Abstract
The antifungal drugs currently available and mostly used for the treatment of candidiasis exhibit the phenomena of toxicity and increasing resistance. In this context, plant materials might represent promising sources of antifungal agents. The aim of this study is to evaluate for the first time the chemical content of the volatile fractions (VFs) along with the antifungal and anti-biofilm of Convolvulus althaeoides L. roots. The chemical composition was determined by gas chromatography coupled to a flame ionization detector and mass spectrometry. In total, 73 and 86 chemical compounds were detected in the n-hexane (VF1) and chloroform (VF2) fractions, respectively. Analysis revealed the presence of four main compounds: n-hexadecenoic acid (29.77%), 4-vinyl guaiacol (12.2%), bis(2-ethylhexyl)-adipate (9.69%) and eicosane (3.98%) in the VF extracted by hexane (VF1). n-hexadecenoic acid (34.04%), benzyl alcohol (7.86%) and linoleic acid (7.30%) were the main compounds found in the VF extracted with chloroform (VF2). The antifungal minimum inhibitory concentrations (MICs) of the obtained fractions against Candida albicans, Candida glabrata and Candida tropicalis were determined by the micro-dilution technique and values against Candida spp. ranged from 0.87 to 3.5 mg/mL. The biofilm inhibitory concentrations (IBF) and sustained inhibition (BSI) assays on C. albicans, C. glabrata and C. tropicalis were also investigated. The VFs inhibited biofilm formation up to 0.87 mg/mL for C. albicans, up to 1.75 mg/mL against C. glabrata and up to 0.87 mg/mL against C. tropicalis. The obtained results highlighted the synergistic mechanism of the detected molecules in the prevention of candidosic biofilm formation.
Keywords: Convolvulus althaeoides L. roots, gas chromatography, mass spectrometry, volatile fractions, antifungal, Candida spp. biofilm
1. Introduction
The plant world has always been considered as a source of many natural compounds, which has prompted researchers to study many plant species. The new findings have demonstrated that plants are enriched with many bioactive secondary metabolites such as terpenoids, fatty acids, phenolics and alkaloids [1,2], which are characterized by high antifungal proprieties [3,4].
Convolvulus is a genus of the family Convolvulaceae which is found in all Mediterranean regions. This genus includes about 250 species such as Convolvulus prostrates, Convolvulus austroaegyptiacus, Convolvulus pilosellifolius Desr., Convolvulus pluricaulis and Convolvulus arvensis L. [5], which have been reported to possess medicinal effects and in particular antioxidant, anti-inflammatory, antimicrobial and anticancer properties [5,6,7,8]. In Tunisia, Convolvulus althaeoides L. has been the most characterized species in terms of chemical composition, and previous studies demonstrated its fruitful content of its extracts in terms of flavonoids, phenol terpenes, carotenoids, polyphenols, chorophylls, and carotenoids [9,10]. Moreover, oxygenated monoterpenes, oxygenated sesquiterpenes and sesquiterpene hydrocarbons, determined by gas chromatography, have been reported as constituens of the flowers of such a species [11]. A new acylated anthocyanintrioside was isolated by extraction with methanol solution from flowers of C. althaeoides L. from Portugal and identified from MALDI-TOF and NMR spectrophotometric technologies [12]. Our previous study revealed that ethanolic extract of C. althaeoides L. leaves were rich in antioxidant polyphenols, while dichloromethane extract showed the highest rate of carotenoids [8]. Ethyl acetate and ethanol extracts were the most active against dermatophytes (T. rubrum, T. menthagrophytes and M. canis), with inhibition percentages reaching 100% at the concentration of 50 mg/mL. Furthermore, ethyl acetate and ethanolic extracts showed maximum inhibition potential with minimum inhibitory fungicidal concentrations (MCFs) ranging from 0.78 to 6.25 mg/mL when tested on Candida spp. cultures. Moreover, extracts of winter leaves of the C. athaeoides L. showed inhibitory effects up to 90% of Candida albicans germ tube formation, at a concentration of 3.1 mg mL−1.
The genus Candida consists of more than 200 species, and only some of them are pathogenic to humans. These are yeast fungi, and the best-known species are represented by C. albicans, C. tropicalis, C. glabrata and C. krusei, generally associated with pathological conditions [13].
Candida spp. are part of natural microbiota of immunocompetent individuals, but in case of health weakness, Candida isolates can become opportunist and cause major common hospital-acquired systemic infections with high mortality rates [14].
According to the US Center of Disease Control and Prevention (CDC) in 2019, a report on the antibiotic resistance threat reveled more than 34,000 cases and 1700 deaths annually were due to drug-resistant Candida spp. In addition, an emerging multidrug resistance in Candida spp. infections has been reported, making them difficult to treat. In fact, fluconazole resistance has been demonstrated in 7% of Candida sepsis and C. glabrata resistance to Echinocandine was constantly reported over the past two decades [15].
The capacity of pathogenic species of the Candida genus to infect and harm is highly related to its virulence factors including adhesion, phenotypic switching germ tube formation, dimorphism, production of hydrolytic enzymes and biofilm formation [16]. The formation of Candida biofilms has a significant clinical impact due to their increased resistance to antifungal treatments and the ability of biofilms to resist immune mechanisms [17].
For many years, a range of different synthetic chemicals (aromatic hydrocarbons, amphotericin B, benzimidazoles and sterol) have been used as antifungal agents to inhibit the growth of plant pathogenic fungi [18]. Plant components with antifungal properties are considered as a valuable resource in the treatment of fungal diseases. There is a growing interest in the use of natural products such as essential oils, which are volatile and non-volatile natural compounds comprising a complex mixture of terpenoids, alcohol compounds, phenylpropanoids, aldehydes and acidic compounds, among others [19]. Due to their strong odor, they have been traditionally used as natural flavorings and recently as natural antimicrobials.
The essential oil composition of the roots of C. althaeoides L. and its potential biomedical value have not been scientifically investigated. Therefore, the present study aims to investigate for the first time the volatile profile and the antifungal and anti-biofilm properties of the essential oil of the Tunisian cultivar of C. althaeoides L., as well as to elucidate the role and the relative importance of their dominant constituents to ensure the maintenance of biological activity.
2. Results and Discussion
2.1. Yields and Chemical Composition of the VFs
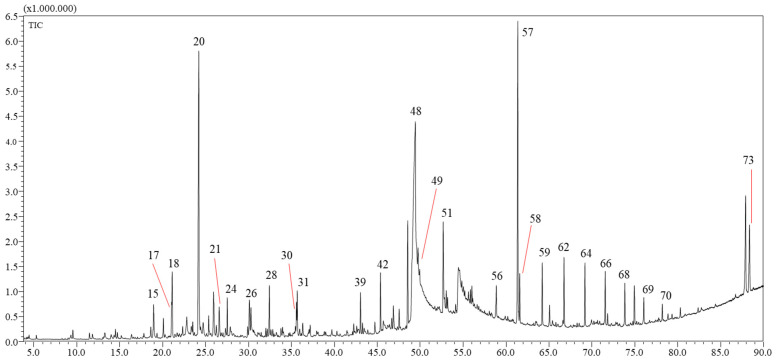
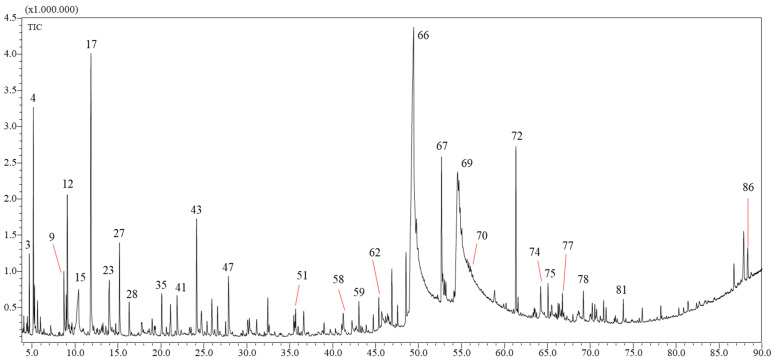
The n-hexane (VF1) and chloroform (VF2) VFs were obtained in yields of 0.012 ± 0.005% and 0.009 ± 0.003 (w/w) of the dry mass of C. althaoides L. roots. This is comparable to the yield of the essential oil obtained from the flowers of this species 0.007% [11]. Chemical profiles of root C. althaeoides L. volatiles fractions were determined by GC-MS, and results are presented in Table 1 and Table 2, in Tables S1 and S2 and in Figure 1 and Figure 2. Seventy-three compounds (Table 1 and Table S1) and eighty-six compounds (Table 2 and Table S2) were identified from VF1 and VF2, respectively.
Table 1.
List of major volatile compounds detected in the hexane fraction (VF1) of C. althaeoides L. roots by GC-MS.
| N. Peak | Compound | Area% | LRI (Lib) | LRI (Exp) | Library | Compound Class | Formula |
|---|---|---|---|---|---|---|---|
| 15 | Myrtenol | 0.93 | 1202 | 1198 | FFNSC 4.0 | Oxygenated monoterpene | C10H16O |
| 17 | Cuminaldehyde | 0.79 | 1243 | 1245 | FFNSC 4.0 | Oxygenated monoterpene | C10H12O |
| 18 | Carvone | 1.77 | 1246 | 1246 | FFNSC 4.0 | Oxygenated monoterpene | C10H14O |
| 20 | 4-Vinylguaiacol | 12.2 | 1309 | 1315 | FFNSC 4.0 | Alcohol | C9H10O2 |
| 21 | Methyl 4-formylbenzoate | 1.03 | - | 1370 | W11N17 | Ester | C9H8O3 |
| 24 | Tetradec-1-ene | 0.9 | 1379 | 1391 | FFNSC 4.0 | Alkene | C14H28 |
| 26 | α-Methoxynaphthalene | 1.52 | 1450 | 1453 | FFNSC 4.0 | Alkene | C11H10O |
| 28 | 2,4-bis(1,1-dimethylethyl)-phenol | 1.47 | - | 1509 | W11N17 | Alcohol | C14H22O |
| 31 | n-Hexadecene | 1.1 | 1593 | 1592 | FFNSC 4.0 | Alkene | C16H32 |
| 39 | Octadec-1-ene | 1.03 | 1793 | 1792 | FFNSC 4.0 | Alkene | C18H36 |
| 42 | diisobutyl phthalate | 1.55 | 1858 | 1860 | FFNSC 4.0 | Ester | C16H22O4 |
| 48 | n-Hexadecanoic acid | 29.77 | 1977 | 1983 | FFNSC 4.0 | Fatty acid | C16H32O2 |
| 49 | n-Eicosene | 3.98 | 1994 | 1993 | FFNSC 4.0 | Alkene | C20H40 |
| 50 | n-Eicosane | 2.11 | 2000 | 1999 | FFNSC 4.0 | Alkane | C20H42 |
| 51 | n-Octadecanol | 2.89 | 2081 | 2087 | FFNSC 4.0 | Alcohol | C18H38O |
| 53 | Ethyl linoleate | 1.75 | 2164 | 2160 | FFNSC 4.0 | Fatty acid | C20H36O2 |
| 54 | Ethyl linolenate | 1.47 | 2165 | 2166 | FFNSC 4.0 | Fatty acid | C20H34O2 |
| 56 | n-Tricosane | 0.81 | 2300 | 2299 | FFNSC 4.0 | Alkane | C23H48 |
| 57 | Bis(2-ethylhexyl)-adipate | 9.69 | 2392 | 2391 | FFNSC 4.0 | Ester | C22H42O4 |
| 58 | n-Tetracosane | 1.23 | 2400 | 2399 | FFNSC 4.0 | Alkane | C24H50 |
| 59 | n-Pentacosane | 1.59 | 2500 | 2499 | FFNSC 4.0 | Alkane | C25H52 |
| 62 | n-Hexacosane | 1.77 | 2600 | 2598 | FFNSC 4.0 | Alkane | C26H54 |
| 64 | n-Heptacosane | 1.64 | 2700 | 2699 | FFNSC 4.0 | Alkane | C27H56 |
| 66 | n-Octacosane | 1.37 | 2800 | 2798 | FFNSC 4.0 | Alkane | C28H58 |
| 68 | n-Nonacosane | 1.12 | 900 | 2898 | FFNSC 4.0 | Alkane | C29H60 |
| 73 | 3,5-bis(1,1-dimethylthyl)-4-hydroxy-, octadecyl ester | 2.71 | - | 3596 | W11N17 | Carboxylic acid | C35H62O3 |
FFNSC: Flavor and Fragrance Natural and Synthetic Compounds; LRI: Linear Retention Indices; W11N17: Wiley11-Nist17.
Table 2.
List of major volatile compounds detected in the chloroform fraction (VF2) of C. althaeoides L. roots by GC-MS.
| N. Peak | Compound | Area% | LRI (Lib) | LRI (Exp) | Library | Compound Class | Formula |
|---|---|---|---|---|---|---|---|
| 3 | Hexan-2-ol | 0.91 | 802 | 812 | FFNSC 4.0 | Alcohol | C6H14O |
| 4 | Furfural | 3.24 | 845 | 832 | FFNSC 4.0 | Aldehyde | C5H4O2 |
| 9 | β-Lutidine | 1.29 | 955 | 955 | FFNSC 4.0 | Pyridine | C7H9N |
| 12 | 3-ethenyl- Pyridine | 2.43 | - | 966 | W11N17 | Pyridine | C7H7N |
| 15 | n-Hexanoic acid | 1.91 | 997 | 1004 | FFNSC 4.0 | Carboxylic acid | C6H12O2 |
| 17 | Benzyl alcohol | 7.86 | 1040 | 1037 | FFNSC 4.0 | Alcohol | C7H8O |
| 23 | 2-Methoxyphenol | 1.27 | - | 1088 | W11N17 | Alcohol | C7H8O2 |
| 27 | Phenethyl alcohol | 2.07 | 1113 | 1115 | FFNSC 4.0 | Alcohol | C8H10O |
| 35 | 4-Vinylphenol | 0.98 | 1217 | 1223 | FFNSC 4.0 | Alcohol | C8H8O |
| 41 | Benzopyridine | 1 | 1259 | 1263 | FFNSC 4.0 | Pyridine | C9H7N |
| 43 | 4-Vinylguaiacol | 2.87 | 1309 | 1314 | FFNSC 4.0 | Alcohol | C7H8O2 |
| 47 | Vanillin | 1.49 | 1394 | 1399 | FFNSC 4.0 | Aldehyde | C8H8O3 |
| 66 | n-Hexadecanoic acid | 34.01 | 1977 | 1983 | FFNSC 4.0 | Fatty acid | C16H32O2 |
| 67 | n-Octadecanol | 3.53 | 2081 | 2087 | FFNSC 4.0 | Alcohol | C18H38O |
| 69 | Linoleic acid | 7.3 | 2144 | 2154 | FFNSC 4.0 | Fatty acid | C18H32O2 |
| 70 | n-Docosane | 2.43 | 2200 | 2199 | FFNSC 4.0 | Alkane | C22H46 |
| 72 | Bis(2-ethylhexyl)-adipate | 3.57 | 2392 | 2390 | FFNSC 4.0 | Ester | C22H42O4 |
| 74 | n-Pentacosane | 1.47 | 2500 | 2499 | FFNSC 4.0 | Alkane | C25H52 |
| 86 | 3,5-bis(1,1-dimethylthyl)-4-hydroxy-, octadecyl ester | 0.95 | - | 3595 | W11N17 | Carboxylic acid | C35H62O3 |
FFNSC: Flavor and Fragrance Natural and Synthetic Compounds; LRI: Linear Retention Indices; W11N17: Wiley11-Nist17.
Figure 1.
GC-MS profile of the hexane fraction (VF1) of C. althaeoides L. roots.
Figure 2.
GC-MS profile of the chloroform fraction (VF2) of C. althaeoides L. roots.
The content of VF1, achieved by the GC-MS technique, was characterized as fatty acids (33.50%), alcohols (17.83%), alkanes (15.33%), esters (14.54%), alkenes (8.73%), oxygenated monoterpenes (3.85%), carboxylic acids (2.71%), apocarotenes (1.29%), ketones (0.84%), aldehyde (0.57%), sesquiterpene hydrocarbons (0.42%) and triterpenes (0.39%). The most abundant components are: n-hexadecanoic acid (29.77%) among fatty acids; 4-vinylguaiacol (12.2%) and octadecanol (2.89%); eicosane (2.11%) among alkanes; bis-(2-ethylhexyl)adipate (9.69%) and diisobutyl phthalate (1.55%) among the esters; eicosene (3.98%) among the alkenes; carvone (1.77%) among the oxygenated monoterpenes; benzenepropanoic acid, 3,5-bis(1,1-dimethylthyl)-4-hydroxy-, octadecyl ester (2.71%) among carboxylic acids; 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (0.65%) among apocarotenes; 5-dodecyldihydro-2(3H)-furanone (0.52%) among ketones; n-nonanal (0.14%) among aldehydes; pentadecylic acid (0.42%) among sesquiterpene hydrocarbons; squalene (0.28%) among triterpenes and cadalene (0.11%) among sesquiterpene hydrocarbons.
The content of VF2, achieved by the GC-MS technique, was characterized by fatty acids (41.74%), alcohols (21.01%), alkanes (8.51%), esters (7.65%), aldehydes (6.51%), pyridine (5.06%), carboxylic acids (2.86%), oxygenated monoterpenes (2.16%), alkenes (1.55%), ketones (1.17%), phenylpropanoids (0.64%), oxygenated sesquiterpenes (0.46%), triterpenes (0.34%), apocarotenes (0.17%) and sesquiterpene hydrocarbons (0.09%). The most abundant components were n-hexadecanoic acid (34.04%) and linoleic acid (7.30%) among the fatty acids; benzyl alcohol (7.86%) and octadecanol (3.53%) among the alcohols; docosan (2.43%) among alkanes; bis-(2-ethylhexyl)adipate (3.57%) among esters; furfural (3.24%) and vanillin (1.49%) among aldehydes; 3-ethenyl-pyridine (2.43%) and beta-lutidine (1.29%) among pyridine; n-hexanoic acid (1.91%) among carboxylic acids; myrtenol (0.46%) among oxygenated monoterpenes; octadec-1-ene (0.67%) among alkenes; 5-dodecyldihydro-2(3H)-furanone (0.52%) among ketones; (E)-coniferyl alcohol (0.67%) among phenylpropanoids; pentadecylic acid (0.36%) among oxygenated sesquiterpenes; squalene (0.34%) among triterpenes; benzophenone (0.1%) among apocarotenes and 1,6-dimethyl-4-(1-methylethyl)-naphthalene (0.09%) among sesquiterpene hydrocarbons.
n-Hexadecanoic (palmitic) acid (C16H32O2) was the most abundant compound, which has several biological activities: antimicrobial, antieczematous, antiseborrheic, sclerosing, antihypoxic, antimutagenic, fibrinolytic, anti-inflammatory, antisecretory, cytoprotective and anesthetic [20]. In fact, n-hexadecanoic acid is one of the primary metabolites produced during microbial degradation because of the oxidized end of the molecule is used as the initial site for the β-oxidation process, whereas microbial oxidation of saturated hydrocarbons (n-hexadecane) cannot be initiated as easily as for n-hexadecanoic acid [21]. 4-vinyl guaiacol is a vinyl phenol produced by the decarboxylation of ferulic acids. Its phenolic structure allows it to play the role of a natural antioxidant. Indeed, the position of the methoxy group on the electron-donating phenolic ring is also a factor that increases the stability of the phenoxy radical and thus its antioxidant effectiveness [22].
The chemical composition of the VFs of the roots of C. althaeoides L. are herein reported for the first time. Only one study on the essential oil of the flower of C. althaeoides L. grown in Tunisia has been carried out [11], which reveled 24 compounds, representing 95.50% of the total compositions. The oil was characterized by a high proportion of sesquiterpene hydrocarbons (36.30%), followed by oxygenated sesquiterpenes (34.70%) and oxygenated monoterpenes (24.50%). The main compounds are germacrene D (12.50%), T-cadinol (11.80%) and verbenone (6.90%).
2.2. Antifungal Activity
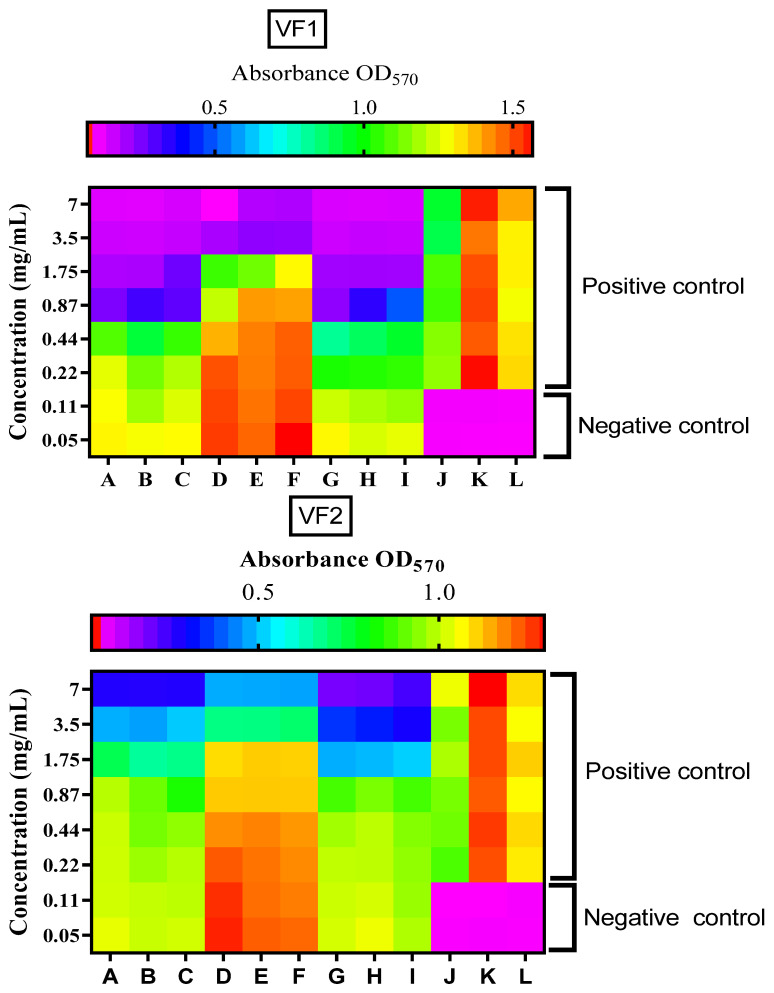
The outputs of the microdilution test of VF1 and VF2 from C. althaeoides L. roots against Candida spp. at different concentrations, read with Multiskan™ FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA), are represented as heatmaps (Figure 3). These illustrations demonstrated a good level of reproducibility in our repeats and a difference of inhibition for both VF1 and VF2 fractions against each fungal strain. C. albicans and C. tropicalis demonstrated the same susceptibility level to VF1 and VF2 fractions with MICs at 0.87 and 1.75 mg/mL, respectively. The MICs for the Amphotericin B were 0.125 × 10−3 mg/mL, 0. 062 × 10−3 mg/mL and 0.250 × 10−3 mg/mL for C. albicans, C. glabrata and C. tropicalis, respectively (data not shown). The MFCs against these strains were reported at 1.75 mg/mL for both VF1 and VF2 fractions of C. albicans and C. tropicalis. However, C. glabrata seems to be less susceptible to VF1 and VF2 fractions with MICs/MFCs at 3.5 mg/mL and 7 mg/mL, respectively. In agreement with the photochemical results of the two VFs, the diversity and the richness of the two VFs in volatile compounds can explain their antifungal potential. Indeed, the main active compounds of the VFs of C. althaeoides L. roots were n-hexadecenoic acid [23], bis (2-ethylhexyl) adipate [24], benzyl alcohol [25] and linoleic acid [26] which are known as antifungal agents. In fact, it was reported previously that the n-hexadecanoic acid or palmitic acid, a saturated fatty acid, abundant compound in VF1 and VF2, showed antifungal activities against mycelial growth and spore production reaching 37.20% and 71.70% by 2 mM concentration [27], respectively. Eicosane (C20H42), a straight chain alkane composed of 20 carbon atoms [28]; carvone (C10H14O), a p-menthane monoterpenoid that consists of cyclohex-2-enone having methyl and isopropenyl substituents at positions 2 and 5, respectively [29], vanillin (C8H8O3) a phenolic aldehyde [30] and guaiacol (C7H8O2), a monomethoxybenzene that consists of phenol with a methoxy substituent at the ortho position [31], were proven to have high antifungal potential at a limited concentration.
Figure 3.
Heatmaps representing spectrophotometric reads (OD) of the microdilution plates at 570 nm using the Multiskan™ FC Microplate Photometer after susceptibility test on Candida spp. to VF1 and VF2 fractions.
Furthermore, it has been demonstrated by several studies that extracts, VFs and essential oils extracted from plants of the Convolvulus genus strongly inhibit the growth of various fungal pathogens [7,9,10,11]. A previous study showed an inhibitory effect of up to 0.31 ± 0.10 mg/mL for C. althaeoides oil against Pseudomonas aeruginosa and Enterococcus faecalis [11].
Columns A, B and C: testing the susceptibility of C. albicans to VFs different concentrations (0.05–7 mg/mL). Columns D, E and F: testing the susceptibility of C. glabrata to different concentrations (0.05–7 mg/mL). Columns G, H and I: testing the susceptibility of C. tropicalis to VFs different concentrations (0.05–7 mg/mL). J, K and L: upper C. albicans, C. glabrata and C. tropicalis positive control wells, respectively; down: negative control wells (RPMI 1640-2% glucose).
2.3. Anti-Biofilm Activity
2.3.1. Inhibition of Biofilm Formation (IBF)
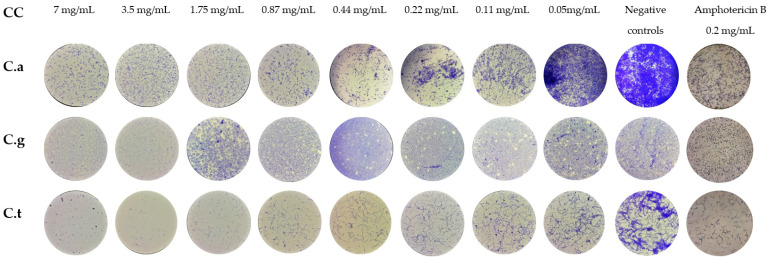
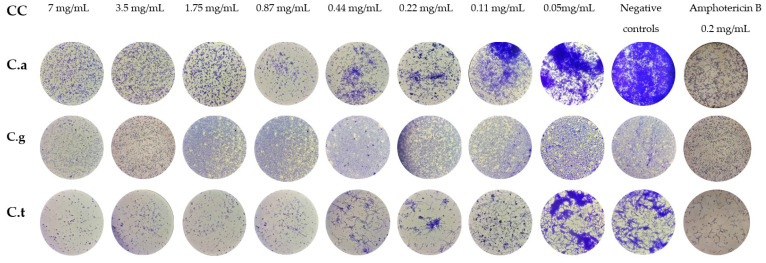
Microscopic observation of biofilm plates permitted us to evaluate the IBF potential of VF1 and VF2 at different concentrations on Candida spp. (Figure 4 and Figure 5). Compared to the positive controls of growth; both VFs demonstrated a high inhibition potential on Candida Biofilm formation. In fact, VF1 and VF2 induced 80% of reduction of C. albicans biofilm biomass up to 0.21 mg/mL and 0.43 mg/mL, respectively. The filamentation was inhibited by both of VFs at concentration of 1.75 mg/mL. Thymus capitatus (0.12 mg/mL) and Cinnamomum verum (0.62 mg/mL) essential oils were efficient against C. albicans biofilm formation at a similar level with 80.60% and 85.57% of inhibition, respectively. Moreover, micrographs of biofilm formed after 24 h incubation demonstrated a filamentation inhibition at half MICs for both of them [32]. Candida albicans biofilms are inherently resistant to antifungal drugs, mainly azoles and polyenes [33]. Thus, essential oils can offer new perspectives as an alternative treatment.
Figure 4.
Micrographs of Candida spp. cells treated with various concentrations of VF1 for Inhibition of Biofilm Formation (IBF). CC: Concentration, C.a: Candida albicans, C.g: Candida glabrata. C.t: Candida tropicalis.
Figure 5.
Micrographs of Candida spp. cells treated with various concentrations of VF2 for Inhibition of Biofilm Formation (IBF). CC: Concentration, C.a: Candida albicans, C.g: Candida glabrata. C.t: Candida tropicalis.
A higher potential of IBFs has been observed on C. tropicalis for both VF1 and VF2 fractions with biomass inhibitions of 100% and 90% up to 3.5 mg/mL and 0.87 mg/mL, respectively. A total filamentation inhibition on C. tropicalis was observed for VF1 and VF2 at 0.87 mg/mL and 1.5 mg/mL, respectively. C. tropicalis have been demonstrated sensitive to treatment with essential oils of Thymbra capitata (0.62 µL/ml) with percentages of reduction of biofilm biomass and reduction of biofilm metabolism of 67.82% and 97.47%, respectively [34]. Essential oils from Pelargonium graveolens have been described to possess a high continence in geraniol and linalool, which are α-terpineol present in both VF1 and VF2 fractions from this study and were 90% inhibitory to C. tropicalis biofilm formation at 8 mg/mL and reduced only 25% of the biofilm at a concentration similar to our effective concentration (0.87 mg/mL).
Both VF1 and VF2 demonstrated a reduced inhibition potential with a reduction of 50% of C. glabrata biofilm biomass up to 3.5 mg/mL concentration. With the exception of Candida spp., Candida glabrata biofilms were more resistant as the reduction in biomass by both VF1 and VF2 remained below 60%. This limited effect can be explained by the particularity of this yeast, which possesses a structure in different multilayers making its biofilm more compact than other Candida strains [35].
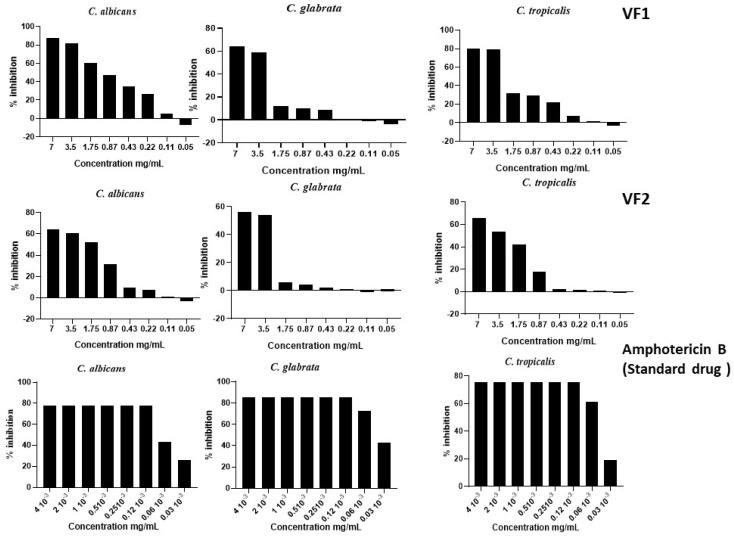
2.3.2. Biofilm Sustained Inhibition (BSI)
The evaluation of the effect of the two VFs of C. althaeoides L. roots on the biofilm formation of C. albicans, C. glabrata and C. tropicalis demonstrated that both of them had an inhibitory potential on biofilm formation (Figure 6). One-way ANOVA computed a p-value < 0.05 (significant effect of different treatments) as percentages of the untreated control. At a concentration of 7 mg/mL, the inhibition percentage of VF1 on C. albicans biofilm formation was 87.23%, while the inhibition rate of VF2 was 65.91%. VF1 and VF2 showed 63.98% and 56.06% inhibition on the BSI of C. glabrata at a concentration of 7 mg/mL, respectively (Figure 4). Similar yields were reported against the biofilm formation of the C. tropicalis strain, which was inhibited by 79.74% and 65.91% for VF1 and VF2, respectively. The BSI on Candida spp. can be explained by several factors. Indeed, inhibition of adhesion is the first significant step in preventing biofilm formation. The anti-biofilm capability of the two VFs with a preference for VF1 can be attributed to its ability to inhibit the attachment of Candida spp. to surfaces. For pathogens, biofilm plays a key role in nullifying the effect of antifungal and anti-biofilm agents. The BSI is the key step in reducing the pathogenic effect of Candida spp. The anti-biofilm effect of these fractions can be attributed to specific components. The two VFs tested in this study have been demonstrated to harbor a wide range of phytochemical components that could be considered responsible for a more or less biological effect. The activity of these tested VFs varied according to its formulation. Indeed, the high amounts of fatty acids and terpenoids were attributed to a better anti-biofilm activity [36,37]. n-Hexadecanoic acid (C16H32O2), namely, palmitic acid, a saturated long-chain fatty acid with a 16-carbon backbone [23]; cuminaldehyde (C10H12O), a benzaldehyde substituted by an isopropyl group at position 4 [38]; linoleic acid (C18H32O2), an octadecadienoic acid in which the two double bonds are at positions 9 and 12 and have Z (cis) stereochemistry [39]; and benzyl alcohol (C7H8O), an aromatic alcohol that consists of benzene bearing a single hydroxymethyl substituent [25] were the main compounds present in these fractions that were associated with anti-biofilm activities. The synergistic effects of such compounds might be responsible for the observed anti-biofilm activity [40]. The antimicrobial activity of these compounds is based on different mode of action, as described for cuminaldehyde, which has the propriety to disrupt Staphylococcus aureus membranes and bind to DNA through the groove to impact normal cellular function [41]. Benzyl Alcohol has been proven to induce attack on bacterial membranes and protein denaturation, following an increase of reactive oxygen species levels [42]. Linoleic acid as fatty acid was involved in affecting membrane permeability (pore formation and membrane destabilization), reducing hydrophobicity but being able to interfere with microbial metabolism or signaling [43]. Palmitic acid was observed to enhance hyphal growth for C. tropicalis through upregulation of hyphal wall protein (HWP1) and enhanced filamentous growth (EFG1) genes [44].
Figure 6.
Effect of different concentrations of VFs on the biofilm of Candida spp. (BSI).
3. Materials and Methods
3.1. Plant Material
The roots of C. althaeoides L. were collected in the region of Kondar (TUNISIA) in January 2017, a rural area of the Tunisian Sahel located about 30 km north-west of the governorate of Sousse. The plant material was naturally dried in the shade at room temperature for 2–3 weeks, ground into a fine powder and later used for extractions.
3.2. Chemicals and Reagents
All solvents used in the experiments (hexane and chloroform) were analytical grade (Merck Life Science, Merck KGaA, Darmstadt, Germany). Dimethyl sulfoxide (DMSO) was purchased from BIO BASIC INC (Desk, Markham, ON, Canada). Culture media were purchased from Sigma-Aldrich (CHEMIE GmBH, Riedstr, Germany). RPMI-1640 medium was purchased from Gibco and stored at 4 °C. Glucose solution 30% (Siphal, Tunisia).
3.3. Preparation of VF from Roots of C. althaeoides L.
The whole process from preparing VFs from root powder was followed according to the method described by Hrichi et al. [40]. Briefly, 100 g of root powder was subjected to hydrodistillation for 3 h. The collected hydrodistillates were subjected to a liquid–liquid extraction with hexane and chloroform, successively. The two VFs, VF1 (hexane) and VF2 (chloroform), were dried over anhydrous sodium sulphate (Na2SO4) and stored in a refrigerator at −4 °C until analysis. The yield of VFs was calculated following the next formula:
| % Yield of VFs = [Weight of VFs/Weight of dried roots] × 100% |
3.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
The composition of VFs of C. althaeoides L. was identified by GC-MS analysis. GC-MS analysis was carried out on a GC–MS-QP2020 system (Shimadzu, Kyoto, Japan) equipped with an “AOC-20i” system auto-injector, and separation was attained in a column SLB-5ms (30 m in length × 0.25 mm in diameter × 0.25 µm in thickness of film, Merck Life Science, Merck KGaA, Darmstadt, Germany). The temperature of the injection port was set at 50 °C and afterwards increased up to 350 °C (increase rate: 3 °C/min; holding time: 5 min). The GC parameters were as follows: injection temperature, 280 °C; injection volume, 1.0 µL (split ratio: 10:1); pure helium gas, 99.9%; linear velocity, 30.0 cm/s; inlet pressure, 26.7 KPa. The MS conditions included an interface temperature of 220 °C, a source temperature of 250 °C and a mass scan range of 40–660 amu. The peak of the samples was identified by using the “FFNSC 3.01” (Shimadzu Europa GmbH, Duisburg, Germany) and “W11N17” (Wiley11-Nist17, Wiley, Hoboken, NJ, USA; Mass Finder 3). Each compound was identified applying a MS similarity match and an LRI filter. Linear retention indices (LRI) were calculated by using a C7-C40 saturated alkanes reference mixture (49452-U, Merck Life Science, Merck KGaA, Darmstadt, Germany). A relative quantity on the basis of peak area percentages was carried out.
3.5. Antifungal Activity
3.5.1. Fungal Strains
Reference strains of Candida spp. used in this study belonged to the American Type Culture Collection (Candida albicans ATCC 90028, Candida tropicalis ATCC 66029 and Candida glabrata ATCC 64677). These fungal strains were supplied by the laboratory of Parasitology-Mycology of the Fattouma Bourguiba teaching hospital of Monastir. Yeast strains were maintained on Sabouraud-Chloramphenicol Agar (SCA).
3.5.2. Determination of the Minimum Inhibitory Concentrations (MICs)
MICs of the VFs were determined using the microdilution technique, as previously described by Hrichi et al. [10], with some modification. Briefly, microtiter plates with 96 flat-bottomed wells were used, and serial dilutions of the test substance (7.00, 3.50, 1.75, 0.87, 0.44, 0.22, 0.11 and 0.05 mg/mL) were prepared using RPMI-1640 media supplemented with 2% glucose. Candida spp. strains were subjected to a susceptibility test face to Amphotericin B concentrations ranging from 16 × 10−3 mg/mL to 0.03 × 10−3 mg/mL as drug standard. Inoculums for the assay were prepared for each strain using diluting a 24 h fresh colony from sabouraud chloramphenicol agar into RPMI-1640-2% glucose and adjusting it to 2.5–5 × 105 CFU mL−1. The final concentration was confirmed using a Malassez counting chamber. Working inoculums (100 µL) were added to the 96-well plates, which were incubated at 37 °C for 24 h. A fungal suspension in the medium was used as growth positive control, and non-inoculated medium RPMI-G 2% (200 µL) was included as a negative control. All experiments were repeated in triplicate. MICs were determined by spectrophotometic lecture at 570 nm to control the growth of fungal strains and defined as the lowest concentration of the VFs produced growth inhibition compared with the growth in the untreated control well.
3.5.3. Minimum fungicidal concentrations (MFCs)
The MFCs were determined using a subculture of 10 μL from MICs wells onto sabouraud chloramphenicol agar plates. The plates were incubated at 37 °C for 24 h. The concentration that induces no visible colony growth after subsequent 24 h incubation was accepted as MFC, which is the lowest concentration without visible biomass growth and corresponding to the death of 99.90% of the original inoculum.
3.6. Inhibition of Biofilm Formation (IBF)
The VFs VF1 and VF2 were tested for their capacity to inhibit Candida spp. biofilm formation. Serial dilutions of (from 7 to 5 × 10−2 mg/mL) were incubated with 5–2.5 × 105 CFU mL−1 fungal cells in RPMI 1640-2% glucose. Inhibition of biofilm formation was determined after crystal violet stain at its sub-inhibitory concentrations [45]. Briefly, serial dilutions of VFs were added to a 96-well plate (Orange Scientific, Braine-l’Alleud, Belgium) and incubated with different Candida spp. inoculums. The media with inoculums were used as positive controls of growth, and the Amphotericin B was used as drug standard at a concentration of 0.2 mg/mL. The morphology of Candida spp. was monitored using an inverted microscope (Olympus CK2) at 40× magnification. The minimum biofilm inhibitory concentration was also determined as the minimum concentration of substance without fungal development [46].
3.7. Biofilm Sustained Inhibition (BSI)
The standard optical density of biofilm sustained inhibition assay of C. althaeoides L. root VFs followed the previously reported protocol for the 96-well format of the biofilm screening assay [47] with some modification. Volatile fractions at different concentrations (7, 3.5, 1.75, 0.87, 0.44, 0.22, 0.11 and 0.05 mg/mL) were added during the 90 min adherence and 24 h growth steps of the Sustained Inhibition Biofilm Assay. Simultaneously different concentrations of Amphotericin B were tested ranging from 4 × 10−3 mg/mL to 0.03 × 10−3 mg/mL as standard drugs. The MIC concentrations for our fungi strains were run many times but not for this plate, if it is possible to mention it as follows. In brief, inoculums were adjusted to 2 × 106 CFU mL−1 from overnight Candida spp. cultures. One hundred microliters of suspension were added to the 96-well plates and shaken at 37 °C for 90 min at 350 rpm in an APELEX shaker. Media was then removed, wells were washed with PBS, and fresh media (or media with VFs) was added back to wells. Plates were then resealed and shaken for a further 24 h at 37 °C. Finally, media was removed, and the absorbance (OD) at 570 nm was determined on a Multiskan™ FC Microplate Photometer.
3.8. Statistical Analysis
Results were reported as means ± standard deviation. All assays were performed considering three analytical replications. Based on the replications, one-way analysis of variance (ANOVA, followed by Turkey’s multiple range test at p < 0.05 level) was employed to assess the significant difference among the biological activities of the samples.
All statistical tests were performed by GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA, USA). Heatmaps graphical representations were guided on OD of microdilution 96 plates at 570 nm.
4. Conclusions
The present research investigates for the first time the chemical profiles of the two VFs of C. althaeoides L. roots and their biological activities. A large number of volatile chemical compounds in the two VFs were identified reaching a value as high as 86 in the chloroformic extarct. These two VFs showed a good antifungal activity against three strains of Candida genus which are pathogenic years. Anti-biofilm activity in Candida spp. with up to 90% was IBF/BSI, indicating that they are promising sources of natural antifungal agents. The richness of the biologically active compounds in these two VFs may be responsible for this activity. Therefore, on the basis of the cytotoxicity study of these two VFs, such a species might be suitable for pharmaceutical applications.
Acknowledgments
The authors gratefully acknowledge Shimadzu Corporation and Merck Life Science for the continuous support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206834/s1, Table S1: List of volatile compounds detected in the hexane fraction (VF1) of C. althaeoides L. roots by GC-MS; Table S2: List of volatile compounds detected in the chloroform fraction (VF2) of C. althaeoides L. roots by GC-MS.
Author Contributions
S.H.: conceptualization, investigation, validation, formal analysis, writing—original draft. R.C.-B.: investigation, validation, formal analysis. F.A.: investigation, data curation. A.B.A.: writing—review and editing. O.B.: investigation. Y.O.E.M.: investigation. H.N.: resources, project administration. L.M.: resources, project administration. H.B.: resources, project administration. Z.M.: resources, project administration, supervision. F.C.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Teodoro G.R., Ellepola K., Seneviratne C.J., Koga-Ito C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015;6:1420. doi: 10.3389/fmicb.2015.01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Hamdani N., Filali-Ansari N., Fdil R., El Abbouyi A., Khyari S. Antifungal activity of the alkaloids extracts from aerial parts of Retama monosperma. Res. J. Pharm. Biol. Chem. Sci. 2016;7:965–971. [Google Scholar]
- 3.Matin M., Bhattacharjee S., Hoque M., Ahamed F. Antibacterial activity of some medicinal plants against carbapenem-resistant Acinetobacter baumannii isolated from patients. Eur. J. Pharm. Med. Res. 2019;6:111–116. [Google Scholar]
- 4.Matin P., Rahman M., Huda D., Bakri M.K., Uddin J., Yurkin Y., Burkov A., Kuok K., Matin M. Application of Synthetic Acyl Glucopyranosides for White-rot and Brown-rot Fungal Decay Resistance in Aspen and Pine Wood. Bioresources. 2022;17:3025–3041. doi: 10.15376/biores.17.2.3025-3041. [DOI] [Google Scholar]
- 5.Hamedi S., Shojaosadati S.A., Mohammadi A. Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 2017;167:36–44. doi: 10.1016/j.jphotobiol.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Semwal D.K., Kumar A., Semwal R.B., Andola H.C. Chapter 3.2.19—Convolvulus prostratus. In: Belwal T., Nabavi S.M., Nabavi S.F., Dehpour A.R., Shirooie S., editors. Naturally Occurring Chemicals Against Alzheimer’s Disease. Academic Press; Cambridge, MA, USA: 2021. pp. 409–424. [DOI] [Google Scholar]
- 7.Al-Rifai A., Aqel A., Al-Warhi T., Wabaidur S.M., Al-Othman Z.A., Badjah-Hadj-Ahmed A.Y. Antibacterial, Antioxidant Activity of Ethanolic Plant Extracts of Some Convolvulus Species and Their DART-ToF-MS Profiling. Evid. Based Complement. Altern. Med. 2017;2017:5694305. doi: 10.1155/2017/5694305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta G.L., Fernandes J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed. Pharmacother. 2019;109:1698–1708. doi: 10.1016/j.biopha.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Azman N., Gallego M., Juliá L., Fajari L., Almajano M. The Effect of Convolvulus arvensis Dried Extract as a Potential Antioxidant in Food Models. Antioxidants. 2015;4:170–184. doi: 10.3390/antiox4010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrichi S., Chaabane-Banaoues R., Giuffrida D., Mangraviti D., Oulad El Majdoub Y., Rigano F., Mondello L., Babba H., Mighri Z., Cacciola F. Effect of seasonal variation on the chemical composition and antioxidant and antifungal activities of Convolvulus althaeoides L. leaf extracts. Arab. J. Chem. 2020;13:5651–5668. doi: 10.1016/j.arabjc.2020.04.006. [DOI] [Google Scholar]
- 11.Hassine M., Zardi-Berguaoui A., Znati M., Flamini G., Ben Jannet H., Hamza M.A. Chemical composition, antibacterial and cytotoxic activities of the essential oil from the flowers of Tunisian Convolvulus althaeoides L. Nat. Prod. Res. 2014;28:769–775. doi: 10.1080/14786419.2013.879476. [DOI] [PubMed] [Google Scholar]
- 12.Cabrita L. A Novel Acylated Anthocyanin with a Linear Trisaccharide from Flowers of Convolvulus althaeoides. Nat. Prod. Commun. 2015;10:1965–1968. doi: 10.1177/1934578X1501001140. [DOI] [PubMed] [Google Scholar]
- 13.Granato D., Shahidi F., Wrolstad R., Kilmartin P., Melton L.D., Hidalgo F.J., Miyashita K., van Camp J., Alasalvar C., Ismail A.B., et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018;264:471–475. doi: 10.1016/j.foodchem.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller M.A., Diekema D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya S., Sae-Tia S., Fries B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics. 2020;9:312. doi: 10.3390/antibiotics9060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deorukhkar S.C., Saini S., Mathew S. Virulence Factors Contributing to Pathogenicity of Candida tropicalis and Its Antifungal Susceptibility Profile. Int. J. Microbiol. 2014;2014:456878. doi: 10.1155/2014/456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage G., Saville S.P., Thomas D.P., López-Ribot J.L. Candida Biofilms: An Update. Eukaryot. Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto E., Salgueiro L.R., Cavaleiro C., Palmeira A., Gonçalves M.J. In vitro susceptibility of some species of yeasts and filamentous fungi to essential oils of Salvia officinalis. Ind. Crops Prod. 2007;26:135–141. doi: 10.1016/j.indcrop.2007.02.004. [DOI] [Google Scholar]
- 19.Achimón F., Brito V.D., Pizzolitto R.P., Ramirez Sanchez A., Gómez E.A., Zygadlo J.A. Chemical composition and antifungal properties of commercial essential oils against the maize phytopathogenic fungus Fusarium verticillioides. Rev. Argent. Microbiol. 2021;53:292–303. doi: 10.1016/j.ram.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Karthikeyan M., Subramanian P., Ramalingam S. Phytochemical analysis in economically important Ficus Benghalensis L. and Ficus Krishnae C.DC. using GC-MS. Int. J. Pharma. Bio. Sci. 2019;10:5–13. doi: 10.22376/ijpbs.2019.10.4.p5-13. [DOI] [Google Scholar]
- 21.Zyakun A., Nii-Annang S., Franke G., Fischer T., Buegger F., Dilly O. Microbial Activity and 13C/12C Ratio as Evidence of N-Hexadecane and N-Hexadecanoic Acid Biodegradation in Agricultural and Forest Soils. Geomicrobiol. J. 2012;29:570–584. doi: 10.1080/01490451.2011.598407. [DOI] [Google Scholar]
- 22.Azadfar M., Gao A.H., Bule M.V., Chen S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015;75:58–66. doi: 10.1016/j.ijbiomac.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Behairy A., Abd El-Rahman G.I., Aly S.S.H., Fahmy E.M., Abd-Elhakim Y.M. Di(2-ethylhexyl) adipate plasticizer triggers hepatic, brain, and cardiac injury in rats: Mitigating effect of Peganum harmala oil. Ecotoxicol. Environ. Saf. 2021;208:111620. doi: 10.1016/j.ecoenv.2020.111620. [DOI] [PubMed] [Google Scholar]
- 24.Al-Akeel R.A., El-Kersh T.A., Al-Sheikh Y.A., Al-Ahmadey Z.Z. Heparin-benzyl alcohol enhancement of biofilms formation and antifungal susceptibility of vaginal Candida species isolated from pregnant and nonpregnant Saudi women. Bioinformation. 2013;9:357–362. doi: 10.6026/97320630009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu M., Wang Y., Sun L., Deng Q., Zhao J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins. 2021;13:852. doi: 10.3390/toxins13120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma K., Kou J., Rahman M.K.U., Du W., Liang X., Wu F., Li W., Pan K. Palmitic acid mediated change of rhizosphere and alleviation of Fusarium wilt disease in watermelon. Saudi J. Biol. Sci. 2021;28:3616–3623. doi: 10.1016/j.sjbs.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahsan T., Chen J., Zhao X., Irfan M., Wu Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express. 2017;7:54. doi: 10.1186/s13568-017-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morcia C., Malnati M., Terzi V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012;29:415–422. doi: 10.1080/19440049.2011.643458. [DOI] [PubMed] [Google Scholar]
- 29.Li Q., Zhu X. Vanillin and its derivatives, potential promising antifungal agents, inhibit Aspergillus flavus spores via destroying the integrity of cell membrane rather than cell wall. Grain Oil Sci. Technol. 2021;4:54–61. doi: 10.1016/j.gaost.2021.03.002. [DOI] [Google Scholar]
- 30.Gao T., Zhang Y., Shi J., Mohamed S.R., Xu J., Liu X. The Antioxidant Guaiacol Exerts Fungicidal Activity Against Fungal Growth and Deoxynivalenol Production in Fusarium graminearum. Front. Microbiol. 2021;12:762844. doi: 10.3389/fmicb.2021.762844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anum Mengal S., Baqi A. 19. Antibacterial and antifungal activities of extracts of Convolvulus leiocalycinus and Haloxylon griffithii of Balochistan, Pakistan. Pure Appl. Biol. 2019;8:2286–2294. [Google Scholar]
- 32.Amin H., Dhiman K., Sharma R., Vyas M., Prajapati P. Shankhapushpi (Convolvulus pluricaulis Choisy): Validation of the Ayurvedic therapeutic claims through contemporary studies. Int. J. Green Pharm. 2014;8:193. doi: 10.1016/j.ijpharm.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Essid R., Gharbi D., Abid G., Karkouch I., Hamouda T.B., Fares N., Trabelsi D., Mhadhbi H., Elkahoui S., Limam F., et al. Combined effect of Thymus capitatus and Cinnamomum verum essential oils with conventional drugs against Candida albicans biofilm formation and elucidation of the molecular mechanism of action. Ind. Crops Prod. 2019;140:111720. doi: 10.1016/j.indcrop.2019.111720. [DOI] [Google Scholar]
- 34.Gulati M., Nobile C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016;18:310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somma A.D., Moretta A., Canè C., Cirillo A., Duilio A. Inhibition of Bacterial Biofilm Formation. IntechOpen; London, UK: 2020. [DOI] [Google Scholar]
- 36.Tits J., Cammue B.P.A., Thevissen K. Combination Therapy to Treat Fungal Biofilm-Based Infections. Int. J. Mol. Sci. 2020;21:8873. doi: 10.3390/ijms21228873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y.-G., Lee J.-H., Lee J. Antibiofilm activities of fatty acids including myristoleic acid against Cutibacterium acnes via reduced cell hydrophobicity. Phytomedicine. 2021;91:153710. doi: 10.1016/j.phymed.2021.153710. [DOI] [PubMed] [Google Scholar]
- 38.Raut J.S., Shinde R.B., Chauhan N.M., Karuppayil S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29:87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- 39.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36:869–888. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hrichi S., Chaabane-Banaoues R., Bayar S., Flamini G., Oulad El Majdoub Y., Mangraviti D., Mondello L., El Mzoughi R., Babba H., Mighri Z., et al. Botanical and Genetic Identification Followed by Investigation of Chemical Composition and Biological Activities on the Scabiosa atropurpurea L. Stem from Tunisian Flora. Molecules. 2020;25:5032. doi: 10.3390/molecules25215032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Zhang M., Addo K.A., Yu Y., Xiao X. Action mode of cuminaldehyde against Staphylococcus aureus and its application in sauced beef. LWT. 2022;155:112924. doi: 10.1016/j.lwt.2021.112924. [DOI] [Google Scholar]
- 42.Yano T., Miyahara Y., Morii N., Okano T., Kubota H. Pentanol and Benzyl Alcohol Attack Bacterial Surface Structures Differently. Appl. Environ. Microbiol. 2016;82:402–408. doi: 10.1128/AEM.02515-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guimarães A., Venâncio A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins. 2022;14:188. doi: 10.3390/toxins14030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasath K.G., Tharani H., Kumar M.S., Pandian S.K. Palmitic Acid Inhibits the Virulence Factors of Candida tropicalis: Biofilms, Cell Surface Hydrophobicity, Ergosterol Biosynthesis, and Enzymatic Activity. Front. Microbiol. 2020;11:864. doi: 10.3389/fmicb.2020.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aswathanarayan J.B., Vittal R.R. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018;8:36133–36141. doi: 10.1039/C8RA06413J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair R.B., Lennartsson P.R., Taherzadeh M.J. Mycelial pellet formation by edible ascomycete filamentous fungi, Neurospora intermedia. AMB Express. 2016;6:31. doi: 10.1186/s13568-016-0203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohse M.B., Gulati M., Craik C.S., Johnson A.D., Nobile C.J. Combination of Antifungal Drugs and Protease Inhibitors Prevent Candida albicans Biofilm Formation and Disrupt Mature Biofilms. Front. Microbiol. 2020;11:1027. doi: 10.3389/fmicb.2020.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.