Abstract
The genus Alchemilla, belonging to the Rosaceae family, is a rich source of interesting secondary metabolites, including mainly flavonoids, tannins, and phenolic acids, which display a variety of biological activities, such as anti-inflammatory, antimicrobial, and antioxidant. Alchemilla species are used in traditional medicine for treatment of acute diarrhea, wounds, dysmenorrhea, and menorrhagia. In this review, we focus on the phenolic compound composition and antioxidative activity of Alchemilla species. We can assume that phytomedicine and natural products chemistry are of significant importance due to the fact that extract combinations with various bioactive compounds possess the activity to protect the human body rather than disturb damaging factors.
Keywords: Alchemilla, Rosaceae, polyphenols, flavonoids, tannins, antioxidant activity
1. Introduction
Alchemilla is commonly called “Lady’s Mantle” or “lion’s foot” and representatives of this genus occur mostly across Europe and Asia which include northeastern Anatolia (Turkey), north of Iraq, and northwest of Iran. More than 300 species have been described from Europe, where large mountain ranges such as the Caucasus, the Alps, the Carpathians, and others with many endemic species are probably their main distribution centers [1].
The genus Alchemilla L. (F. Rosaceae Juss., subfam. Rosoidae Focke) includes a large number of forms that are not easy to identify. All Alchemilla species were considered for many years as one species—Alchemilla vulgaris L., within which at most a few vaguely defined varieties were distinguished. Alchemilla species are apparently very similar to each other, but apart from morphological diversity, they differ greatly in ecological, phytosociological, and geographic terms.
One of the earliest mentions of the Alchemilla species in scientific literature is dated to 1929 and was published by Harvard University Herbaria [2]. As reported by Ergene and co-authors [3], the Alchemilla genus consists of approximately 1000 species. Furthermore, according to The Plant List [4], 1722 plant name records match the search criteria for Alchemilla.
It has been reported that in Turkish folk medicine, species belonging to the Alchemilla are known locally as fındık out or aslan pençesi [5]. As reported by Afshar et al. [6], 24 species have been discovered within Iran and 14 among them are known to be endemic.
Aerial parts of miscellaneous Alchemilla species (Figure 1) are known to be excellent healing agents towards asthma, bronchitis, cough, and disorders connected with skin and liver inflammation [5].
Figure 1.
Alchemilla peristerica Pawł. aerial parts.
Because of the fact that Alchemilla vulgaris possesses significant anti-inflammatory as well as astringent properties, it is a valued remedy for such ailments as ulcers, eczema, and menstrual disorders [7,8,9].
Moreover, Alchemilla species diminish symptoms of sore throat and alleviate nausea and vomiting [10]. Alchemilla species have been reported to possess a wide variety of biological activities, such as antioxidant, antibacterial, antiviral, anti-inflammatory, and ability to heal wounds in rats [11]. European Pharmacopoeia 6.0 describes Alchemillae herba as a medicinal agent with a variety of pharmacodynamics properties [12].
Among Alchemilla species that are the most widely researched for antimicrobial and antioxidant properties, A. vulgaris, A. xanthochlora, A. diademata, A. rizeensis, and A. mollis can be distinguished [13,14].
Various studies showed that Alchemilla species include miscellaneous compounds such as terpenes, hydrocarbons, fatty acids, and their esters as well as aldehydes, responsible for their pharmacological activities [15].
Other active compounds responsible for antioxidant and antimicrobial activities are tannins (composed of gallic and ellagic acid) and flavonoids (quercetin, luteolin, and proanthocyanidins) [16].
Due to the pharmaceutical and cosmetic importance of some Alchemilla species, in the present review, phenolic compounds occurring in the genus and antioxidant activity is discussed.
2. Methodology of Evidence Acquisition
This review focuses primarily on the content of phenolic compounds as well as antioxidant activity within plants belonging to the Alchemilla genus. All relevant literature databases were searched up to 19 June 2022. The database Google Scholar was used for searching articles with definite search terms, namely: Alchemilla phenolic compounds, Alchemilla polyphenols, Alchemilla antioxidant activity. Moreover, evidence was acquired with the use of the database Pubmed. Publications were identified using the search terms Alchemilla (all fields) and phenolic (all fields) or antioxidant (all fields). Articles that focused on and discussed the phenolic compounds and antioxidant activity of Alchemilla species, dating up to June 2022, were selected.
This review was done to highlight the diversity of phenolic compounds in the Alchemilla genus, and thus to reveal its healing potential. Furthermore, the aim of this paper was to provide up-to-date information on antioxidant activity of Alchemilla species, available in the scientific literature. Finally, we decided to discuss occurring knowledge gaps and propose recommendations concerning future research directions.
To the best of our knowledge, such a review has previously not been undertaken, thus the aim of the present study was to fill this knowledge gap.
3. Phenolic Compounds in the Alchemilla Species
Phenolic compounds are the most widely distributed secondary metabolites, ubiquitously present in the plant kingdom. Higher plants synthesize several thousand different phenolic compounds. The leaves contain, among others, amides and glycosides of hydroxycinnamic acids, esters, glycosylated flavonoids, and proanthocyanins and their relatives. Some soluble phenolics are widely distributed, but the distribution of many others is restricted to specific genera or families, making them easy biomarkers for taxonomic studies [17,18].
According to Choi and co-authors, plants from the genus Alchemilla, like other representatives of the Rosaceae family, are rich in polyphenols which are responsible for various pharmacological activities [7].
Another noteworthy observation is that various studies revealed the presence of phenolic compounds within the aerial parts of Alchemilla species, namely tannins, phenolic acids, flavonoids, and coumarins [6,19].
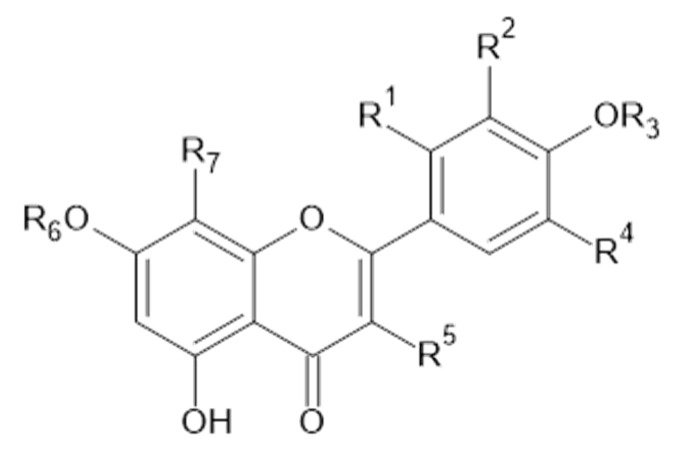
Flavonoids are an exceptionally large group of natural products (over 8000) that are found in many plant tissues, present inside the cells or on the surfaces of different plant organs [17].
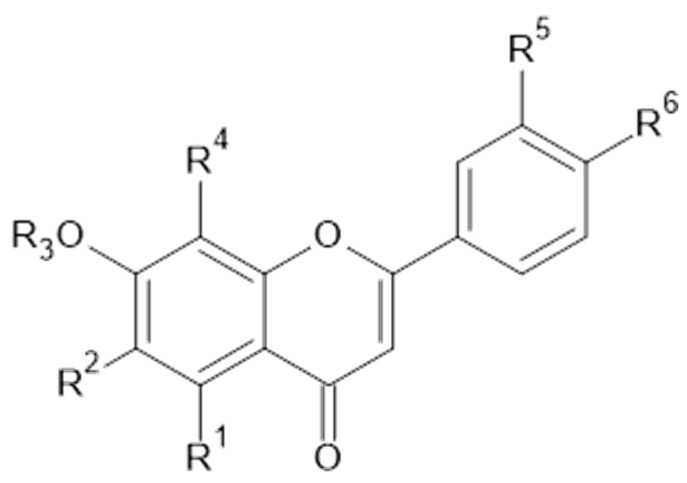
Phytochemical investigations of Alchemilla species have led to the isolation diverse types of flavonoids, represented mostly by flavonols and flavones. Their structures are summarized in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11 and Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6.
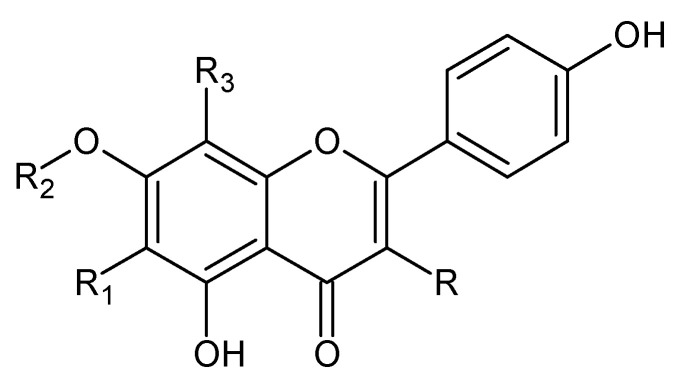
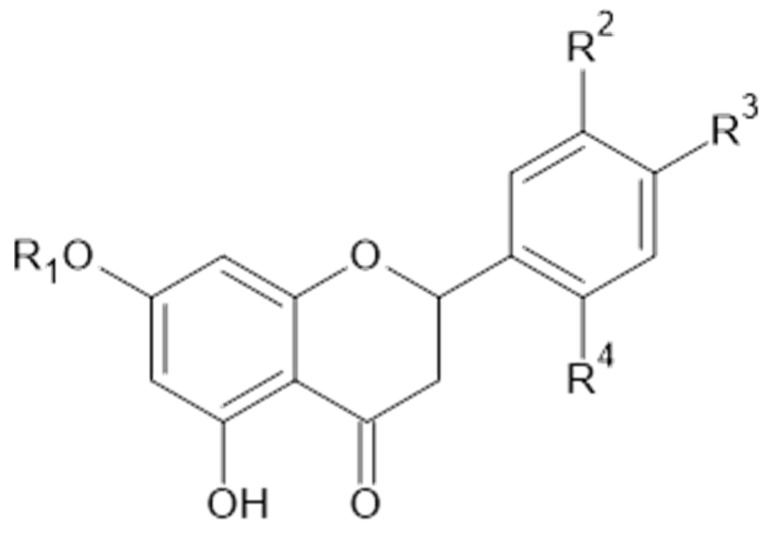
Figure 2.
Kaempferol and kaempferol O-glycosides.
Figure 3.
Quercetin and derivatives.
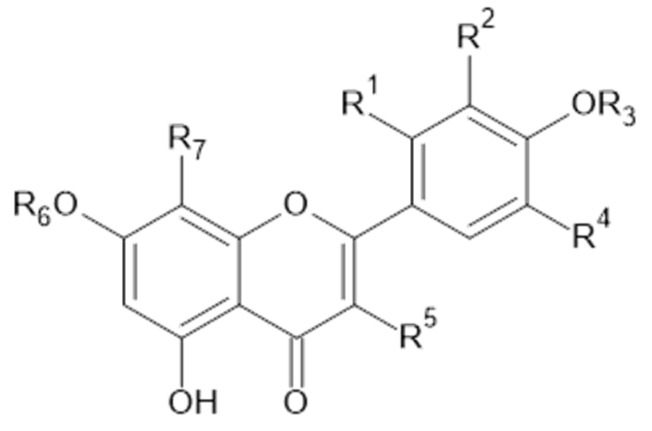
Figure 4.
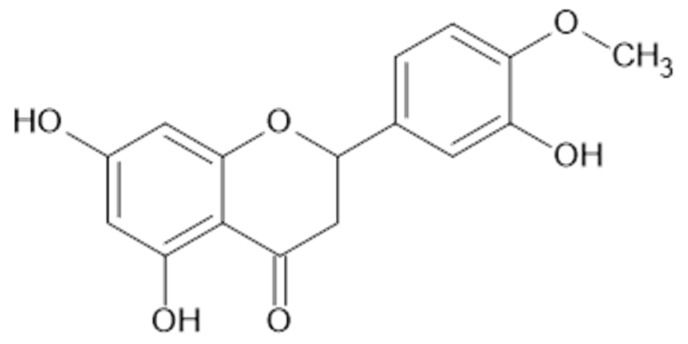
Rhodiolgin (36, MW 464).
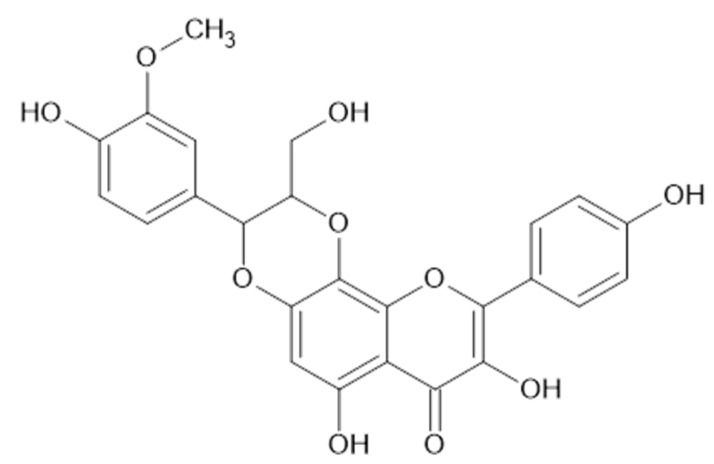
Figure 5.
Chemical structure of compound 38 (R = H; MW = 318).
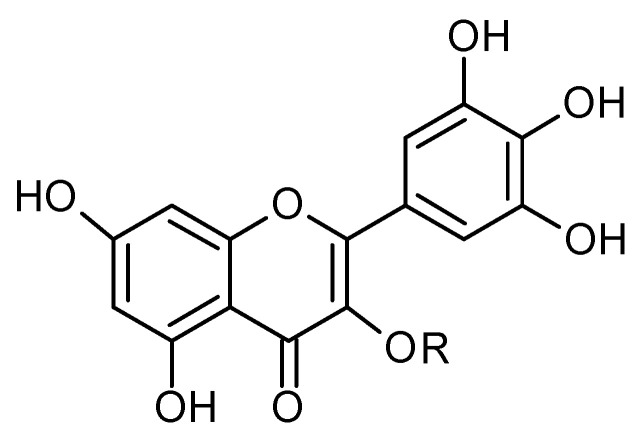
Figure 6.
Luteolin and isorhamnetin, and derivatives.
Figure 7.
Chemical structure of compound 39 (R1 = OH; MW = 270) and 40 (R1 = H; MW = 254).
Figure 8.
Catechin and epicatechin.
Figure 9.
Apigenin and derivatives.
Figure 10.
Chemical structure of compound 50 (MW = 302).
Figure 11.
Chemical structure of compound 43 and 44.
Table 1.
Kaempferol and kaempferol O-glycosides.
| Compound | R | R1 | R2 | R3 | MW (g/mol) |
|---|---|---|---|---|---|
| 1 | OH | H | H | H | 286 |
| 2 | O-glc | H | H | H | 448 |
| 3 | p-coumaroyl-robinobioside | H | H | H | 918 |
| 4 | xyl | H | H | H | 418 |
| 5 | 2″-O-α-L-rha-β-D-glc | H | H | H | 594 |
| 6 | glcA | H | H | H | 462 |
| 7 | 6″-O-(E)-p-coumaroyl)glc | H | H | H | 594 |
| 8 | O-rha-glc | H | H | H | 594 |
| 51 | O-rha | H | rha | H | 578 |
| 53 | 2-p-coumaroyl-glc | H | H | H | 594 |
Table 2.
Quercetin and derivatives.
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | MW (g/mol) |
|---|---|---|---|---|---|---|---|---|
| 9 | H | OH | H | H | OH | H | H | 302 |
| 10 | H | H | H | OH | O-α-L-ara | H | H | 434 |
| 11 | H | OH | H | H | O- glc(6→1)rha | H | H | 610 |
| 12 | H | H | H | OH | O-glucuronide | H | H | 478 |
| 13 | H | OH | H | H | O- gal | H | H | 464 |
| 14 | H | OH | H | H | O- glc | H | H | 464 |
| 15 | H | OH | H | H | O- rha | H | H | 448 |
| 19 | H | OH | H | H | O-β-D-xyl-(2→1)- β-D-glc | H | H | 596 |
| 20 | H | OH | H | H | O-β-D-xyl-(2→1)- β-D-glc | glc | H | 758 |
| 21 | H | OH | H | H | O- α-D-ara-furanoside | H | H | 434 |
| 54 | H | OH | H | H | OH | CH3 | H | 316 |
| 56 | H | OH | H | H | O-ara-furanoside | H | H | 434 |
Table 3.
Luteolin and isorhamnetin, and derivatives.
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | MW (g/mol) |
|---|---|---|---|---|---|---|---|---|
| 24 | H | OH | H | H | H | H | H | 286 |
| 25 | H | OH | H | H | H | H | C-β-D-glc | 448 |
| 26 | H | OH | H | H | H | O-β-D-glc | H | 448 |
| 27 | H | OH | H | H | H | rha-glc | H | 594 |
| 28 | H | OCH3 | H | H | OH | H | H | 316 |
| 29 | H | OCH3 | H | H | O-glc | H | H | 478 |
| 30 | H | OCH3 | H | H | O-rha glc | H | H | 624 |
| 42 | H | OCH3 | H | H | H | H | H | 300 |
Table 4.
Catechin and epicatechin.
| Compound | R1 | R2 | R3 | MW (g/mol) |
|---|---|---|---|---|
| 34 |
 OH OH |
H | OH | 290 |
| 35 |
 OH OH |
H | OH | 290 |
Table 5.
Apigenin and derivatives.
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | MW (g/mol) |
|---|---|---|---|---|---|---|---|
| 31 | OH | H | H | H | H | OH | 270 |
| 32 | OH | H | H | glc | H | OH | 432 |
| 33 | OH | H | glc | H | H | OH | 432 |
| 57 | OH | H | H | H | H | OCH3 | 284 |
Table 6.
Chemical structure of compound 43 and 44.
| Compound | R1 | R2 | R3 | R4 | MW (g/mol) |
|---|---|---|---|---|---|
| 43 | H | H | OH | H | 272 |
| 44 | H | OH | OH | H | 288 |
D’Agostino et al. (1998) isolated four flavonoid glycosides, namely 3-O-kaempferol-6”-O-(p-coumaroyl)-β-D-glucopyranoside (7), quercetin-3-O-β-D-rutinoside (11), quercetin-3-O-β-D-glucopyranoside (14), and quercetin-3-O-α-D-arabinofuranoside (21) from the methanol extract of Alchemilla vulgaris L. (Campania region, Italy) [20].
From the aqueous methanolic extract from the leaves of A. speciosa (Germany) astragalin (2), kaempferol 3-O-β-(2″-O-α-L-rhamnopyranosyl)-glucopyranoside uronic acid (5), kaempferol 3-O-β-D-glucuronide (6), 7, 11, miquelianin (12), 14, quercitrin (15), quercetin 3-O-β-(2″-O-α-L-rhamnopyranosyl)-glucopyranoside uronic acid (18), hyperin (=hyperoside) (13), quercetin 3-O-β-D-sambubioside (19), quercetin 3-O-β-ʋ-sambubioside-7-O-β-D-glucoside (20), cynaroside (26), and scolymoside (27) were isolated [21].
Kaya et al. used TLC and HPLC techniques to separation of 11, 13, 14, 15, orientin (25), and vitexin (32) in 50% aqueous ethanol extracts from leaves of A. hirtipedicellata, A. procerrima, A. sericata, and A. stricta collected in the northeastern Black Sea region of Turkey [22]. The same authors identified 11, 13–15, 25, and 32 in the extracts from the aerial parts of A. bursensis, A. cimilensis, A. hirsutiflora, A. ikizdereensis, A. orduensis, and A. oriturcica [23].
Lamaison and co-authors isolated miquelianin (12) from the aerial parts of A. xanthochlora [24]. This glucuronide of quercetin was later found also in the aerial parts of A. barbatiflora [25], A. caucasica [10], A. achtarowii [26], A. mollis [27], A. persica [6], A. vulgaris [28], A. coriacea, A. filicaulis, and A. glabra [29].
From the aerial flowering parts of A. mollis (Bulgaria), 7, 13, 14, rhodiolgin (36, Figure 4), gossypetin-3-O-β-D-galactopyranosyl-7-O-α-L-rhamnopyranoside (37, MW 626), and sinocrassoside D2 (45, MW 626) were isolated [27]. One year later, Trendafilova et al. isolated astragalin (2), kaempferol 3-O-(4”-E-p-coumaroyl)-robinobioside (variabiloside G, 3), 7, quercetin-3-O-α-D-arabinopyranoside (10), 13, and 14 from the aerial parts of A. achtarowii [26].
Phytochemical studies on active fractions of the water subextract led to the isolation of kaempferol-3-O-β-D-xylopyranoside (4), 7, 10, 12, 13, and 34 from the aerial parts of A. barbatiflora (Turkey) [25]. Guaijaverin (10) was also identified in the aerial parts of A. xanthochlora (France) [30].
Neagu et al. applied liquid chromatography coupled to tandem mass spectrometry to identify polyphenolic compounds extracted by water and aqueous ethanol (70% v/v) from A. vulgaris. Flavonols (kaempferol 1, quercetin 9, 11, myricetin 38, Figure 5), flavanols (35), flavones (luteolin 24, Figure 6), isoflavones (genistein 39, daidzein 40, Figure 7), and flavonoid glucosides (14) were detected in the plant samples [31].
Using high performance liquid chromatography (HPLC) analysis, Denev et al. (2014) found that aerial parts of A. glabra (Plovdiv, Bulgaria) contained 11, 34, and 35 [32]. Akkol and co-authors used the same analytical method to identify 13 and 14 in the aerial parts of A. mollis and A. persica (Turkey) [33].
Duckstein et al. investigated acetone/water extracts from the leaves, including stalks, of A. vulgaris and A. mollis (Germany) for their phenolic composition by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Compounds 12, methyl-quercetin glucuronide (16), quercetin hexoside (17), quercetin-feruloyl hexose (22), and quercetin hexoside-deoxyhexoside (23) were reported [34].
Compounds 1, 9, 11, and 34 were found in the ethanol extracts from the aerial parts of A. vulgaris (Russia) [35]. Karatoprak et al. reported that different (hexane, ethyl acetate, methanol, butanol) extracts from the aerial parts of A. mollis (Turkey) contained 11, 26, cosmosiin (33), 34, and 35 (Figure 8) [36].
Twenty flavonoids were identified in aqueous ethanol (80% v/v) from the leaves of A. vulgaris (Egypt). Among them, 1, 9, 11, 24, 31 (Figure 9), 33, 34, 35, naringenin (43), luteolin 6-arabinose 8-glucose (46, MW 610), luteolin 6-glucose 8-arabinose (47, MW 610), apigenin 6-arabinose 8-galactose (48), apigenin 6-rhamnose 8-glucose (49), apigenin 7-O-neohespiroside (50, MW 578; Figure 10), kaempferol 3,7-dirhamoside (51), hesperetin (52), kaempferol 3-(2-p-comaroyl)glucose (53), and rhamnetin (54) were determined [37].
In the aerial parts of A. vulgaris, 1, 2, 7, 9, 11, 13, 14, 15, 24, 26, 31, 33, 34, 39, morin (41, MW 302), chrysoeriol (42), and 43 were reported [38,39]. Afshar et al. used HR-MS Q-TOF for the identification of nicotiflorin (8), catechin (34), epicatechin (35), and aromadendrin glucoside (55) in the aerial parts of A. persica (Eastern Azarbaijan) [6]. Catechin is regarded as one of the most powerful antioxidants. Moreover, some research has shown the a strong relationship between this compound and inhibition of carcinogenesis (e.g., breast and ovarian cancer cell growth) [39].
In the latest study from 2022, Dos Santos Szewczyk et al. reported that the aerial parts and roots of A. acutiloba Opiz (Poland) contained 1, 2, 8, 9, 11, 13, 14, 15, 24, isorhamnetin (28), isorhamnetin-3-glucoside (29), narcissoside (30), and eriodictyol (44, Figure 11) [40].
Recent studies have revealed the presence of such flavonoids as 2, 7, 11–14, and 16 in methanol extracts from the aerial parts of A. viridiflora (Bulgaria) [41].
The phenolic compounds identified in the Alchemilla genus are summarized in Table 7.
Table 7.
The overview on the phenolic compounds identified in the Alchemilla genus.
| Constituent Name | Species | Part of Plant | References |
|---|---|---|---|
| 1. Kaempferol | A. acutiloba | aerial parts, roots | [40] |
| A. vulgaris | aerial parts | [31,35,37,38,39] | |
| 2. Astragalin | A. acutiloba | aerial parts, roots |
[40] |
| A. achtarowii | aerial parts | [26] | |
| A. speciosa | leaves | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [38,39] | |
| 3. Variabiloside G | A. achtarowii | aerial parts | [26] |
| 4. Kaempferol-3-O-β-D-xylopyranoside | A. barbatiflora | aerial parts | [25] |
| 5. Kaempferol 3-O-β-(2″-O-α-L-rhamnopyranosyl)-glucopyranoside uronic acid | A. speciosa | leaves | [21] |
| 6. Kaempferol 3-O-β-D-glucuronide | A. speciosa | leaves | [21] |
| 7. Kaempferol 3-O-β-D-(6”-O-(E)-p-coumaroyl) glucopyranoside | A. achtarowii | aerial parts | [26] |
| A. barbatiflora | aerial parts | [25] | |
| A. mollis | aerial parts | [27] | |
| A. speciosa | aerial parts | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [20,38] | |
| 8. Nicotiflorin | A. acutiloba | aerial parts, roots |
[40] |
| A. persica | aerial parts | [6] | |
| 9. Quercetin | A. acutiloba | aerial parts, roots |
[40] |
| A. vulgaris | aerial parts leaves |
[31,35,37,38,39] | |
| 10. Guaijaverin | A. achtarowii | aerial parts | [26] |
| A. barbatiflora | aerial parts | [25] | |
| A. xanthochlora | aerial parts | [30] | |
| 11. Rutin | A. acutiloba | aerial parts | [40] |
|
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] | |
| A. glabra | aerial parts | [32] | |
|
A. bursensis
A. cimilensis A. hirsutiflora A. ikizdereensis A. orduensis A. oriturcica |
aerial parts | [23] | |
| A. mollis | aerial parts | [27,36] | |
| A. speciosa | aerial parts | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [20,31,35,37,38,39] | |
| 12. Miquelianin | A. barbatiflora | aerial parts | [25] |
| A. caucasica | aerial parts | [10] | |
| A. achtarowii | aerial parts | [26] | |
| A. mollis | aerial parts | [27] | |
| A. persica | aerial parts | [6] | |
| A. speciosa | aerial parts | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [28] | |
| A. xanthochlora | aerial parts | [24] | |
| 13. Hyperoside | A. achtarowii | aerial parts | [26] |
| A. acutiloba | aerial parts, roots |
[40] | |
|
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] | |
| A. barbatiflora | aerial parts | [25] | |
|
A. coriacea
A. filicaulis A. glabra |
aerial parts | [29] | |
|
A. armeniaca
A. bursensis A. cimilensis A. hirsutiflora A. ikizdereensis A. orduensis A. oriturcica |
aerial parts | [23] | |
| A. mollis | aerial parts | [27,33] | |
| A. persica | aerial parts | [33] | |
| A. speciosa | leaves | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [38] | |
| 14. Isoquercitrin | A. achtarowii | aerial parts | [26] |
| A. acutiloba | aerial parts, roots |
[40] | |
|
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] | |
|
A. bursensis
A. cimilensis A. erzincanensis A. orduensis A. oriturcica |
aerial parts | [23] | |
| A. mollis | aerial parts | [27,33] | |
| A. persica | aerial parts | [33] | |
| A. speciosa | aerial parts | [21] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | aerial parts | [20,31,38] | |
| 15. Quercitrin | A. acutiloba | aerial parts, roots |
[40] |
|
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] | |
|
A. hirsutiflora
A. orduensis |
aerial parts | [23] | |
| A. speciosa | aerial parts | [21] | |
| A. vulgaris | aerial parts | [39] | |
| 16. Methyl-quercetin glucuronide | A. mollis | leaves | [34] |
| A. viridiflora | aerial parts | [41] | |
| 17. Quercetin hexoside | A. mollis | leaves | [34] |
| 18. Quercetin 3-O-β-(2″-O-α-L-rhamnopyranosyl)-glucopyranoside uronic acid | A. speciosa | leaves | [21] |
| 19. Quercetin 3-O-β-D-sambubioside | A. speciosa | leaves | [21] |
| 20. Quercetin 3-O-β-ʋ-sambubioside-7-O-β-D-glucoside | A. speciosa | leaves | [21] |
| 21. Quercetin-3-O-α-D-arabinofuranoside | A. vulgaris | aerial parts | [20] |
| 22. Quercetin-feruloyl hexose | A. vulgaris | leaves | [34] |
| 23. Quercetin hexoside-deoxyhexoside | A. vulgaris | leaves | [34] |
| 24. Luteolin | A. acutiloba | aerial parts, roots |
[40] |
| A. vulgaris | aerial parts | [31,37,38,39] | |
| 25. Orientin |
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] |
|
A. armeniaca
A. cimilensis A. hirsutiflora A. ikizdereensis A. orduensis |
aerial parts | [23] | |
| 26. Cynaroside | A. mollis | aerial parts | [36] |
| A. speciosa | aerial parts | [21] | |
| A. vulgaris | aerial parts | [38,39] | |
| 27. Scolymoside | A. speciosa | aerial parts | [21] |
| 28. Isorhamnetin | A. acutiloba | aerial parts, roots |
[40] |
| 29. Isorhamnetin-3-glucoside | A. acutiloba | aerial parts | [40] |
| 30. Narcissoside | A. acutiloba | aerial parts, roots |
[40] |
| 31. Apigenin | A. caucasica | aerial parts | [10] |
| A. vulgaris | aerial parts leaves |
[37,39] | |
| 32. Vitexin |
A. hirtipedicellata
A. procerrima A. sericata A. stricta |
leaves | [22] |
|
A. armeniaca
A. erzincanensis A. ikizdereensis A. orduensis |
aerial parts | [23] | |
| 33. Cosmosiin | A. mollis | aerial parts | [36] |
| A. vulgaris | aerial parts | [38,39] | |
| leaves | [37] | ||
| 34. Catechin | A. barbatiflora | aerial parts | [25] |
| A. caucasica | aerial parts | [10] | |
| A. glabra | aerial parts | [32] | |
| A. mollis | aerial parts | [36] | |
| A. persica | aerial parts | [6] | |
| A. vulgaris | aerial parts leaves roots |
[35,37,39] | |
| 35. Epicatechin | A. glabra | aerial parts | [32] |
| A. mollis | aerial parts | [36] | |
| A. persica | aerial parts | [6] | |
| A. vulgaris | aerial parts leaves |
[31,37] | |
| 36. Rhodiolgin | A. mollis | aerial parts | [27] |
| 37. Gossypetin-3-O-β-D-galactopyranosyl-7-O-α-L-rhamnopyranoside | A. mollis | aerial parts | [27] |
| 38. Myricetin | A. vulgaris | aerial parts | [31] |
| 39. Genistein | A. vulgaris | aerial parts | [31,39] |
| 40. Daidzein | A. vulgaris | aerial parts | [31] |
| 41. Morin | A. vulgaris | aerial parts | [38] |
| 42. Chrysoeriol | A. vulgaris | aerial parts | [39] |
| 43. Naringenin | A. vulgaris | aerial partsleaves | [37,39] |
| 44. Eriodictyol | A. acutiloba | aerial parts | [40] |
| 45. Sinocrassoside D2 | A. mollis | aerial parts | [27] |
| 46. Luteolin 6-arabinose 8-glucose | A. vulgaris | leaves | [37] |
| 47. Luteolin 6-glucose 8-arabinose | A. vulgaris | leaves | [37] |
| 48. Apigenin 6-arabinose 8-galactose | A. vulgaris | leaves | [37] |
| 49. Apigenin 6-rhamnose 8-glucose | A. vulgaris | leaves | [37] |
| 50. Apigenin 7-O-neohesperidoside | A. vulgaris | leaves | [37] |
| 51. Kaempferol 3,7-dirhamoside | A. vulgaris | leaves | [37] |
| 52. Hesperetin | A. vulgaris | leaves | [37] |
| 53. Kaempferol 3-(2-p-comaroyl)glucose | A. vulgaris | leaves | [37] |
| 54. Rhamnetin | A. vulgaris | leaves | [37] |
| 55. Aromadendrin glucoside | A. persica | aerial parts | [6] |
| 56. Avicularin | A. vulgaris | aerial parts | [35] |
| 57. Acacetin | A. vulgaris | leaves | [37] |
| 58. Agrimoniin | A. mollis | leaves | [34] |
| A. persica | aerial parts | [6] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | leaves | [34] | |
| A. xanthochlora | aerial parts | [42] | |
| 59. Pedunculagin | A. mollis | leaves | [34] |
| A. persica | aerial parts | [6] | |
| A. vulgaris | leaves | [34] | |
| A. viridiflora | aerial parts | [41] | |
| A. xanthochlora | aerial parts | [42] | |
| 60. Laevigatin F | A. xanthochlora | aerial part | [42] |
| 61. Castalagin/vescalagin isomer | A. mollis | leaves | [34] |
| A. vulgaris | leaves | [34] | |
| 62. Galloyl-HHDP hexose | A. mollis | leaves | [34] |
| A. persica | aerial parts | [6] | |
| A. vulgaris | leaves | [34] | |
| 63. Trigalloyl hexose | A. mollis | leaves | [34] |
| 64. Sanguiin | A. mollis | leaves | [34] |
| A. persica | aerial parts | [6] | |
| A. viridiflora | aerial parts | [41] | |
| A. vulgaris | leaves | [34] | |
| 65. Methyl gallate | A. mollis | aerial parts | [36] |
| A. persica | aerial parts | [6] | |
| 66. Casuarictin | A. persica | aerial parts | [6] |
| 67. Digalloyl-galloyl galloside | A. persica | aerial parts | [6] |
| 68. HHDP-hexoside | A. viridiflora | aerial parts | [41] |
| 69. Brevifolincarboxylic acid | A. viridiflora | aerial parts | [41] |
| 70. Tellimagrandin I | A. viridiflora | aerial parts | [41] |
| 71. Tellimagrandin II | A. viridiflora | aerial parts | [41] |
| 72. Benzoic acid | A. vulgaris | leaves | [37,43] |
| A. jumrukczalica | leaves | [43] | |
| 73. Caffeic acid | A. acutiloba | aerial parts, roots |
[40] |
| A. glabra | aerial parts | [32] | |
| A. jumrukczalica | leaves | [43] | |
| A. mollis | aerial parts | [36] | |
| A. vulgaris | aerial parts leaves |
[37,39,43] | |
| 74. Chlorogenic acid | A. glabra | aerial parts | [32] |
| A. mollis | leaves | [34] | |
| A. persica | aerial parts | [6] | |
| A. vulgaris | aerial parts leaves |
[31,34,37,39] | |
| 75. 2,5-Dihydroxybenzoic acid | A. vulgaris | aerial parts | [39] |
| 76. 3,4-Dihydroxybenzoic acid | A. glabra | aerial parts | [32] |
| 77. Ellagic acid | A. mollis | leaves | [34] |
| A. persica | aerial parts | [6] | |
| A. vulgaris | aerial parts leaves |
[31,38,44] [31,34,37] |
|
| 78. Ferulic acid | A. acutiloba | aerial parts | [40] |
| A. vulgaris | aerial parts leaves |
[37,38,39] | |
| 79. Gallic acid | A. acutiloba | aerial parts roots |
[40] |
| A. glabra | aerial parts | [32] | |
| A. jumrukczalica | leaves | [43] | |
| A. mollis | aerial parts leaves |
[34,36] | |
| A. persica | aerial parts | [6] | |
| A. vulgaris | aerial parts leaves roots |
[31,34,35] [37,39,43] [45] |
|
| 80. Gentisic acid | A. acutiloba | aerial parts, roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. mollis | aerial parts | [36] | |
| A. vulgaris | leaves | [43] | |
| 81. Protocatechuic acid | A. acutiloba | aerial parts, roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | aerial parts leaves |
[37,39,43] | |
| 82. p-Coumaric acid | A. acutiloba | aerial parts roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | aerial parts leaves |
[31,37,39,43] | |
| 83. 4-Hydroxybenzoic acid | A. acutiloba | aerial parts roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | aerial parts leaves |
[37,39,43] | |
| 84. Mandelic acid | A. jumrukczalica | leaves | [43] |
| A. vulgaris | leaves | [43] | |
| 85. 3,4,5-Methoxycinnamic acid | A. vulgaris | leaves | [37] |
| 86. β-Resorcylic acid | A. jumrukczalica | leaves | [43] |
| A. vulgaris | leaves | [43] | |
| 87. Rosmarinic acid | A. acutiloba | aerial parts roots |
[40] |
| A. vulgaris | aerial parts leaves |
[31,37] | |
| 88. Salicylic acid | A. acutiloba | aerial parts roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | leaves | [37,43] | |
| 89. Sinapic acid | A. jumrukczalica | leaves | [43] |
| A. vulgaris | aerial parts leaves |
[31,43] | |
| 90. Syringic acid | A. acutiloba | aerial parts roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | leaves | [43] | |
| 91. Trans-cinnamic acid | A. jumrukczalica | leaves | [43] |
| A. vulgaris | leaves | [37,43] | |
| 92. 3,4,5-Trimethoxymandelic acid | A. jumrukczalica | leaves | [43] |
| A. vulgaris | leaves | [43] | |
| 93. Vanillic acid | A. acutiloba | aerial parts roots |
[40] |
| A. jumrukczalica | leaves | [43] | |
| A. vulgaris | leaves | [37,43] | |
| 94. Quinic acid | A. vulgaris | aerial parts | [39] |
The plant tannins are a unique group of phenolics of relatively high molecular weight with the ability to complex strongly with carbohydrates and proteins. In higher plants, tannins consist of two major groups of metabolites: the hydrolysable tannins and condensed tannins [17]. Alchemilla species as members of Rosaceae family also produce, apart from flavonoids, tannins.
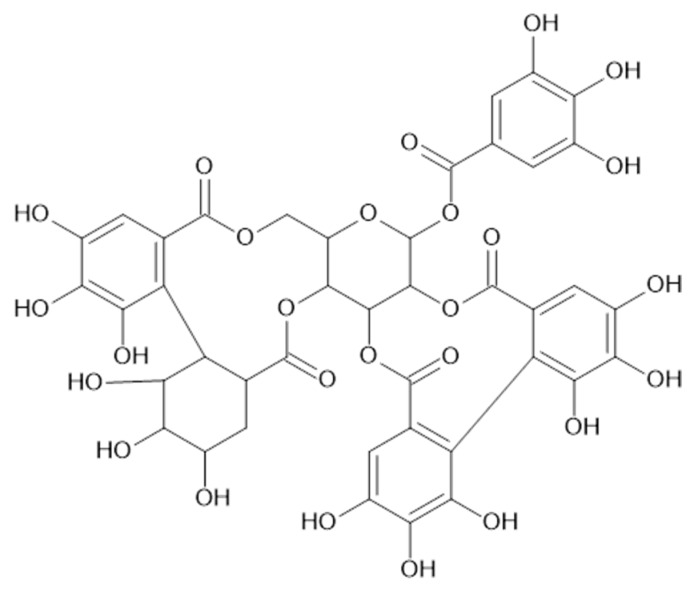
Geiger et al. identified in the aerial parts of A. xanthochlora (Germany) ellagitannins such as agrimoniin (58, MW 1871), pedunculagin (59, MW 784)), and laevigatin F (60, MW 802) [42].
Duckstein and co-authors used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify tannins extracted by acetone/water (8/2, v/v) from the leaves (including stalks) from A. vulgaris and A. mollis (Germany). Compounds 58, 59, castalagin/vescalagin isomer (61, MW 934), galloyl-HHDP hexose (62, MW 618), trigalloyl hexose (63, MW 636), and sanguiin (64, MW 1871) were detected in studied plant samples [34].
In the aerial parts of A. mollis [36] and A. persica [6], methyl gallate (65, Figure 12) was identified. The authors [6] found also that extracts of A. persica contained 58, 59, 62, 64, casuarictin (66, Figure 13), and digalloyl-galloyl galloside (67, MW 1084).
Figure 12.
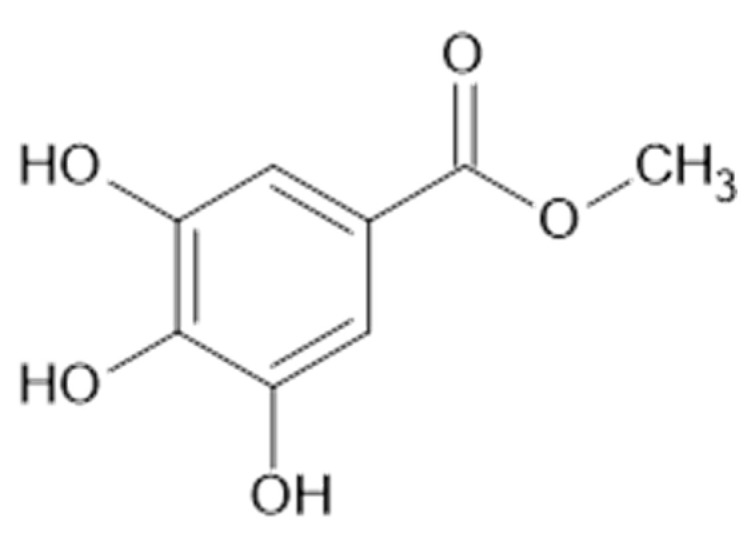
Chemical structure of compound 65 (MW = 184).
Figure 13.
Chemical structure of compound 66 (MW = 936).
Recent studies have revealed the presence in the aerial parts of A. viridiflora such tannins as HHDP-hexoside (68), brevifolincarboxylic acid (69, Figure 14), and tellimagrandin I (70, Figure 15) and II (71, MW 938) which have been reported for the first time in Alchemilla species [41].
Figure 14.
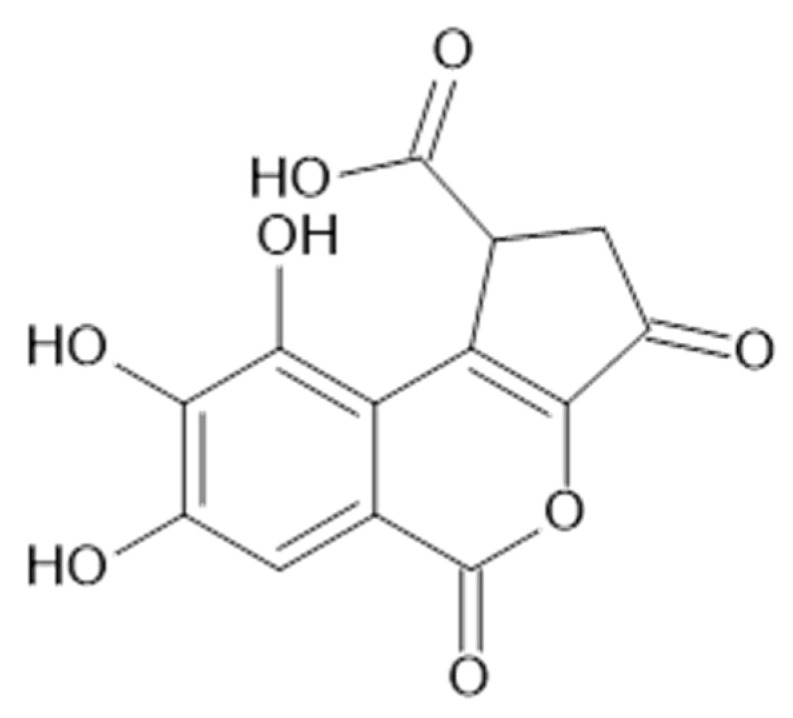
Chemical structure of compound 69 (MW = 292).
Figure 15.
Chemical structure of compound 70 (MW = 786).
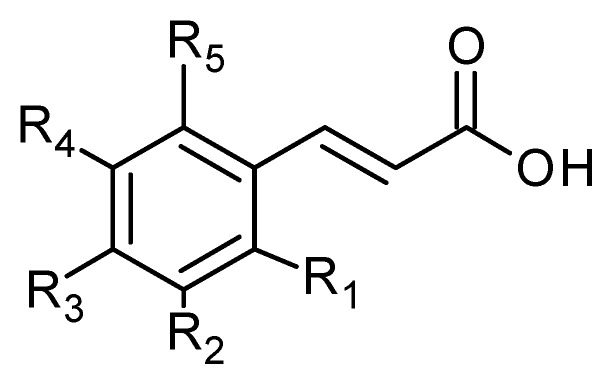
Phenolic acids are also common in higher plants, and they are usually present in the bound soluble form conjugated with sugars or organic acids [17] (Figure 16 and Figure 17, Table 8 and Table 9).
Figure 16.
Hydroxycinnamic acid derivatives.
Figure 17.
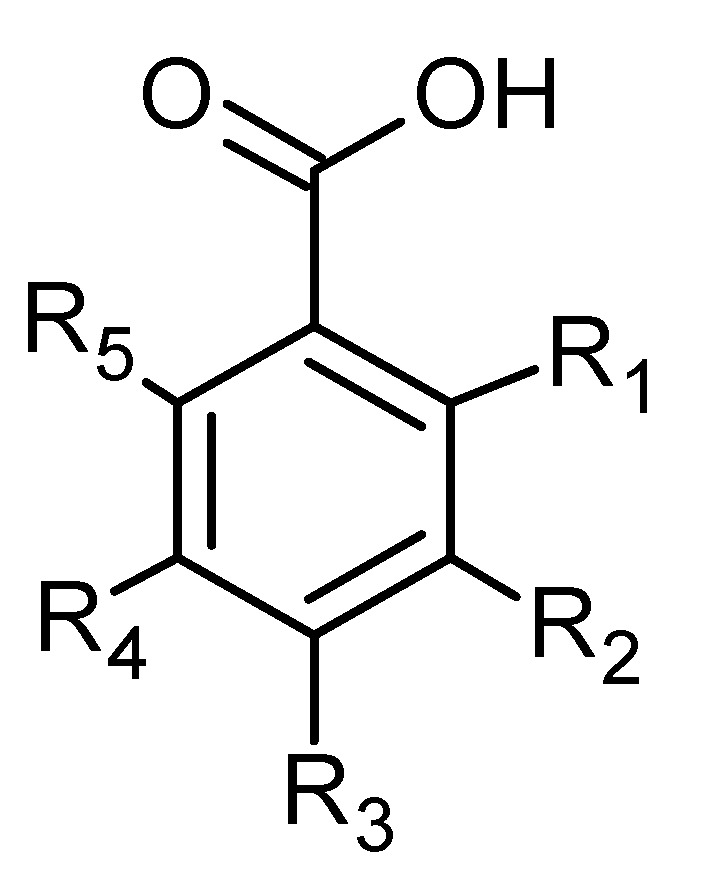
Benzoic acid derivatives.
Table 8.
Hydroxycinnamic acid derivatives.
| Compound | R1 | R2 | R3 | R4 | R5 | MW |
|---|---|---|---|---|---|---|
| 73 | H | OH | OH | H | H | 180 |
| 78 | H | OCH3 | H | OH | H | 194 |
| 82 | H | H | OH | H | H | 164 |
| 85 | H | OCH3 | OCH3 | OCH3 | H | 238 |
| 89 | H | OCH3 | OH | OCH3 | H | 224 |
| 91 | H | H | H | H | H | 148 |
Table 9.
Benzoic acid derivatives.
| Compound | R1 | R2 | R3 | R4 | R5 | MW |
|---|---|---|---|---|---|---|
| 72 | H | H | H | H | H | 122 |
| 79 | H | OH | OH | OH | H | 170 |
| 80 | OH | H | H | OH | H | 154 |
| 81 | H | OH | OH | H | H | 154 |
| 83 | H | H | OH | H | H | 138 |
| 86 | OH | H | OH | H | H | 154 |
| 88 | OH | H | H | H | H | 138 |
| 90 | OH | OCH3 | OH | OCH3 | H | 198 |
| 93 | H | OCH3 | OH | H | H | 168 |
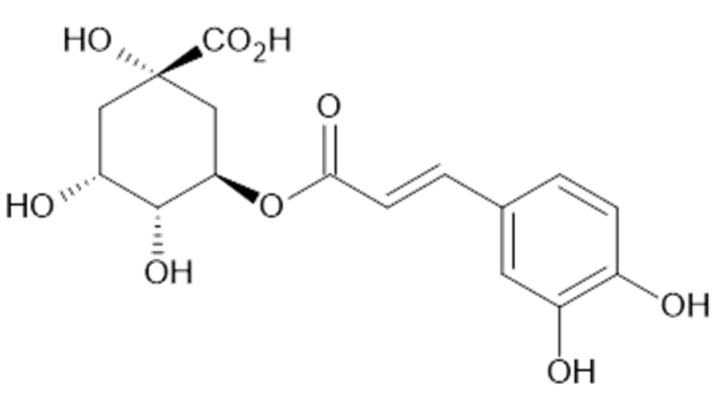
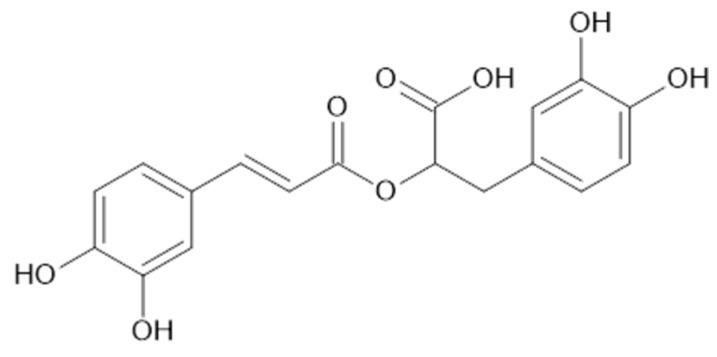
In the aqueous extracts and ethanolic extracts (70% (v/v) ethanol) of A. vulgaris caffeic acid (73), chlorogenic acid (74, Figure 18), elagic acid (77), ferulic acid (78), gallic acid (79), p-coumaric acid (82), rosmarinic acid (87, Figure 19), and sinapic acid (89) were identified using HPLC-MS analysis [31].
Figure 18.
Chemical structure of compound 74 (MW = 354).
Figure 19.
Chemical structure of compound 87 (MW = 360).
Denev et al. reported that the aerial parts of A. glabra contained 73, 74, 3,4-dihydroxybenzoic acid (76), and 79 [32]. In the leaves, including stalks, of A. vulgaris and A. mollis, three phenolic acids (74, 77, 79) were found [34]. Moreover, in different extracts from the aerial parts of A. mollis, 73, 79, and gentisic acid (80) were noticed [36].
In the 80% methanol extracts from the leaves of A. jumrukczalica and A. vulgaris (Bulgaria), free and bonded phenolic acids were identified. Among reported phenolic acids, 73, 80, protocatechuic (81), salicylic (88), trans-cinnamic (91), and vanilic (93) acids were the major compounds [43].
Fifteen phenolic acids (benzoic acid (72), 73, 74, 78, 79, 81, 82, 4-hydroxybenzoic acid (83), 3,4,5-methoxycinnamic acid (85), 87, 88, 91, 93) in the leaves [37] and 77 [38], and 73, 74, 2,5-dihydroxybenzoic acid (75), 78, 79, 81–83, as well as quinic acid (94, Figure 20) [39] were found in the aerial parts of A. vulgaris. The advantages of identified phenolic acids are associated with several health benefits such as antioxidant, anti-diabetic, and anticancer effects [39].
Figure 20.
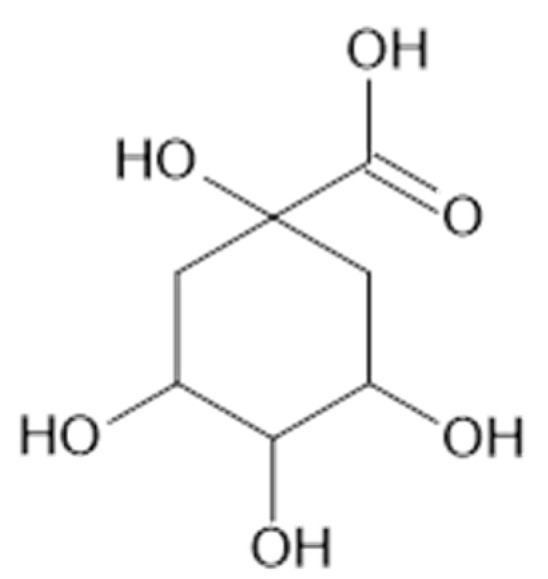
Chemical structure of compound 94 (MW = 192).
Moreover, Dos Santos Szewczyk et al. [40] reported that aerial parts and roots of A. acutiloba contained 73, 78–83, 87, 88, syringic acid (90), and 93.
4. Antioxidant Activity
It has been proven that oxidative stress participates in the formation of various diseases such as chronic obstructive pulmonary disease (COPD), Alzheimer’s disease, atherosclerosis, and cancer [46].
The evidence described above confirms the importance of searching for new effective and safe antioxidant agents. As reported by Forman and Zhang [46], two major mechanisms connected with diseases formation which contribute to cellular damage can be distinguished, namely: generation of reactive oxygen species (•OH, ONOO−, HOCl) which directly oxidize macromolecules, especially membrane lipids, enzymes, proteins, as well as nucleic acids, and leads to death resulting from aberrant cell function. Furthermore, the second pathway relates with aberrant redox signaling.
Based on all the above, in this review, we sought to clarify in more detail the antioxidant potential of Alchemilla species (Table 10).
Table 10.
The overview on the antioxidant activities in Alchemilla species.
| Species | Plant Part/Extract | Antioxidant Assay | Antioxidant Effect | References |
|---|---|---|---|---|
| A. acutiloba | aerial parts, 60% methanol |
DPPH | IC50 = 18.69 µg/mL DE | [40] |
| aerial parts, 60% methanol |
ABTS | IC50 = 6.17 µg/mL DE | ||
| aerial parts, 60% methanol |
CHEL | IC50 = 21.60 µg/mL DE | ||
| roots, 60% methanol |
DPPH | IC50 = 29.87 µg/mL DE | ||
| roots, 60% methanol |
ABTS | IC50 = 14.29 µg/mL DE | ||
| roots, 60% methanol |
CHEL | IC50 = 25.76 µg/mL DE | ||
| aerial parts, butanol fraction |
DPPH | IC50 = 8.96 µg/mL DE | ||
| aerial parts, butanol fraction |
ABTS | IC50 = 1.42 µg/mL DE | ||
| aerial parts, butanol fraction |
CHEL | IC50 = 11.43 µg/mL DE | ||
| roots, butanol fraction |
DPPH | IC50 = 12.08 µg/mL DE | ||
| roots, butanol fraction |
ABTS | IC50 = 8.78 µg/mL DE | ||
| roots, butanol fraction |
CHEL | IC50 = 12.33 µg/mL DE | ||
| aerial parts, diethyl acetate fraction |
DPPH | IC50 = 8.83 µg/mL DE | ||
| aerial parts, diethyl acetate fraction |
ABTS | IC50 = 6.54 µg/mL DE | ||
| aerial parts, diethyl acetate fraction |
CHEL | IC50 = 18.89 µg/mL DE | ||
| roots, diethyl acetate fraction |
DPPH | IC50 = 15.37 µg/mL DE | ||
| roots, diethyl acetate fraction |
ABTS | IC50 =10.39 µg/mL DE | ||
| roots, diethyl acetate fraction |
CHEL | IC50 = 19.30 µg/mL DE | ||
| aerial parts, diethyl ether fraction |
DPPH | IC50 = 41.46 µg/mL DE | ||
| aerial parts, diethyl ether fraction |
ABTS | IC50 = 16.28 µg/mL DE | ||
| aerial parts, diethyl ether fraction |
CHEL | IC50 = 25.51 µg/mL DE | ||
| roots, diethyl ether fraction |
DPPH | IC50 = 51.42 µg/mL DE | ||
| roots, diethyl ether fraction |
ABTS | IC50 = 24.82 µg/mL DE | ||
| roots, diethyl ether fraction |
CHEL | IC50 = 44.12 µg/mL DE | ||
| A. alpina | aerial parts, methanol |
DPPH | % Inhibition = 45.4–94.4% | [47] |
| A. arvensis | leaves, methanol |
DPPH | IC50 = 97.72 µg/mL | [48] |
| leaves, hexane |
IC50 = 11.22 µg/mL | |||
| leaves, acetone |
IC50 = 4.86 µg/mL | |||
| A. barbatiflora | aerial parts, methanol |
DPPH | % Inhibition = 83.44–95.35% | [25] |
| aerial parts, hexane fraction |
% Inhibition = 18.6–59.62% | |||
| aerial parts, chloroform fraction |
% Inhibition = 67.17–91.11% | |||
| aerial parts, water fraction |
% Inhibition = 83.06–97.17% | |||
| aerial parts, methanol |
SOD | % Inhibition = 83.34–85.83% | ||
| aerial parts, hexane fraction |
% Inhibition = 9.80% | |||
| aerial parts, chloroform fraction |
% Inhibition = 12.84–42.73% | |||
| aerial parts, water fraction |
% Inhibition = 81.07% | |||
| aerial parts, methanol |
PRA | Absorbance 0.932–1.280 | ||
| aerial parts, hexane fraction |
Absorbance 0.355–0.612 | |||
| aerial parts, chloroform fraction |
Absorbance 0.640–0.820 | |||
| aerial parts, water fraction |
Absorbance 1.158–1.516 | |||
| aerial parts, methanol |
FRAP | 44.32 mg BHAE/g DE | ||
| aerial parts, chloroform fraction |
15.76 mg BHAE/g DE | |||
| aerial parts, water fraction |
93.46 mg BHAE/g DE | |||
| A. bulgarica | aerial parts, 80% methanol |
DPPH | IC50 = 75.63 µg/mL | [49] |
| A. crinita | aerial parts, 80% methanol |
DPPH | IC50 = 46.03 µg/mL | [49] |
| A. ellenbergiana | aerial parts, hexane |
DPPH | IC50 = 7.1 µg/mL | [50] |
| A. ellenbergiana | aerial parts, ethanol |
DPPH | IC50 = 243.6 µg/mL | [51] |
| aerial parts, methanol |
IC50 = 243.1 µg/mL | |||
| A. erythropoda | aerial parts, 80% methanol |
DPPH | IC50 = 30.67 µg/mL | [50] |
| A. glabra | aerial parts, 80% acetone in 0.2% formic acid |
ORAC | IC50 = 1337 μmol TE/g | [32] |
| TRAP | IC50 = 1815 μmol TE/g | |||
| HORAC | IC50 = 1999 μmol GAE/g | |||
| A. glabra | aerial parts, 80% methanol |
DPPH | IC50 = 34.89 µg/mL | [49] |
| A. glaucescens | aerial parts, 80% methanol |
DPPH | IC50 = 36.10 µg/mL | [49] |
| A. jumrukczalica | leaves, 80% methanol |
DPPH | IC50 = 12.09 µg/mL | [43] |
| A. mollis | shoots grown in vitro on different nutrient media | DPPH | IC50 = 18.6–38.1 μg/mL | [52] |
| leaves of ex vitro adapted plants in Bulgarian mountains Vitosha | IC50 = 13.1 μg/mL | |||
| one year old in vivo plantsgrown in Bulgarian mountains Viotsha | IC50 = 27.5 μg/mL | |||
| one year old in vivo plantsgrown in Bulgarian mountains Rhodopes | IC50 = 22.2 μg/mL | |||
| A. mollis | leaves, 50% ethanol |
DPPH | IC50 = 42.4 μg/mL | [53] |
| ABTS | IC50 = 7.8 μg/mL | |||
| A. mollis | aerial parts, water |
DPPH | IC50 = 0.264 mg/mL | [54] |
| aerial parts, deodorized water |
IC50 = 0.146 mg/mL | |||
| aerial parts, 50% methanol |
IC50 = 0.161 mg/mL | |||
| aerial parts, water |
ABTS | 0.90 mmol/L/Trolox | ||
| aerial parts,deodorized water | 0.4 mmol/L/Trolox | |||
| aerial parts,50% methanol | 0.4 mmol/L/Trolox | |||
| A. mollis | aerial parts, 70% methanol |
DPPH | IC50 = 0.21 mg/mL | [36] |
| aerial parts,water | IC50 = 0.24 mg/mL | |||
| aerial parts, 70% methanol |
ABTS | TEAC = 0.75 mmol/Trolox | ||
| aerial parts,water | TEAC = 0.83 mmol/Trolox | |||
| aerial parts,hexane, ethyl acetate, methanol, butanol, 70% methanol, water | Inhibition of β-carotene/linoleic acid co-oxidation | no data | ||
| A. mollis | dry stalks, aqueous ethanol |
FRAP | TEAC = 382.78 mmol TE/g DW | [55] |
| dry stalks, aqueous ethanol |
CUPRAC | TEAC = 363.79 mmol TE/g DW | ||
| dry stalks, aqueous ethanol |
DPPH | TEAC = 247.58 mmol TE/g DW | ||
| dry stalks, aqueous ethanol |
ABTS | TEAC = 308.44 mmol TE/g DW | ||
| A. mollis | aerial parts, methanol |
DPPH | IC50 = 31.7 μg/mL | [27] |
| aerial parts, ethyl acetate fraction |
IC50 = 9.8 μg/mL | |||
| aerial parts, petroleum fraction |
IC50 = > 200 μg/mL | |||
| aerial parts, chloroform fraction |
IC50 = > 200 μg/mL | |||
| aerial parts, water residue fraction |
IC50 = 42.5 μg/mL | |||
| A. persica | aerial parts, 80% methanol |
DPPH | IC50 = 0.055 M | [3] |
| roots, 80% methanol |
IC50 = 0.151 M | |||
| aerial parts, 80% methanol |
TBARS | MDA = 5.9 nmol/mL | ||
| roots, 80% methanol |
MDA = 19.08 nmol/mL | |||
| A. monticola | aerial parts, 80% methanol |
DPPH | IC50 = 32.72 μg/mL | [49] |
| A. obtusa | aerial parts, 80% methanol |
DPPH | IC50 = 26.35 μg/mL | [49] |
| A. sericata | aerial parts, hexane |
DPPH | IC50 = 185 μg/mL | [56] |
| A. vulgaris | leaves, 50% ethanol |
DPPH | % Inhibition = 71.8% | [57] |
| A. vulgaris | aerial parts, methanol |
DPPH | IC50 = 5.40 µg/mL | [58] |
| ABTS | IC50 = 60.10 µg/mL | |||
| A. vulgaris | aerial parts, methanol |
DPPH | IC50 = 5.96 µg/mL | [11] |
| roots, methanol |
IC50 = 11.86 µg/mL | |||
| aerial parts, methanol |
ABTS | IC50 = 14.80 µg/mL | ||
| roots, methanol |
IC50 = 32.49 µg/mL | |||
| aerial parts, methanol |
Hydroxyl radical scavenging activity | IC50 = 13.06 µg/mL | ||
| roots, methanol |
IC50 = 18.44 µg/mL | |||
| aerial parts, methanol |
Inhibition of lipid peroxidation | IC50 = 31.91 µg/mL | ||
| roots, methanol |
IC50 = 475.13 µg/mL | |||
| aerial parts, methanol |
Reducing power | IC50 = 632.99 mg TE/g DE | ||
| roots, methanol |
IC50 = 607.52 mg TE/g DE | |||
| aerial parts, methanol |
Total antioxidant activity | IC50 = 265.62 mg AA/g DE | ||
| roots, methanol |
IC50 = 316.47 mg AA/g DE | |||
| A. vulgaris | leaves, 80% ethanol |
DPPH | % inhibition = 131.74% | [37] |
| A. vulgaris | roots, 50% ethanol |
TEAC | 68.21 mmol TE/g DW | [59] |
| FRAP | 40.12 mmol TE/g DW | |||
| A. vulgaris | aerial parts, cyclohexane |
DPPH | IC50 = 23.12 µg/mL | [44] |
| A. vulgaris | aerial parts, 80% methanol |
DPPH | 153.30 mg TE/g DE | [39] |
| ABTS | 143.55 mg TE/g DE | |||
| CUPRAC | 216.14 mg TE/g DE | |||
| PRAP | 1.77 mmol TE/g DE | |||
| CHEL | 42.58 mg EDTAE/g DE | |||
| FRAP | 7899.45 mg AAE/g DE | |||
| aerial parts, 70% ethanol |
DPPH | 95.99 mg TE/g DE | ||
| ABTS | 119.62 mg TE/g DE | |||
| CUPRAC | 203.53 mg TE/g DE | |||
| PRAP | 1.57 mmol TE/g of DE | |||
| CHEL | 42.32 mg EDTAE/g DE | |||
| FRAP | 6405.75 mg AAE/g DE | |||
| aerial parts, 70% ethyl-acetate |
DPPH | 502.56 mg TE/g DE | ||
| ABTS | 174.05 mg TE/g DE | |||
| CUPRAC | 283.16 mg TE/g DE | |||
| PRAP | 2.22 mmol TE/g DE | |||
| CHEL | 37.96 mg EDTAE/g DE | |||
| FRAP | 8745.31 AAE/g DE | |||
| aerial parts, water |
DPPH | 89.25 mg TE/g DE | ||
| ABTS | 37.50 mg TE/g DE | |||
| CUPRAC | 78.56 mg TE/g DE | |||
| PRAP | 0.53 mmol TE/g DE | |||
| CHEL | 39.23 mg EDTAE/g DE | |||
| FRAP | 3240.09 mg AAE/g DE | |||
| A. vulgaris | aerial parts, ethanol |
DPPH | IC50 = 0.11 μg/mL | [38] |
| aerial parts, water |
IC50 = 27.22 μg/mL | |||
| aerial parts, propylene glycolic |
IC50 = 2.88 μL/mL | |||
| A. vulgaris | aerial parts, 70% ethanol |
DPPH | 87.95% (at 3 mg/mL) and 80.71% (at 1.5 mg/mL) | [31] |
| A. vulgaris | leaves, 80% methanol |
DPPH | IC50 = 19.62 µg/mL | [43] |
| A. xanthochlora | aerial parts, 80% methanol |
DPPH | IC50 = 41.78 µg/mL | [49] |
| A. xanthochlora | leaves,hexane | TLC-DPPH analysis, DPPH | no data | [13] |
| leaves, chloroform |
no data | |||
| leaves, ethylacetate |
no data | |||
| leaves, methanol |
no data | |||
| leaves, water |
no data |
DPPH, 2.2-diphenyl-1-picryl-hydrazyl free radical scavenging activity; ABTS, 2,2′-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid decolorization assay; CHEL, metal chelating activity; DE, dry extract; SOD, superoxide radical scavenging; PRAP, phosphomolibdenum-reducing antioxidant power; FRAP, ferric-reducing antioxidant power; BHAE, butylated hydroxyanisole equivalents; ORAC, oxygen radical absorbance capacity; TE, Trolox equivalents; TRAP, total peroxyl-radical antioxidant parameter; HORAC, hydroxyl radical averting capacity; GAE, gallic acid equivalents; CUPRAC, cupric reducing antioxidant capacity; DW, dry weight; TBARS, thiobarbituric acid reactive substances assay; MDA, malondialdehyde level; TEAC, Trolox equivalent antioxidant capacity; EDTAE, ethylenediaminetetraacetic acid equivalent; AAE, ascorbic acid equivalents; no data—results on the figures, without values.
Ondrejovič et al. evaluated the antioxidant activities of various solvent extracts and fractions acquired by solid-liquid and liquid-liquid extraction and column chromatography from leaves of A. xanthochlora. The most prominent antioxidant activity was observed in methanolic extract. Isolated fraction showed antioxidant activity of 535.2 mg DPPH per g of fraction residue [13].
Antioxidant activity of the extracts from the aerial parts and roots of A. persica (Turkey) was evaluated using the DPPH radical scavenging assay and measurement of malondialdehyde (MDA) levels. The extracts were found to exhibit DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging activity with IC50 values of 0.055 M and 0.151 M for the aerial parts and roots, respectively. The MDA level of the aerial parts was found to be 5.9 nmol/mL, and 19.08 nmol/mL for the roots [3].
Nikolova et al. evaluated antioxidant activity of the methanol extracts from leaves of A. jumrukczalica and A. vulgaris by the scavenging effect on DPPH radicals. The extracts showed good antiradical activity with IC50 values of 12.09 μg/mL and 19.62 μg/mL, respectively. Obtained values were comparable with those of butylated hydroxytoluene (BHT)—12.65 μg/mL and syringic acid—4.40 μg/mL, used as standard substances [43].
Boroja and co-authors [58] analyzed antioxidant activity of the methanol extract from aerial parts of A. vulgaris. They found that studied extract possessed DPPH inhibition activity with IC50 = 5.40 µg/mL. Moreover, the ABTS results demonstrated IC50 value of 60.10 µg/mL. The same authors evaluated antioxidant efficacy of extracts from the roots and aerial parts of A. vulgaris (Central Serbia) as total antioxidant capacity, metal chelation and reducing power ability, inhibition of lipid peroxidation, as well as their potential to neutralize DPPH, ABTS, and OH radicals. They found that roots exert a higher total antioxidant activity than aerial parts (316.5 and 265.6 mg ascorbic acid/g, respectively). Comparable results for both extracts were obtained in the reducing power assay (633.0—aerial parts and 607.5 mg Trolox/g—roots). In the ferrous ion chelating test, all studied samples failed to chelate Fe2+ at concentration 1 mg/mL [11].
Antioxidant capacity of the methanolic extract and fractions of A. mollis was also measured by their ability to scavenge the DPPH radical. The EtOAc fraction was found to be the most active radical scavenger (IC50 = 9.8 ± 1.8 μg/mL) but this value was less than that of quercetin (IC50 = 3.2 ± 0.4 μg/mL) [27]. The antioxidant capacity of stalks of A. mollis aqueous ethanol extracts was also investigated. The activity was determined by four different assays (FRAP (ferric-reducing antioxidant power), CUPRAC (cupric ion reducing antioxidant capacity), DPPH, and ABTS) and was expressed as mmol Trolox equivalent per dm3 extract. The maximum values of extracts were 382.78 ± 1.16; 363.79 ± 0.74; 247.58 ± 2.26; and 308.44 ± 6.74 for FRAP, CUPRAC, DPPH, and ABTS assays, respectively [55]. Karatoprak et al. evaluated antioxidant activity of hexane, ethyl acetate, methanol, butanol, water, and 70% methanol extracts from the aerial parts of A. mollis. In the DPPH assay, the IC50 values were found to be 0.21 mg/mL and 0.24 mg/mL, respectively for 70% MeOH and water extracts. ABTS+• radical scavenging effects of the extracts were studied at the doses of 0.25 and 0.5 mg/mL. All the extracts showed the highest level of activity at 0.5 mg/mL. The TEAC values of 70% methanol and water extracts (0.75 and 0.83 mM/Trolox) were found higher than the methanol, ethyl acetate, and hexane extracts [36]. The authors studied antiradical activity of various extracts from the herb of A. mollis also in different research. The IC50 values in the DPPH test were found 0.264 mg/mL, 0.146, and 0.161 mg/mL, respectively, for water, deodorized water, and 50% MeOH extracts. All extracts also managed to inhibit the ABTS●+ radical. The TEAC values of water extract (0.90 and 1.55 mM/L/Trolox) were found higher than the deodorized water and 50% MeOH extracts [54].
Denev et al. evaluated extracts of the aerial parts of A. glabra by means of several assays, including ORAC, TRAP, HORAC, and inhibition of lipid peroxidation. Between all extracts studied, A. glabra extract revealed the second highest chelating ability expressed as a HORAC value of 1999.4 μmol GAE/g [32].
Hamid and co-authors analyzed antioxidant activities of A. vulgaris roots using Trolox equivalent antioxidant capacity (TEAC), ferric reducing antioxidant power (FRAP), and TBARS (thiobarbituric acid reactive substances) assays. The antioxidant activity measured with TEAC was 68.21 mmol of Trolox (TE)/g of dry weight (DW). Whereas FRAP assay was 40.12 mmol of TE/g DW [59].
Vlaisavljević et al. evaluated the various extracts (80% methanol, 70% ethanol, 70% ethylacetate, and distilled water) of the aerial parts of A. vulgaris by means of different in vivo assays. The authors found that ethylacetate extract demonstrated the highest antioxidant potential, where the most pronounced and significant antioxidant effect of tested extracts was observed for DPPH and FRAP assays (DPPH: 502.56 mg TE per g extract; FRAP: 8745.31 mg EAA per g of dry extract), followed by methanol extract [39].
Antioxidant effects of the extracts, fractions, and isolated compounds from the aerial parts of A. barbatiflora were estimated using several methods as 1,1-diphenyl-2-picryl-hydrazyl (DPPH), superoxide radical scavenging (SOD), phosphomolibdenum-reducing antioxidant power (PRAP), and ferric-reducing antioxidant power (FRAP) assays. Crude methanol extract showed remarkable DPPH and SOD radical scavenging activities with 83.44% and 83.34% at 125 μg/mL. Among the tested sub-extracts, water sub-extract showed the best results with 83.06%, 96.08%, and 97.17% at 125, 250, and 500 μg/mL, respectively, for DPPH scavenging activities. Hexane sub-extract had moderate DPPH scavenging activities as compared to gallic acid. In SOD assay at 125 μg/mL, water sub-extract displayed significant SOD radical scavenging activities with 81.07%. Moreover, water sub-extract showed higher absorbance than methanol extract in PRAP assay. In FRAP assay, the result of methanol extract was found as 44.32 mg BHAE/g extract while the result of water sub-extract was as 93.46 mg BHAE/g extract [25].
The antioxidant activities of various extracts of the aerial parts and roots of A. acutiloba were determined using the DPPH• and ABTS•+ radical scavenging assays. It was found that at a dose of 50.0 µg/mL, the DPPH• scavenging abilities were the highest for the ethyl acetate (94.85%) and butanol (87.31%) fraction of the aerial parts, and in the ABTS•+ assay for butanol fraction (80.56%). Among studied extracts, butanol fraction of the aerial parts was also the most active ones interfering with the formation of iron and ferrozine complexes (IC50 = 11.43 µg/mL of DE) [40].
5. Conclusions and Research Gaps/Future Investigations
The available data suggest that recent times have brought a fundamental change in classical medicine considering the treatment of diseases. The above-mentioned phenomenon is related to large-scale application of drug combinations, thus the multidrug way of treatment is currently of great significance. Simultaneously, mono-substance therapies are becoming less and less popular. Moreover, a gradual development of drugs that activate natural defense and protective as well as repair mechanisms instead of impairing disadvantageous agents (such as cancer cells and microorganisms) can be observed [60].
Taking into consideration the above-mentioned facts, we can assume that phytomedicine and natural products chemistry are of significant importance due to the fact that extract combinations with various bioactive compounds can protect the human body rather that disturb damaging factors.
The World Health Organization (WHO) indicates that application of 74% of the curatives with plant origin using in modern medicine correlates with their traditional usages in various traditional medicines [10].
Author Contributions
Conceptualization, K.D.S.S. and S.K.; writing—original draft preparation, K.D.S.S., S.K., B.K., R.C. and M.B.; writing—review and editing, K.D.S.S., B.K. and S.K.; supervision, K.D.S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sepp S., Paal J. Taxonomic continuum of Alchemilla (Rosaceae) in Estonia. Nord. J. Bot. 1998;18:519–535. doi: 10.1111/j.1756-1051.1998.tb01532.x. [DOI] [Google Scholar]
- 2.Perry L.M. A tentative revision of Alchemilla § Lachemilla. Contrib. Gray Herb. Harv. Univ. 1929;84:1–57. doi: 10.5962/p.336113. [DOI] [Google Scholar]
- 3.Ergene B., Bahadir Acikara Ö., Bakar F., Saltan G., Nebioǧlu S. Antioxidant activity and phytochemical analysis of Alchemilla persica Rothm. Ankara Univ. Eczac. Fak. Derg. 2010;39:145–154. doi: 10.1501/eczfak_0000000560. [DOI] [Google Scholar]
- 4. [(accessed on 31 August 2022)]. Available online: http://theplantlist.org/1.1/browse/A/Rosaceae/Alchemilla/
- 5.Ozbek H., Acikara O.B., Keskin I., Kirmizi N.I., Ozbilgin S., Oz B.E., Kurtul E., Ozrenk B.C., Tekin M., Saltan G. Evaluation of hepatoprotective and antidiabetic activity of Alchemilla mollis. Biomed. Pharmacother. 2017;86:172–176. doi: 10.1016/j.biopha.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Afshar F.H., Maggi F., Ferrari S., Peron G., Dall’Acqua S. Secondary metabolites of Alchemilla persica growing in Iran (East Azarbaijan) Nat. Prod. Commun. 2015;10:1705–1708. doi: 10.1177/1934578X1501001018. [DOI] [PubMed] [Google Scholar]
- 7.Choi J., Park Y.G., Yun M.S., Seol J.W. Effect of herbal mixture composed of Alchemilla vulgaris and Mimosa on wound healing process. Biomed. Pharmacother. 2018;106:326–332. doi: 10.1016/j.biopha.2018.06.141. [DOI] [PubMed] [Google Scholar]
- 8.Jarić S., Mačukanović-Jocić M., Djurdjević L., Mitrović M., Kostić O., Karadžić B., Pavlović P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia) J. Ethnopharmacol. 2015;175:93–108. doi: 10.1016/j.jep.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Masullo M., Montoro P., Mari A., Pizza C., Piacente S. Medicinal plants in the treatment of women’s disorders: Analytical strategies to assure quality, safety and efficacy. J. Pharm. Biomed. 2015;113:189–211. doi: 10.1016/j.jpba.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Karaoglan E.S., Bayir Y., Albayrak A., Toktay E., Ozgen U., Kazaz C., Kahramanlar A., Cadirci E. Isolation of major compounds and gastroprotective activity of Alchemilla caucasica on indomethacin induced gastric ulcers in rats. Eurasian J. Med. 2020;52:249–253. doi: 10.5152/eurasianjmed.2020.19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boroja T., Mihailović V., Katanić J., Pan S.P., Nikles S., Imbimbo P., Monti D.M., Stanković N., Stanković M.S., Bauer R. The biological activities of roots and aerial parts of Alchemilla vulgaris L. S. Afr. J. Bot. 2018;116:175–184. doi: 10.1016/j.sajb.2018.03.007. [DOI] [Google Scholar]
- 12.European Pharmacopoeia 6.0 (Volume 2) Alchemillae herba. Druckerei C. H. Beck; Nördlingen, Germany: 2008. p. 1123. [Google Scholar]
- 13.Ondrejovıč M., Ondrıgová Z., Kubincová J. Isolation of antioxidants from Alchemilla xanthochlora. Nova Biotechnol. Chim. 2009;9:313–318. doi: 10.36547/nbc.1140. [DOI] [Google Scholar]
- 14.Usta C., Yildirim A.B., Turker A.U. Antibacterial and antitumour activities of some plants grown in Turkey. Biotechnol. Biotechnol. Equip. 2014;28:306–315. doi: 10.1080/13102818.2014.909708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaoglan E.S., Yilmaz B. Identification of bioactive compounds of Alchemilla caucasica using gas chromatography-mass spectrometry. Int. J. Pharmacogn. 2018;5:287–293. [Google Scholar]
- 16.Shrivastava R., Cucuat N., John G.W. Effects of Alchemilla vulgaris and glycerine on epithelial and myofibroblast cell growth and cutaneus lesion healing in rats. Phytother. Res. 2007;21:369–373. doi: 10.1002/ptr.2060. [DOI] [PubMed] [Google Scholar]
- 17.Lattanzio V. Phenolic compounds: Introduction 50. Nat. Prod. 2013:1543–1580. doi: 10.1007/978-3-642-22144-6_57. [DOI] [Google Scholar]
- 18.Harborne J.B. Plant phenolics. In: Bell E.A., Charlwood B.V., editors. Encyclopedia of Plant Physiology. Secondary Plant Products. Volume 8. Springer; Berlin, Germany: 1980. pp. 329–402. [Google Scholar]
- 19.Shilova I.V., Suslov N.I., Samylina I.A., Baeva V.M., Lazareva N.B., Mazin E.V. Neuroprotective properties of common lady’s mantle infusion. Pharm. Chem. J. 2020;53:1059–1062. doi: 10.1007/s11094-020-02122-0. [DOI] [Google Scholar]
- 20.D’Agostino M., Dini I., Ramundo E., Senatore F. Flavonoid glycosides of Alchemilla vulgaris L. Phyther. Res. 1998;12:1997–1998. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S162::AID-PTR284>3.0.CO;2-P. [DOI] [Google Scholar]
- 21.Felser C., Schimmer O. Flavonoid glycosides from Alchemilla speciosa. Planta Med. 1999;65:1987–1989. doi: 10.1055/s-2006-960845. [DOI] [PubMed] [Google Scholar]
- 22.Kaya B., Menemen Y., Saltan F.Z. Flavonoid compounds identified in Alchemilla L. species collected in the north-eastern Black Sea region of Turkey. Afr. J. Tradit. Complement. Altern. Med. 2012;9:418–425. doi: 10.4314/ajtcam.v9i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaya B., Menemen Y., Saltan F.Z. Flavonoids in the endemic species of Alchemilla L., (section Alchemilla L. subsection Calycanthum Rothm. Ser. Elatae Rothm.) from north-east Black Sea region in Turkey. Pakistan J. Bot. 2012;44:595–597. [Google Scholar]
- 24.Lamaison J.L., Carnat A., Petitjean-Freytet C., Carnat A.P. Quercetin-3-glucuronide, the main flavonoid of Lady’s Mantle, Alchemilla xanthochlora Rothm. (Rosaceae) Ann. Pharm. Fr. 1991;49:186–189. [PubMed] [Google Scholar]
- 25.Renda G., Özel A., Barut B., Korkmaz B., Šoral M., Kandemir Ü., Liptaj T. Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec. Nat. Prod. 2018;12:76–85. doi: 10.25135/rnp.07.17.07.117. [DOI] [Google Scholar]
- 26.Trendafilova A., Todorova M., Gavrilova A., Vitkova A. Flavonoid glycosides from Bulgarian endemic Alchemilla achtarowii Pawl. Biochem. Syst. Ecol. 2012;43:156–158. doi: 10.1016/j.bse.2012.03.013. [DOI] [Google Scholar]
- 27.Trendafilova A., Todorova M., Nikolova M., Gavrilova A., Vitkova A. Flavonoid constituents and free radical scavenging activity of Alchemilla mollis. Nat. Prod. Commun. 2011;6:1851–1854. doi: 10.1177/1934578X1100601216. [DOI] [PubMed] [Google Scholar]
- 28.Mandrone M., Coqueiro A., Poli F., Antognoni F., Choi Y.H. Identification of a collagenase-inhibiting flavonoid from Alchemilla vulgaris using NMR-based metabolomics. Planta Med. 2018;84:941–946. doi: 10.1055/a-0630-2079. [DOI] [PubMed] [Google Scholar]
- 29.Fraisse D., Carnat A., Carnat A.P., Lamaison J.L. Standardisation des parties aériennes d’alchémille. Ann. Pharm. Fr. 1999;57:401–405. [PubMed] [Google Scholar]
- 30.Fraisse D., Heitz A., Carnat A., Carnat A.P., Lamaison J.L. Quercetin 3-arabinopyranoside, a major flavonoid compound from Alchemilla xanthochlora. Fitoterapia. 2000;71:463–464. doi: 10.1016/S0367-326X(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 31.Neagu E., Paun G., Albu C., Radu G.L. Assessment of acetylcholinesterase and tyrosinase inhibitory and antioxidant activity of Alchemilla vulgaris and Filipendula ulmaria extracts. J. Taiwan Inst. Chem. Eng. 2015;52:1–6. doi: 10.1016/j.jtice.2015.01.026. [DOI] [Google Scholar]
- 32.Denev P., Kratchanova M., Ciz M., Lojek A., Vasicek O., Blazheva D., Nedelcheva P., Vojtek L., Hyrsl P. Antioxidant, antimicrobial and neutrophil-modulating activities of herb extracts. Acta Biochim. Pol. 2014;61:359–367. doi: 10.18388/abp.2014_1907. [DOI] [PubMed] [Google Scholar]
- 33.Akkol E.K., Demirel M.A., Acıkara O.B., Süntar I., Ergene B., Ilhan M., Ozbilgin S., Saltan G., Keleş H., Tekin M. Phytochemical analyses and effects of Alchemilla mollis (Buser) Rothm. and Alchemilla persica Rothm. in rat endometriosis model. Arch. Gynecol. Obstet. 2015;292:619–628. doi: 10.1007/s00404-015-3665-6. [DOI] [PubMed] [Google Scholar]
- 34.Duckstein S.M., Lotter E.M., Meyer U., Lindequist U., Stintzing F.C. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Zeitschrift Naturforsch. Sect. C J. Biosci. 2012;67:529–540. doi: 10.1515/znc-2012-11-1201. [DOI] [PubMed] [Google Scholar]
- 35.Filippova E.I. Antiviral activity of Lady’s Mantle (Alchemilla vulgaris L.) extracts against Orthopoxviruses. Bull. Exp. Biol. Med. 2017;163:374–377. doi: 10.1007/s10517-017-3807-x. [DOI] [PubMed] [Google Scholar]
- 36.Karatoprak G.S., Ilgun S., Kosar M. Phenolic composition, anti-inflammatory, antioxidant, and antimicrobial activities of Alchemilla mollis (Buser) Rothm. Chem. Biodivers. 2017;14:e1700150. doi: 10.1002/cbdv.201700150. [DOI] [PubMed] [Google Scholar]
- 37.El-Hadidy E.M., Refat O.G., Halaby M.S., Elmetwaly E.M., Omar A.A. Effect of Lion’s Foot (Alchemilla vulgaris) on liver and renal functions in rats induced by CCl4. Food Nutr. Sci. 2018;09:46–62. doi: 10.4236/fns.2018.91004. [DOI] [Google Scholar]
- 38.Tasić-Kostov M., Arsić I., Pavlović D., Stojanović S., Najman S., Naumović S., Tadić V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019;238:111789. doi: 10.1016/j.jep.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Vlaisavljević S., Jelača S., Zengin G., Mimica-Dukić N., Berežni S., Miljić M., Stevanović Z.D. Alchemilla vulgaris agg. (Lady’s mantle) from central Balkan: Antioxidant, anticancer and enzyme inhibition properties. RSC Adv. 2019;9:37474–37483. doi: 10.1039/C9RA08231J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dos Santos Szewczyk K., Pietrzak W., Klimek K., Gogacz M. LC-ESI-MS/MS identification of biologically active phenolics in different extracts of Alchemilla acutiloba Opiz. Molecules. 2022;27:621. doi: 10.3390/molecules27030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radović J., Suručić R., Niketić M., Kundaković-Vasović T. Alchemilla viridiflora Rothm.: The potent natural inhibitor of angiotensin I converting enzyme. Moll. Cell. Biochem. 2022;477:1893–1903. doi: 10.1007/s11010-022-04410-7. [DOI] [PubMed] [Google Scholar]
- 42.Geiger C., Scholz E., Rimpler H. Ellagitannins from Alchemilla xanthochlora and Potentilla erecta. Planta Med. 1994;60:384–385. doi: 10.1055/s-2006-959510. [DOI] [PubMed] [Google Scholar]
- 43.Nikolova M., Dincheva I., Vitkova A., Badjakov I. Phenolic acids and free radical scavenging activity of Bulgarian endemic—Alchemilla jumrukczalica Pawl. Planta Med. 2011;77:802–804. doi: 10.1055/s-0031-1282722. [DOI] [Google Scholar]
- 44.Ilić-Stojanović S., Nikolić V., Kundaković T., Savić I., Savić-Gajić I., Jocić E., Nikolić L. Thermosensitive hydrogels for modified release of ellagic acid obtained from Alchemilla vulgaris L. extract. Int. J. Polym. Mater. Polym. Biomater. 2018;67:553–563. doi: 10.1080/00914037.2017.1354202. [DOI] [Google Scholar]
- 45.Condrat D., Mosoarca C., Zamfir A.D., Crişan F., Szabo M.R., Lupea A.X. Qualitative and quantitative analysis of gallic acid in Alchemilla vulgaris, Allium ursinum, Acorus calamus and Solidago virga-aurea by chip-electrospray ionization mass spectrometry and high performance liquid chromatography. Cent. Eur. J. Chem. 2010;8:530–535. doi: 10.2478/s11532-010-0012-4. [DOI] [Google Scholar]
- 46.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inci Ş., Eren A., Kirbağ S. Determination of antimicrobial and antioxidant activity of Alchemilla alpina L. Turk J. Food Agric. Sci. 2021;9:2260–2264. [Google Scholar]
- 48.Hazar A., Raed A., Nidal J., Motasem M. Evaluation of phytochemical and pharmacological activities of Taraxacum syriacum and Alchemilla arvensis. Jordan J. Pharm. Sci. 2021;14:457–471. [Google Scholar]
- 49.Vitkova A., Nikolova M., Delcheva M., Tashev A., Gavrilova A., Aneva I., Dimitrov D. Influence of species composition on total phenolic content and antioxidant properties of Herba Alchemillae. Bulg. J. Agric. Sci. 2015;21:990–997. [Google Scholar]
- 50.Acet T., Özcan K. Determination of antioxidant and antimicrobial properties of lady’s mantle (Alchemilla ellenbergiana) extracts. GÜFBED/GUSTIJ. 2018;8:113–121. doi: 10.17714/gumusfenbil.308430. [DOI] [Google Scholar]
- 51.Uçar Sözmen E., Eruygur N., Akpolat A., Çetin M.D., Durukan H., Demirbaş A., Karaköy T. Sivas İli Doğal Florasından Toplanan Sarı Kantaron (Hypericum scabrum L.) ve Aslan Pençesi (Alchemilla mollis (Buser) Rothm) Bitkilerinin Bazı Kalite Kriterlerinin Belirlenmesi. J. Inst. Sci. Tech. 2020;10:1410–1418. doi: 10.21597/jist.655128. [DOI] [Google Scholar]
- 52.Stanilova M., Gorgorov R., Trendafilova A., Nikolova M., Vitkova A. Influence of nutrient medium composition on in vitro growth, polyphenolic content and antioxidant activity of Alchemilla mollis. Nat. Prod. Commun. 2012;7:761–766. [PubMed] [Google Scholar]
- 53.Hwang E., Ngo H.T.T., Seo S.A., Park B., Zhang M., Yi T.-H. Protective effect of dietary Alchemilla mollis on UVB-irradiated premature skin aging through regulation of transcription factor NFATc1 and Nrf2/ARE pathways. Phytomedicine. 2018;39:125–136. doi: 10.1016/j.phymed.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 54.Karatoprak G.S., Ilgun S., Kosar M. Antiradical, antimicrobial and cytotoxic activity evaluations of Alchemilla mollis (Buser) Rothm. Int. J. Herb. Med. 2018;6:33–38. [Google Scholar]
- 55.Nedyalkov P., Kaneva M., Mihaylova D., Kostov G., Kemilev S. Influence of the ethanol concentration on the antioxidant capacity and polyphenol content of Alchemilla mollis extracts. Comptes Rendus Acad. Bulg. Sci. Sci. Math. Nat. 2015;68:1491–1502. [Google Scholar]
- 56.Shafaghat A. Chemical constituents, antioxidant and antibacterial activities of the hexane extract of Alchemilla sericata Reichenb. J. Food Biochem. 2019;43:9–14. doi: 10.1111/jfbc.12913. [DOI] [Google Scholar]
- 57.Oktyabrsky O., Vysochina G., Muzyka N., Samoilova Z., Kukushkina T., Smirnova G. Assessment of antioxidant activity of plant extracts using microbial test systems. J. Appl. Microbiol. 2009;106:1175–1183. doi: 10.1111/j.1365-2672.2008.04083.x. [DOI] [PubMed] [Google Scholar]
- 58.Boroja T., Mihailović V., Katanić J., Stanković N., Mladenović M. Alchemilla vulgaris L. as a potential source of natural antioxidants. Zb. Rad. 2014;19:233–237. [Google Scholar]
- 59.Hamid K., Azman N., Sharaani S., Zain N., Ahmad N., Sulaiman A., Chik S., Ishak W., Pablos M. Alchemilla vulgaris and Filipendula ulmaria extracts as potential natural preservatives in beef patties. Malaysian J. Anal. Sci. 2017;21:986–995. doi: 10.17576/mjas-2017-2104-26. [DOI] [Google Scholar]
- 60.Tadić V., Krgović N., Žugić A. Lady’s mantle (Alchemilla vulgaris L., Rosaceae): A review of traditional uses, phytochemical profile, and biological properties. Lek. Sirovine. 2020;40:66–74. doi: 10.5937/leksir2040066T. [DOI] [Google Scholar]