Abstract
Pseudomonas biofilms have been studied intensively for several decades and research outcomes have been successfully implemented in various medical and agricultural applications. Research on biofilm synthesis and composition has also overlapped with the objectives of environmental sciences, since biofilm components show exceptional physicochemical properties applicable to remediation techniques. Especially, exopolysaccharides (ExPs) have been at the center of scientific interest, indicating their potential in solving the environmental issues of heavy metal land and water contamination via sorptive interactions and flocculation. Since exposure to heavy metal via contaminated water or soil poses an imminent risk to the environment and human health, ExPs provide an interesting and viable solution to this issue, alongside other effective and green remedial techniques (e.g., phytostabilization, implementation of biosolids, and biosorption using agricultural wastes) aiming to restore contaminated sites to their natural, pollution-free state, or to ameliorate the negative impact of heavy metals on the environment. Thus, we discuss the plausible role and performance of Pseudomonas ExPs in remediation techniques, aiming to provide the relevant available and comprehensive information on ExPs’ biosynthesis and their usage in heavy metal remediation or other environmental applications, such as wastewater treatment via bioflocculation and soil remediation.
Keywords: exopolysaccharides, Pseudomonas, biosorption, bioremediation, heavy metals
1. Introduction
Bacterial exopolysaccharides (ExPs), a group of extracellular polymeric substances (EPSs), are the structural and functional components of microbial biofilms that display exceptional physicochemical properties. Thus, bacterial ExPs with unique attributes have found their way into biomedical science practice (e.g., tissue engineering) and have been successfully implemented into a myriad of industrial and medical applications [1,2].
Interest in ExP-producing bacteria has also expanded into the research areas of environmental sciences, including studies on eco-friendly municipal and wastewater treatment processes [3]. This is because many standard remediation techniques require the usage of reagents that may be hard to degrade, or various environmentally harmful by-products are produced during their utilization at contaminated sites. This includes chemical treatment, which usually detoxifies metals via redox transformation or neutralization by application of the reagents, such as potassium permanganate, hydrogen peroxide, hypochlorite, synthetic surfactants, or chlorine gas, to precipitate, immobilize, or preconcentrate the hazardous contaminants [4,5,6]. The chemical leaching of soils and sediments by applying strong inorganic and organic acids and persistent synthetic chelating agents (e.g., ethylenediaminetetraacetic acid or its derivatives) to solubilize contaminants has also been successfully tested for heavy metal removal [7]. Reactive solid inorganic and biological substances, as well as materials with active surfaces (e.g., zero-valent iron, ferric oxides and oxohydroxides, nanomaterials, zeolite, biological waste) have been studied as potential components of permeable treatment barriers to restrict the movement of the contaminant in the environment [8,9,10,11]. However, in some cases, unpredictable effects regarding the toxicity and mobility of generated species can be expected since these interactions are usually non-specific. The application of electrochemical and electrokinetic remediation methods, engineered to site-specific requirements, has been performed for heavy metal removal [12], showing promising results in combination with other remediation approaches, including novel biochemical methods [13]. More prominent green approaches include phytoremediation, phytoextraction, and biosorption. They are usually performed in conjunction with other methods, e.g., chemical leaching [14]. Still, the application of microbial ExPs is environmentally advantageous since these biogenic polymers are usually water soluble, susceptible to natural degradation, and less harmful than synthetic polymers [15].
Bacterial ExPs find their successful applications in heavy metal removal, oil recovery, and various in situ remediation techniques such as emulsifiers, sorbents, biofilters, surfactants, and bioflocculants [16,17,18]. The interest of environmental researchers in ExP-producing bacteria is also highlighted in several patent deposits focusing on the prosperous application of bacteria in the remediation of contaminated sites. Villela et al. [19] reported that there are 114 patents describing the degradation of oil compounds exclusively by Pseudomonas, thus highlighting the leading role of this bacterial genus in hydrocarbon-contaminated site remediation.
The utilization of Pseudomonas strains in remediation is not limited solely to the biodegradation of hydrocarbons; they have also been successfully applied for the decontamination of heavy metal-polluted waters, soils, and sediments [20]. This is primarily due to their ability to produce metal-chelating siderophores and surface-active extracellular polymeric substances [21]. Regarding the latter, Pseudomonas species are considered high-ExP-producing organisms [22], and since they are ubiquitous, being isolated from various types of environments, including industrial waste and activated sludge [23], they are considered potent in solving the issue of heavy metal contamination [24]. Unfortunately, to our knowledge, no exhaustive review has been published that specifically explores the interaction of heavy metals with ExPs of Pseudomonas. Therefore, this review aims to sum up this topic by providing comprehensive information on ExPs’ biosynthesis and the usage of these Gram-negative, aerobic bacilli in heavy metal remediation as well as some other environmental applications (e.g., soil stabilization and turbidity decrease).
2. Biosynthesis of Extracellular Polysaccharides in Pseudomonas
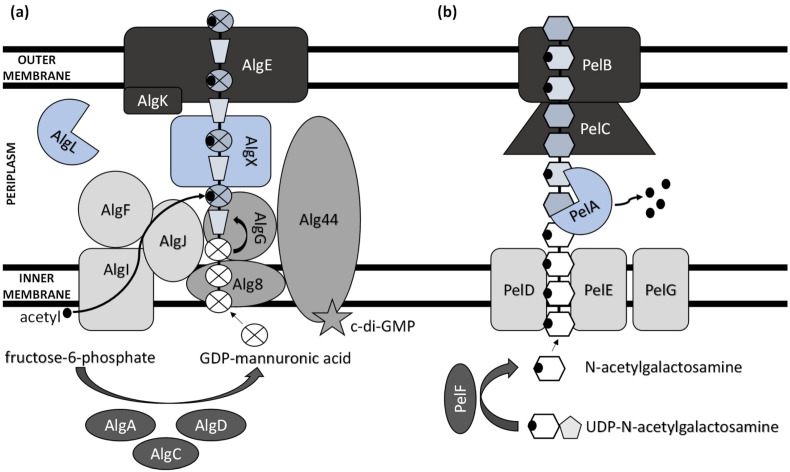
Extensive progress has been made in elucidating the synthesis of bacterial extracellular homopolysaccharides and heteropolysaccharides in recent years (Figure 1). They are synthesized by bacteria either extracellularly (outside the cell membrane and the cell wall), within the cell wall, or intracellularly [25].
Figure 1.
Schematic model of (a) alginate and (b) Pel polysaccharide biosynthetic machinery.
While heteropolysaccharides are mostly synthesized intracellularly and transported outside the cell, homopolysaccharide production generally involves the activity of enzymes secreted by the bacterium to the extracellular environment. ExP production comprises several steps, including the synthesis of ExP precursors, repeat-unit assembly on a lipid carrier located at the cytoplasmic membrane, modification (e.g., acylation, acetylation, sulphation, and methylation), membrane translocation, polymerization, and export [26]. Thus, there are several functionally distinguished enzymes required for ExPs’ synthesis and development [27]. Several other enzymes, which are not unique to ExP production and serve as intermediates in protein regulation and central carbon metabolism, are also involved in ExPs’ biosynthesis process [28].
The biosynthesis of ExPs requires the involvement of activated monosaccharides derived from catabolized sugars. These include sugar nucleosides (nucleoside diphosphate sugars) or their derivatives (e.g., uridine diphosphate (UDP)-N-acetylglucosamine and guanosine diphosphate (GDP)-mannuronic acid) [29].
The main ExP component in the bacteria of Pseudomonas genera is alginate, an anionic linear polymer composed of beta-1,4-linked mannuronic acids (M-blocks) and C5-epimer α-L-guluronic acid (G-blocks) [30]. Alginate’s viscosifying, gelling, and stabilizing properties make this biopolymer an important industrial polysaccharide with great prospects in applications such as drug and protein delivery systems and food encapsulation [31,32]. Thirteen proteins are directly involved in the biosynthesis of alginate, and except for AlgC, they are all encoded by the alg operon [33]. The alginate precursor is synthesized by three enzymes including AlgA (bifunctional enzyme phosphomannose isomerase/guanosine 5′-diphospho-D-mannose pyrophosphorylase), AlgC (phosphomannomutase) and AlgD (GDP-mannose dehydrogenase), which allow the conversion of fructose-6-phosphate to GDP-mannuronic acid via four steps [34,35,36]. Alginate is first synthesized as a linear homopolymer from the GDP-mannuronic acid to polymannuronic acid by catalytic subunit Alg8 (alpha-1,3-glucosyltransferase) which interacts with Alg44 co-polymerase located at the cytoplasmatic membrane [37], with most of the latter being exposed to the periplasm [38]. These enzymes allowed the movement of the alginate precursor across the inner membrane for polymerization [39]. Alg44 also demonstrated the capability of binding the second messenger cyclic dimeric guanosine monophosphate (c-di-GMP) synthesized by MucR, a membrane-anchored protein, which is required for alginate biosynthesis [40].
In the periplasm, the polymannuronate is modified by epimerization or acetylation. AlgI, AlgJ, and AlgF are required for the addition of O-acetyl groups to the alginate polymer at O2 and/or O3 positions, which is an essential process for the stabilization of the intracellular alginate matrix for microcolony formation [41]. Acetylation can also affect epimerization reactions, since the activity of AlgG, a C5-epimerase that directly converts D-mannuronate to L-guluronate, has been detected only when the acetyl groups are removed from the polymannuronate substrate [42]. Newly formed macromolecules are most likely transported within the periplasm by the periplasmic protein AlgX that surrounds and protects the polymers from degradation by AlgL, a periplasmic alginate lyase [43,44]. Alginate is then secreted by the putative export protein AlgE [45]. The proper localization of AlgE for the periplasmic components of the alginate’s biosynthetic machinery is facilitated by AlgK [46].
The acid hydrolysis method is usually applied for the determination of ExPs’ monomeric components (e.g., glucose, fructose, galactose, and arabinose). Myszka and Czaczyk [47] reported that, under starvation conditions (ABPG medium reduced by 90% (w/v) of optimal nutrient availability), the EPS matrix of Pseudomonas aeruginosa ATCC 10145 consisted solely of glucosyl units. Grob, et al. [48] suggested that the survival of P. aeruginosa SG41 under highly chlorinated conditions was enabled by the overproduction of alginate, a major component of the SG41 strain’s ExPs (109.8 µg·g−1 cell dry mass) [49]. Thus, alginate overproduction is advantageous in harsh environments. Still, the nonmucoid P. aeruginosa strains that are the predominant environmental phenotype do not need to express the alginate biosynthetic genes to form the nonmucoid biofilms [50]. These use either Pel or Psl as the primary matrix structural polysaccharide [51].
A previously reported analysis of Pel polymer suggested that it is rich in cationic amino sugars, N-acetylgalactosamine, and N-acetylglucosamine, in a 5:1 ratio [52]. However, just recently, Le Mauff et al. [53] characterized the configuration and structure of Pel and suggested that it is a polymer of partially de-N-acetylated α-1,4-N-acetylgalactosamine, and it does not contain N-acetylglucosamine.
Pel synthesis requires protein products of a seven-gene operon pelABCDEFG [54]. The protein complex of PelD, PelE, PelF, and PelG is very likely a Pel-polysaccharide synthase whose activity is dependent on the localization of cytosolic glycosyltransferase PelF [55] to the inner membrane protein complex PelDEG [56]. PelDEG is also competent for the transport of the Pel polymer across the cytoplasmatic membrane.
PelA is a multi-domain protein that localizes to both the periplasm and membrane and exhibits both hydrolase and de-N-acetylase activity [57,58]. The PelBC complex is responsible for the transport of the matured polymer into the extracellular milieu. PelB is located at the outer membrane and contains a transmembrane β-barrel porin towards which the lipoprotein PelC, which is localized to the inner leaflet of the outer membrane, guides the positively charged Pel and, thus, acts as a charged molecular funnel facilitating Pel export [59].
Psl is a neutral branched pentasaccharide comprising D-mannose, D-glucose, and L-rhamnose. It is synthesized by the polysaccharide synthesis locus (psl). The psl gene cluster consists of 15 genes, of which 11 are necessary for Psl polysaccharide synthesis (pslACDEFGHIJKL) [60]. However, the specific function of each protein is not completely understood.
pslB likely encodes a bifunctional enzyme with GDP-mannose pyrophosphorylase/phosphomannose isomerase dual activities, which is the only enzyme from the psl operon involved in sugar nucleotide precursor production [61]. The inner membrane-associated glycoside hydrolase PslG can be involved in the biosynthesis of Psl polysaccharide [60], although its role in this process is controversial [62]. Since the five PslAEJKL proteins have inner membrane-spanning domains, it was hypothesized that they make up the Psl polymerization complex [33]. Regarding Psl translocation and export, the complex of PslD and PslE helps transport Psl across the outer membrane [63].
3. Environmental Applications of ExPs
3.1. Heavy Metal Removal
The heavy metal removal from various environmental matrices by microbial biomass has been studied extensively in recent years. In the case of Pseudomonas strains, the application of bacterial extracellular polymeric substances, including purified ExPs, is at the center of interest as an alternative approach for the remediation of contaminated waters, since these extracellular biomolecules are capable of adsorbing and flocculating various metal ions effectively and with enormous environmental and economic advantages, as discussed in the following text.
Lau et al. [64] partially purified the capsular ExPs of Pseudomonas sp. CU-1 and monitored the copper(II) binding onto the cells via the dye displacement method. They concluded that the ExPs efficiently prevented the contact of copper with the cell surfaces since the 0.32 mmol·g−1 sorption capacity for copper(II) was only slightly lower than the removal capacity of the cells’ pellets (0.33 mmol·g−1). This is in good agreement with Kazy et al. [65], who noted that the production of ExPs was more pronounced in the copper-resistant Pseudomonas aeruginosa strain (4.78 mg mg−1 cell dry wt.) in comparison with the copper-sensitive strain (2.78 mg mg−1 dry wt.). Furthermore, the ExPs of the resistant strain could accumulate 1.2-fold higher amounts of copper(II) in comparison with its copper-sensitive counterpart.
The sequestration of copper(II) in EPSs efficiently prevents its access to the cytoplasm since the cell fractionation revealed that the cytoplasmic copper(II) was significantly higher in sensitive cells of P. aeruginosa [66]. Therefore, the optimization of ExP production by P. aeruginosa is a reasonable strategy for the sequestration of heavy metals. Chug et al. [67] reported that up to 26 mg (dry wt.) of P. aeruginosa strains’ ExPs can be obtained after 96 h incubation at pH 6 and 32 °C temperature in 50 mL of culture media. It was also tested against nickel(II) and chromium(VI); however, the outcomes were not satisfactory since only 26% and 9% of chromium(VI) and nickel(II) were removed from the aqueous systems at pH 7 when the initial concentration of metals in the solution was 10 mg·L−1.
The ExPs with high flocculating activity produced during submerged fermentation with high metal resistance bacterium P. aeruginosa Al-Dhabi144 [68] have been exploited for the removal of heavy metals from industrial wastewater. They exhibited various levels of metal removal efficiencies since the metal-binding activities varied with the type of metal being used in this study, including 1 mM concentrations of copper(II), cadmium(II), lead(II), cobalt(II), and zinc(II). The sorption capacities of ExPs for lead, copper, cadmium, zinc, and cobalt cations reached 380 mg·g−1, 300 mg·g−1, 250 mg·g−1, 250 mg·g−1, and 225 mg·g−1 of ExPs, respectively. Interestingly, Kumari and Das [69] observed that the densities of biofilm-associated polysaccharide components were reduced considerably in the EPS of P. aeruginosa N6P6 after being treated with lead(II). This ultimately reduced the overall biofilm density. This is especially important for heavy metal removal since high-volumetric-density biofilms generally exhibit higher sorption capacities in comparison with low-density biofilms that are characterized by the low content of ExPs [70]. Similarly, Abinaya Sindu and Gautam [71] noted that the exposure of the P. aeruginosa MTCC 2297 strain to metal fatty acid salts that included cadmium(II) stearate led to poor development of the biofilm with the least amount of ExPs. Therefore, only 1.1% of cadmium(II) was absorbed by the biofilm ExPs, while almost 58%, 52%, and 48.5% of zinc(II), copper(II), and iron(III) were removed by the ExPs, respectively. The complexation of metal ions with the carboxyl and phosphate functional groups of ExPs using FTIR analysis has been revealed.
The critical role of ExPs in metal sequestration was also highlighted by Rizvi and Saghir Khan [72], who suggested that these biomolecules maintain the metabolic activity of Gram-negative P. aeruginosa CPSB1 (and Azotobacter chroococcum CAZ3) even under stressful conditions of heavy metal contamination, and prevent desiccation as well. Thus, ExP production by P. aeruginosa CPSB1 increased by at least 34% when it was exposed to 200 µg·mL−1 of copper(II), cadmium(II), chromium(VI), nickel(II), or lead(II), relative to the untreated control.
Ferreira et al. [73] noted that the soluble ExP fraction of EPSs produced by Pseudomonas veronii 2E was composed of fucose, galactosamine, glucosamine, galactose, glucose, mannose, and glucuronic acid. It was capable of removing 82.8% of 1 mM copper(II) and the registered sorption capacity was 0.066 mmol·g−1 after 96 h in the kinetic studies [74]. It was even successfully applied in an effluent biotreatment method for the removal of copper(II) from copper-loaded effluents [75]. Although it was suggested that the metal–siderophore interaction may have also contributed to the binding of copper(II) or some other heavy metals via the P. veronii 2E strain [76], copper(II) did not promote siderophore genesis. Thus, the interaction of copper(II) in biofilm matrix and complexation by EPSs should be considered the primary detoxification mechanism in P. veronii 2E, since the production of soluble ExPs in the presence of divalent cationic heavy metals was enhanced. However, the produced siderophores, which belong to the family of pyoverdines, can be used as an efficient extracting agent for bacteria since they are effective in solubilizing and mobilizing numerous metals with variable affinities [77].
The ATR-FTIR analysis of the P. veronii 2E strain’s soluble ExPs displayed the presence of N-acetylaminosugars. The multivariate analysis indicated that the amino groups in these aminosugars were primarily involved in the complexing of copper(II) and zinc(II). However, the carboxylates of polysaccharides in the EPS of P. veronii 2E chelated cadmium(II) [78]. The purified ExP fraction of this autochthonous bacterium [73], isolated from sediments associated with the Reconquista River Basin (Buenos Aires Metropolitan Area), was fractionated by anion exchange chromatography resulting in three acidic and one neutral polysaccharide fractions. While these included glucose as the main sugar in all fractions, other components were listed as mannose, glucosamine, fucose, and glucuronic acid [79]. The ATR-FTIR analysis revealed the presence of the amino and carboxylic groups that are most likely responsible for metal complexation in the most abundant ExP fraction (65%), which is α-1-4-glucan, highly substituted with N-acetylglucosamine units.
The composition of the heteropolysaccharide component of P. stutzeri AS22, designated as EPS22, has been studied by Maalej et al. [80], who noted that it was mainly composed of mannose, glucose, and carboxyethyl-substituted rhamnose in an approximate ratio of 1.1:1:0.7. It was also suggested that it has enormous potential for biotechnological application due to its advantageous properties over synthetic polysaccharides. Thus, in his later research, Maalej et al. [81] studied the optimal conditions for ExP production by submerged cultures of P. stutzeri AS22. The optimal culture conditions were determined to be 30 °C, 250 rpm, 10% inoculum size, initial pH of 8.0, and 24 h of incubation time. The extracted crude ExP’s performance in heavy metal removal was exceptionally good, and it has shown high selectivity for lead(II) (460 mg·g−1 ExPs) in a mixed metal solution consisting of 0.1 mmol·L−1 lead(II), cobalt(II), iron(II), copper(II), and cadmium(II). Interestingly, the zinc(II) and cadmium(II) uptakes in one-metal sorption experiments were negligible, reaching up to 8 mg·g−1 ExP sorption capacity.
Meena et al. [82] tested ten bacterial isolates collected at the rhizosphere of a leguminous plant. Only the P. stutzeri TN_AlgSyn showed the capability of synthesizing ExPs whose major component was uronic acid (83%). Further analysis using NMR and FTIR spectroscopy revealed guluronic and mannuronic acid residues, which are the two building blocks of alginate. The synthesized bacterial alginate was then successfully tested for the removal of chromium(VI), lead(II), and cobalt(II) from aqueous media with an adsorption rate over 96%.
Thorgersen et al. [83] noted that exopolysaccharide production via P. stutzeri RCH2 is linked to uranium(VI) resistance under anaerobic conditions, since the expression of genes involved in the formation of exopolysaccharides is induced upon strain exposure to U(VI)O22+. Marqués et al. [84] recovered ExPs from the culture of Pseudomonas sp. EPS-5028 by precipitation with ethanol. The following batch sorption experiment showed that the sorption efficiency of 0.1% (w/v) polysaccharide was independent of temperature but highly pH-sensitive, since the uptake declined significantly below pH 5. The maximum uranium(IV) removal was 96 µg·mg−1. More importantly, the desorption with 0.1 M sodium carbonate released up to 98% of metal bound to ExPs, indicating that uranium(IV) is coupled with the ligands that are easily substituted with carbonate. However, when living cells are involved, uranium is accumulated intracellularly as needle-like fibrils [85]. Nevertheless, this strain belongs to procaryotes, which have shown unique efficiency in trapping uranium, alongside the species of Bacillus mucilaginosus ACCC 10012, Pseudomonas sp. MGF-48, Streptomyces sp., and Bacillus licheniformis ATCC 14580 [86].
The FTIR analysis of EPS and the cell wall of P. putida that was exposed to the 10 mg·L−1 of cadmium(II) revealed that it is directly complexed with phosphoryl and polysaccharide groups [87]. Therefore, the presence of ExPs increased the viability of the cell. Similar outcomes have been presented by Wei et al. [88], who concluded that the P. putida strain’s carboxyl and phosphate groups in EPS (comprising 128 mg·g−1 polysaccharides and 290 mg·g−1 proteins) increased the total binding sites concentrations for cadmium(II). Unfortunately, the EPS-free cells’ maximum sorption capacity derived from Langmuir isotherm decreased by only 9.7% in comparison to the untreated cells. Furthermore, Ueshima et al. [87] highlighted that there was no significant difference on a per mass basis in the extent of cadmium(II) binding between the components of the EPS and cell wall.
The extended X-ray absorption fine-structure (EXAFS) spectroscopy and equilibrium titration studies performed by Guiné et al. [89] indicated that zinc(II) is primarily complexed with phosphoester, carboxyl, and sulfhydryl ligands in P. putida ATCC 12633 biomass and, more importantly, EPSs play the dominant role in zinc retention compared with other cell components (e.g., periplasmic space, outer membrane) with reactive site densities of 16 zinc·nm−2. Additionally, Lin et al. [90] noted that the K-edge X-ray absorption near-edge structure (XANES) analysis showed that, besides the cell walls’ and cell membranes’ phospholipids and intracellular thiol-rich proteins, the acidic polysaccharide alginate in EPS played a crucial role in copper(II) binding in P. putida CZ1. The authors suggested that the carboxyl functional groups of alginate played a key role in the adsorption process since they predominantly complexed with copper in EPS. Furthermore, reduced copper(I) species were identified in the biofilm microenvironment. Andreazza et al. [91] reported that the Pseudomonas sp. strain NA was capable of reducing 23.4 mg·L−1 of initial 100 mg·L−1 copper(II) after 24 h incubation. This can be linked to the toxicity response of the biofilm to copper(II) exposure [92].
Upadhyay and Srivastava [93] highlighted the role of ExPs in zinc(II) accumulation via the plant-growth-promoting Gram-negative bacterium P. fluorescens Psd [94]. Zinc was capable of promoting the ExP synthesis by the bacterium and the exposure to 5 mM zinc(II) enhanced the ExP levels by six times. The PsD strain could immobilize up to 265 mg·g−1 (approximately 70%) of zinc(II). Such a positive effect on ExP synthesis can be used in the mitigation of drought or salinity stress in plants to increase agricultural productivity [95]. The biotechnological potential of the genus Pseudomonas for heavy metal removal was also highlighted by Vélez et al. [96], who employed P. nitroreducens, P. alcaligenes, and P. aeruginosa for lead(II) sorption.
3.2. Wastewater Treatment via Bioflocculation
One of the most common processes in biological wastewater treatment includes the usage of activated sludge for organic or inorganic contaminant removal and decreasing turbidity of biological or non-biological origin. Therefore, the EPS production of the sludge bacteria plays a crucial role in floc formation. Therefore, ExPs of Pseudomonas bacilli isolated from the activated sludge have been widely studied for their role in flocculating properties.
The strain P. aeruginosa IASST201 isolated from the activated oil sludge could remove up to 68% straight chain hydrocarbon through the entrapment mechanism via bioflocs during the treatment of oil field formation water [97]. Furthermore, the crude bioflocculant, consisting of 62% carbohydrates, showed exceptional 80%, 83%, and 90% removal capacity for nickel, iron, and chromium, respectively. Subramanian et al. [98] noted that the ExP fraction in EPS played the leading role in sludge flocculation via the Pseudomonas strain BS2. Similarly, Pseudomonas sp. strain 38 A, isolated from a wastewater treatment plant, was used to produce bioflocculant and subsequently the bioflocculation of kaolin clay suspension [99]. The bioflocculant consisted of carbohydrates with little presence of protein and, at optimal conditions, it reached the flocculation activity of 99.9%. Liu et al. [100] successfully tested the reduction in artificial turbidity using the EPS of P. veronii L918, whose composition was mainly polysaccharide (77%), and the turbidity reduction reached 93%.
The superiority of the bioflocculant application in contrast to the standard usage of inorganic flocculants (e.g., alum) has been noted by Buthelezi et al. [101], who highlighted the satisfactory performance of various Gram-negative and Gram-positive bacterial bioflocculants, including biopolymers produced by P. pseudoalcaligenes and P. plecoglossicida. On the other hand, the presence of ExPs may enhance the flocculation efficiency of inorganic-based flocculants. Such is the case of a composite based on the EPS of P. aeruginosa ZJU, whose outstanding performance in the flocculation of harmful algal blooms was reported by Sun et al. [102].
The performance of ExPs, however, is highly affected by the synthesis medium composition since it influences the content of biopolymeric substances in bacterial bioflocculant. However, the outcomes can be confusing. Drakou et al. [103] noted that while the ExPs produced by the P. aeruginosa strain LVD-10 cultivated on crude glycerol reached flocculation efficiency higher than 80%, and the performance of ExPs collected from the bilge wastewater was inferior, the emulsification properties of the isolated exopolysaccharide matrix were significantly higher in the strain cultivated on bilge wastewaters.
Another critical issue in treating wastewater and effluents is the removal of reactive (azo)dyes. Decolorization usually utilizes both the sorptive properties of ExPs and the degradative capabilities of bacterial consortia dwelling in the EPS. Mao et al. [104] studied the optimal conditions to produce a biopolymer via P. fluorescens in brewery wastewater and its use for the removal of reactive light-yellow K-4G and reactive turquoise-blue KN-G. The single-strain performance (P. alcaligenes PS-25) of the removal of these dyes has also been studied by Wang et al. [105]. The bioflocculant, comprising primarily of saccharides, reached a satisfactory decolorization of over 90% in both cases.
The usage of bacterial consortia may be less efficient in dye removal, but they can be applied in a wide range of environmental conditions. The consortia of Klebsiella sp. PCH427, Enterobacter sp. PCH428, and Pseudomonas sp. PCH429 degraded only 77% of the synthetic dye mixture [106]. However, the performance was relatively consistent at a wide range of pHs, at high salt concentrations, and low nutrient availability as well.
3.3. Soil Reclamation and Remediation
The contamination of soil and the degradation of its physicochemical properties are issues that are the top priorities of remediation strategies worldwide. Thus, the usage of purified bacterial ExPs or the direct in situ application of ExP-producing strains is extensively studied. This is due to their excellent sorptive properties, ability to enhance phytoremediation performance, and capacity to stabilize the soil environment. In the case of the latter, Yi et al. [107] used the ExPs of Pseudomonas sp. as a biocement to increase the soil stability and decrease the water permeability of sandy soils. The inoculation by bacteria changed the penetration stress characteristics of subjected soil samples since the ExPs attached to the sand soil particles and, thus, filled, patched, and closed the pores between them. However, when the bacilli are introduced directly, it should be taken into consideration that the production and quality of ExPs alter through time in response to environmental stressors such as drought, salinity, and temperature. Sandhya and Ali [108] reported that the production of ExPs by the P. putida GAP-P45 strain was high under drought stress, and increased with increasing stress levels (e.g., up to 1.4 M salinity and temperature of 50 °C). Additionally, the soil aggregation stability increased under stress conditions.
Interesting research about the usage of ExPs in soils for biostimulation was conducted by Fatima and Arora [109]. They studied the plant growth-promoting traits of ExPs isolated from P. entomophila PE3, and their effect on salinity tolerance and growth, oil production, and the biochemical aspects of the common sunflower (Helianthus annuus) under salinity stress. The field experiments with isolated ExPs introduced to the soil showed significant improvement in the salinity tolerance of the plant and promoted the growth attributes of H. annuus in saline fields. Similarly, Tewari and Arora [110] concluded that the ExPs of P. aeruginosa PF23 have an important role in stress amelioration under saline conditions, serve as plant growth promoters, and participate in biological control against pathogens.
4. Conclusions
In microorganisms, extracellular polymeric substances have a strong protective role against various environmental stressors, e.g., they allow the efficient sequestration of contaminants and potentially harmful substances. Since ExPs are a major component of biofilm comprising extracellular polymeric substances, their immobilizing capabilities and potential applications in environmental protection and the remediation of contaminated sites have been studied extensively. ExPs are also a key constituent of extracellular matrix material secreted by Pseudomonas species; thus, we have reviewed the prospects of this ubiquitous genus in the remediation of matrices contaminated with metals and metalloids via sorption and bioflocculation. Here, we have highlighted that ExPs have enormous potential in remediation technologies, including wastewater treatment and soil reclamation. Still, further work will be needed for revealing their full potential in these processes and the successful transfer of experimental outcomes to other industrial branches, such as agriculture and biotechnology.
Author Contributions
Writing—original draft preparation, K.B. and E.D.; writing—review and editing, H.K., H.V., P.M. and M.U. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by The Project for Specific University Research (SGS) No. SP2022/8 from the Faculty of Mining and Geology of VSB—Technical University of Ostrava; and by the Scientific Grant Agency of the Slovak Republic Ministry of Education and the Slovak Academy of Sciences under VEGA contract No. 1/0175/22.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mohd Nadzir M., Nurhayati R.W., Idris F.N. Biomedical applications of bacterial exopolysaccharides: A review. Polymers. 2021;13:530. doi: 10.3390/polym13040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nwodo U.U., Green E., Okoh A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012;13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanmani P., Yuvapriya S. Exopolysaccharide from Bacillus sp. YP03: Its properties and application as a flocculating agent in wastewater treatment. Int. J. Environ. Sci. Technol. 2018;15:2551–2560. doi: 10.1007/s13762-017-1416-x. [DOI] [Google Scholar]
- 4.Mulligan C.N., Yong R.N., Gibbs B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001;60:193–207. doi: 10.1016/S0013-7952(00)00101-0. [DOI] [Google Scholar]
- 5.Hagarová I., Kudrík I. Optimization of extraction procedure with nonionic surfactant for determination of trace lead in waters. Chem. Listy. 2016;110:504–510. [Google Scholar]
- 6.Hagarová I. Utilization of Supramolecular Solvents in the Extraction of Metals. Chem. Listy. 2014;108:949–955. [Google Scholar]
- 7.Zhang T., Liu J.-M., Huang X.-F., Xia B., Su C.-Y., Luo G.-F., Xu Y.-W., Wu Y.-X., Mao Z.-W., Qiu R.-L. Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. J. Hazard. Mater. 2013;262:464–471. doi: 10.1016/j.jhazmat.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 8.Hiller E., Jurkovič Ľ., Faragó T., Vítková M., Tóth R., Komárek M. Contaminated soils of different natural pH and industrial origin: The role of (nano) iron- and manganese-based amendments in As, Sb, Pb, and Zn leachability. Environ. Pollut. 2021;285:117268. doi: 10.1016/j.envpol.2021.117268. [DOI] [PubMed] [Google Scholar]
- 9.Chmielewska E., Tylus W., Bujdoš M. Study of Mono- and Bimetallic Fe and Mn Oxide-Supported Clinoptilolite for Improved Pb(II) Removal. Molecules. 2021;26:4143. doi: 10.3390/molecules26144143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudová J., Bujdoš M. Study of selenium sorption on iron oxide hydroxides. Chem. Listy. 2015;109:770–774. [Google Scholar]
- 11.Hagarová I., Nemček L. Application of Metallic Nanoparticles and Their Hybrids as Innovative Sorbents for Separation and Pre-concentration of Trace Elements by Dispersive Micro-Solid Phase Extraction: A Minireview. Front. Chem. 2021;9:672755. doi: 10.3389/fchem.2021.672755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pamukcu S. Handbook of Environmental Engineering. John Wiley & Sons; Hoboken, NJ, USA: 2018. In Situ Soil and Sediment Remediation; pp. 209–248. [DOI] [Google Scholar]
- 13.Song T.-S., Zhang J., Hou S., Wang H., Zhang D., Li S., Xie J. In situ electrokinetic remediation of toxic metal-contaminated soil driven by solid phase microbial fuel cells with a wheat straw addition. J. Appl. Chem. Biotechnol. 2018;93:2860–2867. doi: 10.1002/jctb.5638. [DOI] [Google Scholar]
- 14.You Y., Dou J., Xue Y., Jin N., Yang K. Chelating Agents in Assisting Phytoremediation of Uranium-Contaminated Soils: A Review. Sustainability. 2022;14:6379. doi: 10.3390/su14106379. [DOI] [Google Scholar]
- 15.Zhou W., Shen B., Meng F., Liu S., Zhang Y. Coagulation enhancement of exopolysaccharide secreted by an Antarctic sea-ice bacterium on dye wastewater. Sep. Purif. Technol. 2010;76:215–221. doi: 10.1016/j.seppur.2010.10.011. [DOI] [Google Scholar]
- 16.Kumar A.S., Mody K., Jha B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Yu Y., Fang A., Feng D., Du M., Tang A., Chen S., Li A. Insight into biosorption of heavy metals by extracellular polymer substances and the improvement of the efficacy: A review. Lett. Appl. Microbiol. 2021 doi: 10.1111/lam.13563. [DOI] [PubMed] [Google Scholar]
- 18.Hagarová I. Utilization of biosurfactants in remediation of environmental media contaminated with heavy metals. Chem. Listy. 2015;109:431–436. [Google Scholar]
- 19.Villela H.D.M., Peixoto R.S., Soriano A.U., Carmo F.L. Microbial bioremediation of oil contaminated seawater: A survey of patent deposits and the characterization of the top genera applied. Sci. Total Environ. 2019;666:743–758. doi: 10.1016/j.scitotenv.2019.02.153. [DOI] [PubMed] [Google Scholar]
- 20.Al Disi Z., Al-Ghouti M.A., Zouari N. Investigating the simultaneous removal of hydrocarbons and heavy metals by highly adapted Bacillus and Pseudomonas strains. Environ. Technol. Innov. 2022;27:102513. doi: 10.1016/j.eti.2022.102513. [DOI] [Google Scholar]
- 21.Braud A., Jézéquel K., Bazot S., Lebeau T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere. 2009;74:280–286. doi: 10.1016/j.chemosphere.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Chlebek D., Płociniczak T., Gobetti S., Kumor A., Hupert-Kocurek K., Pacwa-Płociniczak M. Analysis of the genome of the heavy metal resistant and hydrocarbon-degrading rhizospheric Pseudomonas qingdaonensis zcr6 strain and assessment of its plant-growth-promoting traits. Int. J. Mol. Sci. 2022;23:214. doi: 10.3390/ijms23010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore E.R.B., Tindall B.J., Martins Dos Santos V.A.P., Pieper D.H., Ramos J.-L., Palleroni N.J. Nonmedical: Pseudomonas. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes: A Handbook on the Biology of Bacteria Volume 6: Proteobacteria: Gamma Subclass. Springer; New York, NY, USA: 2006. pp. 646–703. [DOI] [Google Scholar]
- 24.Abdelbary S., Elgamal M.S., Farrag A. Trends in Heavy Metals Tolerance and Uptake by Pseudomonas aeruginosa. In: Sriramulu D., editor. Pseudomonas aeruginosa—An Armory Within. IntechOpen; London, UK: 2018. [Google Scholar]
- 25.Nouha K., Kumar R.S., Balasubramanian S., Tyagi R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018;66:225–245. doi: 10.1016/j.jes.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Hay I.D., Rehman Z.U., Moradali M.F., Wang Y., Rehm B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013;6:637–650. doi: 10.1111/1751-7915.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q., Tun H.M., Leung F.C.-C., Shah N.P. Genomic insights into high exopolysaccharide-producing dairy starter bacterium Streptococcus thermophilus ASCC 1275. Sci. Rep. 2014;4:4974. doi: 10.1038/srep04974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osemwegie O.O., Adetunji C.O., Ayeni E.A., Adejobi O.I., Arise R.O., Nwonuma C.O., Oghenekaro A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon. 2020;6:e04205. doi: 10.1016/j.heliyon.2020.e04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehm B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010;8:578–592. doi: 10.1038/nrmicro2354. [DOI] [PubMed] [Google Scholar]
- 30.Ohman D.E. Molecular genetics of exopolysaccharide production by mucoid Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. 1986;5:6–10. doi: 10.1007/BF02013452. [DOI] [PubMed] [Google Scholar]
- 31.Kasak P., Sasová J., Shoheeduzzaman R., Baig M.T., Alyafei A.A.H.A., Tkac J. Influence of direct electric field on PMCG-alginate-based microcapsule. Emergent Mater. 2021;4:769–779. doi: 10.1007/s42247-021-00166-w. [DOI] [Google Scholar]
- 32.Kasak P., Danko M., Zavahir S., Mrlik M., Xiong Y., Yousaf A.B., Lai W.-F., Krupa I., Tkac J., Rogach A.L. Identification of molecular fluorophore as a component of carbon dots able to induce gelation in a fluorescent multivalent-metal-ion-free alginate hydrogel. Sci. Rep. 2019;9:15080. doi: 10.1038/s41598-019-51512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin M., Nivens D., Weadge J., Howell P. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011;2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May T.B., Shinabarger D., Boyd A., Chakrabarty A.M. Identification of amino acid residues involved in the activity of phosphomannose isomerase-guanosine 5′-diphospho-D-mannose pyrophosphorylase. A bifunctional enzyme in the alginate biosynthetic pathway of Pseudomonas aeruginosa. J. Biol. Chem. 1994;269:4872–4877. doi: 10.1016/S0021-9258(17)37625-1. [DOI] [PubMed] [Google Scholar]
- 35.Zielinski N.A., Chakrabarty A.M., Berry A. Characterization and regulation of the Pseudomonas aeruginosa algC gene encoding phosphomannomutase. J. Biol. Chem. 1991;266:9754–9763. doi: 10.1016/S0021-9258(18)92885-1. [DOI] [PubMed] [Google Scholar]
- 36.Tavares I.M., Leitão J.H., Fialho A.M., Sá-Correia I. Pattern of changes in the activity of enzymes of GDP-D-mannuronic acid synthesis and in the level of transcription of algA, algC and algD genes accompanying the loss and emergence of mucoidy in Pseudomonas aeruginosa. Res. Microbiol. 1999;150:105–116. doi: 10.1016/S0923-2508(99)80028-X. [DOI] [PubMed] [Google Scholar]
- 37.Oglesby L.L., Jain S., Ohman D.E. Membrane topology and roles of Pseudomonas aeruginosa Alg8 and Alg44 in alginate polymerization. Microbiology. 2008;154:1605–1615. doi: 10.1099/mic.0.2007/015305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remminghorst U., Rehm B.H.A. Alg44, a unique protein required for alginate biosynthesis in Pseudomonas aeruginosa. FEBS Lett. 2006;580:3883–3888. doi: 10.1016/j.febslet.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 39.Maharaj R., May T.B., Shang-Kwei W., Chakrabarty A.M. Sequence of the alg8 and alg44 genes involved in the synthesis of alginate by Pseudomonas aeruginosa. Gene. 1993;136:267–269. doi: 10.1016/0378-1119(93)90477-K. [DOI] [PubMed] [Google Scholar]
- 40.Hay I.D., Remminghorst U., Rehm B.H.A. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2009;75:1110–1120. doi: 10.1128/AEM.02416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nivens D.E., Ohman D.E., Williams J., Franklin M.J. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 2001;183:1047–1057. doi: 10.1128/JB.183.3.1047-1057.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franklin M.J., Chitnis C.E., Gacesa P., Sonesson A., White D.C., Ohman D.E. Pseudomonas aeruginosa AlgG is a polymer level alginate C5-mannuronan epimerase. J. Bacteriol. 1994;176:1821–1830. doi: 10.1128/jb.176.7.1821-1830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robles-Price A., Wong Thiang Y., Sletta H., Valla S., Schiller Neal L. AlgX is a periplasmic protein required for alginate biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 2004;186:7369–7377. doi: 10.1128/JB.186.21.7369-7377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiller N.L., Monday S.R., Boyd C.M., Keen N.T., Ohman D.E. Characterization of the Pseudomonas aeruginosa alginate lyase gene (algL): Cloning, sequencing, and expression in Escherichia coli. J. Bacteriol. 1993;175:4780–4789. doi: 10.1128/jb.175.15.4780-4789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitney J.C., Hay I.D., Li C., Eckford P.D.W., Robinson H., Amaya M.F., Wood L.F., Ohman D.E., Bear C.E., Rehm B.H., et al. Structural basis for alginate secretion across the bacterial outer membrane. Proc. Natl. Acad. Sci. USA. 2011;108:13083–13088. doi: 10.1073/pnas.1104984108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keiski C.-L., Harwich M., Jain S., Neculai A.M., Yip P., Robinson H., Whitney J.C., Riley L., Burrows L.L., Ohman D.E., et al. AlgK is a TPR-containing protein and the periplasmic component of a novel exopolysaccharide secretin. Structure. 2010;18:265–273. doi: 10.1016/j.str.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myszka K., Czaczyk K. Characterization of adhesive exopolysaccharide (EPS) produced by Pseudomonas aeruginosa under starvation conditions. Curr. Microbiol. 2009;58:541–546. doi: 10.1007/s00284-009-9365-3. [DOI] [PubMed] [Google Scholar]
- 48.Grobe S., Wingender J., Flemming H.-C. Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Int. J. Hyg. Environ. Health. 2001;204:139–142. doi: 10.1078/1438-4639-00085. [DOI] [PubMed] [Google Scholar]
- 49.Grobe S., Wingender J., Trüper H.G. Characterization of mucoid Pseudomonas aeruginosa strains isolated from technical water systems. J. Appl. Bacteriol. 1995;79:94–102. doi: 10.1111/j.1365-2672.1995.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak D.J., Wyckoff T.J.O., Starkey M., Keyser R., Azadi P., O’Toole G.A., Parsek M.R. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA. 2003;100:7907–7912. doi: 10.1073/pnas.1231792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colvin K.M., Irie Y., Tart C.S., Urbano R., Whitney J.C., Ryder C., Howell P.L., Wozniak D.J., Parsek M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jennings L.K., Storek K.M., Ledvina H.E., Coulon C., Marmont L.S., Sadovskaya I., Secor P.R., Tseng B.S., Scian M., Filloux A., et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Mauff F., Razvi E., Reichhardt C., Sivarajah P., Parsek M.R., Howell P.L., Sheppard D.C. The Pel polysaccharide is predominantly composed of a dimeric repeat of α-1,4 linked galactosamine and N-acetylgalactosamine. Commun. Biol. 2022;5:502. doi: 10.1038/s42003-022-03453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman L., Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2004;51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- 55.Ghafoor A., Jordens Z., Rehma H.A.B. Role of pelf in pel polysaccharide biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2013;79:2968–2978. doi: 10.1128/AEM.03666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitfield Gregory B., Marmont Lindsey S., Ostaszewski A., Rich Jacquelyn D., Whitney John C., Parsek Matthew R., Harrison Joe J., Howell P.L. Pel Polysaccharide Biosynthesis Requires an Inner Membrane Complex Comprised of PelD, PelE, PelF, and PelG. J. Bacteriol. 2020;202:e00684-19. doi: 10.1128/JB.00684-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Low K.E., Howell P.L. Gram-negative synthase-dependent exopolysaccharide biosynthetic machines. Curr. Opin. Struct. Biol. 2018;53:32–44. doi: 10.1016/j.sbi.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Colvin Kelly M., Alnabelseya N., Baker P., Whitney John C., Howell P.L., Parsek Matthew R. PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2013;195:2329–2339. doi: 10.1128/JB.02150-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marmont L.S., Rich J.D., Whitney J.C., Whitfield G.B., Almblad H., Robinson H., Parsek M.R., Harrison J.J., Howell P.L. Oligomeric lipoprotein PelC guides Pel polysaccharide export across the outer membrane of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2017;114:2892–2897. doi: 10.1073/pnas.1613606114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Byrd M.S., Sadovskaya I., Vinogradov E., Lu H., Sprinkle A.B., Richardson S.H., Ma L., Ralston B., Parsek M.R., Anderson E.M., et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 2009;73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee H.-J., Chang H.-Y., Venkatesan N., Peng H.-L. Identification of amino acid residues important for the phosphomannose isomerase activity of PslB in Pseudomonas aeruginosa PAO1. FEBS Lett. 2008;582:3479–3483. doi: 10.1016/j.febslet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Baker P., Whitfield G.B., Hill P.J., Little D.J., Pestrak M.J., Robinson H., Wozniak D.J., Howell P.L. Characterization of the Pseudomonas aeruginosa glycoside hydrolase PslG reveals that Its levels are critical for Psl polysaccharide biosynthesis and biofilm formation. J. Biol. Chem. 2015;290:28374–28387. doi: 10.1074/jbc.M115.674929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H., Wang D., Tang M., Ma L.Z. The advance of assembly of exopolysaccharide Psl biosynthesis machinery in Pseudomonas aeruginosa. MicrobiologyOpen. 2019;8:e857. doi: 10.1002/mbo3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau T.C., Wu X.A., Chua H., Qian P.Y., Wong P.K. Effect of exopolysaccharides on the adsorption of metal ions by Pseudomonas sp. CU-1. Water. Sci. Technol. 2005;52:63–68. doi: 10.2166/wst.2005.0182. [DOI] [Google Scholar]
- 65.Kazy S.K., Sar P., Singh S.P., Sen A.K., D’Souza S.F. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 2002;18:583–588. doi: 10.1023/A:1016354713289. [DOI] [Google Scholar]
- 66.Kazy S.K., Sar P., Asthana R.K., Singh S.P. Copper uptake and its compartmentalization in Pseudomonas aeruginosa strains: Chemical nature of cellular metal. World J. Microbiol. Biotechnol. 1999;15:599–605. doi: 10.1023/A:1008997718811. [DOI] [Google Scholar]
- 67.Chug R., Mathur S., Kothari S.L., Harish, Gour V.S. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem. Biophys. Rep. 2021;26:100972. doi: 10.1016/j.bbrep.2021.100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Dhabi N.A., Esmail G.A., Valan Arasu M. Sustainable conversion of palm juice wastewater into extracellular polysaccharides for absorption of heavy metals from Saudi Arabian wastewater. J. Clean. Prod. 2020;277:124252. doi: 10.1016/j.jclepro.2020.124252. [DOI] [Google Scholar]
- 69.Kumari S., Das S. Expression of metallothionein encoding gene bmtA in biofilm-forming marine bacterium Pseudomonas aeruginosa N6P6 and understanding its involvement in Pb(II) resistance and bioremediation. Environ. Sci. Pollut. Res. 2019;26:28763–28774. doi: 10.1007/s11356-019-05916-2. [DOI] [PubMed] [Google Scholar]
- 70.Rezić T., Rezić I., Zeiner M., Šantek B. Application of mixed microbial culture biofilms for manganese (II), cobalt (II), and chromium (VI) biosorption by horizontal rotating tubular bioreactor. In: Farooq R., Ahmad Z., editors. Biological Wastewater Treatment and Resource Recovery. IntechOpen; London, UK: 2017. [DOI] [Google Scholar]
- 71.Abinaya Sindu P., Gautam P. Studies on the biofilm produced by Pseudomonas aeruginosa grown in different metal fatty acid salt media and its application in biodegradation of fatty acids and bioremediation of heavy metal ions. Can. J. Microbiol. 2017;63:61–73. doi: 10.1139/cjm-2015-0384. [DOI] [PubMed] [Google Scholar]
- 72.Rizvi A., Saghir Khan M. Putative role of bacterial biosorbent in metal sequestration revealed by SEM–EDX and FTIR. Indian J. Microbiol. 2019;59:246–249. doi: 10.1007/s12088-019-00780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferreira M.L., Casabuono A.C., Stacchiotti S.T., Couto A.S., Ramirez S.A., Vullo D.L. Chemical characterization of Pseudomonas veronii 2E soluble exopolymer as Cd(II) ligand for the biotreatment of electroplating wastes. Int. Biodeterior. Biodegrad. 2017;119:605–613. doi: 10.1016/j.ibiod.2016.10.013. [DOI] [Google Scholar]
- 74.Busnelli M.P., Lazzarini Behrmann I.C., Ferreira M.L., Candal R.J., Ramirez S.A., Vullo D.L. Metal-Pseudomonas veronii 2E interactions as strategies for innovative process developments in environmental biotechnology. Front. Microbiol. 2021;12:622600. doi: 10.3389/fmicb.2021.622600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Busnelli M.P., Vullo D.L. Copper removal mediated by Pseudomonas veronii 2E in batch and continuous reactors. J. Sustain. Dev. Energy Water Environ. Syst. 2022;10:1080351. doi: 10.13044/j.sdewes.d8.0351. [DOI] [Google Scholar]
- 76.Ferreira M.L., Ramirez S.A., Vullo D.L. Chemical characterization and ligand behaviour of Pseudomonas veronii 2E siderophores. World J. Microbiol. Biotechnol. 2018;34:134. doi: 10.1007/s11274-018-2519-3. [DOI] [PubMed] [Google Scholar]
- 77.Schalk I.J., Hannauer M., Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011;13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira M.L., Gerbino E., Cavallero G.J., Casabuono A.C., Couto A.S., Gomez-Zavaglia A., Ramirez S.A.M., Vullo D.L. Infrared spectroscopy with multivariate analysis to interrogate the interaction of whole cells and secreted soluble exopolimeric substances of Pseudomonas veronii 2E with Cd(II), Cu(II) and Zn(II) Spectrochim. Acta Part A. 2020;228:117820. doi: 10.1016/j.saa.2019.117820. [DOI] [PubMed] [Google Scholar]
- 79.Cavallero G.J., Ferreira M.L., Casabuono A.C., Ramírez S.A., Vullo D.L., Couto A.S. Structural characterization and metal biosorptive activity of the major polysaccharide produced by Pseudomonas veronii 2E. Carbohydr. Polym. 2020;245:116458. doi: 10.1016/j.carbpol.2020.116458. [DOI] [PubMed] [Google Scholar]
- 80.Maalej H., Boisset C., Hmidet N., Buon L., Heyraud A., Nasri M. Purification and structural data of a highly substituted exopolysaccharide from Pseudomonas stutzeri AS22. Carbohydr. Polym. 2014;112:404–411. doi: 10.1016/j.carbpol.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Maalej H., Hmidet N., Boisset C., Buon L., Heyraud A., Nasri M. Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J. Appl. Microbiol. 2015;118:356–367. doi: 10.1111/jam.12688. [DOI] [PubMed] [Google Scholar]
- 82.Meena S., Vidya Kalaivani M., Tripathi A.D., Ramyaa Lakshmi T. Optimization and characterization of alginic acid synthesized from a novel strain of Pseudomonas stutzeri. Biotechnol. Rep. 2020;27:e00517. doi: 10.1016/j.btre.2020.e00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thorgersen M.P., Andrew Lancaster W., Ge X., Zane G.M., Wetmore K.M., Vaccaro B.J., Poole F.L., Younkin A.D., Deutschbauer A.M., Arkin A.P., et al. Mechanisms of chromium and uranium toxicity in Pseudomonas stutzeri RCH2 grown under anaerobic nitrate-reducing conditions. Front. Microbiol. 2017;8:01529. doi: 10.3389/fmicb.2017.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marqués A.M., Bonet R., Simon-Pujol M.D., Fusté M.C., Congregado F. Removal of uranium by an exopolysaccharide from Pseudomonas sp. Appl. Microbiol. Biotechnol. 1990;34:429–431. doi: 10.1007/BF00170074. [DOI] [Google Scholar]
- 85.Marqués A.M., Roca X., Simon-Pujol M.D., Fuste M.C., Congregado F. Uranium accumulation by Pseudomonas sp. EPS-5028. Appl. Microbiol. Biotechnol. 1991;35:406–410. doi: 10.1007/BF00172734. [DOI] [PubMed] [Google Scholar]
- 86.Quintero N.Y., Bruggemann R., Restrepo G. Ranking of 38 prokaryotes according to their uranium uptake capacity in aqueous solutions: An approach from order theory through the Hasse diagram technique. Toxicol. Environ. Chem. 2017;99:1242–1269. doi: 10.1080/02772248.2017.1312401. [DOI] [Google Scholar]
- 87.Ueshima M., Ginn B.R., Haack E.A., Szymanowski J.E.S., Fein J.B. Cd adsorption onto Pseudomonas putida in the presence and absence of extracellular polymeric substances. Geochim. Cosmochim. Acta. 2008;72:5885–5895. doi: 10.1016/j.gca.2008.09.014. [DOI] [Google Scholar]
- 88.Wei X., Fang L., Cai P., Huang Q., Chen H., Liang W., Rong X. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011;159:1369–1374. doi: 10.1016/j.envpol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Guiné V., Spadini L., Sarret G., Muris M., Delolme C., Gaudet J.P., Martins J.M.F. Zinc sorption to three gram-negative bacteria: Combined titration, modeling, and EXAFS study. Environ. Sci. Technol. 2006;40:1806–1813. doi: 10.1021/es050981l. [DOI] [PubMed] [Google Scholar]
- 90.Lin H., Wang C., Zhao H., Chen G., Chen X. A subcellular level study of copper speciation reveals the synergistic mechanism of microbial cells and EPS involved in copper binding in bacterial biofilms. Environ. Pollut. 2020;263:114485. doi: 10.1016/j.envpol.2020.114485. [DOI] [PubMed] [Google Scholar]
- 91.Andreazza R., Pieniz S., Wolf L., Lee M.-K., Camargo F.A.O., Okeke B.C. Characterization of copper bioreduction and biosorption by a highly copper resistant bacterium isolated from copper-contaminated vineyard soil. Sci. Total Environ. 2010;408:1501–1507. doi: 10.1016/j.scitotenv.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 92.Coutaud M., Méheut M., Glatzel P., Pokrovski G.S., Viers J., Rols J.-L., Pokrovsky O.S. Small changes in Cu redox state and speciation generate large isotope fractionation during adsorption and incorporation of Cu by a phototrophic biofilm. Geochim. Cosmochim. Acta. 2018;220:1–18. doi: 10.1016/j.gca.2017.09.018. [DOI] [Google Scholar]
- 93.Upadhyay A., Srivastava S. Mechanism of zinc resistance in a plant growth promoting Pseudomonas fluorescens strain. World J. Microbiol. Biotechnol. 2014;30:2273–2282. doi: 10.1007/s11274-014-1648-6. [DOI] [PubMed] [Google Scholar]
- 94.Upadhyay A., Srivastava S. Evaluation of multiple plant growth promoting traits of an isolate of Pseudomonas fluorescens strain Psd. Indian J. Exp. Biol. 2010;48:601–609. [PubMed] [Google Scholar]
- 95.Bhagat N., Raghav M., Dubey S., Bedi N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021;31:1045–1059. doi: 10.4014/jmb.2105.05009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vélez J.M.B., Martínez J.G., Ospina J.T., Agudelo S.O. Bioremediation potential of Pseudomonas genus isolates from residual water, capable of tolerating lead through mechanisms of exopolysaccharide production and biosorption. Biotechnol. Rep. 2021;32:e00685. doi: 10.1016/j.btre.2021.e00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pathak M., Devi A., Sarma H.K., Lal B. Application of bioflocculating property of Pseudomonas aeruginosa strain IASST201 in treatment of oil-field formation water. J. Basic Microbiol. 2014;54:658–669. doi: 10.1002/jobm.201301011. [DOI] [PubMed] [Google Scholar]
- 98.Subramanian B.S., Yan S., Tyagi R.D., Surampalli R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010;44:2253–2266. doi: 10.1016/j.watres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 99.Farag S., Zaki S., Elkady M., Abd-El-Haleem D. Production and characteristics of a bioflocculant produced by Pseudomonas sp. strain 38A. J. Adv. Microbiol. 2011;4:286–295. [Google Scholar]
- 100.Liu W., Hao Y., Jiang J., Zhu A., Zhu J., Dong Z. Production of a bioflocculant from Pseudomonas veronii L918 using the hydrolyzate of peanut hull and its application in the treatment of ash-flushing wastewater generated from coal fired power plant. Bioresour. Technol. 2016;218:318–325. doi: 10.1016/j.biortech.2016.06.108. [DOI] [PubMed] [Google Scholar]
- 101.Buthelezi S., Olaniran A., Pillay B. Turbidity and microbial load removal from river water using bioflocculants from indigenous bacteria isolated from wastewater in South Africa. Afr. J. Biotechnol. 2009;8:3261–3266. [Google Scholar]
- 102.Sun P.-F., Lin H., Wang G., Lu L.-L., Zhao Y.-H. Preparation of a new-style composite containing a key bioflocculant produced by Pseudomonas aeruginosa ZJU1 and its flocculating effect on harmful algal blooms. J. Hazard. Mater. 2015;284:215–221. doi: 10.1016/j.jhazmat.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 103.Drakou E.-M., Amorim C.L., Castro P.M.L., Panagiotou F., Vyrides I. Wastewater valorization by pure bacterial cultures to extracellular polymeric substances (EPS) with high emulsifying potential and flocculation activities. Waste Biomass Valoriz. 2018;9:2557–2564. doi: 10.1007/s12649-017-0016-9. [DOI] [Google Scholar]
- 104.Mao Y., Xiao X., Liu Y., Zhao E.L., Zhai L.B. Production of a novel biopolymer by culture of Pseudomonas fluorescens using brewery wastewater and its use for dye removal. Adv. Mater. Res. 2010;171–172:45–48. doi: 10.4028/www.scientific.net/AMR.171-172.45. [DOI] [Google Scholar]
- 105.Wang Y.H., Liu R.Q., Liu W.F., Tong L.B., Wang Y.Q., Wang R.N. Production of a novel bioflocculant by culture of Pseudomonas alcaligenes using brewery wastewater and its application in dye removal; Proceedings of the 2009 International Conference on Energy and Environment Technology, ICEET 2009; Guilin, China. 16–18 October 2009; pp. 678–682. [Google Scholar]
- 106.Kumar V., Jamwal A., Kumar V., Singh D. Green bioprocess for degradation of synthetic dyes mixture using consortium of laccase-producing bacteria from Himalayan niches. J. Environ. Manage. 2022;310:114764. doi: 10.1016/j.jenvman.2022.114764. [DOI] [PubMed] [Google Scholar]
- 107.Yi H.W., Yuliani E., Handayani M., Sseng H.C., Ching C.S. Affectivity of biological cement’s application to sandy soil for geotechnical engineering; Proceedings of the MATEC Web of Conferences, The Sixth International Multi-Conference on Engineering and Technology Innovation 2017, IMETI2017; Hualien, Taiwan. 27–31 October 2017. [Google Scholar]
- 108.Sandhya V., Ali S.Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation. Microbiology. 2015;84:512–519. doi: 10.1134/S0026261715040153. [DOI] [Google Scholar]
- 109.Fatima T., Arora N.K. Pseudomonas entomophila PE3 and its exopolysaccharides as biostimulants for enhancing growth, yield and tolerance responses of sunflower under saline conditions. Microbiol. Res. 2021;244:126671. doi: 10.1016/j.micres.2020.126671. [DOI] [PubMed] [Google Scholar]
- 110.Tewari S., Arora N.K. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 2014;69:484–494. doi: 10.1007/s00284-014-0612-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.