Abstract
Background:
In our center, large vestibular schwannoma (VS) is typically managed by a planned partial resection through the translabyrinthine route. Here, we report on a rare complication of VS surgery and severe neurogenic pulmonary edema.
Case Description:
A 33-year-old male was referred to our skull-base center with a large VS. A planned partial resection was performed. The surgery was without complications and the patient showed good recovery without facial nerve dysfunction. In the evening of the 2nd day after surgery, the patient showed rapid neurological deterioration, accompanied by cardiac arrest. After the patient was resuscitated, a computed tomography (CT) was made, which showed generalized (infra- and supratentorial) brain edema and hematoma in the resection cavity. Despite rapid removal of the hematoma, there was no change in the neurological situation. The next CT scan showed a further increase of brain edema and the patient died eventually. Autopsy revealed generalized lung edema, brain edema, and Hashimoto’s thyroiditis. The pathologist diagnosed neurogenic lung edema.
Conclusion:
Neurogenic lung edema can occur on the 2nd day after surgery and induce rapid deterioration of the patient with massive brain edema.
Keywords: Hashimoto’s thyroiditis, Hematoma, Large vestibular schwannoma, Neurogenic pulmonary edema

CASE REPORT
A 33-year-old patient was referred to our skull-base center because of a large vestibular schwannoma (VS) (diameter of 4.4 cm, Figure 1: magnetic resonance imaging). The patient presented with unilateral hearing loss, mild ataxia, and signs of cerebellar coordination dysfunction after discussing the case in our multidisciplinary team, we advised a planned partial resection via the translabyrinthine approach. The patient gave informed consent and was admitted for surgery, which was performed by an ENT/Neurosurgery team. The facial nerve was EMG-monitored during surgery. The surgery was without complications and a substantial amount of tumor was removed. The patient showed no additional deficits soon after the surgery and displayed good recovery in the next 24 h. However, in the evening of the 2nd day, the patient developed a cardiac arrest which was followed by a Glasgow Coma Score of 1-1-1 in a short period of time. The patient was swiftly resuscitated and a computed tomography (CT) scan was made. CT showed generalized brain edema (supratentorial and infratentorial, Figure 2) and the presence of blood in the resection cavity. Venous sinus thrombosis was ruled out as a cause of delayed cerebellar hemorrhage and swelling. The blood in the resection cavity was decompressed immediately and the patient was transferred to the intensive care unit, after which another CT was made. The second CT showed evacuation of the hematoma but progression of the generalized brain edema. Subsequently, the patient died and an autopsy was performed. The autopsy indicated generalized lung edema, brain edema, and Hashimoto’s thyroiditis (HT), and a diagnosis of neurogenic lung edema was made. Histological examination of the tumor confirmed the diagnosis of schwannoma.
Figure 1:
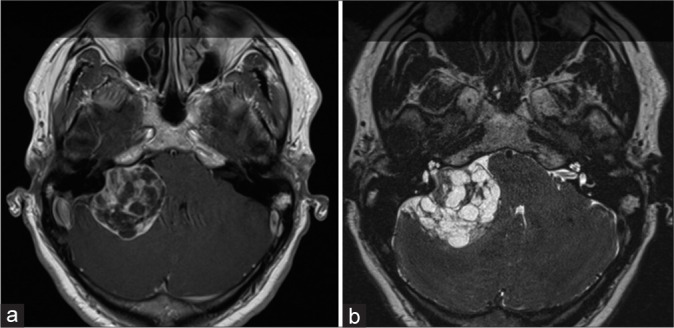
An axial T1-weighed contrast-enhanced (a) and T2-weighed (b) Magnetic resonance image showing the vestibular schwannoma in the right cerebellopontine angle. There is severe brainstem compression.
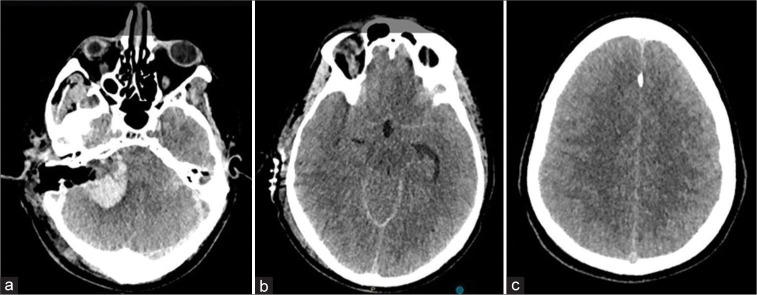
Figure 2:
Computed tomography images showing the hematoma in the resection cavity (a), compression of the basal cistern (b), and supratentorial edema causing sulcal effacement (c).
DISCUSSION
Here, our patient developed an unusually rapid deterioration in the 2nd day after VS surgery with cardiac arrest. CT imaging showed hematoma in the resection cavity and generalized edema. The time course of a typical hemorrhage after a posterior fossa surgery is different. This occurs in the 1st h after surgery and is characterized by neurological deterioration without hemodynamic instability. However, in our case, the neurological and hemodynamic deterioration occurred relatively late, was very fast, and had a catastrophic clinical course. Rapid evacuation of the hematoma in the resection cavity had no effect at all.
The Berlin definition defines neurogenic pulmonary edema (NPE) as a type of acute respiratory distress syndrome, defined by marked, acute-onset, and extravascular accumulation of pulmonary interstitial fluid.[3] NPE is typically triggered by severe stress from acute insults in the central nervous system (CNS), mostly located in the brainstem or the hypothalamus. NPE was first discovered in 1908, since then two forms have been demarcated depending on the onset relative to acute CNS insult. This includes a more common acute onset (<4 h – usually within 30–60 min after CNS event) and a delayed onset (12–72 h after CNS event).[3] On its discovery, several other triggers have been proposed, such as enterovirus-71-induced brainstem encephalitis, subarachnoid bleeding, and cerebral/spinal surgery.[2,3]
What distinguishes NPE from other pulmonary edemas (PE) is that there are two basic mechanisms of PE development at play: (1) increased intravascular and interstitial pressure and (2) an increased permeability of the pulmonary capillaries.[8] It is likely that the increase in hydrostatic pressure and permeability operate through separate mechanisms in NPE.[10] The injury to the CNS elicits a major sympathetic overstimulation (a catecholamine storm), which leads to profound hemodynamic alterations. These changes alter the Starling forces between the pulmonary capillaries and interstitium and/or increase the permeability of these capillaries.[8] The mechanism by which pulmonary permeability is changed include neuropeptide-Y action and inflammation.[8,9]
Certain CNS centers are involved in the regulation of sympathetic innervation and are able to change hemodynamic functions.[8] CNS areas responsible for the sympathetic hyperstimulation can be attributed to NPE trigger zones. Studies indicate that mid-collicular decerebration does not inhibit the development of NPE, indicating that the hypothalamus and higher CNS structures may not be involved in the pathogenesis of NPE.[8] Furthermore, a pivotal role in the pathogenesis of NPE is executed by the rostral ventrolateral medulla, the regulator of sympathetic and baroreflex activity.[8] Further vasomotor centers that are crucial for NPE development are found in the medulla oblongata and include, for instance the dorsal motor vagus nucleus, nucleus tractus solitarii, area A1 and A5, and the area postrema.[2,8]
Local edema is a common pathological consequence following surgery. Edema occurs as a result of endothelial cell damage, abnormal tight junctions, and disrupted transcellular transport. The damaged cells and blood vessels activate a plethora of cellular mechanisms, which worsen the injury.[9]
The present understanding suggests that the elevated intracranial pressure plays a key role in the development of NPE.[2] A catecholamine storm elicits direct myocyte injury resulting in the development of PE.[2] The PE is characterized by acute distention of the capillaries and interstitial edema causing thickened alveolar walls, eventually the alveolar lumen is replaced by transudate.[7] The sympathetic discharge is thought to cause vasoconstriction which causes blood to shift from the high-resistance systemic circulation to the low-resistant pulmonary circulation. This essentially results in a fluid overload in the lungs, resulting in damaged pulmonary capillaries and altered permeability. The presence of red blood cells and high protein edema fluid suggest the presence of a damaged and disrupted capillary endothelium.[4] Most cases of NPE resolve within 48–72 h; however, the development of the edema is associated with a worse prognosis.[5]
The patient’s autopsy report also revealed an underlying HT which, we believe, may have further exacerbated the PE.[1] HTs is the most common cause of hypothyroidism in developed countries, and this deficit in the thyroid hormone can lead to cardiogenic PE.[6] This autoimmune disease equips both cell and antibody-mediated immune processes to destroy thyroid follicular cells. The exact pathophysiological mechanism behind the non-cardiogenic PE formation due to hypothyroidism is not fully elucidated.
Another possible etiology of the PE could have a cardiogenic origin despite the “non-cardiogenic” label given to NPE.[1] In fact, there is evidence that in a group of patients, neurologic insult leads to Takotsubo’s cardiomyopathy, which can depress lusitropy, cause diastolic dysfunction. The hypokinetic state can leave the lungs vulnerable to cardiogenic PE.[2] However, the autopsy study only revealed cardiomegaly (460 g) with a right dominant circulation, normally functioning valves, no arterial narrowing, and a normal lactate dehydrogenase staining pattern indicating normal postmortem and agonal changes.
CONCLUSION
The hematoma in the resection cavity and the HT probably have triggered CNS mechanisms to cause NPE in our case.
Footnotes
How to cite this article: Ravindran P, Kunst D, Waterval J, Hovinga K, Temel Y. A rare complication after vestibular schwannoma surgery: Neurogenic pulmonary edema. Surg Neurol Int 2022;13:441.
Contributor Information
Pawan Kishore Ravindran, Email: pk.ravindran@student.maastrichtuniversity.nl.
Dirk Kunst, Email: dirk.kunst@mumc.nl.
Jerome Waterval, Email: j.waterval@mumc.nl.
Koos Hovinga, Email: koos.hovinga@mumc.nl.
Yasin Temel, Email: y.temel@maastrichtuniversity.nl.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Al-Sofiani M, Nikolla D, Metta VV. Hypothyroidism and noncardiogenic pulmonary edema: Are we missing something here? Endocrinol Diabetes Metab Case Rep. 2015;2015:150014. doi: 10.1530/EDM-15-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16:212. doi: 10.1186/cc11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finsterer J. Neurological perspectives of neurogenic pulmonary edema. Eur Neurol. 2019;81:94–102. doi: 10.1159/000500139. [DOI] [PubMed] [Google Scholar]
- 4.Gundogdu C, Kizilkaya M, Aydin MD, Atalay C, Sevimli ZU. Histopathologic evaluation of neurogenic pulmonary edema after subarachnoid hemorrhage in rabbits. Neurosciences (Riyadh) 2006;11:158–61. [PubMed] [Google Scholar]
- 5.Junttila E, Ala-Kokko T, Ohtonen P, Vaarala A, Karttunen A, Vuolteenaho O, et al. Neurogenic pulmonary edema in patients with nontraumatic intracerebral hemorrhage: Predictors and association with outcome. Anesth Analg. 2013;116:855–61. doi: 10.1213/ANE.0b013e3182811cc7. [DOI] [PubMed] [Google Scholar]
- 6.Mincer DL. Maryland: US National Library of Medicine; 2021. Hashimoto Thyroiditis. [Google Scholar]
- 7.Murray JF. Pulmonary edema: Pathophysiology and diagnosis. Int J Tuberc Lung Dis. 2011;15:155–60. [PubMed] [Google Scholar]
- 8.Šedý J, Kuneš J, Zicha J. Pathogenetic mechanisms of neurogenic pulmonary edema. J Neurotrauma. 2015;32:1135–45. doi: 10.1089/neu.2014.3609. [DOI] [PubMed] [Google Scholar]
- 9.Temple M, Hallman M, Luks AM. Neurogenic Pulmonary Edema. 2021. Available from: https://www.uptodate.com/contents/neurogenic-pulmonary-edema [Last accessed on 2021 Dec 05]
- 10.Travis ZD, Sherchan P, Hayes WK, Zhang JH. Surgically-induced brain injury: Where are we now? Chin Neurosurg J. 2019;5:29. doi: 10.1186/s41016-019-0181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]