Abstract
Study Design:
Uncontrolled retrospective observational study.
Objectives:
Surgery for patients with back pain and degenerative disc disease is controversial, and studies to date have yielded conflicting results. We evaluated the effects of lumbar fusion surgery for patients with this indication in the Canadian Spine Outcomes and Research Network (CSORN).
Methods:
We analyzed data that were prospectively collected from consecutive patients at 11 centers between 2015 and 2019. Our primary outcome was change in patient-reported back pain at 12 months of follow-up, and our secondary outcomes were satisfaction, disability, health-related quality of life, and rates of adverse events.
Results:
Among 84 patients, we observed a statistically significant improvement of back pain at 12 months that exceeded the threshold of Minimum Clinically Important Difference (MCID) (mean change -3.7 points, SD 2.6, p < 0.001, MCID = 1.2; 77% achieved MCID), and 81% reported being “somewhat” or “extremely” satisfied. We also observed improvements of Oswestry Disability Index (-17.3, SD 16.6), Short Form-12 Physical Component Summary (10.3, SD 9.6) and Short Form-12 Mental Component Summary (3.1, SD 8.3); all p < 0.001). The overall rate of adverse events was 19%.
Conclusions:
Among a highly selective group of patients undergoing lumbar fusion surgery for degenerative disc disease, most experienced a clinically significant improvement of back pain as well as significant improvements of disability and health-related quality of life, with high satisfaction at 1 year of follow-up. These findings suggest that surgery for this indication may provide some benefit, and that further research is warranted.
Keywords: back pain, spine surgery, fusion, lumbar, degenerative disc disease, operation
Introduction
Low back pain causes more global disability and socioeconomic impact than any other condition, and the burden of low back pain is increasing as populations age.1,2 Best available evidence estimates a point prevalence of up to 20%, lifetime incidence of up to 70%, and considerable direct and indirect costs due to healthcare utilization and economic losses.3-5 Current thinking characterizes low back pain according to a biopsychosocial framework, and the development of strategies to diagnose and treat specific causes of low back pain is of urgent importance to a broad group of patients, clinicians, researchers, funding agencies, and policy-makers.6,7
Intervertebral discs are complex structures that allow multiplanar spinal motion while transmitting large loads. 8 They are composed of hydrated proteoglycan and collagen-rich tissues, and their osmotic pressure is an important contributor to their structure and function. “Degenerative disc disease” refers to consequences of aging, injury, or genetic predisposition that lead to desiccation, collapse, and ultimately mechanical failure. 9 Associations between this diagnosis and symptoms of back pain remain controversial, but lumbar fusion surgery has been proposed as a treatment option.10-13 Whereas clinical trials to date have yielded conflicting results, further research is warranted to optimally inform shared clinical decision-making. Analyses of observational data from high-quality prospective registries could offer insight about generalizable “real world” outcomes.14,15
In this study, we aimed to determine the effects of lumbar fusion surgery for patients with back pain and degenerative disc disease from the Canadian Spine Outcomes and Research Network (CSORN). Our primary objective was to evaluate changes in patient-reported back pain at 12 months of follow-up, and our secondary objectives were to evaluate post-operative satisfaction, changes in disability and health-related quality of life, and rates of adverse events.
Methods
We performed an uncontrolled retrospective observational study using data that were prospectively collected from consecutive patients enrolled in the CSORN registry. CSORN is a group of over 50 neurosurgical and orthopedic spine surgeons from 18 tertiary care academic and nonacademic hospitals across Canada.16-19 Teams of surgeons and local research coordinators collected patient data at each site, which were then tracked and audited by a national coordinator. We obtained Clinical Research Ethics Board approval at each participating site prior to enrolling patients, collecting data, and performing this study. All patients provided written informed consent to participate.
Patient Sample
We included all patients who presented with primary symptoms of back-dominant pain, were diagnosed with degenerative disc disease (single level or multi-level), and underwent lumbar fusion surgery. We did not standardize any aspects of decision-making with respect to patient selection, surgical indications, or surgical techniques. The presence of back pain as a primary symptom was determined by the treating fellowship-trained spine surgeons based on their initial consultations with each patient and recorded prospectively. Diagnoses of degenerative disc disease were not standardized but were generally made on the basis of magnetic resonance imaging findings that included features such as loss of homogeneous disc structure, loss of distinction between nucleus pulposus and annulus fibrosis, altered disc signal intensity, and/or collapse of disc height. 20
We excluded those with primary symptoms other than back pain such as radiculopathy, neurogenic claudication, or deformity; diagnoses of stenosis, spondylolisthesis, deformity, fracture, disc herniation, tumor, infection, or inflammation; and those who underwent procedures other than fusion, such as lumbar disc arthroplasty or decompression alone. Absence of deformity was defined in the CSORN registry as: no sagittal or coronal imbalance and a lumbar coronal cobb angle of less than 20 degrees.
We also excluded patients who underwent lumbar fusion with concurrent lumbar disc arthroplasty at adjacent levels. We did not record the presence or absence of concurrent facet arthropathy and thus did not exclude patients on this basis.
Data Sources
We used standardized case report forms to collect the following pre-operative baseline characteristics: age, sex, body mass index (BMI), living status (alone versus not alone), education (post-secondary or greater), smoking status, presence of disability or insurance claims, number of comorbidities, daily opioid use, and duration of symptoms. We also collected baseline measures of disability with the Oswestry Disability Index (ODI), general health-related quality of life with the Short Form 12 (SF-12) Physical and Mental Component Summaries (PCS and MCS), and depressive symptoms with the nine-item Patient Health Questionnaire (PHQ-9).21-24 Our primary outcome measure was change in patient-reported back pain from pre-operative to 12-months post-operative according to a 11 item numeric pain rating scale (NPRS), where 0 represents no pain and 10 represents worst imaginable pain. Secondary outcomes included satisfaction, ODI, SF-12 PCS and MCS, and adverse events. Adverse events were collected prospectively using the Spinal Adverse Events Severity (SAVES) protocol.25-27 Imaging studies were not available for review or analysis.
We also used standardized case report forms to collect the following surgical and clinical data: number of lumbar levels (i.e. disc spaces) operated on, concurrent discectomy or direct posterior decompression, anterior versus posterior approaches, interbody cages, fixation, bone grafting, operating room (OR) time, blood loss, and length of stay. Anterior approaches included trans- and retro-peritoneal procedures, including lateral trans- or pre-psoas techniques. Posterior approaches included open midline, open paraspinal, and posterior minimally invasive (MIS).
Statistical Analysis
We report discrete variables as counts or proportions, normally distributed continuous variables as means with standard deviations (SDs), and skewed continuous variables as medians with interquartile ranges (IQRs). We used parametric tests for data with normal distributions and nonparametric tests for data without normal distributions.
We compared mean changes in patient-reported outcome measures (PROMs) from baseline to 12-months with paired samples’ t-tests, and we interpreted changes using Minimal Clinically Important Differences (MCIDs). MCIDs are the smallest treatment effects that informed patients are likely to perceive as beneficial (or harmful) enough to justify changes in their management. 28 We implemented the following MCIDs, which were established elsewhere in the literature: NPRS back pain—1.2 points, 22 ODI—12.8 points, 22 SF12 PCS—3.3 points, 29 SF12 MCS—3.8 points. 29
We tested for associations between patient characteristics or surgical techniques and achievement of the MCID for back pain using conventional univariate and multiple binomial logistic regression. Candidate variables were initially selected based on established clinical relevance, and variables with univariate p-values <0.2 were included in an adjusted model, from which we report Odds Ratios (OR) with 95% Confidence Intervals (CIs). We performed sensitivity analyses in which categorical and continuous versions of variables were exchanged, study site was controlled for, and alternative model building strategies were tested (full model, backward elimination, and forward selection). We removed or combined independent variables that were highly correlated and measured similar constructs. We evaluated model fit using the coefficient of determination (adjusted R 2 ).
All of our analyses were complete case analyses in which patients with missing data were excluded and imputations were not performed. All tests of significance were 2-tailed and p-values <0.05 were considered statistically significant. We used IBM SPSS (version 25.0.0.1, IBM Corp.) and Microsoft Excel 2011 (Microsoft Corp.).
Results
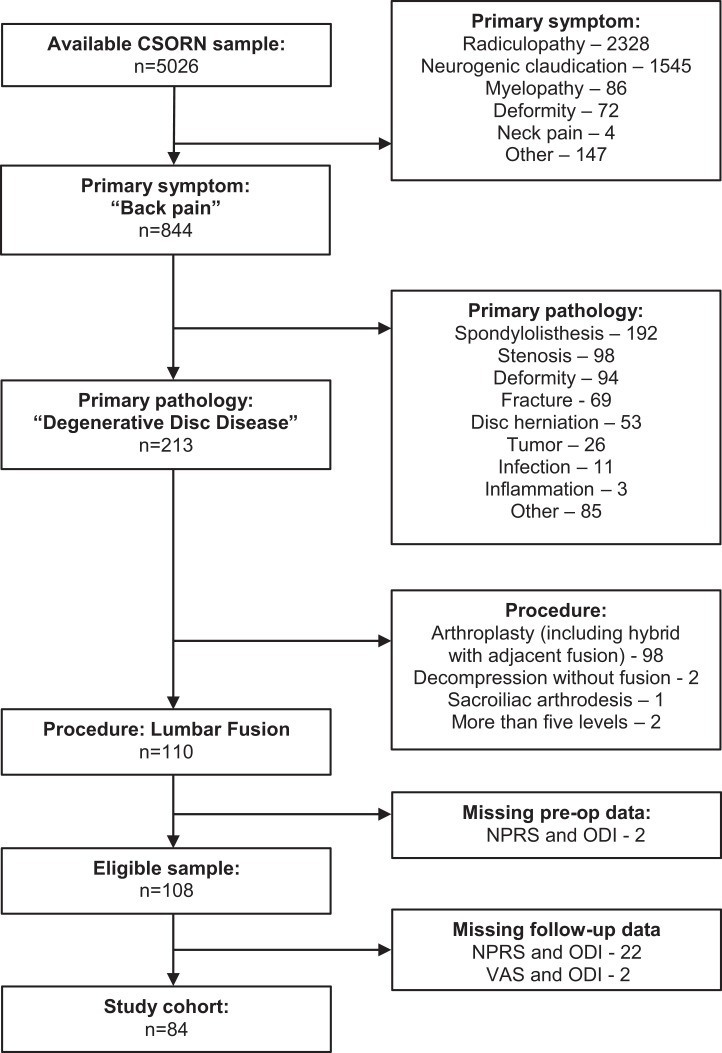
From the available CSORN database of patients with elective thoracolumbar pathology and at least 12 months of follow-up, we identified 108 who presented with primary symptoms of back pain, were diagnosed with degenerative disc disease, completed baseline PROMs, and underwent lumbar fusion surgery (Figures 1 and 2). Of these, we excluded 24 (22%) because of missing 12-month PROMs, which yielded a final study sample of 84 patients that were enrolled from 11 sites between 2015 and 2019.
Figure 1.
Identification of the study cohort: 84 patients who underwent lumbar fusion surgery for back pain and degenerative disc disease.
Figure 2.
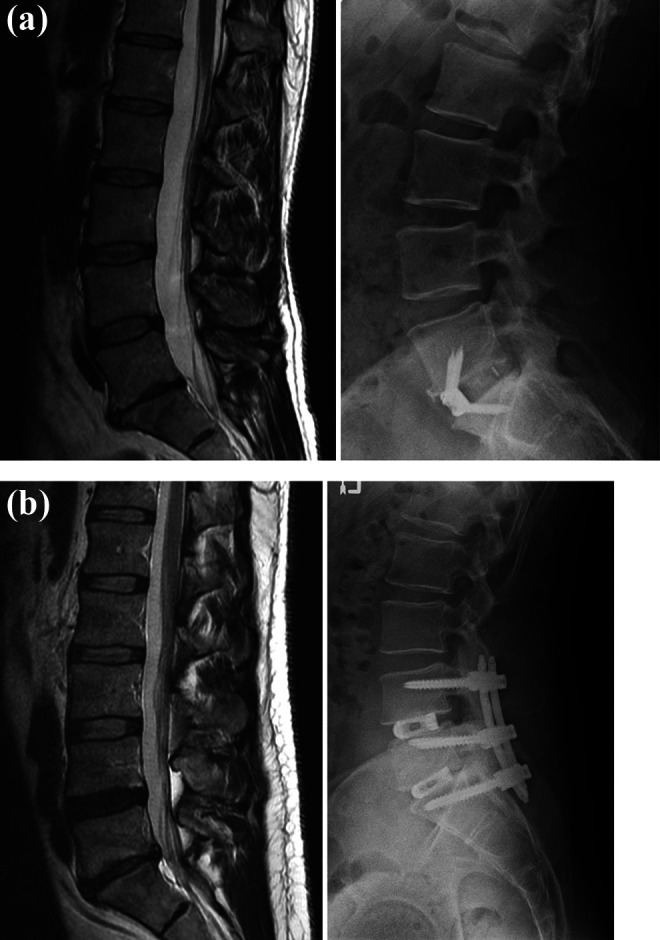
Illustrative cases: pre-operative magnetic resonance imaging (MRI, T2 sagittal) and 12-month post-operative xrays of (a) a 36-year old female who presented with back pain and degenerative disc disease at L5-S1, and (b) a 41-year old male who presented with back pain and degenerative disc disease at L4-5 and L5-S1. Both patients experienced improvement of their back pain that exceeded the Minimum Clinically Important Difference.
Of note, the baseline demographics of those 24 patients excluded for missing 12-month PROMS did not differ substantially from our final study sample (Appendix 1). Likewise, 22 of these excluded patients actually completed 3-month PROMs, which demonstrated improvements in back pain and disability that were statistically and clinically significant (back pain mean change (SD): -3.4 (2.9), p < 0.001, MCID = 1.2 points; ODI mean change (SD): -16.0 (20.2), p < 0.001, MCID 12.8).
We report the baseline characteristics of our final study sample in Table 1, and their surgical data in Table 2. Mean age was 50.8 (SD 12.2), 48% were male, mean BMI was 27.8 (SD 5.3), and 20% were current smokers. Most (79%) reported a pre-operative symptom duration of greater than 2 years, and 41% reported pre-operative daily opioid use. Mean pre-operative back pain was 7.6 (SD 1.8) and mean pre-operative ODI was 48.2 (SD 14.1). Eighty-five percent of patients underwent 1- or 2-level fusions, and 68% underwent fusion alone (ie. without a direct posterior decompression). Interbody cages were placed from anterior approaches in 33 (39%) and posterior approaches in 21 (25%), while 30 (36%) did not receive interbody cages at all. Median operating time was 141 minutes (IQR 107 to 221), blood loss was 200 mL (IQR 100 to 500), and length of stay was 3 days (IQR 1 to 4).
Table 1.
Baseline Characteristics of 84 Patients Who Underwent Lumbar Fusion Surgery for Back Pain and Cegenerative Disc Disease.
| Variable | Sample (n = 84) |
|---|---|
| Age: mean (SD) | 50.8 (12.2) |
| Sex (males) | 40 (48%) |
| Body Mass Index: mean (SD) | 27.8 (5.3) |
| Living status (lives alone) | 10 (12%) |
| Education (post-secondary or greater) | 52 (61%) |
| Smoking status (current smoker) | 17 (20%) |
| Current disability or insurance claims (yes) | 35 (41%) |
| Number of comorbidities: mean (SD) | 2.8 (1.8) |
| Pre-operative daily opiate use (yes) | 35 (41%) |
| Pre-operative use of over-the-counter analgesics (yes) | 38 (45%) |
| Pre-operative use of non-steroidal anti-inflammatories (yes) | 14 (17%) |
| Pre-operative use of muscle relaxants (yes) | 21 (25%) |
| Pre-operative use of anti-depressants (yes) | 17 (20%) |
| Pre-operative use of neuroleptic (yes) | 17 (20%) |
| Duration of symptoms: | |
| Less than 3 months | 0 (0%) |
| 3 to 6 months | 1 (1%) |
| 6 to 12 months | 4 (5%) |
| 1 to 2 years | 12 (14%) |
| Greater than 2 years | 67 (79%) |
| Pre-operative Patient-Reported Outcome Measures | |
| Numerical Pain Rating Scale—back pain: mean (SD) | 7.6 (1.8) |
| Oswestry Disability Index: mean (SD) | 48.2 (14.1) |
| Short Form 12—Physical Component Summary | 31.4 (8.7) |
| Short Form 12—Mental Component Summary | 46.4 (7.9) |
| Patient Health Questionnaire | 11.9 (5.8) |
SD = standard deviation.
Table 2.
Surgical Characteristics of 84 Patients Who Underwent Lumbar Fusion Surgery for Back Pain and Degenerative Disc Disease.
| Variable | Sample (n = 84) |
|---|---|
| Number of levels: median (IQR) | 1 (1 to 2) |
| 1 | 51 (61%) |
| 2 | 20 (24%) |
| 3 | 7 (8%) |
| 4 | 5 (6%) |
| 5 | 1 (1%) |
| Decompression (i.e. direct) | |
| Posterior midline | 25 (30%) |
| Posterior minimally invasive | 2 (2%) |
| None | 57 (68%) |
| Discectomy | |
| Anterior | 33 (39%) |
| Posterior | 27 (32%) |
| None | 24 (29%) |
| Interbody cage | |
| Anterior | 33 (39%) |
| Posterior | 21 (25%) |
| None | 30 (36%) |
| Fixation | |
| Anterior alone | 26 (31%) |
| Posterior alone | 51 (60%) |
| Anterior plus posterior | 7 (8%) |
| Bone Graft | |
| Autograft | 49 (58%) |
| Allograft | 23 (27%) |
| Bone Morphogenic Protein | 32 (38%) |
| Other synthetic | 24 (29%) |
| Operating time (mins): median (IQR) | 141 (107 to 221) |
| Blood loss (mL): median (IQR) | 200 (100 to 500) |
| Length of stay (days): median (IQR) | 3 (1 to 4) |
IQR = interquartile range.
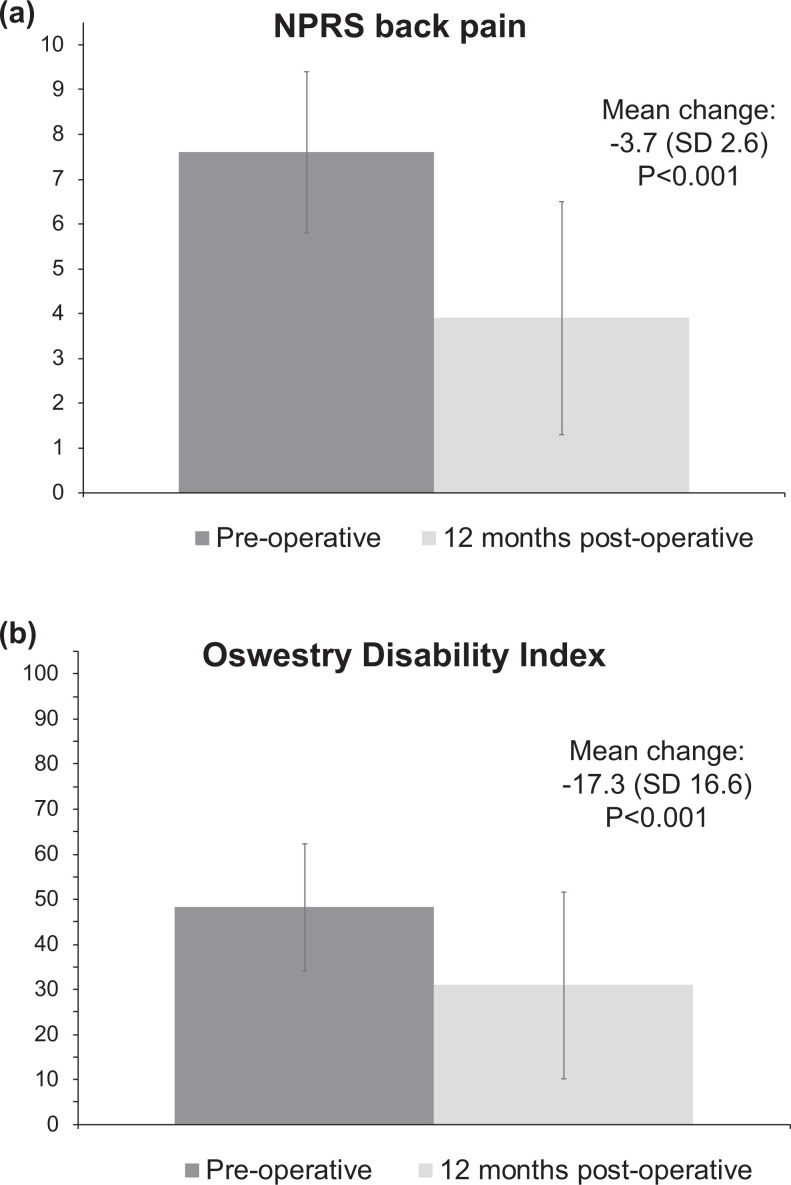
At 12-months of follow-up, we observed a statistically significant improvement in back pain across our sample that exceeded the threshold of MCID (mean change (SD): -3.7 points (2.6), p < 0.001, MCID = 1.2 points, Figure 3). Further, the proportion of individual patients that experienced improvements in back pain equal or greater than the MCID was 77%. We also observed statistically significant improvements in the secondary outcomes ODI and SF12 PCS and MCS, with proportions meeting MCID that varied from 40 to 65% (Table 3). Eighty-one percent of patients reported being either “somewhat” (35%) or “extremely” (46%) satisfied; 77% reported feeling overall “better” (27%) or “much better” (50%); 79% reported that they would “probably” (23%) or “definitely” (56%) choose to have surgery again; and 80% reported that their experienced change in back pain met their expectations “somewhat” (46%) or “completely” (33%).
Figure 3.
Pre-operative and 12-month post-operative Numerical Pain Rating Scale back pain (a) and Oswestry Disability Index (ODI) (b) scores of 84 patients who underwent lumbar fusion surgery for back pain and degenerative disc disease.
Table 3.
Patient-Reported Outcome Measures of 84 Patients Who Underwent Lumbar Fusion Surgery for Back Pain and Degenerative Disc Disease.
| Outcome | Before surgery | After lumbar fusion surgery at 12 months of follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Change (SD) | p-value | MCID | % achieved MCID | |
| NPRS back pain | 84 | 7.6 (1.8) | 84 | 3.9 (2.6) | -3.7 (2.6) | <0.001 | 1.2 22 | 77% |
| ODI | 84 | 48.2 (14.1) | 84 | 30.9 (20.7) | -17.3 (16.6) | <0.001 | 12.8 22 | 61% |
| SF12 PCS | 84 | 31.4 (8.7) | 84 | 41.6 (10.7) | 10.3 (9.6) | <0.001 | 3.3 29 | 65% |
| SF12 MCS | 84 | 46.4 (7.9) | 84 | 49.5 (8.5) | 3.1 (8.3) | <0.001 | 3.8 29 | 40% |
NPRS = Numerical Pain Rating Scale; ODI = Oswestry Disability Index; SF12 = Short Form 12; PCS = Physical Component Summary; MCS = Mental Component Summary; SD = standard deviation; MCID = Minimum Clinically Important Difference.
In unadjusted analyses, we failed to identify statistically significant associations between MCID for back pain and any of age, sex, BMI, education, smoking status, disability or insurance claims, number of comorbidities, daily opioid use, PHQ9 score, anterior versus posterior surgery, number of levels, or use of interbody cages. After adjusting for potential confounding, we also failed to confirm associations with living status or duration of symptoms (Table 4). However, we identified a significant independent association with pre-operative back pain, whereby patients with worse pain were more likely to achieve the MCID (OR 1.72, 95% CI 1.17 to 2.52, p < 0.01; model fit: R2 = 0.24). These findings were consistent across sensitivity analyses in which categorical and continuous versions of variables were exchanged, study site was controlled for, and alternative model building strategies were tested.
Table 4.
Unadjusted and Adjusted Associations of Patient or Surgical Characteristics and Achievement of the Minimal Clinically Important Difference for Back Pain Improvement Among 84 Patients Who Underwent Lumbar Fusion Surgery for Back Pain and Degenerative Disc Disease.
| Variable | Unadjusted univariable associations | Adjusted multiple binomial regression Model fit: R2 = 0.24 |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 0.98 | 0.94-1.02 | 0.39 | - | - | - |
| Sex | 1.33 | 0.48-3.74 | 0.59 | - | - | - |
| Body Mass Index | 1.02 | 0.92-1.31 | 0.72 | - | - | - |
| Living status | 0.38 | 0.10-1.53 | 0.17 | 0.38 | 0.83-1.78 | 0.22 |
| Education | 0.69 | 0.23-2.05 | 0.51 | - | - | - |
| Smoking status | 1.46 | 0.37-5.75 | 0.59 | - | - | - |
| Claims | 0.56 | 0.20 –1.58 | 0.27 | - | - | - |
| Comorbidities | 0.84 | 0.64-1.11 | 0.22 | - | - | - |
| Daily opiate use | 1.30 | 0.45-3.72 | 0.63 | - | - | - |
| Duration of symptoms | 0.90 | 0.79-1.03 | 0.14 | 0.83 | 0.70-0.99 | 0.37 |
| Anterior vs posterior | 0.55 | 0.15-2.05 | 0.37 | - | - | - |
| Direct decompression | 0.76 | 0.26-2.22 | 0.62 | - | - | - |
| Number of levels | 0.76 | 0.46-1.25 | 0.28 | - | - | - |
| Interbody cage | 1.00 | 0.51-1.93 | 0.99 | - | - | - |
| PHQ9 score | 1.00 | 0.91-1.09 | 0.94 | - | - | - |
| NPRS back pain* | 1.33 | 1.07-1.96 | 0.02 | 1.72 | 1.17-2.52 | <0.01 |
| ODI score* | 0.97 | 0.93-1.00 | 0.08 | - | - | - |
| Study site | 0.98 | 0.91-1.06 | 0.66 | - | - | - |
OR = odds ratio; CI = confidence interval; PHQ9 = Patient Health Questionnaire 9; NPRS = Numerical Pain Rating Scale; ODI = Oswestry Disability Index.
*Pre-operative ODI score was omitted from the final multiple binomial regression model because it was highly colinear with pre-operative NPRS back pain score (Pearson Correlation Coefficient 0.41, p < 0.001).
The overall rate of intra- and peri-operative adverse events was 19%. Two patients required reoperations during their index admission: one for evacuation of a symptomatic hematoma after a posterior procedure, and one for excision of a symptomatic new disc herniation after a posterior procedure. Other adverse events included: incidental durotomy in 6 patients; minor wound problems not requiring re-operation in 4 patients (2 excessive drainage, one superficial infection, one minor dehiscence); post-operative ileus in 2 patients; unexpected blood loss greater than 2000 mL in 1 patient during an anterior retroperitoneal procedure; and a retained sponge requiring re-opening of an anterior wound while under the index anesthetic in 1 patient. There were no reoperations after discharge within 12 months of follow-up.
Discussion
We performed an observational study to determine the effects of lumbar fusion surgery for patients with back pain and degenerative disc disease from the Canadian Spine Outcomes and Research Network (CSORN). We found that 77% of these patients experienced an improvement of their back pain after surgery that was statistically significant and clinically important at 12 months of follow-up. After adjusting for potential confounders, we failed to observe any important associations between patient characteristics or surgical techniques and achievement of the MCID for back pain except that patients with worse pre-operative pain were more likely to achieve MCID. We also found high patient-reported satisfaction, statistically significant improvements in disability and health-related quality of life, and low rates of adverse events.
Strengths and Limitations
The major strength of this study is our use of the CSORN platform to evaluate the outcomes of a controversial surgical indication with “real world” data that are high-quality and relatively generalizable. Our PROMs were collected prospectively by teams of research coordinators such that they provide accurate and reliable outcomes, and our strict inclusion and exclusion criteria yielded a selected group of patients without competing pathology. Our study is also strengthened by our use of MCIDs to aid interpretability, and our reporting of potential harms via adverse event data. Although we excluded 24 otherwise eligible patients due to incomplete follow-up, we demonstrated that their baseline demographics and 3-month PROMs did not differ substantially from those included. We required 12-months of follow-up because patient-reported changes in back pain have been shown to plateau by this time-point. 30
It should be clarified that our research question was posed without any prior data exploration. We performed a retrospective observational study using data that had been collected prospectively, and we sought to only study patients with back pain and degenerative disc disease (without disc herniation, other abnormalities, or other symptoms). We defined our study eligibility criteria and then applied our inclusion and exclusion criteria to identify the eligible sample, and then performed the statistical analyses. We did not analyze the entire CSORN registry or seek to identify this group on the basis of having the best outcomes.
The major limitation of this study is that it is an uncontrolled observational study. Invasive surgical interventions are known to have substantial placebo effects, and well-designed trials are essential to evaluate comparative effectiveness.31,32 Our data do not inform about the effects of lumbar fusion on back pain in comparison to non-operative treatment or other surgical interventions, nor do they inform about the natural history of degenerative disc disease without treatment. Likewise, we acknowledge that our results inherently reflect a selection bias among the patients and the participating surgeons because patient selection, decision-making, and surgical techniques were not standardized. It is entirely plausible that patients who were not offered (or declined) surgery for their back pain and degenerative disc disease (and thus not enrolled in CSORN) could have a fundamentally different prognosis for improvement in comparison to our sample.
Our study was also limited by lack of an imaging analysis, because imaging studies are not a part of the current CSORN registry platform. For example, it would have been helpful to investigate whether particular changes in spinal alignment parameters might have been associated with outcomes because many parameters have been correlated with quality of life already. Likewise, it would have also been helpful to evaluate whether specific findings of degenerative disc disease, modic changes, disc heights, disc angles or other features could aid reproducible patient selection. Although we would hypothesize that patients in our sample who were lacking lordosis pre-operatively were most likely corrected, we do not have any data to evaluate this possibility. Detailed imaging analyses are an important future research priority for CSORN, and our group is currently working on multi-center data management strategies to enable this possibility.
Another important limitation of this study is our relatively small sample size. However, it is worth noting that only 2% of enrolled CSORN registry patients with elective thoracolumbar pathology and at least 12 months of follow-up met our eligibility criteria, which suggests that our multicenter registry study design was optimal to maximize the sample size as much as possible. We interpret this finding as evidence to support that surgery for back pain in patients with degenerative disc disease seems to be very uncommon among participating Canadian surgeons. This could relate to national practice patterns and actual differences in per capita utilization of elective spine surgery for this indication compared to other settings, but it is also plausible that underreporting or selective enrolment could play a role. In a population-level study of administrative data, Cram et al reported significantly lower utilization of spine surgery per capita among patients in Ontario, Canada versus New York State, United States, with almost all of the difference being driven by elective fusion procedures. 33 Potential explanations for this finding include differences in the values and preferences of surgeons and patients involved in shared clinical decision-making, or differences in health care funding, insurance coverage, and other economic incentives. 34
Our study has limited ability to make inferences about specific surgical techniques. The standardized case report forms were not developed to clearly identify less invasive “anterior” procedures, such as direct lateral and oblique lumbar interbody fusions, nor did they reliably differentiate between open midline, paramedian, and minimally invasive posterior procedures. 28 Although one might suggest that the 26 patients (31%) who underwent anterior fixation alone most likely had supine retroperitoneal anterior lumbar interbody fusions (ALIF), our dataset did not include detailed operative reports, device records, or complete radiographic series for confirmation. Further studies that include this information along with long-term follow-up are imperative to inform about reoperations for pseudarthrosis, implant failure, and adjacent segment deterioration. Our adjusted analysis failed to suggest an effect attributable to the use of interbody devices in this population.
Although our observed 19% rate of adverse events may seem high in comparison to prior literature, we note that it is actually similar to other studies of patients with lumbar degenerative pathology in which adverse event data have been prospectively collected using the SAVES protocol. For example, in a prospective cohort study of 92 patients with lumbar degenerative spondylolisthesis from an academic quaternary care spine center, Kelly et al found that at least 1 adverse event occurred in 34.8% of patients, with dural tears and post-operative urinary tract infections being most common. 35 More recently, Ayling et al reported a 21.6% rate of major or minor adverse events among 3556 consecutive CSORN patients from 18 tertiary care centers across Canada and found that major adverse events were associated with worse functional outcomes and lower satisfaction. 36
Our study could also be limited by confounding due to variable use of effective co-interventions, either pre-operatively or post-operatively. Although we collected data about the use of opiate and non-opiate analgesic medications pre-operatively, we did not collect data about the use of analgesic medications post-operatively and we did not collect data about the use of non-pharmacological non-surgical interventions such as physical therapy at any time point. If, for example, patients only underwent physical therapy after surgery, one might consider that at least some of their symptomatic improvement could be due to the physical therapy alone rather than surgery. However, 93% of patients had a pre-operative symptom duration of at least 1 year (and 79% greater than 2 years), which suggests that most patients would have likely exhausted a reasonable course of conservative management in the Canadian public health care system prior to undergoing surgery.
Relation to Previous Literature
There exists a large and growing body of literature reporting on the outcomes of lumbar fusion surgery. However, surgery for patients with primary symptoms of back pain and diagnoses of degenerative disc disease without instability, deformity, or stenosis remains controversial.10,37 Population-based studies have consistently shown increasing fusion utilization despite limited evidence of effectiveness, which has appropriately led to criticism.13,38-42 Our study makes an important pragmatic contribution to this field by documenting outcomes from a collaborative group of surgeons in a unique universal healthcare environment. 43
In a recent systematic review and meta-analysis, Yavin et al reported statistically significant improvements of back pain and disability attributed to lumbar fusion among patients with chronic low back pain from 5 randomized controlled trials. 10 None of these trials were blinded, and some were at risk of bias due to suboptimal allocation concealment, crossovers, and losses to follow-up. In one of the largest trials, Fritzell et al randomized 294 patients to either lumbar fusion performed according to the preferences of the treating surgeons or an outpatient program of physiotherapy. MCIDs were not implemented, but patients in the surgical group experienced statistically significantly greater improvements in back pain that were maintained at 24 months of follow-up. 12 In contrast, Fairbank et al observed only a marginal difference in favor of surgery for ODI score in their trial of 349 patients, and concluded that surgery was not more beneficial than intensive rehabilitation. 11 Although often quoted as evidence against surgery, poor availability of intensive cognitive behavioral therapy rehabilitation programs such as was used in that trial limits generalizability.
Notwithstanding differences in reporting, patients in our study appeared to have similar baseline characteristics and disability to those in the above trials, but slightly worse pre-op back pain (mean 7.6, SD 1.8 versus mean 6.4, SD 1.4 in Fritzell et al, adjusted for a 10-point scale; not reported in Fairbank et al). The effect sizes observed among surgical patients were greater in our study for both back pain (-3.7 versus -2.1 in Fritzell et al) and ODI (-17.3 versus -11.6 in Fritzell et al and -12.5 in Fairbank et al).
Other studies from CSORN have demonstrated varying effects of lumbar spine surgery on back pain. In a comparative analysis of decompression alone versus decompression plus fusion among 306 patients with neurogenic claudication secondary to lumbar stenosis without spondylolisthesis or deformity, Thomas et al failed to identify a significant benefit attributable to fusion at 24 months despite overall improvements that met MCID. 19 This finding is not surprising because a related study by Srinivas et al found that decompression alone was associated with achievement of the MCID for back pain in 74% of patients. 18 Similar to our study, adjusted analyses showed that patients with higher pre-operative back pain scores were more likely to experience benefit. It is possible that these results reflect ceiling and floor effects of the NPRS scale, or that they relate to differences in expectations for improvement. Decompression (in addition to fusion) was not associated with a significant independent effect in our regression analysis. Finally, Ailon et al compared CSORN patients treated surgically for degenerative spondylolisthesis to those from the Spine Patient Outcomes Research Trial (SPORT) and found no significant differences between cohorts in pre-operative or 12-month back pain scores, which supports that CSORN patients are likely similar to those elsewhere. 43
Lumbar fusion can be provided via many different surgical techniques, but current literature does not clearly support superiority of any one technique over the others. Phan et al reviewed 12 observational studies that compared ALIF to transforaminal lumbar interbody fusion (TLIF) for various indications, and reported a trade-off of increased vascular injuries in the ALIF group versus increased incidental durotomies in the TLIF group. 44 Pain scores, ODI scores, rates of fusion, OR time, and blood loss were similar between groups, but most studies were small and retrospective, which limits confidence in overall pooled effect estimates. Studies of MIS versus open TLIF have also yielded similar patient-reported outcomes, but with less blood loss and shorter lengths of stay with MIS techniques. 45 Other surgical options for degenerative disc disease in the lumbar spine include Lateral Lumbar Interbody Fusion (LLIF) and Lumbar Disc Arthroplasty.46,47
Implications
Low back pain is known to result from diverse underlying anatomical and biopsychosocial pathology, and the development of strategies to manage patients with specific diagnoses is an important public health priority.1,2,6 Our results suggest that some patients with primary symptoms of back pain and a diagnosis of degenerative disc disease might benefit substantially from lumbar fusion surgery, but caution is warranted. Surgeons, patients, and other evidence users must recognize the uncontrolled nature of our observations, consider our findings in context with the totality of the literature, and integrate values and preferences through a process of shared decision-making.48,49 We suggest that those considering this indication should perform a thorough clinical evaluation, exhaust a reasonable course of non-operative management, and participate in a frank discussion of the various risks, uncertain benefits, and non-operative alternatives.
Further research is warranted to address several important questions. First, the relationships between findings of degenerative disc disease and presentations of pain or disability deserve further investigation. Some studies have shown increased rates of disc degeneration among patients with back pain, but others have documented high proportions in asymptomatic controls.50,51 There is a major knowledge gap in understanding whether differences in severity or patterns of disc degeneration could contribute this problem, and current research exploring novel advanced imaging, molecular biomarkers, and psychological factors may prove helpful in refining patient selection.52-54 Second, surgical techniques and innovative biological therapies are rapidly evolving. Although randomization controlled trials of novel surgical and other non-pharmacological therapies are challenging to design and conduct, rigorous methodology is possible and paramount to avoid spurious or misleading conclusions.31,55 Finally, evidence alone is never enough: decisions about elective spine surgery invariably require attention to individual patients’ values and preferences, and further study of how these factors influence decision-making could optimize outcomes for patients and surgeons alike.48,56-59
Conclusions
Among a highly selective group of patients undergoing lumbar fusion surgery for degenerative disc disease, most experienced a clinically important improvement of back pain as well as significant improvements of disability and health-related quality of life, with high satisfaction and low rates of adverse events. These findings suggest that surgery for this indication may provide some benefit. Caution and further research are warranted when considering application to clinical practice because this was an uncontrolled study and patient selection was not standardized.
Acknowledgement
The authors thank all of the subjects who participated in the study and the support and research coordinator staff and investigators from the Canadian Spine Outcomes and Research Network (CSORN) contributing sites.
Appendix A
Baseline Characteristics of 24 Patients Who Underwent Lumbar Fusion Surgery for Back Pain and Degenerative Disc Disease, but Were Excluded From the Study Cohort due to Incomplete Follow-Up
Twenty-two of these patients (92%) completed 3-month patient-reported outcome measures, which demonstrated improvements in back pain and disability that were statistically and clinically significant (Numerical Pain Rating Scale for back pain mean change [SD]: −3A (2.9), P < .001, MCID = 1.2; ODI score mean change [SD]: −16.0 (20.2), P < .001, MCID = 12.8).
| Variable | Sample (n = 24) |
|---|---|
| Age: mean (SD) | 50.5 (13.2) |
| Sex (males) | 10 (42%) |
| Body Mass Index: mean (SD) | 27.6 (5.6) |
| Living status (lives alone) | 6 (25%) |
| Education (post-secondary or greater) | 18 (75%) |
| Smoking status (current smoker) | 6 (25%) |
| Current disability or insurance claims (yes) | 6 (24%) |
| Number of comorbidities: mean (SD) | 3.0 (2.3) |
| Pre-operative daily opiate use (yes) | 9 (38%) |
| Duration of symptoms: | |
| Less than 3 months | 2 (8%) |
| 3 to 6 months | 0 (0%) |
| 6 to 12 months | 1 (4%) |
| 1 to 2 years | 2 (8%) |
| Greater than 2 years | 19 (79%) |
| Pre-operative Patient-Reported Outcome Measures | |
| Numerical Pain Rating Scale - back pain: mean (SD) | 7.1 (2.0) |
| Oswestry Disability Index: mean (SD) | 43.7 (17.5) |
| Short Form 12 - Physical Component Summary | 32.5 (9.7) |
| Short Form 12 - Mental Component Summary | 47.3 (7.0) |
| Patient Health Questionnaire | 11.3 (6.6) |
SD = Standard Deviation.
Authors’ Note: The Canadian Spine Outcomes and Research Network (CSORN) is funded by the Canadian Spine Research & Education Foundation (CSREF) fund grant. CSREF had no involvement in the development of this study, analysis or interpretation of data, writing of the manuscript, or decision to submit for publication. This study was approved by the Research Ethics Boards at each participating institution (approval numbers not applicable): University of Calgary, Canada East Spine Centre, University of Toronto, Université Laval, Western University, University of Manitoba, and University of British Columbia.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nathan Evaniew, MD, PhD, FRCSC  https://orcid.org/0000-0003-1974-5224
https://orcid.org/0000-0003-1974-5224
Greg McIntosh, MSc  https://orcid.org/0000-0002-0268-6523
https://orcid.org/0000-0002-0268-6523
References
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet Lond Engl. 2018;391(10137):2384–2388. [DOI] [PubMed] [Google Scholar]
- 3.Fatoye F, Gebrye T, Odeyemi I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int. 2019;39(4):619–626. [DOI] [PubMed] [Google Scholar]
- 4.Kim LH, Vail D, Azad TD, et al. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw Open. 2019;2(5): e193676–e193676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rampersaud YR, Power JD, Perruccio AV, et al. Healthcare utilization and costs for spinal conditions in Ontario, Canada—opportunities for funding high-value care: a retrospective cohort study. Spine J. 2020:20(6):878–881. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. Spine J. 2014;14(8):1375–1391. [DOI] [PubMed] [Google Scholar]
- 7.Waddell G.1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine. 1987;12(7):632–644. [DOI] [PubMed] [Google Scholar]
- 8.Alini M, Grad S, Wilke H-J, et al. Biology, mechanics, and genetics of the disk: state of the art. In: Vialle LRG, Wang JC, Lamartin C, eds. AOSpine Masters Series—Volume 8: Back Pain. Thieme; 2017:55–72. [Google Scholar]
- 9.Battié MC, Joshi AB, Gibbons LE; ISSLS Degenerative Spinal Phenotypes Group. Degenerative disc disease: what is in a name? Spine. 2019;44(21):1523–1529. [DOI] [PubMed] [Google Scholar]
- 10.Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80(5):701–715. [DOI] [PubMed] [Google Scholar]
- 11.Fairbank J, Frost H, Wilson-MacDonald J, et al. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: The MRC Spine Stabilisation Trial. BMJ. 2005;330(7502):1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritzell P, Hägg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26(23):2521–2532; discussion 2532-2534. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA. Fusion surgery for lumbar degenerative disc disease: still more questions than answers. Spine J. 2015;15(2):272–274. [DOI] [PubMed] [Google Scholar]
- 14.Castillo RC, Scharfstein DO, MacKenzie EJ. Observational studies in the era of randomized trials: finding the balance. J Bone Joint Surg Am. 2012;94(suppl 1):112–117. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe DJ, Schemitsch EH, Morshed S, et al. Hierarchy of evidence: where observational studies fit in and why we need them. J Bone Jt. Surg Am. 2009;91(suppl 3):2–9. [DOI] [PubMed] [Google Scholar]
- 16.Evaniew N, Cadotte D, Dea N, et al. Clinical predictors of achieving the minimal clinically important difference after surgery for cervical spondylotic myelopathy: an external validation study from the Canadian Spine Outcomes and Research Network. J Neurosurg Spine. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Bond M, McIntosh G, Fisher C, et al. Treatment of mild cervical myelopathy: factors associated with decision for surgical intervention. Spine. 2019;44(22):1606–1612. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas S, Paquet J, Bailey C, et al. Effect of spinal decompression on back pain in lumbar spinal stenosis: a Canadian Spine Outcomes Research Network (CSORN) study. Spine J. 2019;19(6):1001–1008. [DOI] [PubMed] [Google Scholar]
- 19.Thomas K, Faris P, McIntosh G, et al. Decompression alone vs. decompression plus fusion for claudication secondary to lumbar spinal stenosis. Spine J. 2019;19(10):1633–1639. [DOI] [PubMed] [Google Scholar]
- 20.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. [DOI] [PubMed] [Google Scholar]
- 21.Bakhsheshian J, Scheer JK, Gum JL, et al. Impact of poor mental health in adult spinal deformity patients with poor physical function: a retrospective analysis with a 2-year follow-up. J Neurosurg Spine. 2017;26(1):116–124. [DOI] [PubMed] [Google Scholar]
- 22.Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study Questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968–974. [DOI] [PubMed] [Google Scholar]
- 23.Theologis AA, Ailon T, Scheer JK, et al. Impact of preoperative depression on 2-year clinical outcomes following adult spinal deformity surgery: the importance of risk stratification based on type of psychological distress. J Neurosurg Spine. 2016;25(4):477–485. [DOI] [PubMed] [Google Scholar]
- 24.Tuck AN, Scribani MB, Grainger SD, et al. The 9-Item Patient Health Questionnaire (PHQ-9): an aid to assessment of patient-reported functional outcomes after spinal surgery. Spine J. 2018;18(8):1398–1405. [DOI] [PubMed] [Google Scholar]
- 25.Glennie RA, Noonan VK, Fallah N, et al. Reliability of the Spine Adverse Events Severity System (SAVES) for individuals with traumatic spinal cord injury. Spinal Cord. 2014;52(10):758–763. [DOI] [PubMed] [Google Scholar]
- 26.Street JT, Lenehan BJ, DiPaola CP, et al. Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J. 2012;12(1):22–34. [DOI] [PubMed] [Google Scholar]
- 27.Rampersaud YR, Anderson PA, Dimar JR, et al. Spinal adverse events severity system, version 2 (SAVES-V2): inter- and intraobserver reliability assessment. J Neurosurg Spine. 2016;25(2):256–263. [DOI] [PubMed] [Google Scholar]
- 28.Evaniew N, Khan M, Drew B, Kwok D, Bhandari M, Ghert M. Minimally invasive versus open surgery for cervical and lumbar discectomy: a systematic review and meta-analysis. CMAJ Open. 2014;2(4):E295–E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Díaz-Arribas MJ, Fernández-Serrano M, Royuela A, et al. Minimal clinically important difference in quality of life for patients with low back pain. Spine. 2017;42(24):1908–1916. [DOI] [PubMed] [Google Scholar]
- 30.Ayling OGS, Ailon T, McIntosh G, et al. Clinical outcomes research in spine surgery: what are appropriate follow-up times? J Neurosurg Spine. 2018;30(3):1–8. [DOI] [PubMed] [Google Scholar]
- 31.Evaniew N, Carrasco-Labra A, Devereaux PJ, et al. How to use a randomized clinical trial addressing a surgical procedure: users’ guide to the medical literature. JAMA Surg. 2016;151(7):657–662. [DOI] [PubMed] [Google Scholar]
- 32.Wartolowska K, Judge A, Hopewell S, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cram P, Landon BE, Matelski J, et al. Utilization and outcomes for spine surgery in the United States and Canada. Spine. 2019;44(19):1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evaniew N, Fisher CG. Point of view: commentary on “utilization and outcomes for spine surgery in the United States and Canada.” Spine. 2019;44(19):1381. [DOI] [PubMed] [Google Scholar]
- 35.Kelly AM, Batke JNN, Dea N, Hartig DP, Fisher CG, Street JT. Prospective analysis of adverse events in surgical treatment of degenerative spondylolisthesis. Spine J. 2014;14(12):2905–2910. [DOI] [PubMed] [Google Scholar]
- 36.Ayling OGS, Ailon T, Street JT, et al. The Effect of perioperative adverse events on long-term patient-reported outcomes after lumbar spine surgery. Neurosurgery. 2020;nyaa427. [DOI] [PubMed] [Google Scholar]
- 37.Harris IA, Traeger A, Stanford R, Maher CG, Buchbinder R. Lumbar spine fusion: what is the evidence? Intern Med J. 2018;48(12):1430–1434. [DOI] [PubMed] [Google Scholar]
- 38.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369–376. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15(2):265–271. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Yen D, Whitehead M, Xu J, Johnson AP. Use of instrumented lumbar spinal surgery for degenerative conditions: trends and costs over time in Ontario, Canada. Can J Surg. 2019;62(6):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makanji H, Schoenfeld AJ, Bhalla A, Bono CM. Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J. 2018;27(8):1868–1876. [DOI] [PubMed] [Google Scholar]
- 42.Mannion AF, Brox J-I, Fairbank JC. Consensus at last! Long-term results of all randomized controlled trials show that fusion is no better than non-operative care in improving pain and disability in chronic low back pain. Spine J. 2016;16(5):588–590. [DOI] [PubMed] [Google Scholar]
- 43.Ailon T, Tee J, Manson N, et al. Patient-reported outcomes following surgery for degenerative spondylolitshtesis: comparison of a universal and multitier health care system. Spine J. 2019;19(1):24–33. [DOI] [PubMed] [Google Scholar]
- 44.Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29(5):705–711. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein CL, Phillips FM, Rampersaud YR. Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: a systematic review. Spine. 2016;41(suppl 8):S74–S89. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs W, Van der Gaag NA, Tuschel A, et al. Total disc replacement for chronic back pain in the presence of disc degeneration. Cochrane Database Syst Rev. 2012;(9):CD008326. [DOI] [PubMed] [Google Scholar]
- 47.Walker CT, Farber SH, Cole TS, et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine. 2019:1–15. [DOI] [PubMed] [Google Scholar]
- 48.Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet Lond. Engl. 2017;390(10092):415–423. [DOI] [PubMed] [Google Scholar]
- 49.GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinjikji W, Diehn FE, Jarvik JG, et al. MRI Findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J. 2015;15(9):1919–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci. 2017;1410(1):68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strøm J, Bjerrum MB, Nielsen CV, et al. Anxiety and depression in spine surgery-a systematic integrative review. Spine J. 2018;18(7):1272–1285. [DOI] [PubMed] [Google Scholar]
- 55.Slobogean GP, Sprague S, Bhandari M. The tactics of large randomized trials. J Bone Jt Surg Am. 2012;94(suppl 1):19–23. [DOI] [PubMed] [Google Scholar]
- 56.Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neumann I, Santesso N, Akl EA, et al. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016;72:45–55. [DOI] [PubMed] [Google Scholar]
- 58.Bederman SS, Mahomed NN, Kreder HJ, McIsaac WJ, Coyte PC, Wright JG. In the eye of the beholder: preferences of patients, family physicians, and surgeons for lumbar spinal surgery. Spine. 2010;35(1):108–115. [DOI] [PubMed] [Google Scholar]
- 59.Bederman SS, Coyte PC, Kreder HJ, Mahomed NN, McIsaac WJ, Wright JG. Who’s in the driver’s seat? The influence of patient and physician enthusiasm on regional variation in degenerative lumbar spinal surgery: a population-based study. Spine. 2011;36(6):481–489. [DOI] [PubMed] [Google Scholar]