expression suggests differences in signaling transduction pathways in monocytes. In support of this notion, Dziarski et al. (13) reported that LPS and soluble PepG activate similar but not identical mitogen-activated protein kinases in a mouse macrophage cell line. Furthermore, the expression of CD69, an early activation marker on lymphoid cells, was recently found to be differently regulated by LPS and MDP (23), substantiating the assertion that important differences in cellular signaling exist between LPS and PepG. The recent discovery of human TLRs may provide an explanatory tool for these stimulatory differences between G+ and G− bacterial products. TLR2 was recently shown to play a major role in G+ bacterial recognition, whereas TLR4 plays a critical role in LPS signaling (34, 41, 49); however, whether our findings can be explained by signaling through these receptors remains to be determined.
Alternatively, the decrease in mCD14 expression subsequent to LPS stimulation could be due to increased shedding of this membrane molecule. Release from the cell surface has been demonstrated as a mechanism of down-modulation of CD14 on human monocytes stimulated with LPS (4). We were, however, unable to demonstrate any changes in the plasma concentration of soluble CD14. The plasma concentration of this molecule was, however, high in unstimulated blood, and we cannot rule out minor changes in response to stimulation.
In contrast to other authors, we could not document any regulation of the costimulatory molecules B7.1 and B7.2, the adhesion molecule LFA-3, or the IgG receptor FcγRI. We do not, however, exclude such an effect since we analyzed the expression of these molecules only 6 h after stimulation. In this respect, B7.2 was shown by Heinzelmann et al. (23) to be up-regulated by both LPS and MDP by 1 h after stimulation, but regulation of B7.2 was not different from the unstimulated control after 6 and 18 h. LPS has, however, also been reported to down-regulate the expression of B7.2 on human monocytes (8). Schmittel et al. (33) analyzed freshly separated human monocytes and found that B7.1 was not constitutively expressed but became up-regulated after 48 h of stimulation with LPS.
In conclusion, our results indicate that G+ and G− bacterial cell wall products have different effects on the phenotype of human whole-blood monocytes, which substantiates the hypothesis that CD14 plays different roles in G+ and G− bacterial sepsis.
ACKNOWLEDGMENTS
We are indebted to K. Murato, Fujisawa GmbH, Munich, Germany, and S. Sehgal, Wyeth-Ayerst Research, Princeton, N.J., for kindly providing tacrolimus and sirolimus, respectively. We gratefully appreciate the effort of T. Espevik and his associates at the Institute for Cancer Research and Molecular Biology, Norwegian University of Science and Technology, Trondheim, Norway, in analyzing the plasma samples for sCD14.
This work was supported by UNIFOR and the Harry W. Holm's Foundation.
REFERENCES
- 1.Abraham R T, Wiederrecht G J. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 2.Adams D O, Hamilton T A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 3.Atrih A, Zollner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazil V, Strominger J L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 5.Blease K, Chen Y, Hellewell P G, Burke-Gaffney A. Lipoteichoic acid inhibits lipopolysaccharide-induced adhesion molecule expression and IL-8 release in human lung microvascular endothelial cells. J Immunol. 1999;163:6139–6147. [PubMed] [Google Scholar]
- 6.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couturier C, Haeffner-Cavaillon N, Caroff M, Kazatchkine M D. Binding sites for endotoxins (lipopolysaccharides) on human monocytes. J Immunol. 1991;147:1899–1904. [PubMed] [Google Scholar]
- 8.Creery W D, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7–1 and B7–2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–1277. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 9.Damle N K, Klussman K, Linsley P S, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992;148:1985–1992. [PubMed] [Google Scholar]
- 10.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dentener M A, Bazil V, Von Asmuth E J, Ceska M, Buurman W A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 12.Dustin M L, Rothlein R, Bhan A K, Dinarello C A, Springer T A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 13.Dziarski R, Jin Y P, Gupta D. Differential activation of extracellular signal-regulated kinase (ERK) 1, ERK2, p38, and c-Jun NH2-terminal kinase mitogen-activated protein kinases by bacterial peptidoglycan. J Infect Dis. 1996;174:777–785. doi: 10.1093/infdis/174.4.777. [DOI] [PubMed] [Google Scholar]
- 14.Engel P, Gribben J G, Freeman G J, Zhou L J, Nozawa Y, Abe M, Nadler L M, Wakasa H, Tedder T F. The B7–2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood. 1994;84:1402–1407. [PubMed] [Google Scholar]
- 15.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 16.Foster S J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantz B, Nordby E C, Bren G, Steffan N, Paya C V, Kincaid R L, Tocci M J, O'Keefe S J, O'Neill E A. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994;13:861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giese M J, Shum D C, Rayner S A, Mondino B J, Berliner J A. Adhesion molecule expression in a rat model of Staphylococcus aureus endophthalmitis. Investig Ophthalmol Vis Sci. 2000;41:145–153. [PubMed] [Google Scholar]
- 19.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 20.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–379. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 21.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 22.Haziot A, Hijiya N, Schultz K, Zhang F, Gangloff S C, Goyert S M. CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-alpha production. J Immunol. 1999;162:4801–4805. [PubMed] [Google Scholar]
- 23.Heinzelmann M, Polk H C, Chernobelsky A, Stites T P, Gordon L E. Endotoxin and muramyl dipeptide modulate surface receptor expression on human mononuclear cells. Immunopharmacology. 2000;48:117–128. doi: 10.1016/s0162-3109(00)00195-8. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen P F, Wang J E, Almlöf M, Solberg R, Okkenhaug C, Scholz T, Thiemermann C, Foster S J, Aasen A O. Sirolimus interferes with the innate response to bacterial products in human whole blood by attenuation of IL-10 production. 2001. Scand. J. Immunol., in press. [DOI] [PubMed] [Google Scholar]
- 25.Kopp E B, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee J D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leturcq D J, Moriarty A M, Talbott G, Winn R K, Martin T R, Ulevitch R J. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Investig. 1996;98:1533–1538. doi: 10.1172/JCI118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien E, Aukrust P, Sundan A, Muller F, Froland S S, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 29.Maio M, Tessitori G, Pinto A, Temponi M, Colombatti A, Ferrone S. Differential role of distinct determinants of intercellular adhesion molecule-1 in immunologic phenomena. J Immunol. 1989;143:181–188. [PubMed] [Google Scholar]
- 30.McLeish K R, Wellhausen S R, Dean W L. Biochemical basis of HLA-DR and CR3 modulation on human peripheral blood monocytes by lipopolysaccharide. Cell Immunol. 1987;108:242–248. doi: 10.1016/0008-8749(87)90209-7. [DOI] [PubMed] [Google Scholar]
- 31.Moingeon P, Chang H C, Sayre P H, Clayton L K, Alcover A, Gardner P, Reinherz E L. The structural biology of CD2. Immunol Rev. 1989;111:111–144. doi: 10.1111/j.1600-065x.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 32.Ruetten H, Thiemermann C, Perretti M. Upregulation of ICAM-1 expression on J774.2 macrophages by endotoxin involves activation of NF-kappaB but not protein tyrosine kinase: comparison to induction of iNOS. Mediators Inflamm. 1999;8:77–84. doi: 10.1080/09629359990568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittel A, Scheibenbogen C, Keilholz U. Lipopolysaccharide effectively up-regulates B7–1 (CD80) expression and costimulatory function of human monocytes. Scand J Immunol. 1995;42:701–704. doi: 10.1111/j.1365-3083.1995.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz R H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal S N, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 37.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 38.Springer T A, Dustin M L, Kishimoto T K, Marlin S D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 39.Sriskandan S, Cohen J. Gram-positive sepsis: mechanisms and differences from gram-negative sepsis. Infect Dis Clin N Am. 1999;13:397–412. doi: 10.1016/s0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- 40.Sykes M. Immunobiology of transplantation. FASEB J. 1996;10:721–730. doi: 10.1096/fasebj.10.7.8635689. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 42.Unkeless J C, Scigliano E, Freedman V H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 43.van Langevelde P, Ravensbergen E, Grashoff P, Beekhuizen H, Groeneveld P H, van Dissel J T. Antibiotic-induced cell wall fragments of Staphylococcus aureus increase endothelial chemokine secretion and adhesiveness for granulocytes. Antimicrob Agents Chemother. 1999;43:2984–2989. doi: 10.1128/aac.43.12.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J E, Jorgensen P F, Almlof M, Thiemermann C, Foster S J, Aasen A O, Solberg R. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect Immun. 2000;68:3965–3970. doi: 10.1128/iai.68.7.3965-3970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J E, Solberg R, Okkenhaug C, Jørgensen P F, Krohn C D, Espevik T, Aasen A O. Cytokine modulation in experimental endotoxemia: characterisation of an ex vivo whole blood model. Eur Surg Res. 2000;32:65–73. doi: 10.1159/000008743. [DOI] [PubMed] [Google Scholar]
- 46.Wassmuth R. HLA/MHC class II gene regulation. In: Browning M, McMichael A, editors. HLA and MHC genes, molecules and function. London, United Kingdom: BIOS Scientific Publishers Ltd.; 1996. pp. 159–191. [Google Scholar]
- 47.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Young D W, Gusovsky F, Chow J C. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]