Abstract
Various countries and organizations call for banning the use of antibiotic growth promoters (AGPs) as prophylaxis and for growth promotion in the livestock industry. Hence, seeking a substitute for antibiotics is strongly required by the livestock industry to maintain the productivity level and profits. Probiotics could represent one viable solution because of their beneficial effects on host health and maintaining the intestinal microbiota balance. In the present study, we aimed to isolate bacterial strains with probiotics properties from JinHua pig (a Chinese native pig breed) gastrointestinal tract that have antagonistic activity against to common disease-causing bacteria on farms. The four most potent strains were isolated (PP31, BA11, BA40, BV5) by the agar well diffusion method and further characterized by acid, bile salt, trypsin tolerance, whole genome sequencing (WGS), and suppressing Clostridium perfringens adhesion to IPEC-J2 cells. According to these results, BA40 had the highest number and variety of probiotic secondary metabolic secretion genes and capacity to exclude the attachment of Clostridium perfringens to IPEC-J2 cells as same as PB6. The animal experiment in vivo illustrated that BA40 and PB6 could reduce the phenomenon induced by Clostridium perfringens challenge of body weight loss, colon length decrease, pro-inflammatory cytokine increase, and Clostridium perfringens and Escherichia coli increase. The present study provides evidence that BA40 could represent a novel probiotic candidate as PB6, which exhibited some probiotic features and mitigated the burden of Clostridium perfringens associated gut disease.
Keywords: JinHua pig, probiotics, whole genome sequencing, mice model, immunity, gut microbiota
1. Introduction
There are many common pathogens that could cause foodborne diseases, such as Escherichia coli, Salmonella enterica, Staphylococcus aureus, and Clostridium perfringens, which also cause diseases in animals, including cattle [1], poultry [2], and pigs [3,4]. As for Clostridium perfringens, it always involves intestinal problems in animals with a huge economic loss in the livestock industry because of the high mortality rate [5,6]. These animal products with security risks may cause many problems, including an increase in livestock mortality and a decline in production and foodborne illnesses. Using antibiotics can reduce bacterial infections. However, the use of antibiotics for feed purpose is banned and for other purpose is strictly regulated because the spread of antibiotic-resistant pathogens has become a serious problem [7,8]. Therefore, the development of a safe and efficient additive with antimicrobial properties has attracted scholars’ attention. Numerous alternative choices of substitutes emerged, including phytogenic feed additives, antimicrobial peptides, bacteriophages, prebiotics, and probiotics [9,10,11]. Among these alternatives, probiotics represent one viable alternative because of its beneficial effects to host health and maintaining the intestinal microbial balance [12]. Probiotics were defined by World Health Organization (WHO) in 2001—”Probiotics are live microorganisms in sufficient numbers that, when administered, are beneficial to the health of the host” and the International Scientific Association for Probiotics and Prebiotics (ISAPP) strengthen this concept of probiotics [13]. One mechanism for probiotics playing important roles is that it can inhibit the proliferation of pathogenic bacterial and stimulate the growth of beneficial microorganism. Probiotics can interact with gut microbiota and improve the microbial barrier function [14]. Besides, many bacterial genera have been described as probiotic properties with anti-pathogenic activity, such as bacillus and lactobacillus [15,16,17,18]. Now, some bacterial of the host’s gut community, such as many bacillus and lactobacillus strains, have been good probiotic candidates.
Generally, native breeds of livestock have higher disease resistance than commercial breeds. PB6 was obtained from health broilers and broadly used for inhibiting Clostridium perfringens in the livestock industry [19,20,21,22]. JinHua pigs (a Chinese native pig breed, named China panda pig) have stronger resistance to bacterial invasion than Landrace × Yorkshire (commercial breed) pigs [23]. Hence, screening the isolates from the JinHua pig’s intestinal mucosa as probiotics candidates is a good way to find antibiotic alternatives in the livestock industry. Probiotics can achieve the best effects when they are alive and in a similar environment to where they were isolated from in the same species [24]. Although these probiotics have advantages, we have to evaluate the safety of them and isolate them from the healthy organism. Therefore, we aimed to isolate potential probiotic candidates from the ceca mucosa of finishing JinHua pig, based on anti-pathogenic capacity (especially for Clostridium perfringens), acid tolerance, bile salt tolerance, trypsin tolerance, whole genome sequencing, and capacity to exclude the attachment of Clostridium perfringens to IPEC-J2 cells. Then we verified the safety and efficacy of the isolated strains in mice model.
2. Materials and Methods
We carried out all the procedures in accordance with the university’s regulations on animal experimentation and strictly enforced the guidelines of the Institutional Animal Care and Use Committee of Zhejiang University (ZJU20220164) during the experimental process.
2.1. Isolation of Microbes from JinHua Pig Intestines
JinHua pig (Chinese panda pigs, well known for their resistance to disease) feces were collected and sterile swabs (Biosigma Inc., Cona, Italy) were used to collect the fecal samples. Sterile swabs can avoid contamination and the fecal samples were maintained in a solution with 25% (v/v) glycerol and stored in liquid nitrogen to preserve the microorganisms as the previous study [25].
We mixed 1 g of fecal samples with normal phosphate-buffered saline (PBS) to get suspension. Furthermore, 10-fold serial dilutions by sterilized water were performed in our laboratory. 100 μL of 10-fold dilutions samples was incubated anaerobically in Man, Rogosa, and Sharpe (MRS) plate, and aerobically in Luria Bertani (LB) plate at 37 °C for 24 h till a single colony of appropriate size was grown, respectively. Representative colonies were selected, and the colonies were purified by placing them on new agar plates. The purified colonies were cultured in the corresponding broths and stored at −80 °C with 25% (v/v) glycerol.
2.2. Bacterial Strains Preparation
The following strains were used to detect the antimicrobial activity of probiotics candidates: Escherichia coli (ATCC), Salmonella enterica (ATCC29628), Staphylococcus aureus (ATCC6538), and Clostridium perfringens (ATC13124) were purchased through China Center of Industrial Culture Collection (CCICC). Salmonella enterica, Escherichia coli, and Staphylococcus aureus were cultured in LB broth overnight at 37 °C. Clostridium perfringens was cultured under an anaerobic environment in Reinforced Clostridium Medium (RCM) for 24 h at 37 °C. The Bacillus subtills PB6 (ATCC-PTA 6737) were purchased from Kemin Industries and cultured in LB broth at 37 °C for 12 h. PB6 was used as a positive probiotic control. Isolated strains from JinHua pig feces were cultured in LB broth or RCM broth at 37 °C under aerobic or anaerobic conditions as previous study described [26]. All isolated strains were purified three times since the probiotics were isolated from the intestinal mucosa on agar plates. Then all probiotics candidates were stored at −80 °C.
2.3. Preparation of Culture Supernatant and Agar-Well Diffusion Method
We used agar well diffusion to test antimicrobial activity of isolates from JinHua pigs [27,28]. Briefly, the broth (LB or RCM) with 1.5% agar (10 mL) was poured onto the sterile Petri plates (10 × 10 mm). When the agar solidified, we seeded it with 1% of pathogen cultures (approximately 1 × 108 CFU/mL) in autoclaved LB or RCM broth (containing 0.75% agar and cooled about 45 °C) 10 mL. When the plate solidified, 8-mm diameter Oxford Cups were placed on the plate to make five wells, then each well was filled with 100 μL cell-free supernatant (CFS) extracted from isolates. These plates were refrigerated at 4 °C for 4 h. Then the plates were incubated at 37 °C for 12–24 h according to the growth requirements of each pathogen. We obtained CFS by centrifuging at 6000 rpm for 5 min, filtration through a 0.22 μm pore size filter (Millipore, China). Finally, the zone of inhibition could be visibly observed as a clear location where there was no obvious pathogenic growth. The diameters of the inhibition zone were measured by calipers. Each test was carried out in triplicate.
2.4. Identification of the Selected Isolates by 16S rRNA Sequencing
16S rRNA analysis method were used to test the isolated probiotic strain candidates First, extracting Genomic DNA by DNeasy Blood and Tissue Kit (Qiagen, Toronto, ON, Canada) as previous study [29]. For isolates, the amplified gene of 16S rRNA using the 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-CTACGGCTACCTTGTTACGA-3′) universal primer sets. Amplifications by PCR and sequenced by the Shanghai Majorbio Bio-Pharm Technology Co.,Ltd (Shanghai, China). The results of sequences were aligned against 16S ribosomal RNA database by using the National Centre for Biotechnology Information (NCBI) BLAST platform. Finally, the sequencing results were uploaded to the GeneBank database: ON227058 for Bacillus amyloliquefaciens 40 (BA40), ON227093 for Bacillus amyloliquefaciens 11 (BA11), ON227128 for Bacillus velezensis 5 (BV5) and ON228197 for Pediococcus pentosaceus 31 (PP31).
2.5. Growth Characteristics, Acid, Bile Salts and Trypsin Tolerance
Streaking and inoculating PB6, PP31, BA11, BA40, and BV5 onto plates to activate these strains. Briefly, 1% activated strains were anaerobically or aerobically in LB or MRS broth at 37 °C, respectively.
The previous study [30] used the acid, bile, and trypsin to examine the resistance of probiotics candidates. 1% of activated strains was incubated in LB or MRS broth with pH (2.5, 3.5, 4.5), bile salts (0.2%, 0.5%, 0.8%), or trypsin (0.2%, 0.3%, 0.4%). Then, probiotics candidates were incubated at 37 °C for 2 h under anaerobic or aerobic condition (200 rpm shaking). We used the ten-fold serial dilutions method and drop plating method to enumerate the colony counts. The control is 0 h. The survival ratio of probiotics candidates in different pH conditions, different bile salts, or trypsin levels were calculated by the colony counting method, respectively.
2.6. Suppress Pathogen Adhesion to IPEC-J2 Cell
IPEC-J2 cells were cultivated using the method in the previous study [31]. Separately cultured isolated strains and Clostridium perfringens ATCC13124, and collected the bacteria respectively. Then we washed three times with PBS and resuspended by DMEM/F12 medium. Then the isolated strains were adjusted concentration at 1 × 108 CFU/mL, and Clostridium perfringens was adjusted at 1 × 106 CFU/mL. Antibacterial activity experiments were performed by competition, exclusion, and replacement trials, respectively. The co-culture of isolated strains of Clostridium perfringens were placed onto the IPEC-J2 cell plate for 2 h, then we tested their competition ability with pathogens. The exclusion trail process was presented as follows: Isolated strains were added onto the IPEC-J2 cell plate and incubated in an atmosphere of 5% CO2 at 37 °C, 1 h. Then we discarded the supernatant and washed the plates three times with PBS; subsequently, we added Clostridium perfringens to the co-culture for 1 h again to test the exclusion ability. The difference between the replacement and exclusion trails was that the order of lying the isolated strains was different. Briefly, Clostridium perfringens and IPEC-J2 cells were co-cultured for 1 h, and other isolated strains were added to the mixture 1 h. Besides, all the control groups used DMEM/F12 to replace isolated strains. Finally, we calculated the inhibition rate via the numbers of live Clostridium perfringens. The formula is:
2.7. Whole Genome Sequencing and Analysis
We extracted Genomic DNA by SPARKeasy Genome DNA Purification kit (SparkJade, Jinan, China) in accordance with the manufacturer’s instructions. The high purity DNA was sequenced at Shanghai Majorbio Biopharm Technology Co., Ltd. by Illumina sequencing platforms (Hiseq X Ten; Illumina, San Diego, CA, USA), and the method was conducted as the previous study [32]. The low-quality Illumina reads were filtered off to acquire clean data for further analysis. Raw reads quality control was also performed, including base quality, error rate, and distribution. The quality checked DNA samples were constructed with inserts of ~400 bp, and paired-end reads with a length of 150 bp, providing no less than 100× coverage depth of the genome for each sample. Then the Illumina reads were used to estimate the genome size, repeat content, heterozygosity, and finally assembled by SOAPdenovo (Version 2.04 (https://github.com/aquaskyline/SOAPdenovo2 accessed on 24 December 2021)) as previous to generate genome scaffold. Sequenced data were deposited at NCBI GeneBank database under the BioProject ID PRJNA826263, with accession numbers JALMGL000000000 to JALMGO000000000 and accessed on 8 August 2022.
Glimmer (Version 3.02 (http://ccb.jhu.edu/software/glimmer/index.shtml accessed on 24 December 2021)) predicted the coding sequences (CDSs) and Non-Redundant Protein Database (NR), Swiss-Port, Pfam, Gene Ontology (GO), Clusters of orthologous Group (COG), and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotated it. Sequence alignment tools such as antiSMASH (Version 4.0.2 (https://dl.secondarymetabolites.org/releases/4.0.2/ accessed on 24 December 2021)), Virulent Factor Database (VFDB, Version:2016.03 (http://www.mgc.ac.cn/VFs/main.htm accessed on 24 December 2021)), and Comprehensive Antibiotic Resistance Database (CARD, Version 1.1.3 (https://card.mcmaster.ca accessed on 24 December 2021)) were used to classify and predict the gene function and gene annotations were obtained from the best-matched subject (E-value < 10−5). We used the free online Majorbio Cloud Platform (https://cloud.majorbio.com accessed on 24 December 2021) and analyzed all data.
2.8. Animal Experiment
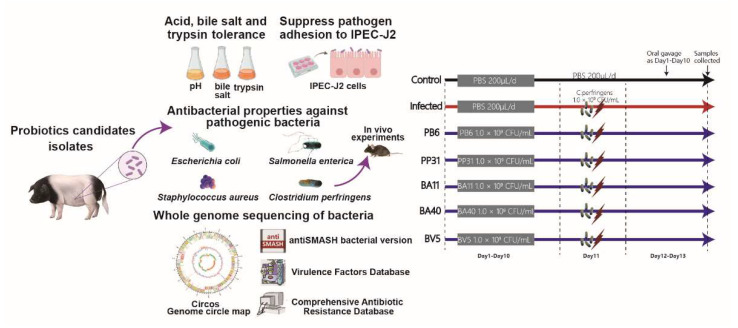
We purchased forty-two mice (five-week, male C57BL/6) from Shanghai Laboratory Animal Co., Ltd. (SLAC), Shanghai, China. We separated all mice randomly into 7 groups (Figure 1) after adaptation: Control, Infected, PB6, PP31, BA11, BA40, BV5. In the Control and Infected group, the mice were orally dosed with 200 μL PBS from day 1 to day 13. The PB6, PP31, BA11, BA40, and BV5 groups were dosed with 200 μL PBS containing 1 × 109 CFU probiotics candidates from day 1 to day 13, respectively. Meanwhile, the mice in Infected, PB6, PP31, BA11, BA40, and BV5 groups were orally challenged with 200 μL resuspension Clostridium perfringens (1 × 109 CFU) on day 11, and the mice in Control group was treated with 200 μL PBS. We weighted all mice every day, and mice were free accessed to the water and feed.
Figure 1.
Experimental design.
2.9. Sample Collection and Treatment
After the last gavage and waiting for 12 h, all the mice were weighted and sacrificed. The weights of spleen and liver were recorded as previous study [33]. We used these results to calculate the organ index. We used a vernier caliper to measure the length of colon and collected the blood samples through cardiac puncture. After centrifugation at 3000× g (10 min at 4 °C), we obtained the serum. Simultaneously, digesta in the ileum and cecum were collected into 2 mL sterile tubes (Sigma-Aldrich, Los Angeles, CA, USA) for determining the microbiota enumeration.
2.10. Bacteria Enumeration of Ileum and Cecum
On day 13, digesta for bacteriological examination were collected aseptically from the ileum and cecum of mice. The population of Clostridium perfringens, Escherichia, and Lactobacillus species in the digesta were detected by the method of absolute quantitative real-time PCR (RT-PCR), as previously described [34], with some modification. Briefly, DNA were isolated from the ileum and cecum. Standard curves for RT-PCR were prepared using DNA extracted from pure cultures to produce a high concentration of the target DNA by normal PCR amplification. Primer sequences were showed in Table 1. We applied Escherichia coli DH5α (Takara Bio Inc., Kusatsu, Japan) to generate plasmid standards. We used PCR purification kit (Biomed Gene Technologies, Beijing, China) to purify PCR products and accessed to clone into pCR 2.1 by TA cloning kit (Invitrogen Corporation, Carlsbad, CA, USA). We exerted Nanodrop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA) to quantify the purified insert-containing plasmids. Then target DNA copies were calculated [35]:
Table 1.
qRT-PCR primers used to quantify intestinal bacteria.
The standard curve was constructed by the ten-fold serial dilutions of plasmid DNA. We used a StepOne Real-Time PCR System (ABI StepOnePlue, Applied Biosystem, Foster City, CA, USA) according to commercial SYBR-Green PCR kit (Takara Biotechnology Inc., Kusatsu, Japan) protocols for absolute qRT-PCR template.
2.11. Determination of Inflammatory Cytokines, Immunoglobulin, DAO, and DLA Concentrations
We used ELISA kits (Enzyme-Labeled, Nanjing, Jiangsu, China) to determine Inflammatory cytokines IL-1β, IL-6, TNF-α and immunoglobulin IgA, IgG and fecal sIgA. We detected D-lactate (DLA) and diamine oxidase (DAO) concentrations in serum and the inducible nitric oxide synthase (iNOS) and nitric oxide (NO) in the jejunum using ELISA kits (Enzyme-Labeled, Nanjing, Jiangsu, China). The protocols were followed by previous studies [29,36].
2.12. Statistical Analysis
We conducted all experiments three or six times. The results were expressed as mean ± standard deviation (SD). One-factor analysis variance (ANOVA) was used ± to statistical analysis by SPSS 20. A p-value < 0.05 was considered to be significant. Graph pad Prism 8 was used to perform a statistical analysis.
3. Results
3.1. Pathogen Inhibition Using Well Diffusion Assay
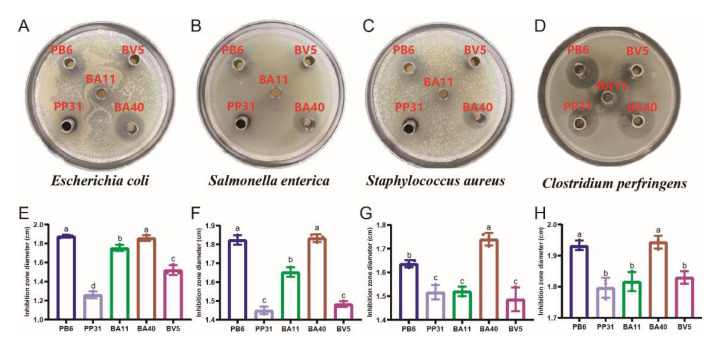
The results of antimicrobial activity detection have shown that the isolated strains can inhibit the growth of Escherichia coli, Salmonella enterica, Staphylococcus aureus, and Clostridium perfringens (Figure 2A–D). The BA40 presented the best antimicrobial activity against these four foodborne disease microorganisms in humans and animals (Figure 2E–H) than other isolated probiotics (p < 0.05). These results indicated that BA40 has the potential as an effective probiotic to resist the pathogens for further studies.
Figure 2.
Agar well diffusion assay illustrating the growth inhibition of pathogenic bacteria by cell-free supernatants extracted from the isolated strains. (A) Escherichia coli plate. (B) Salmonella enterica plate. (C) Staphylococcus aureus plate. (D) Clostridium perfringens plate. (E) Inhibition zone diameter of Escherichia coli (F) Inhibition zone diameter of Salmonella enterica (G) Inhibition zone diameter of Staphylococcus aureus (H) Inhibition zone diameter of Clostridium perfringens. a, b, c Means values with dissimilar letters were significantly different (p < 0.05). All values contained three repetitions.
3.2. Phenotypic Characteristics of Isolated Strains
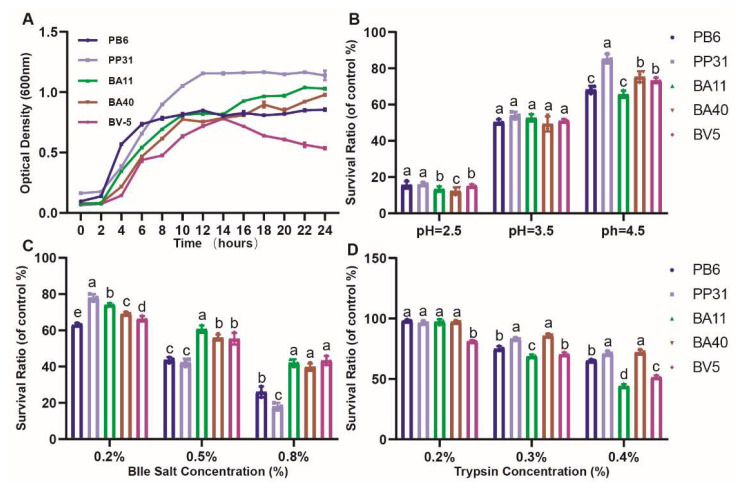
Four isolates were selected by agar diffusion assay in Figure 3. The growth curves (Figure 3A) of 4 isolates started to enter the logarithmic growth phase quickly (4 h), and 6–10 h reached the stable phase except PP31 (12 h). The fastest one arrived plateau was strain PB6, which is the positive control; the slowest one was BV5, with a time of 12 h. It is critical for surviving in the gastrointestinal tract to tolerate acid, bile salt and trypsin as probiotics. The isolated strains’ survival rate increased with the rise of pH (Figure 3B). The bile salt and trypsin tolerance test (Figure 3C,D) presented the different results compared to pH assay, because the survival rate of all strains was negatively correlated with the increase in bile or trypsin concentration. There was the highest survival rate in PP31 and PB6 (16.10%, 15.67%) at pH 2.5. BA40 had the best performance in the bile salt and trypsin tolerance test than others (p < 0.05), which showed the survival rate of 39.67% at 0.8% bile salt and 72.10% at 0.4% trypsin.
Figure 3.
Probiotic properties of isolated strains. (A) The growth curves. (B) The ability of acid tolerance. (C) The ability of bile salt tolerance. (D) The ability of trypsin tolerance. a, b, c, d Means values with dissimilar letters were significantly different (p < 0.05). All values contained three repetitions.
3.3. Adhesion of Isolated Strains to IPEC-J2
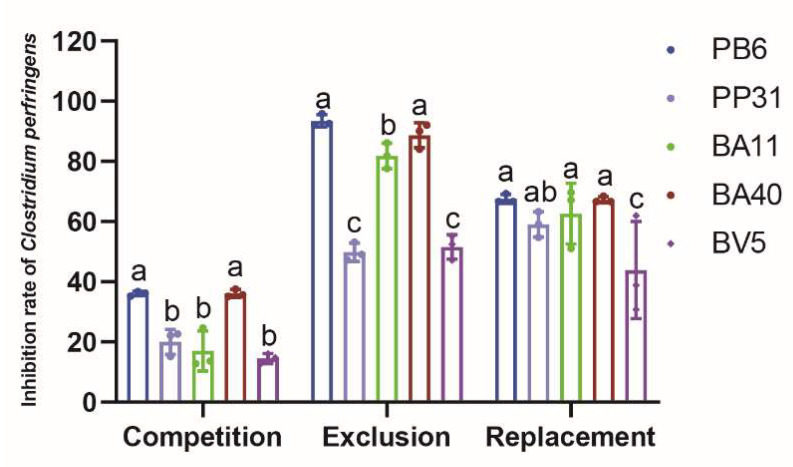
As the data shown in Figure 4, isolate probiotics proved that it inhibited Clostridium perfringens adhering to IPEC-J2 cells in exclusion and replacement trails, effectively. In the competition trail, the BA40 had the same ability (36.10%) as PB6 (36.07%) to compete with Clostridium perfringens. In the exclusion trail, PB6 (93.47%), BA40 (88.68%), and BA11 (81.84%) presented a stronger ability (p < 0.05) to exclude Clostridium perfringens than PP31 (49.81%) and BV5 (51.58%). For the replacement experiment, the isolated probiotics had similar results except BV5. The inhibition rate of Clostridium perfringens of PB6 (67.52%), PP31 (59.04%), BA11 (62.64%), and BA40 (67.25%) were higher than BV5 (43.85%). All results indicated that the BA40 had an identical ability as PB6 to inhibit the pathogenic bacteria and has the potential to become an antibiotic alternative.
Figure 4.
The inhibitory results of isolate strains on the adhesion of Clostridium perfringens. a, b, c Means values with dissimilar letters were significantly different (p < 0.05). All values contained three repetitions.
3.4. Whole-Genome Sequence of the Isolated Probiotics
Table 2 summarizes the genomic information of these isolated probiotics strains. According to genome sequences obtained using Illumina Hiseq, the genome sequence of PP31, BA11, BA40, and BV5 were presented with genome size, genes on forward strand, genes on reverse strand, rRNA, tRNA, GC content, and GC skew (Supplementary Figure S1A–D). There were several secondary metabolic gene clusters via an antiSMASH analysis, while only four gene clusters harbored 100% similarity in BA11, BA40, and BV5, respectively, to known secondary metabolites (Table 3). The metabolites of these gene clusters were Bacillaene, Macrolactin H, Bacilysin, Bacillibactin, Mersacidin, and Amylocyclicin, with antibacterial, antifungal, antiviral, anti-biofilm, and biocontrol activities. Additionally, we found genes with up to 50% similarity after blasting in the database of virulence factors. We also classified these genes into three groups: defensive virulence factors, offensive virulence factors, and non-specific virulence factors. We found no virulence genes but rather regulatory genes that played important roles in regulating biological processes, including virulence in other bacteria (Supplementary Table S1). Moreover, we found genes > 50% similarity through blasting in the Comprehensive Antibiotic Resistance Database (CARD). There are four genes ErmB, ErmA, InuA, and poxtA that are important for the resistance to lincosamide antibiotics, macrolide antibiotics, and streptogramin antibiotics in PP31 with up to 90% similarity (Supplementary Table S2). However, just two genes named clbA and ImrB are important for the resistance to lincosamide antibiotic phenicol antibiotic in BA11 and BA40 with up to 85% similarity.
Table 2.
Genomic features of isolated strains.
| Strains | 16S rRNA Identity |
Scaffold Number |
Genome Size (bp) |
GC % | N50 (bp) | Sequencing Depth (x) |
Completeness (%) |
Genebank Accession Number |
|---|---|---|---|---|---|---|---|---|
| PP31 | Pediococcuspentosaceus | 36 | 1818617 | 37.3 | 260845 | 659.14 | 97.3 | ON228197 |
| BA11 | Bacillus amyloliquefaciens | 36 | 3927418 | 46.42 | 565069 | 317.09 | 99.3 | ON227093 |
| BA40 | Bacillus amyloliquefaciens | 143 | 3969383 | 46.4 | 382464 | 322.08 | 99.3 | ON227058 |
| BV5 | Bacillus velezensis | 36 | 3867471 | 46.49 | 608593 | 328.7 | 99.3 | ON227128 |
Table 3.
Secondary metabolites predicted by the antiSMASH database.
| Strains | Cluster Type | MIBiG Accession | Similarity | Location (Start–End) | Gene Number |
|---|---|---|---|---|---|
| PP31 | Coagulin | BGC0000617 | 40% | 14107–19888 | 6 |
| BA11 | Bacillaene | BGC0001089 | 100% | 142968–252589 | 52 |
| Macrolactin H | BGC0000181 | 100% | 471746–559949 | 44 | |
| Bacilysin | BGC0001184 | 100% | 514804–556223 | 42 | |
| Bacillibactin | BGC0000309 | 100% | 73512–122977 | 44 | |
| Difficidin | BGC0000176 | 53% | 131390–177054 | 31 | |
| Locillomycin | BGC0001005 | 35% | 23265–45904 | 20 | |
| Fengycin | BGC0001095 | 20% | 1–13205 | 3 | |
| BA40 | Bacillibactin | BGC0000309 | 100% | 73491–125283 | 45 |
| Bacilysin | BGC0001184 | 100% | 661592–703011 | 42 | |
| Macrolactin H | BGC0000181 | 100% | 53617–141453 | 44 | |
| Bacillaene | BGC0001089 | 100% | 360981–470835 | 52 | |
| Fengycin | BGC0001095 | 80% | 535580–623198 | 46 | |
| Difficidin | BGC0000176 | 46% | 273837–319624 | 30 | |
| Plipastatin | BGC0000407 | 30% | 1–13991 | 12 | |
| BV5 | Macrolactin H | BGC0000181 | 100% | 507723–595523 | 44 |
| Bacilysin | BGC0001184 | 100% | 265403–306822 | 43 | |
| Mersacidin | BGC0000527 | 100% | 443880–467069 | 19 | |
| Amylocyclicin | BGC0000616 | 100% | 118402–125992 | 9 | |
| Fengycin | BGC0001095 | 80% | 1–86692 | 46 | |
| Bacillaene | BGC0001089 | 71% | 817963–854795 | 25 | |
| Difficidin | BGC0000176 | 53% | 1–45718 | 30 | |
| Surfactin | BGC0000433 | 39% | 681718–707084 | 22 |
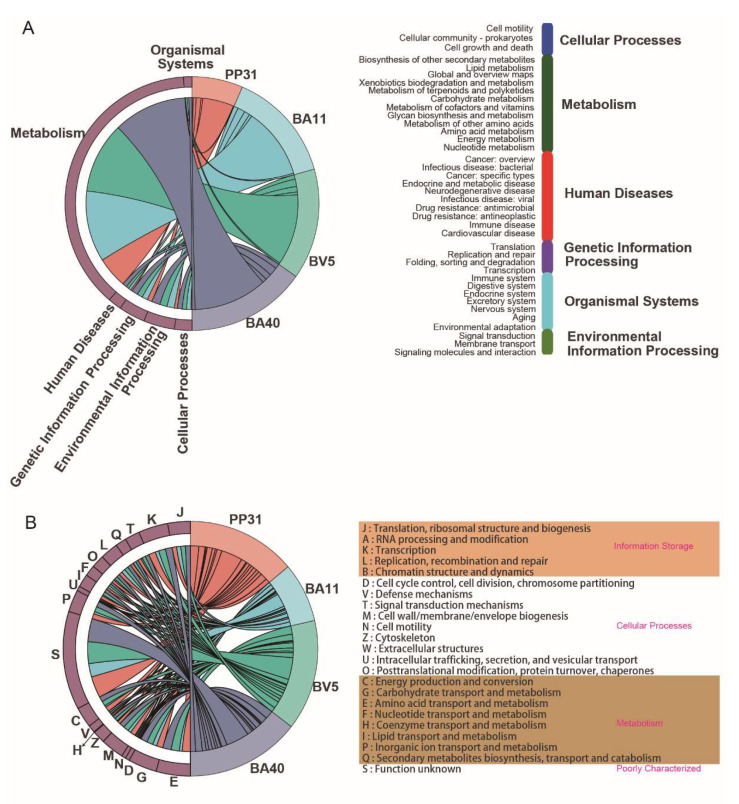
Figure 5 showed the annotated chord diagrams based on KEGG and COG database. BA40 enriched the most genes in first category of KEGG. As the Figure 5A showed, PP31 occupied the minimum genes in KEGG pathway. All isolated probiotics presented the most genes in Carbohydrate metabolism (BA40, 235; BA11, 229; BV5, 226; PP31, 126) which belonged to Metabolism. PP31, BA40 and BV5 were with up 80% of all genes with COG annotation (88.14%, 80.38% and 80.26%). BA40 were annotated the most categories of COG (21) than other isolated probiotics (PP31,19; BA11, 20; BV5, 20) in Figure 5B. E (amino acid transport and metabolism) was the highest abundance annotated in BA40 (289), BA11 (285) and BV5 (284) except S (Function unkonw), while the J (Translation, ribosomal structure and biogenesis) was the highest abundance in PP31 (142).
Figure 5.
Annotated chord diagrams based on database matching for classification. (A) Correspondence between annotated information on bacterial genomes and metabolic pathways obtained by KEGG database. (B) The COG database was compared to classify the predicted proteins into gene families and to give the corresponding functional annotation information for the family.
3.5. Effect of Isolated Probiotics Administration on the Mice
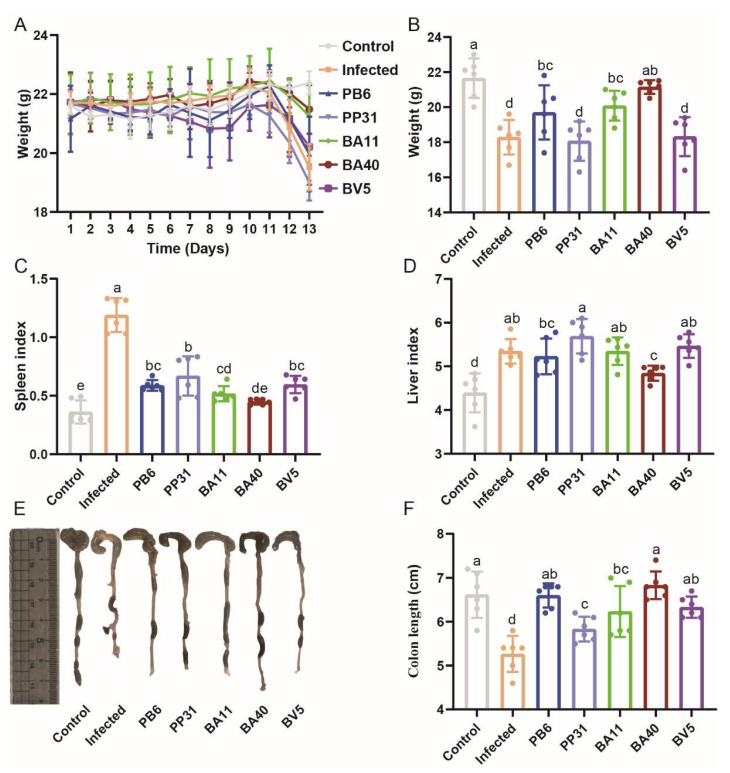
Figure 6 showed the growth performance of the seven groups during the experiment. Patrial data of this figure were published in our previous study [29]. After being challenged with Clostridium perfringens, the body weight (BW) of the Infected group showed a significant decrease (Figure 6A). However, the BW of the BA40 group and the BA11 group almost had no change when compared to the Control group (p > 0.05). At the end of the experiment (Figure 6B), the BW of BA40, BA11, and PB6 groups remained unchanged (p > 0.05) when compared to the Control group; whereas, the Infected group, PP31 group, and BV5 group showed a dramatic decrease (p < 0.05) during the Clostridium perfringens treatment. The spleen index and liver index among seven groups were presented in Figure 6C,D. The spleen index of the Infected group showed a significant increase (p < 0.05) over other groups. When compared to the Control group, the liver index in the other groups increased markedly (p < 0.05). Figure 6E,F showed that the colon length had no difference among the Control, PB6, and BA40 groups (p > 0.05). The other six groups showed a significant difference in colon length when compared to the the Infected group (p < 0.05). The growth performance indicated that the BA40 could effectively protect against Clostridium perfringens infection in mice.
Figure 6.
The protective effect of probiotic candidates against Clostridium perfringens infection in mice. (A) Bodyweight (BW). (B) At the end of experiment, mice weight. (C) The spleen index. (D) The liver index. (E) The colon images (F) The colon length. a, b, c, d Means values with dissimilar letters were significantly different (p < 0.05). All values contained six repetitions. Partial data of this figure were published in previous study. Adapted with permission from ref. [29]. Copyright 2021 Jiang, Li, Su, Wen, Gong, Zhang, Wang, Jin and Lu.
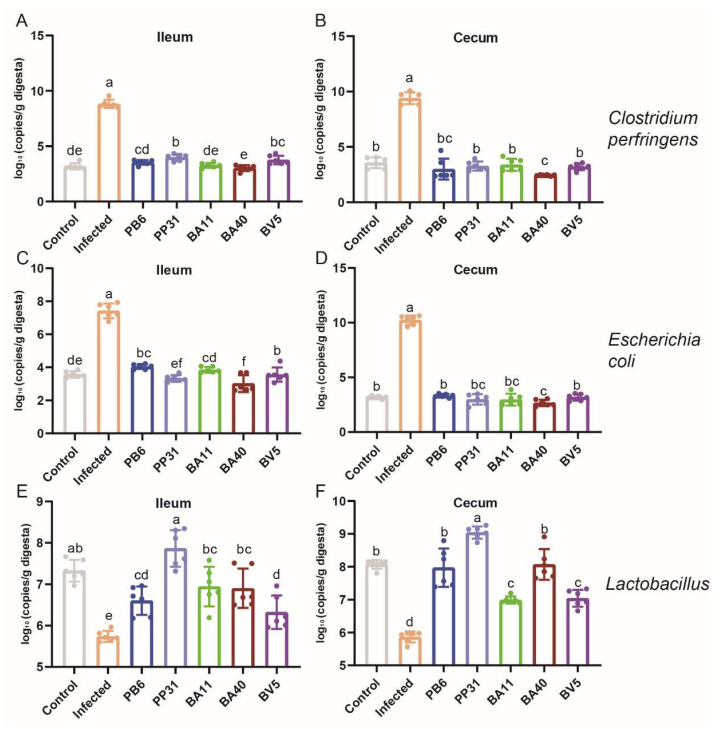
Figure 7 showed Clostridium perfringens, Escherichia coli, and Lactobacillus enumeration in ileum and cecum in mice. After Clostridium perfringens infection in cecum and ileum, the population of Clostridium perfringens and Escherichia coli increased significantly (p < 0.05) when compared to the Control group and other isolated probiotics treatments. The genes copies of Clostridium perfringens and Escherichia coli decreased remarkably (p < 0.05) in BA40. Meanwhile, the population of Lactobacillus reduced dramatically (p < 0.05) in the Infected group in contrast to the Control group in the ileum and cecum of mice. The Lactobacillus slightly increased in the ileum and notably increased (p < 0.05) in cecum of mice in the PP31 group. The population of Lactobacillus in other groups remained steady or reduced.
Figure 7.
The population of intestinal microbiota of mice. (A) Clostridium perfringens in the ileum. (B) Clostridium perfringens in the cecum. (C) Escherichia coli in the ileum. (D) Escherichia coli in the cecum. (E) Lactobacillus in the ileum. (F) Lactobacillus in the cecum. Results are presented as mean ± SD (The data were presented as log10 gene copies/g of intestinal digesta). a, b, c, d Means values with dissimilar letters were significantly different (p < 0.05).
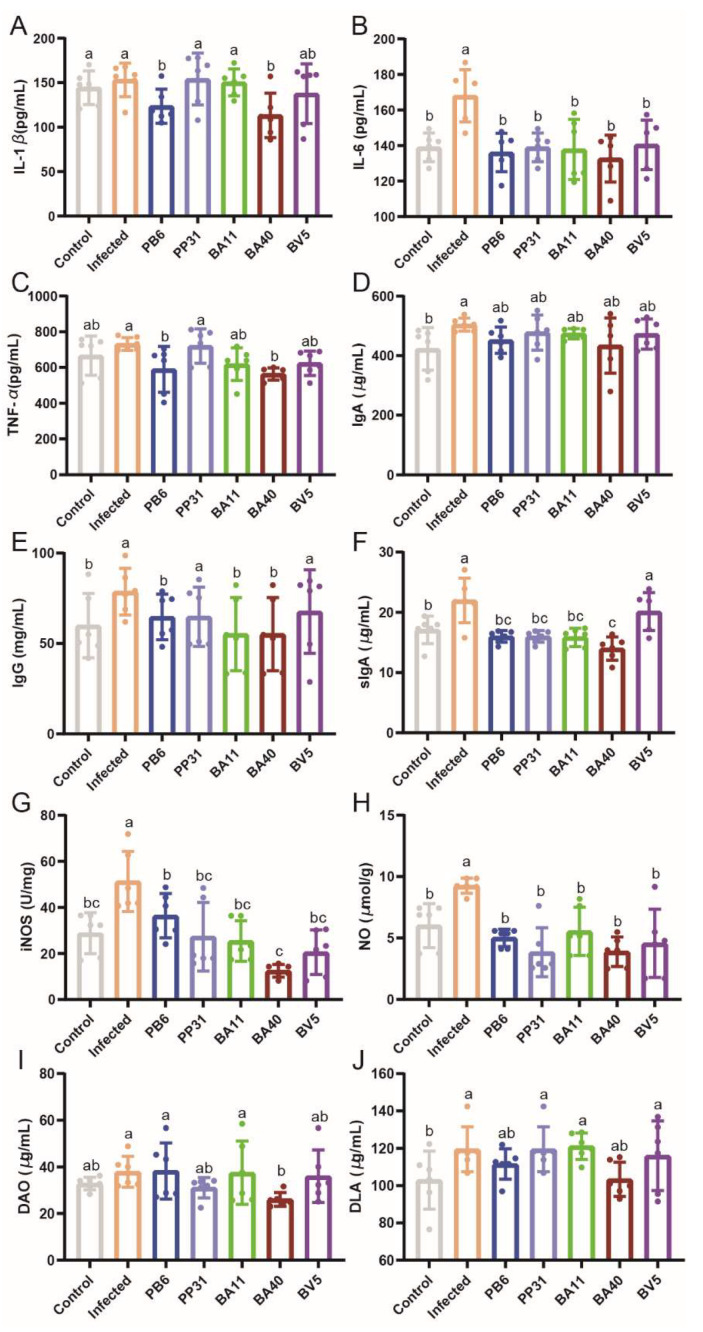
The effect of isolated probiotics on the serum inflammatory cytokines, DAO, and DLA were shown in Figure 8. Compared with the Infected group, the IL-1β, IL-6, TNF-α, and IgG (Figure 8A–E) were reduced by BA40 and PB6 treatment (p < 0.05), while IgA had no difference (p > 0.05). In the Infected group, iNOS, NO concentrations of serum and sIgA (Figure 8F–H) of the jejunum tissue had a significant increase (p < 0.05) in contrast to other groups. Figure 8I,J showed the difference of the DAO and DLA concentrations among seven groups. The DLA concentrations were significantly increased (p < 0.05) in the the Infected group, and the DAO significantly decreased (p < 0.05) by BA40 and pre-treatment compared to the Infected group. The DLA concentrations in the BA40 group (p > 0.05) had no difference in contrast to the Control group.
Figure 8.
The effect of isolated probiotics treatment on inflammatory cytokines, immunoglobulin, DAO and DLA in mice. (A) IL-1β concentrations. (B) IL-6 concentrations. (C) TNF-α concentrations. (D) IgA concentrations. (E) IgG concentrations. (F) sIgA concentrations. (G) iNOS concentrations. (H) NO concentrations. (I) DAO concentrations. (J) DLA concentrations. Results are presented as mean ± SD. a, b, c, d Means values with dissimilar letters were significantly different (p < 0.05). All values contained six repetitions.
4. Discussion
The infectious diseases are caused by pathogenic bacteria (including Escherichia coli, Salmonella enterica, Staphylococcus aureus, and Clostridium perfringens, etc.) and are one of the reasons for losses in the husbandry industry [37]. These pathogens are responsible for contracting diseases in humans (foodborne illnesses) and the decline in the livestock production [38,39,40,41]. Antibiotic bans in the farming process are used to reduce problems, such as antibiotic residues and antibiotic resistance [42]. Probiotics are one of the ideal alternatives to antibiotics. Probiotics have been shown to increase the beneficial bacterial in the gastrointestinal tract (GIT), increase nutrient absorption, and feed conversion efficiency and body weight [14,43,44,45,46]. In our study, we aimed to isolate bacterial strains with probiotics properties from the mucosa in JinHua pigs (Chinese panda pig) that could reduce common pathogenic bacteria in pig farms (Escherichia coli, Salmonella enterica, Staphylococcus aureus, and Clostridium perfringens etc.). Subsequently, isolated strains were subjected to tolerance trails, antibacterial capacity, adhesion to IPEC-J2, whole genome sequencing (WGS), and in vivo experiments. We revealed that BA40 isolated from JinHua pigs had good probiotic potential.
Given that the ability to inhibit the growth of common pathogenic bacteria on farms is often considered in the selection of potential probiotics to replace antibiotics [42,47,48], we selected four of the most active isolates and further tested their antimicrobial ability. Our antimicrobial well diffusion results revealed that the BA40 showed strong ability in inhibiting pathogens and had the proximate inhibition capacity with PB6. The result were similar with previous studies [49,50,51]. This result suggests that the JinHua pig’s isolates are possibly caused by additional production of antimicrobial compounds. For testing the potential probiotic properties of isolated strains, three trials (including acid, bile salt, and trypsin tolerance) were used to examined their tolerance capacity, because the survival of probiotic bacteria is essential for exerting health benefits and they must remain alive in the GIT to reach the large intestines [52]. Our results showed that Bacillus had better growth curve, bile salt, and trypsin tolerance, while Lactobacillus had good acid tolerance ability. For further detecting the ability to bind epithelial cell sites of isolates, in the present study, porcine intestinal epithelial cells (IPEC-J2) and the method was used as previous study described [27,53]. The BA40 showed the strong ability to outcompete pathogens for epithelial cell adhesion sites. As we all know, the adhesion to intestinal lining plays an essential role in Clostridium perfringens pathogenesis [54,55,56,57]. Our results indicated that BA40 could exclude the Clostridium perfringens, and one possible reason is that BA40 has certain adhesion abilities to IPEC-J2. The competition ability was lower than exclusion and replacement ability, which suggested to us that probiotics should be used to prevent the intestinal diseases.
From the WGS results, BA40 had the largest scale of scaffold number and genome size. Bacteriocin genes were identified in isolates strains, suggesting that isolates owned strong pathogen inhibitory activity. Based on the genomic sequencing analysis, BA11, BA40, and BV5 could produce several active compounds such as bacillibactin, marcolactin H, bacilysin, bacillaene, fengycin, and do not contain virulence genes; meanwhile, PP31 only produces coagulin with 40% similarity, and this could illustrate why PP31 had the worst inhibitory activity of pathogenic bacteria among all four isolated strains. Besides, BA11, BA40, and BV5 also possessed different antibacterial abilities, BA40 had the best anti-pathogenic activity. One possible reason that we speculate is the fengycin generated in different quantities (BA11, 20%; BA40, 80%; BV5, 80%). Pipat Piewngam et al. reported that the fengycin secreted by Bacillus could inhibit the pathogens colonized in animal intestines by competitively combining the receptor protein Accessory gene regulator (AgrC) of bacterial quorum-sensing system [58]. Additionally, BA40 is not an antibiotic-resistant bacterium because of susceptibility to the most antibiotics than PP31, BA11, and BV5, including penicillin, cefalexin, ampicillin, streptomycin, kanamycin, gentamicin, ciprofloxacin, chloramphenicol, vancomycin, imipenem, erythromycin, and norfloxacin. Based on KEGG analysis, BA40 were annotated the most genes, 2666 genes, compared other isolates, which were involved in carbohydrate metabolism (8.8%), amino acid metabolism (7.6%), and signal transduction (5.7%) and membrane transport (5.4%). Moreover, there was the same trend with KEGG analysis, the COG analysis annotated the most genes (3177) of BA40 into the most categories (21) than other bacteria. The largest COG group of BA40 was the E (amino acid transport and metabolism), followed by K (transcription), and G (carbohydrate transport and metabolism). Other studies also found that the bacteria possessed potential probiotic ability could enrich these functions based on KEGG and COG database [59,60,61].
As our previous study described, we used the Clostridium perfringens to construct a mouse model to test the effect of isolated strains in vivo [35]. We measured isolated strains’ function by analyzing the growth performance, immune status, and the population of beneficial and harmful bacteria. The animal experiment showed that Clostridium performance can influence the growth status (including body weight, spleen index, liver index, and colon length) of mice, and BA40 and PB6 were able to attenuate the side effects of Clostridium perfringens. In vivo experiments demonstrated that it had the same effect as in vitro experiments. One way the isolated strains exerted the critical role is that it could regulate the gut microbiota of mice. As the reported described, probiotics supplementation could modulate the gut microbiota, improve the immune status, and increase the growth performance. Lactobacillus is one of the biomarkers to measure the balance of gut microbial community. However, many harmful bacterial (including Clostridium perfringens and Escherichia coli etc.) at above normal level also had a negative effect on gut microbiota [1,18,62,63]. Our study results indicated that BA40 had the same ability as PB6 to modulate the balance of gut microbiota, decreasing the population of Clostridium perfringens and Escherichia coli, and increasing the proliferation of Lactobacillus. These results could illustrate how isolated strains to mitigate intestinal microbial disorders by Clostridium perfringens challenge. Another possible reason that isolated strains could mitigate the infection of Clostridium perfringens is isolates can enhance the inflammatory response by stimulating cytokine production [64]. Clostridium perfringens infection induced a strong inflammatory response according to Gong et al. [65] and our results, while isolated strains especially BA40 could reduce the excessive immune response. Meanwhile, BA40 and PB6 also improve the anti-inflammatory cytokine (IL-10, IL-22) concentrations, which can inhibit the immune cells proliferation to decrease the immune response [66,67]. Besides, NO is one biomarker because during the pathogen’s infection process, the immune cells improved pro- IL-1β, TNF-α and INF-γ through promoting NO production [68]. Many studies have revealed that high levels of DAO and DLA can cause intestinal barrier injury or intestinal permeability [69,70]. Our results indicated that BA40 and PB6 can alleviate the intestinal barrier injury.
5. Conclusions
Taken together, microbes were isolated from JinHua pigs and screened through a series of assays Then we processed four isolated strains with the highest probiotic potential. Some isolates contained ideal probiotic properties, such as good anti-pathogenic capacity, high survival ratio in acid, bile salt and trypsin environments, lack of virulence, or AMR genes. Notably, we demonstrated that BA40 had antimicrobial activity against enteric pathogens in well diffusion, and we showed its capacity to exclude the attachment of Clostridium perfringens to IPEC-J2 cells. The WGS suggested that BA40 had strong antibacterial capacity due to secreting a variety of secondary metabolites at high levels. Besides, the animal experiment illustrated that BA40 and PB6 could reduce the phenomenon induced by Clostridium perfringens challenge of body weight loss, colon length decrease, pro-inflammatory cytokine increase, Clostridium perfringens, and Escherichia coli increase. The present study provides evidence that BA40 could represent a novel probiotic candidate as PB6, which can mitigate the burden of Clostridium perfringens associated gut disease or even benefit for human health. Further studies via pig models and clinical studies are required to ascertain the safety and efficacy of JinHua pig-derived probiotics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10102056/s1, Figure S1: Circos genome circle map of probiotic candidate strains; Table S1: Gene with up to 50% similarity found in probiotics candidate genome according to the database of virulence factors; Table S2: Gene with up to 50% similarity found in probiotics candidates genome according to the Comprehensive Antibiotic Resistance Database (CARD).
Author Contributions
Z.J.: Conceptualization, Methodology, Investigation, Writing—original draft. M.Y., W.S., W.L., T.G., Y.Z.: Investigation, Visualization. C.W.: Formal analysis, Visualization. X.W., Y.W. and M.J.: Writing—review and editing. Z.L.: Resources, Writing—review and editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the university’s regulations on animal experimentation, and approved by the Institutional Animal Care and Use Committee of Zhejiang University (ZJU20220164, 1 July 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole genome sequence of the four tested strains had been deposited at GeneBank under the BioProject ID PRJNA826263, with accession numbers JALMGL000000000 to JALMGO000000000.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding Statement
This research was supported by the fund from Science and Technology Projects of Zhejiang (2021C02008, CTZB-2020080127, 2022C02043), National Natural Science Foundation of China (U21A20249), China Agriculture Research System (CARS-35), National Center of Technology Innovation for Pigs and China Postdoctoral Science Foundation (2022M712793), Ji’an Science and Technology Special Project (Taihe Silky Fowl).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lange M.E., Uwiera R.R.E., Inglis G.D. Enteric Escherichia coli O157:H7 in Cattle, and the Use of Mice as a Model to Elucidate Key Aspects of the Host-Pathogen-Microbiota Interaction: A Review. Front. Vet. Sci. 2022;9:937866. doi: 10.3389/fvets.2022.937866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- 3.Xie W., Song L., Wang X., Xu Y., Liu Z., Zhao D., Wang S., Fan X., Wang Z., Gao C., et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes. 2021;13:1956281. doi: 10.1080/19490976.2021.1956281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin D., Bai Y., Li Y., Huang Y., Li L., Wang G., Qu L., Wang J., Yu L.-Y., Hou X. Changes in Gut Microbiota by the Lactobacillus casei Anchoring the K88 Fimbrial Protein Prevented Newborn Piglets From Clinical Diarrhea. Front. Cell. Infect. Microbiol. 2022;12:842007. doi: 10.3389/fcimb.2022.842007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora Z.V., Macias-Rodriguez M.E., Arratia-Quijada J., Gonzalez-Torres Y.S., Nuno K., Villarruel-Lopez A. Clostridium perfringens as Foodborne Pathogen in Broiler Production: Pathophysiology and Potential Strategies for Controlling Necrotic Enteritis. Animals. 2020;10:1718. doi: 10.3390/ani10091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzal F.A., Navarro M.A., Li J., Freedman J.C., Shrestha A., McClane B.A. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe. 2018;53:11–20. doi: 10.1016/j.anaerobe.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh T.R., Wu Y. China bans colistin as a feed additive for animals. Lancet Infect. Dis. 2016;16:1102–1103. doi: 10.1016/S1473-3099(16)30329-2. [DOI] [PubMed] [Google Scholar]
- 8.Cao X., Tang L., Zeng Z., Wang B., Zhou Y., Wang Q., Zou P., Li W. Effects of Probiotics BaSC06 on Intestinal Digestion and Absorption, Antioxidant Capacity, Microbiota Composition, and Macrophage Polarization in Pigs for Fattening. Front. Vet. Sci. 2020;7:570593. doi: 10.3389/fvets.2020.570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayalew H., Zhang H., Wang J., Wu S., Qiu K., Qi G., Tekeste A., Wassie T., Chanie D. Potential Feed Additives as Antibiotic Alternatives in Broiler Production. Front. Vet. Sci. 2022;9:916473. doi: 10.3389/fvets.2022.916473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni R.R., Gaghan C., Gorrell K., Sharif S., Taha-Abdelaziz K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens. 2022;11:692. doi: 10.3390/pathogens11060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbuewu I.P., Mabelebele M., Sebola N.A., Mbajiorgu C. Bacillus Probiotics as Alternatives to In-feed Antibiotics and Its Influence on Growth, Serum Chemistry, Antioxidant Status, Intestinal Histomorphology, and Lesion Scores in Disease-Challenged Broiler Chickens. Front. Vet. Sci. 2022;9:876725. doi: 10.3389/fvets.2022.876725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marco M.L., Sanders M.E., Ganzle M., Arrieta M.C., Cotter P.D., De Vuyst L., Hill C., Holzapfel W., Lebeer S., Merenstein D., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021;18:196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zyl W.F., Deane S.M., Dicks L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 2020;12:1831339. doi: 10.1080/19490976.2020.1831339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y., Ma K., Li J., Ren Z., Zhang J., Shan A. Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022;13:90. doi: 10.1186/s40104-022-00737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse S., Schenk M., Pierre F., Morlock G.E. Bacillus subtilis spores in probiotic feed quantified via bacterial metabolite using planar chromatography. Anal. Chim. Acta. 2022;1221:340124. doi: 10.1016/j.aca.2022.340124. [DOI] [PubMed] [Google Scholar]
- 17.Lu S., Na K., Li Y., Zhang L., Fang Y., Guo X. Bacillus-derived probiotics: Metabolites and mechanisms involved in bacteria-host interactions. Crit. Rev. Food Sci. Nutr. 2022:1–14. doi: 10.1080/10408398.2022.2118659. [DOI] [PubMed] [Google Scholar]
- 18.Shamshirgaran M.A., Golchin M., Mohammadi E. Lactobacillus casei displaying Clostridium perfringens NetB antigen protects chickens against necrotic enteritis. Appl. Microbiol. Biotechnol. 2022;106:6441–6453. doi: 10.1007/s00253-022-12155-y. [DOI] [PubMed] [Google Scholar]
- 19.Aljumaah M.R., Alkhulaifi M.M., Abudabos A.M., Aljumaah R.S., Alsaleh A.N., Stanley D. Bacillus subtilis PB6 based probiotic supplementation plays a role in the recovery after the necrotic enteritis challenge. PLoS ONE. 2020;15:e0232781. doi: 10.1371/journal.pone.0232781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foligne B., Peys E., Vandenkerckhove J., Van Hemel J., Dewulf J., Breton J., Pot B. Spores from two distinct colony types of the strain Bacillus subtilis PB6 substantiate anti-inflammatory probiotic effects in mice. Clin. Nutr. 2012;31:987–994. doi: 10.1016/j.clnu.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Guo S., Xv J., Li Y., Bi Y., Hou Y., Ding B. Interactive effects of dietary vitamin K3 and Bacillus subtilis PB6 on the growth performance and tibia quality of broiler chickens with sex separate rearing. Animal. 2020;14:1610–1618. doi: 10.1017/S1751731120000178. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Wei S., Chen N., Xiang Y., Wang Y., Jin M. Characteristics of gut microbiota in pigs with different breeds, growth periods and genders. Microb. Biotechnol. 2022;15:793–804. doi: 10.1111/1751-7915.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripamonti B., Agazzi A., Bersani C., De Dea P., Pecorini C., Pirani S., Rebucci R., Savoini G., Stella S., Stenico A., et al. Screening of species-specific lactic acid bacteria for veal calves multi-strain probiotic adjuncts. Anaerobe. 2011;17:97–105. doi: 10.1016/j.anaerobe.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Lone A., Mottawea W., Ait Chait Y., Hammami R. Dual Inhibition of Salmonella enterica and Clostridium perfringens by New Probiotic Candidates Isolated from Chicken Intestinal Mucosa. Microorganisms. 2021;9:166. doi: 10.3390/microorganisms9010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu J., Wang T., Xiao X., Cheng Y., Wang F., Jin M., Wang Y., Zong X. Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells. 2021;10:527. doi: 10.3390/cells10030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan L.L., Tan C.H., Ng N.K.J., Tan Y.H., Conway P.L., Loo S.C.J. Potential Probiotic Strains From Milk and Water Kefir Grains in Singapore-Use for Defense Against Enteric Bacterial Pathogens. Front. Microbiol. 2022;13:857720. doi: 10.3389/fmicb.2022.857720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Z., Li W., Su W., Wen C., Gong T., Zhang Y., Wang Y., Jin M., Lu Z. Protective Effects of Bacillus amyloliquefaciens 40 Against Clostridium perfringens Infection in Mice. Front. Nutr. 2021;8:733591. doi: 10.3389/fnut.2021.733591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Z., Zhang J., Li Y., Li K., Gong S., Li F., Wang P., Iqbal M., Kulyar M.F., Li J. Probiotic Potential of Bacillus licheniformis and Bacillus pumilus Isolated from Tibetan Yaks, China. Probiotics Antimicrob. Proteins. 2022;14:579–594. doi: 10.1007/s12602-022-09939-z. [DOI] [PubMed] [Google Scholar]
- 31.Shen J., Zhang J., Zhao Y., Lin Z., Ji L., Ma X. Tibetan Pig-Derived Probiotic Lactobacillus amylovorus SLZX20-1 Improved Intestinal Function via Producing Enzymes and Regulating Intestinal Microflora. Front. Nutr. 2022;9:846991. doi: 10.3389/fnut.2022.846991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng H., Zhang Q., Tan B., Li M., Zhang W., Feng J. A metagenomic view of how different carbon sources enhance the aniline and simultaneous nitrogen removal capacities in the aniline degradation system. Bioresour. Technol. 2021;335:125277. doi: 10.1016/j.biortech.2021.125277. [DOI] [PubMed] [Google Scholar]
- 33.Zong X., Cao X., Wang H., Xiao X., Wang Y., Lu Z. Cathelicidin-WA Facilitated Intestinal Fatty Acid Absorption Through Enhancing PPAR-gamma Dependent Barrier Function. Front. Immunol. 2019;10:1674. doi: 10.3389/fimmu.2019.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinttila T., Kassinen A., Malinen E., Krogius L., Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Z., Su W., Wen C., Li W., Zhang Y., Gong T., Du S., Wang X., Lu Z., Jin M., et al. Effect of Porcine Clostridium perfringens on Intestinal Barrier, Immunity, and Quantitative Analysis of Intestinal Bacterial Communities in Mice. Front. Vet. Sci. 2022;9:881878. doi: 10.3389/fvets.2022.881878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao X., Cheng Y., Fu J., Lu Z., Wang F., Jin M., Zong X., Wang Y. Gut Immunity and Microbiota Dysbiosis Are Associated with Altered Bile Acid Metabolism in LPS-Challenged Piglets. Oxid. Med. Cell Longev. 2021;2021:6634821. doi: 10.1155/2021/6634821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acharjee M., Hasan F., Islam T., Nur I.T., Begum N., Mazumder C., Lubna M.A., Zerin N., Shahriar A., Mahmud M.R. In-vitro antibacterial activity of commercially available probiotics on food-borne pathogens along with their synergistic effects with synthetic drugs. Metab. Open. 2022;14:100187. doi: 10.1016/j.metop.2022.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaper J.B., Nataro J.P., Mobley H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 39.Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A Review of Prebiotics Against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva R.O., Lobato F.C. Clostridium perfringens: A review of enteric diseases in dogs, cats and wild animals. Anaerobe. 2015;33:14–17. doi: 10.1016/j.anaerobe.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Silva R.O.S., Oliveira Junior C.A., Guedes R.M.C., Lobato F.C.F. Clostridium perfringens: A review of the disease in pigs, horses and broiler chickens. Ciência Rural. 2015;45:1027–1034. doi: 10.1590/0103-8478cr20140927. [DOI] [Google Scholar]
- 42.Lo Verso L., Lessard M., Talbot G., Fernandez B., Fliss I. Isolation and Selection of Potential Probiotic Bacteria from the Pig Gastrointestinal Tract. Probiotics Antimicrob. Proteins. 2018;10:299–312. doi: 10.1007/s12602-017-9309-3. [DOI] [PubMed] [Google Scholar]
- 43.Adhikari B., Kwon Y.M. Characterization of the Culturable Subpopulations of Lactobacillus in the Chicken Intestinal Tract as a Resource for Probiotic Development. Front. Microbiol. 2017;8:1389. doi: 10.3389/fmicb.2017.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J., Li Y., Zhang J., Yang Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res. Vet. Sci. 2013;94:62–68. doi: 10.1016/j.rvsc.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Foligne B., Daniel C., Pot B. Probiotics from research to market: The possibilities, risks and challenges. Curr. Opin. Microbiol. 2013;16:284–292. doi: 10.1016/j.mib.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Wan M.L.Y., Forsythe S.J., El-Nezami H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019;59:3320–3333. doi: 10.1080/10408398.2018.1490885. [DOI] [PubMed] [Google Scholar]
- 47.Dowarah R., Verma A.K., Agarwal N., Singh P., Singh B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE. 2018;13:e0192978. doi: 10.1371/journal.pone.0192978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musikasang H., Tani A., H-kittikun A., Maneerat S. Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J. Microbiol. Biotechnol. 2009;25:1337–1345. doi: 10.1007/s11274-009-0020-8. [DOI] [Google Scholar]
- 49.Chen G., Fang Q., Liao Z., Xu C., Liang Z., Liu T., Zhong Q., Wang L., Fang X., Wang J. Detoxification of Aflatoxin B1 by a Potential Probiotic Bacillus amyloliquefaciens WF2020. Front. Microbiol. 2022;13:891091. doi: 10.3389/fmicb.2022.891091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulaw G., Sisay Tessema T., Muleta D., Tesfaye A. In Vitro Evaluation of Probiotic Properties of Lactic Acid Bacteria Isolated from Some Traditionally Fermented Ethiopian Food Products. Int. J. Microbiol. 2019;2019:7179514. doi: 10.1155/2019/7179514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngamsomchat A., Kaewkod T., Konkit M., Tragoolpua Y., Bovonsombut S., Chitov T. Characterisation of Lactobacillus plantarum of Dairy-Product Origin for Probiotic Chevre Cheese Production. Foods. 2022;11:934. doi: 10.3390/foods11070934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WoldemariamYohannes K., Wan Z., Yu Q., Li H., Wei X., Liu Y., Wang J., Sun B. Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020;68:14709–14727. doi: 10.1021/acs.jafc.0c06396. [DOI] [PubMed] [Google Scholar]
- 53.Kelly C.G., Younson J.S. Anti-adhesive strategies in the prevention of infectious disease at mucosal surfaces. Expert Opin. Investig. Drugs. 2000;9:1711–1721. doi: 10.1517/13543784.9.8.1711. [DOI] [PubMed] [Google Scholar]
- 54.Anonye B.O., Hassall J., Patient J., Detamornrat U., Aladdad A.M., Schuller S., Rose F., Unnikrishnan M. Probing Clostridium difficile Infection in Complex Human Gut Cellular Models. Front. Microbiol. 2019;10:879. doi: 10.3389/fmicb.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misawa Y., Kelley K.A., Wang X., Wang L., Park W.B., Birtel J., Saslowsky D., Lee J.C. Staphylococcus aureus Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins. PLoS Pathog. 2015;11:e1005061. doi: 10.1371/journal.ppat.1005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redondo L.M., Carrasco J.M., Redondo E.A., Delgado F., Miyakawa M.E. Clostridium perfringens type E virulence traits involved in gut colonization. PLoS ONE. 2015;10:e0121305. doi: 10.1371/journal.pone.0121305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsilia V., Uyttendaele M., Kerckhof F.M., Rajkovic A., Heyndrickx M., Van de Wiele T. Bacillus cereus Adhesion to Simulated Intestinal Mucus Is Determined by Its Growth on Mucin, Rather Than Intestinal Environmental Parameters. Foodborne Pathog. Dis. 2015;12:904–913. doi: 10.1089/fpd.2014.1926. [DOI] [PubMed] [Google Scholar]
- 58.Piewngam P., Zheng Y., Nguyen T.H., Dickey S.W., Joo H.S., Villaruz A.E., Glose K.A., Fisher E.L., Hunt R.L., Li B., et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu M., Zhang X. Alien species invasion of deep-sea bacteria into mouse gut microbiota. J. Adv. Res. 2022;In press doi: 10.1016/j.jare.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M., Liu L., Jia S., Li S., Zou Y., Zhong C. Complete genome analysis of Gluconacetobacter xylinus CGMCC 2955 for elucidating bacterial cellulose biosynthesis and metabolic regulation. Sci. Rep. 2018;8:6266. doi: 10.1038/s41598-018-24559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W., Ji H., Zhang D., Liu H., Wang S., Wang J., Wang Y. Complete Genome Sequencing of Lactobacillus plantarum ZLP001, a Potential Probiotic That Enhances Intestinal Epithelial Barrier Function and Defense Against Pathogens in Pigs. Front. Physiol. 2018;9:1689. doi: 10.3389/fphys.2018.01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Yi D., Xu H., Tan Z., Meng Y., Wu T., Wang L., Zhao D., Hou Y. Dietary supplementation with sodium gluconate improves the growth performance and intestinal function in weaned pigs challenged with a recombinant Escherichia coli strain. BMC Vet. Res. 2022;18:303. doi: 10.1186/s12917-022-03410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zong X., Fu J., Xu B., Wang Y., Jin M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020;6:389–396. doi: 10.1016/j.aninu.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torchinsky M.B., Garaude J., Martin A.P., Blander J.M. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 65.Gong L., Wang B., Zhou Y., Tang L., Zeng Z., Zhang H., Li W. Protective Effects of Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 Against Clostridium perfringens Infection in Broilers. Front. Immunol. 2020;11:628374. doi: 10.3389/fimmu.2020.628374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Djaldetti M., Bessler H. Probiotic strains modulate cytokine production and the immune interplay between human peripheral blood mononucear cells and colon cancer cells. FEMS Microbiol. Lett. 2017;364:fnx014. doi: 10.1093/femsle/fnx014. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Y., Wang X., Zhu L., Tu Y., Chen W., Gong L., Pan T., Lin H., Lin J., Sun H., et al. Lactobacillus rhamnosus GG combined with inosine ameliorates alcohol-induced liver injury through regulation of intestinal barrier and Treg/Th1 cells. Toxicol. Appl. Pharmacol. 2022;439:115923. doi: 10.1016/j.taap.2022.115923. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Ortiz A., Serrador J.M. Nitric Oxide Signaling in T Cell-Mediated Immunity. Trends Mol. Med. 2018;24:412–427. doi: 10.1016/j.molmed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y., Miao L., Yao Y., Wu W., Wu X., Gong C., Qiu L., Chen J. Dexmedetomidine Ameliorate CLP-Induced Rat Intestinal Injury via Inhibition of Inflammation. Mediat. Inflamm. 2015;2015:918361. doi: 10.1155/2015/918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi H., Zhang L., Gan Z., Xiong H., Yu C., Du H., Wang Y. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 2016;6:25679. doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole genome sequence of the four tested strains had been deposited at GeneBank under the BioProject ID PRJNA826263, with accession numbers JALMGL000000000 to JALMGO000000000.