Abstract
Cardiovascular diseases are associated with gut dysbiosis, but the role of microbe-derived metabolites as biomarkers or modulators of cardiovascular disease are not well understood. This is a targeted metabolomics study to investigate the association of nine microbe-derived metabolites with lower extremity peripheral artery disease (PAD), a form of atherosclerosis, and major adverse cardiac events (MACE). The study cohort consists of individuals with intermittent claudication and ankle-brachial index (ABI) < 0.9 (N = 119) and controls without clinically-apparent atherosclerosis (N = 37). The primary endpoint was MACE, a composite endpoint of myocardial infarction, coronary revascularization, stroke, transient ischemic attack, or cardiac-related death. Plasma metabolite concentrations differed significantly between the PAD and control groups. After adjustment for traditional atherosclerosis risk factors, kynurenine, hippuric acid, indole-3-propionic acid (IPA), and indole-3-aldehyde (I3A) concentrations were negatively associated with PAD, whereas indoxyl sulfate and 3-hydroxyanthranilic acid were positively associated. Hippuric acid, IPA, and I3A correlated with ABI, a surrogate for atherosclerotic disease burden. Those in the highest I3A concentration quartile had significantly improved freedom from MACE during follow-up compared to those in the lowest quartile. This study identifies specific indole- and phenyl-derived species impacted by gut microbial metabolic pathways that could represent novel microbiome-related biomarkers of PAD.
Keywords: peripheral artery disease, ankle-brachial index, microbiome
1. Introduction
Multiple lines of evidence point towards a modulatory role of the gut microbiome in atherosclerosis. Germ-free mice with a genetic predisposition for atherosclerosis (apolipoprotein E deficiency) that were fed a low fat diet had less atherosclerotic plaques compared to conventionally-raised mice [1]; microbial producers of butyrate, a short-chain fatty acid, have a protective effect against atherosclerosis [2]; and trimethylamine-N-oxide, a derivative of the microbial metabolite trimethylamine, is both atherogenic in animal models and associated with cardiovascular events in humans [3,4,5,6]. Patients with symptomatic carotid stenosis requiring revascularization have been found to have altered microbiota compared to age- and gender-matched controls [7]. Other cardiovascular diseases have also recently been associated with gut dysbiosis, including coronary artery disease [8,9,10], hypertension [11,12,13], stroke [14,15,16,17], heart failure [18,19,20].
While metabolomic phenotyping allows for detailed documentation of the global functional output of the gut microbiome through qualitative and quantitative analyses of the mammalian–microbial co-metabolome [21], the functional role of most of the commensal metabolome in cardiovascular disease is not well understood. We previously identified correlations between systemic concentration of microbe-derived indole- and phenyl-derived metabolites [22] and advanced atherosclerosis in a heterogeneous population of vascular surgery patients undergoing procedures for advanced atherosclerosis [23]. The cohort with advanced atherosclerosis had either carotid or lower extremity peripheral artery disease (PAD). Given the heterogeneity in the clinical presentation and severity of atherosclerosis in the previous study, the current study is focused only on people with intermittent claudication, a form of PAD in which pain in the legs occurs with exercise and is relieved by rest (Rutherford category 1–3) [24]. The purpose of this study is to investigate the ability of these selected microbe-derived metabolites to discriminate between a well-characterized cohort of participants with intermittent claudication and non-PAD controls.
2. Materials and Methods
2.1. Study Population
The PAD cohort consists of participants in the placebo arm of the OMEGA PAD trials at the San Francisco VA Medical Center [25,26,27] between 2011 and 2016, all of whom were claudicants (Rutherford category 1–3) with PAD confirmed by the ankle-brachial index (ABI) < 0.9 and were age ≥ 50 years. Exclusion criteria in the OMEGA PAD trials were severe hepatic (Child–Pugh ≥ B) or renal (creatinine ≥ 2 mg/dL) disease, chronic limb-threatening ischemia, or nonvascular inflammatory disease. Patients were also excluded if they had a severe acute illness within 30 days or were taking immunosuppressive medications or steroids. An additional cohort of patients with no history of PAD, no clinical atherosclerotic disease (coronary artery or cerebrovascular disease), and ABI > 0.9 served as non-PAD controls. Demographic and clinical data were collected prospectively. Blood was drawn at the baseline visit and plasma was immediately prepared, aliquoted, and stored at −80 °C. Plasma aliquots had not been thawed before this study. The investigator-initiated protocol was approved by the University of California, San Francisco Committee on Human Research as well as the San Francisco Veterans Affairs Research and Development Office. Written informed consent was obtained. The OMEGA PAD trials were registered with ClinicalTrials.gov (unique identifiers: NCT01310270 and NCT01979874).
2.2. Variables
Sociodemographic variables included sex, age, and race. Inflammatory markers including high-sensitivity C-reactive protein (CRP) were measured as previously described [25,26]. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [28]. Past medical history and medication use in both the PAD and non-PAD groups were collected at the time of original study enrollment. Smoking was defined as current or prior tobacco use. Bilateral resting ABI was measured at the baseline visit in the following manner. Systolic blood pressures of the brachial, posterior tibial, and dorsalis pedis arteries were measured bilaterally. For each lower extremity, the highest systolic pressure of the 2 pedal pulses was divided by the highest systolic pressure of the 2 brachial arteries. The ABI was classified as “incompressible” if ≥ 1.3. The omega-3 index is a measure of omega-3 fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) in red blood cell membranes and is recognized as an independent biomarker for cardiovascular health and disease [29]. The omega-3 index was measured using previously published methods [30].
2.3. Endpoints
The primary endpoint was major adverse cardiovascular event (MACE), defined as a composite endpoint of myocardial infarction (MI), coronary revascularization, stroke, transient ischemic attack, or cardiac-related death [27]. Data on the date of these events were abstracted retrospectively from the electronic medical record. MI was defined according to the American Heart Association universal definition of acute MI [31]. Stroke was defined as any new embolic, thrombotic, or hemorrhagic cerebrovascular event with neurologic deficits that persisted for at least 24 hours, as defined by an attending neurologist.
2.4. Detection and Quantification of Metabolites by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
Plasma concentrations of metabolites (p-cresyl sulfate [PCS], hippuric acid [HA], indole-3-propionic acid [IPA], tryptophan [TRP], kynurenine [KYN], indoxyl sulfate [IS], serotonin, indole-3-aldehyde [I3A], and 3-hydroxyanthranilic acid [HAA]) in 50 μL plasma were measured as previously described [23] in a 96-well plate format.
2.5. Statistical Analysis
Continuous variables are reported as median values with interquartile ranges and were compared using the Wilcoxon signed rank test or the Student’s t-test based on the normality of distribution. Associations between continuous variables were evaluated by Spearman rank correlation. Categorical variables are reported as frequencies and percentages and compared using Chi-squared or Fisher’s exact testing. Metabolite concentrations were natural log (ln) transformed to reduce skewness before regression analyses. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Study Population
Baseline characteristics of the claudication (N = 119) and non-PAD cohorts (N = 37) are shown in Table 1. The claudication cohort had a greater prevalence of coronary artery disease, hypertension, hyperlipidemia, smoking, and history of prior MACE compared to the control cohort. As anticipated, the claudication cohort also had more frequent use of aspirin and statin medication and worse ABI. Interestingly, median low-density lipoprotein cholesterol concentration was lower in the claudication group, likely attributable to statin use. The follow-up period ranged from 2.5 to 3.2 years, with a median of 2.9 years (interquartile range 2.3–3.6)
Table 1.
Baseline characteristics of study population.
| Covariates N (%) or Median [Interquartile Range] |
Control Cohort (N = 37) |
Claudication (N = 119) |
p Value |
|---|---|---|---|
| Age | 67 (60–74) | 68 (65–73) | 0.19 |
| Male sex | 32 (86.5) | 115 (96.6) | 0.02 |
| African-American | 7 (18.9) | 23 (19.3) | 0.54 |
| Body mass index (kg/m2) | 29.4 (26.1–31.3) | 27.8 (24.4–31.2) | 0.18 |
| Coronary artery disease | 0 | 47 (39.5) | <0.0001 |
| Prior coronary revascularization | 33 (75%) | ||
| Prior MACE | 0 | 46 (42.4) | <0.0001 |
| Hypertension | 25 (67.6) | 111 (93.3) | <0.0001 |
| Hyperlipidemia | 24 (64.9) | 100 (84) | <0.01 |
| Diabetes mellitus | 8 (21.6) | 41 (34.5) | 0.14 |
| Current/former smoker | 27 (75) | 119 (93.3) | 0.002 |
| Aspirin use | 19 (51.4) | 86 (72.3) | 0.02 |
| Statin use | 23 (62.2) | 95 (79.3) | 0.03 |
| ABI | 1.1 (1.1–1.2) | 0.73 (0.64–0.81) | <0.0001 |
| Total cholesterol, mg/dL | 170 (142.5–197) | 158 (127–182) | 0.10 |
| LDL cholesterol, mg/dL | 99 (79.5–124.5) | 82 (60–106) | 0.008 |
| HDL cholesterol, mg/dL | 43.5 (38–47.5) | 42 (35–55) | 0.89 |
| eGFR, mL/min/1.73 mm2 | 82 (66–107) | 70.5 (60.5–86.5) | 0.02 |
| hs-CRP, mg/mL | 3.3 (1.2–5.1) | 2.4 (1.5–6) | 0.82 |
| Rutherford classification | <0.0001 | ||
| 0 | 37 (100) | 4 (3.4) | |
| 1 | 0 | 38 (32.5) | |
| 2 | 0 | 34 (29.1) | |
| 3 | 0 | 41 (35.0) | |
| Metabolites, μmol | |||
| Indole derivatives | |||
| Serotonin | 1.3 (.52–2.4) | 0.96 (0.48–1.38) | 0.02 |
| KYN | 3.8 (2.9–5) | 2.3 (1.9–4.2) | 0.0001 |
| TRP | 0.076 (0.047–0.099) | 0.052 (0.038–0.076) | 0.005 |
| KYN/TRP ratio (x100) | 4975 (3717–6783) | 4935 (3949–6743) | 0.69 |
| IPA | 2.7 (1.7–4.1) | 1.07 (0.57–1.86) | <0.0001 |
| I3A | 0.21 (0.14–0.24) | 0.12 (0.10–0.16) | <0.0001 |
| IS | 1.3 (0.6–2.4) | 2.8 (1.8–4.9) | 0.002 |
| HAA | 0 | 0.31 (0.22–0.54) | <0.0001 |
| Phenyl derivatives | |||
| PCS | 0.23 (0.13–0.37) | 0.31 (0.19–0.51) | 0.07 |
| HA | 18.4 (11.7–24.2) | 9.4 (6.3–16.7) | <0.0001 |
| Cytokines, pg/mL | |||
| IL-6 | 0.85 (0.63–1.23) | 1.2 (0.93–1.81) | 0.02 |
| ICAM | 206 (172–300) | 246 (207–300) | 0.06 |
| TNF-α | 1.7 (1.3–2.0) | 1.98 (1.64–2.35) | 0.004 |
| Omega-3 index | 0.064 (0.050–0.072) | 0.046 (0.040–0.055) | <0.0001 |
ABI, Ankle–brachial index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low density lipoprotein. All other abbreviations are as described in the text. Red values indicate p < 0.05.
Claudicants had significantly higher baseline plasma concentrations of inflammatory cytokines IL-6, ICAM, and TNF-α and a significantly lower omega-3 index compared to non-PAD controls. There were also significant unadjusted differences in the concentrations of the microbe-derived metabolites of interest, as shown in Table 1. Claudicants had significantly higher plasma concentrations of IS, HAA, and PCS and lower baseline plasma concentrations of serotonin, KYN, TRP, IPA, I3A, and HA than non-PAD controls. The KYN/TRP ratio, a surrogate for indole-2,3-deoxygenase (IDO1) and tryptophan-2,3- deoxygenase (TDO) activity [32], was not observed to be significantly different between the groups. After adjusting the metabolites for sex and traditional risk factors for atherosclerosis (smoking, hypertension, diabetes, and hyperlipidemia) (Table 2), KYN (OR 0.22; 95% CI 0.086–0.57; p = 0.002), HA (OR 0.42; 95% CI, 0.24–0.72; p = 0.002), IPA (OR 0.36; 95% CI, 0.21–0.61; p = 0.0002), and I3A (OR 0.11; 95% CI, 0.03–0.35; p = 0.0002) remained significantly negatively associated with claudication, while IS (OR 1.8; 95% CI, 1.11–3.00); p = 0.02) and HAA (OR 4.44; 95% CI 2.6–7.5; p < 0.0001) correlated positively with claudication.
Table 2.
Odds ratios (OR) for claudication obtained from logistic models. In each model, individual metabolites are separately adjusted for sex, smoking, diabetes, hypertension, and hyperlipidemia.
| Metabolite | OR | 95% CI | p Value |
|---|---|---|---|
| ln serotonin | 0.69 | 0.45–1.1 | 0.1 |
| ln KYN | 0.22 | 0.086–0.57 | 0.002 |
| ln TRP | 0.69 | 0.34–1.4 | 0.29 |
| ln KYN/TRP | 0.65 | 0.36–1.15 | 0.14 |
| ln HA | 0.42 | 0.24–0.72 | 0.002 |
| ln IPA | 0.36 | 0.21–0.61 | 0.0002 |
| ln IS | 1.8 | 1.11–3.00 | 0.02 |
| ln PCS | 1.1 | 0.72–1.72 | 0.63 |
| ln HAA | 4.44 | 2.6–7.5 | <0.0001 |
| ln I3A | 0.11 | 0.03–0.35 | 0.0002 |
OR, odds ratio. CI, confidence interval. All other abbreviations are as described in the text. Red values indicate p < 0.05.
3.2. Correlation between Metabolites and ABI
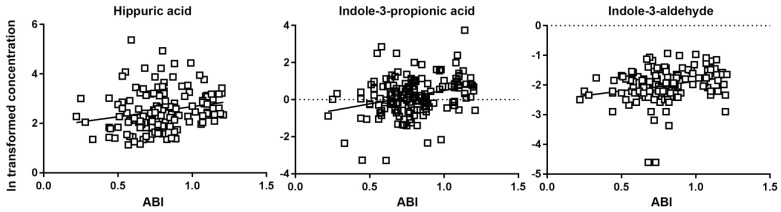
PAD is associated with prevalent cardiovascular disease and adverse cardiovascular disease risk factor profiles [33,34,35]. Prospective studies using ABI have shown that a low ABI predicts both cardiovascular and all-cause mortality in people with and without existing clinical coronary artery disease [35,36,37,38,39,40,41,42,43,44,45,46]. Therefore, we examined the Spearman correlation between each metabolite and ABI. As shown in Figure 1, HA (Spearman r = 0.29; p = 0.0004), IPA (Spearman r = 0.27; p = 0.001), and I3A (Spearman r = 0.3; p = 0.0004) had modest but statistically significant positive correlations with ABI.
Figure 1.
Correlations between ln-transformed metabolite concentrations and ankle-brachial index (ABI). Only correlations with p < 0.05 are shown. Each line represents the linear regression of each metabolite on ABI. Spearman coefficients and p values are provided in the text.
3.3. Relationship between Metabolites and MACE
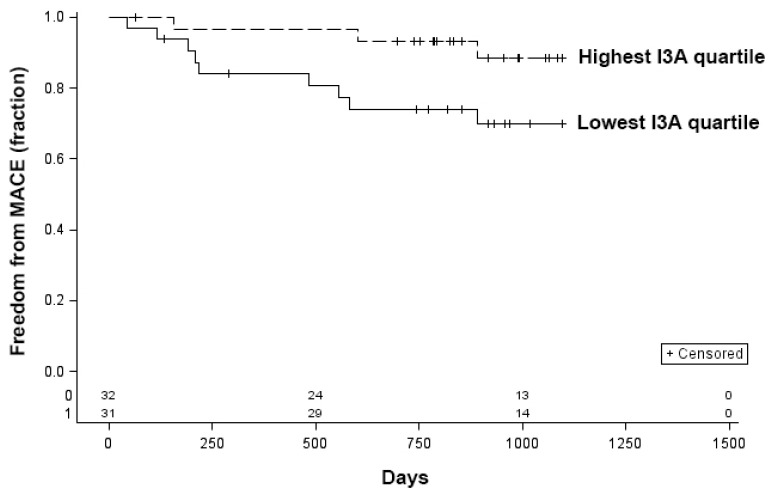
Data on MACE were available on 139 participants. Of these, 28 (20.1%) patients experienced a MACE during the follow-up period. Univariate analysis comparing patients who had a MACE with those without MACE is shown in Table 3. Patients who experienced MACE were more likely to have coronary artery disease (p = 0.0002) and a decreased ABI (p = 0.01) compared to those who did not have a MACE. In addition, baseline unadjusted KYN (p = 0.02), TRP (p = 0.003), and I3A (p = 0.02) were significantly associated with MACE on univariate analysis. When patients were stratified into groups by using the quartiles of metabolite concentrations, patients in the highest I3A quartile had significantly improved freedom from MACE compared to patients in the lowest I3A quartile in a Kaplan–Meier analysis (p = 0.045, log-rank) (Figure 2). Differences in freedom from MACE based on the lowest and highest quartiles of KYN and TRP did not reach statistical significance (KYN, p = 0.45; TRP, p = 0.38; log-rank).
Table 3.
Univariate associations with MACE during the follow-up period.
| Variable | No MACE N = 111 (79.9%) |
MACE N = 28 (20.1%) |
p Value |
|---|---|---|---|
| Age | 67.4 (63.6–73.3) | 68.8 (64.4–75.8) | 0.35 |
| Male sex | 108 (97.3%) | 27 (96.4%) | 0.99 |
| African-American | 24 (21.6%) | 4 (14.3%) | 0.56 |
| Body mass index (kg/m2) | 28.6 (25.0–31.2) | 27.8 (24.6–32.6) | 0.99 |
| Past medical history | |||
| Coronary artery disease | 27 (24.3%) | 17 (60.7%) | 0.0002 |
| Prior coronary revascularization | 18 (66.7%) | 15 (88.2%) | 0.11 |
| Prior MACE | 29 (26.1%) | 17 (60.7%) | 0.0005 |
| Hypertension | 98 (88.3%) | 25 (89.3%) | 0.88 |
| Hyperlipidemia | 92 (82.3%) | 22 (78.6%) | 0.60 |
| Diabetes mellitus | 35 (31.5%) | 10 (35.7%) | 0.67 |
| Current/former smoker | 100 (90.9%) | 25 (89.3%) | 0.79 |
| Aspirin use | 76 (68.5%) | 20 (71.4%) | 0.76 |
| Statin use | 82 (73.9%) | 23 (82.1%) | 0.36 |
| ABI | 0.8 (0.7–1.0) | 0.7 (0.6–0.8) | 0.01 |
| Total cholesterol, mg/dL | 160 (136–185) | 156 (122–189.5) | 0.61 |
| LDL cholesterol, mg/dL | 83 (64–110) | 83 (57.5–108.5) | 0.53 |
| HDL cholesterol, mg/dL | 43 (36–54) | 45 (35.5–50.5) | 0.57 |
| eGFR, mL/min/1.73 mm2 | 73 (63–90) | 72 (60–93) | 0.76 |
| hs-CRP, mg/mL | 2.4 (1.3–5.1) | 3.4 (1.7–7.1) | 0.28 |
| Rutherford classification | 0.08 | ||
| 0 | 29 (26.4%) | 4 (14.3%) | |
| 1 | 30 (27.3%) | 4 (14.3%) | |
| 2 | 25 (22.7%) | 7 (25.0%) | |
| 3 | 26 (23.6%) | 13 (45.4%) | |
| Metabolites, μmol | |||
| Indole derivatives | |||
| Serotonin | 0.96 (0.40–1.49) | 1.14 (0.64–1.51) | 0.46 |
| KYN | 2.84 (2.02–4.57) | 2.89 (1.77–3.21) | 0.02 |
| TRP | 0.06 (0.04–0.09) | 0.04 (0.029–0.057) | 0.003 |
| KYN/TRP ratio (x100) | 4790 (3798–6491) | 4955 (4189–6892) | 0.30 |
| IPA | 1.15 (0.61–2.3) | 1.11 (0.63–1.77) | 0.65 |
| I3A | 0.14 (0.11–0.20) | 0.11 (0.096–0.16) | 0.02 |
| IS | 2.70 (1.55–4.56) | 3.03 (1.81–4.40) | 0.74 |
| HAA | 0.26 (0–0.44) | 0.28 (0.11–0.63) | 0.32 |
| Phenyl derivatives | |||
| PCS | 0.29 (0.17–0.47) | 0.32 (0.21–0.70) | 0.20 |
| HA | 11.0 (7.1–19.7) | 9.1 (5.3–16.8) | 0.12 |
| Cytokines, pg/mL | |||
| IL-6 | 1.1 (0.79–1.49) | 1.5 (1–2.4) | 0.03 |
| ICAM | 230 (196.4–280) | 272.9 (221.5–308.1) | 0.07 |
| TNF-α | 1.86 (1.45–2.24) | 2.05 (1.82–2.31) | 0.04 |
| Omega-3 index | 0.07 (0.06–0.08) | 0.05 (0.04–0.06) | 0.49 |
CI, confidence interval. All other abbreviations are as described in the text. Red values indicate p < 0.05.
Figure 2.
Kaplan–Meier curves for freedom from MACE for patients in the lowest and highest quartiles of indole-3-aldehyde (I3A) concentration. p = 0.045, log-rank.
4. Discussion
We demonstrate that baseline plasma concentrations of multiple gut microbe-derived indole- and phenyl-derived metabolites (KYN, TRP, IPA, I3A, IS, HAA, and HA) are associated with presence of lower extremity PAD and with MACE. Specifically, after adjustment for traditional risk factors for atherosclerosis, KYN, HA, IPA, I3A, and IS are associated with decreased risk of claudication, while IS and HAA are associated with an increased risk. Furthermore, there was a significant positive association between baseline KYN, TRP, and I3A levels and MACE.
These findings are largely in concordance with our previous study describing an association between indole- and phenyl-derived metabolites that are either exclusively or partly produced via microbial metabolic pathways and advanced atherosclerosis [23]. In this study, we observed that claudicants had significantly lower IPA and I3A levels compared to non-PAD controls (OR 0.36 and OR 0.11, respectively, p = 0.0002 for both). However, new associations were made between IS, HAA, HA, and PAD, while previous observations linking TRP and the KYN/TRP ratio to advanced atherosclerosis [23] were not seen in the current study.
Like the previous study [23], we also observed modest yet statistically significant positive correlations between HA, IPA, and I3A and ABI. A diagnosis of PAD can be made clinically or by hemodynamic assessment (i.e., ABI ≤ 0.9). The utility of a novel plasma biomarker for PAD would be greatest in individuals who are not suspected to have this disease or underlying risk. A future larger study will be needed to understand if these metabolites alone, or, more likely, a model of these metabolites interacting with other clinical parameters, predict the probability of PAD.
Our observations are largely consistent with what is currently known about these metabolites and atherosclerosis or atherogenesis. IPA, which is a product of the bacterial indole pyruvate pathway of tryptophan metabolism [47], is a ligand for the pregnane X receptor, which is found in many tissues including the endothelium and modulates vasodilation, innate immune receptor expression/function, and endothelial detoxification processes [48,49,50]. We observed an inverse correlation between IPA and advanced atherosclerosis in the previous study [23] and between IPA and claudication in the current study. Similarly, others have found an inverse correlation between circulating IPA and type 2 diabetes mellitus and low-grade inflammation in human population-based studies [51,52]. IPA was also downregulated in patients with coronary artery disease compared to healthy controls, and dietary IPA supplementation attenuated atherosclerotic plaque in a genetic model of atherosclerosis [53].
IS is a uremic toxin formed through hydroxylation of indole in the liver followed by O-sulfation and by the activity of bacterial tryptophanase [47]. IS activates the aryl hydrocarbon receptor (AHR) pathway in primary human aortic vascular smooth muscle cells to promote thrombosis through the upregulation of tissue factor and inhibition of ubiquitination and degradation of tissue factor [54,55]. In endothelial and adipose cells, IS induces proinflammatory cytokines and monocyte/macrophage activation [56]. Serum concentrations of IS are associated with aortic calcification, arterial stiffness, and increased cardiovascular mortality in patients with chronic kidney disease [57]. Among patients with end-stage renal disease on hemodialysis, IS was associated with incident PAD [58].
As with IPA, I3A is a product of TRP metabolism by bacterial tryptophanase [47]. I3A also acts on the AHR pathway [59]. We observed an inverse correlation between I3A and claudication, and elevated I3A reduces risk of MACE. Others have found that I3A increases anti-inflammatory IL-10 receptor expression [60] and reduction in the type I interferon response [61] and inflammatory cytokine profile [62], thus raising the possibility that I3A reduces risk of PAD by attenuating systemic inflammation.
HAA is a downstream product of TRP metabolism to KYN via IDO1 and tryptophan 2,3-dioxygenase followed by conversion of KYN to 3-hydroxykynurenine and then to HAA. IDO1 is induced in the context of inflammation [63] and is involved in autoimmunity, chronic infection, granulomatous diseases, and cancer [64]. In a cohort of patients with stable angina, plasma HAA, in addition to other kynurenines, was associated with risk of acute MI after multivariable adjustment (hazard ratio 1.48; 95% CI 1.10–1.99) and correlated with phenotypes of metabolic syndrome, suggesting that these metabolites could be used to improve risk estimates [65]. In animal models, HAA regulates the inflammasome and decreases plasma lipids and atherosclerosis [66].
We observed a negative correlation between PAD and HA, although the mechanism by which HA impacts cardiovascular disease is unknown. HA, the glycine conjugate of benzoic acid, is part of the endogenous urinary metabolite profile, but levels can be indicative of microbial metabolism of certain nutrients, and it can be a biomarker of toxic compounds such as toluene [67]. In a rat model of diet-induced atherosclerosis, hippurate excretion was higher in the atherosclerosis group compared to the control group, but differences in diets and cage environments were not explored [68]. Notably, hippurate has been shown to correlate with a lean phenotype and a diet rich in flavanols [69,70], which is beneficial for cardiovascular health [71,72,73]. Interestingly, in a large population-based cohort study of patients with hypertension, low urinary hippurate excretion correlated with high blood pressure [74], a finding that is in concordance with the observation that urinary hippurate is lower in spontaneously hypertensive rats compared to normotensive rats [75]. In contrast, in other animal models, elevated hippurate is associated with endothelial dysfunction and accelerated atherosclerosis [76]. Given these controversies, further research is needed to understand the role of HA in cardiovascular disease, either as a biomarker or direct modulator.
While we previously observed that TRP and the KYN/TRP ratio correlated with advanced atherosclerosis [23], we did not observe a significant link between these metabolites and claudication in the current study. However, there was a significant correlation between these metabolites and development of MACE during the follow-up period. As IDO1 activity correlates with systemic inflammation, we surmise that more pronounced baseline inflammation in the previous cohort of patients with advanced atherosclerosis, all of whom underwent either revascularization or amputation, compared to the claudicants in the current cohort, accounts for the differences in metabolomics profiles. Indeed, patients with chronic limb-threatening ischemia are known to have higher circulating inflammatory cytokine levels than patients with claudication [77], and inflammatory markers improve predictive models for adverse events such as major amputation and death in patients with severe limb ischemia [78].
While MACE endpoints are typically adopted for patients with chronic limb-threatening ischemia, claudication is associated with a high risk of cardiovascular morbidity but a low risk of progression of leg symptoms. In a population-based observational study, patients with claudication had 2.6-fold increased risk of cardiovascular death compared to asymptomatic patients [79]. In patients with large-vessel PAD, there was a nearly six-fold relative risk of cardiovascular death over 10 years compared to those without PAD [40]. In a more contemporary prospective cohort, MACE events occurred in 37% and 64% of claudicants at 5 and 10 years, respectively [80]. Thus, we felt that the MACE metric was relevant for this cohort.
This study provides supportive evidence for further investigation of indole- and phenyl-derived microbial metabolites in PAD and, more generally, in cardiovascular disease. We focused solely on claudication, which is a more clinically homogeneous clinical entity than our previous cohort of patients who were a heterogeneous group undergoing revascularization or amputation for advanced atherosclerotic disease in multiple vascular beds. This allowed for better control of potentially confounding factors. Concordance in findings between these two studies argues for assessment of these associations in larger populations to confirm these findings. Since high-density metabolite profiling systems can simultaneously detect microbial metabolites in biofluids (e.g., blood and urine) and tissue biopsies, which can then be integrated with clinical data, it is possible that future studies could provide novel insights into disease-predictive and/or disease-associated microbe-associated biomarkers. Furthermore, given the known crucial roles of many microbial metabolites in host homeostasis and immunology, an in-depth understanding of the genetic control of microbial metabolic pathways and the repercussions of gut microbial community changes to host biochemical and metabolic pathways is of fundamental importance [81,82].
The limitations of this study include its observational nature and inherent potential for unmeasured confounders, such as lack of direct dietary or microbiome data and lack of information on antibiotic exposure and other medications which could impact the microbiome [83,84]. Furthermore, while this mostly male and Caucasian cohort is reflective of the population served by our medical center, its composition potentially limits the generalizability of the findings. Third, a targeted metabolomic approach inherently restricts the panel of candidate markers and focuses on only a few metabolic pathways. However, focused examination of these metabolites arose from a broad untargeted comparative metabolomic analysis of germ-free and conventional mice which identified metabolites that arise exclusively from microbiota or are significantly impacted by microbiota [22]. Finally, although our analysis cannot determine causality, it provides further supportive evidence that alterations in specific indole- and phenyl-derived metabolic species might represent important pathophysiologic mechanisms in PAD that deserve further investigation.
5. Conclusions
In summary, the present study identifies specific indole- and phenyl-derived microbial metabolites associated with claudication and MACE. Our findings lay the groundwork for future studies that refine the microbial metabolomic signature of PAD and increase our understanding of how these metabolites could add independent value to existing clinical risk scores for diagnosing and prognosticating clinically relevant outcomes in PAD.
Author Contributions
Conceptualization, K.J.H., C.K.O. and S.M.G.; methodology, K.J.H., K.G.H. and S.M.G.; formal analysis, K.J.H., J.L.R., K.G.H., I.H. and S.M.G.; investigation, J.L.R., R.K., K.G.H., L.X. and S.M.G.; resources, K.J.H. and S.M.G.; data curation, K.J.H., J.L.R. and I.H.; writing—original draft preparation, K.J.H., J.L.R., R.K. and S.M.G.; writing—review and editing, K.J.H., J.L.R., R.K., K.G.H., I.H., L.X., C.K.O. and S.M.G.; visualization, K.J.H., J.L.R. and I.H.; supervision, K.J.H. and S.M.G.; project administration, K.J.H.; funding acquisition, K.J.H. and S.M.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no conflict of interest relevant to this study to disclose.
Funding Statement
This work was supported by the National Heart, Lung and Blood Institute (K08HL130601 and R03HL146880 to K.J.H., K23HL122446 to S.M.G., and R01HL133500 to C.K.O.), the National Center for Advancing Translational Sciences (TL1TR001871 to J.R.); National Center for Research Resources (KL2RR024130 to S.M.G.); the American College of Surgeons (Career Development Awards to K.J.H. and S.M.G.); the Society for Vascular Surgery (Career Development Awards to K.J.H. and S.M.G., Clinical Research Seed Grant to S.M.G., Student Research Fellowship Award to J.R.); the American Heart Association (Student Scholarships to J.R. and R.K.); the Northern California Institute for Research and Education (to S.M.G.); Vascular Cures (to K.J.H.); the Northwestern Memorial Foundation, Eleanor Wood-Prince Grants Initiative (to K.J.H.); and the Northwestern University Feinberg School of Medicine, Division of Vascular Surgery (the Vascular Surgery Student Research Award from the Joseph B. & Marjorie M. Lanterman Endowed Research and Education fund to R.K.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stepankova R., Tonar Z., Bartova J., Nedorost L., Rossman P., Poledne R., Schwarzer M., Tlaskalova-Hogenova H. Absence of Microbiota (Germ-Free Conditions) Accelerates the Atherosclerosis in ApoE-Deficient Mice Fed Standard Low Cholesterol Diet. J. Atheroscler. Thromb. 2010;17:796–804. doi: 10.5551/jat.3285. [DOI] [PubMed] [Google Scholar]
- 2.Kasahara K., Krautkramer K.A., Org E., Romano K.A., Kerby R.L., Vivas E.I., Mehrabian M., Denu J.M., Bäckhed F., Lusis A.J., et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018;3:1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W.W., Wang Z., Fan Y., Levison B., Hazen J.E., Donahue L.M., Wu Y., Hazen S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W.H.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., DuGar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.-M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senthong V., Wang Z., Fan Y., Wu Y., Hazen S.L., Tang W.H.W. Trimethylamine N-Oxide and Mortality Risk in Patients With Peripheral Artery Disease. J. Am. Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson F., Fåk F., Nookaew I., Tremaroli V., Fagerberg B., Petranovic D., Bäckhed F., Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Q., Gao R., Zhang Y., Pan D., Zhu Y., Zhang X., Yang R., Jiang R., Xu Y., Qin H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018;50:893–903. doi: 10.1152/physiolgenomics.00070.2018. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Chen X., Hu X., Niu H., Tian R., Wang H., Pang H., Jiang L., Qiu B., Chen X., et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. 2019;7:68. doi: 10.1186/s40168-019-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian R., Liu H., Feng S., Wang H., Wang Y., Wang Y., Liang L., Xu H., Xing H., Zhang S. Gut microbiota dysbiosis in stable coronary artery disease combined with type 2 diabetes mellitus influences cardiovascular prognosis. Nutr. Metab. Cardiovasc. Dis. 2021;31:1454–1466. doi: 10.1016/j.numecd.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Zhao F., Wang Y., Chen J., Tao J., Tian G., Wu S., Liu W., Cui Q., Geng B., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan X., Mushi Z., Baili W., Han L., Enqi W., Huanhu Z., Shuchun L. Differential Analysis of Hypertension-Associated Intestinal Microbiota. Int. J. Med. Sci. 2019;16:872–881. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun S., Lulla A., Sioda M., Winglee K., Wu M.C., Jacobs D.R., Jr., Shikany J.M., Lloyd-Jones D.M., Launer L.J., Fodor A.A., et al. Gut Microbiota Composition and Blood Pressure. Hypertension. 2019;73:998–1006. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., Sita G., Racchumi G., Ling L., Pamer E.G., et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh V., Roth S., Llovera G., Sadler R., Garzetti D., Stecher B., Dichgans M., Liesz A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia G.-H., You C., Gao X.-X., Zeng X.-L., Zhu J.-J., Xu K.-Y., Tan C.-H., Xu R.-T., Wu Q.-H., Zhou H.-W., et al. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated With Brain Injury and Prognosis of Stroke. Front. Neurol. 2019;10:397. doi: 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K., Gao X., Xia G., Chen M., Zeng N., Wang S., You C., Tian X., Di H., Tang W., et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021;70:1486–1494. doi: 10.1136/gutjnl-2020-323263. [DOI] [PubMed] [Google Scholar]
- 18.Sun W., Du D., Fu T., Han Y., Li P., Ju H. Alterations of the Gut Microbiota in Patients With Severe Chronic Heart Failure. Front. Microbiol. 2021;12:813289. doi: 10.3389/fmicb.2021.813289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organ C.L., Otsuka H., Bhushan S., Wang Z., Bradley J., Trivedi R., Polhemus D.J., Tang W.W., Wu Y., Hazen S.L., et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui X., Ye L., Li J., Jin L., Wang W., Li S., Bao M., Wu S., Li L., Geng B., et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018;8:635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson J.K., Holmes E., Wilson I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 22.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cason C.A., Dolan K.T., Sharma G., Tao M., Kulkarni R., Helenowski I.B., Doane B.M., Avram M.J., McDermott M.M., Chang E.B., et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2017;65:1552–1562. doi: 10.1016/j.jvs.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutherford R.B., Baker J., Ernst C., Johnston K., Porter J.M., Ahn S., Jones D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997;26:517–538. doi: 10.1016/S0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 25.Grenon S.M., Owens C.D., Nosova E.V., Hughes-Fulford M., Alley H.F., Chong K., Perez S., Yen P.K., Boscardin J., Hellmann J., et al. Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients with Peripheral Artery Disease (the OMEGA-PAD I Trial) J. Am. Heart Assoc. 2015;4:e002034. doi: 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grenon S.M., Owens C.D., Alley H., Chong K., Yen P.K., Harris W., Hughes-Fulford M., Conte M.S. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: The OMEGA-PAD trial. Vasc. Med. 2013;18:263–274. doi: 10.1177/1358863X13503695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaller M.S., Ramirez J.L., Gasper W.J., Zahner G.J., Hills N.K., Grenon S.M. Frailty is Associated with an Increased Risk of Major Adverse Cardiac Events in Patients with Stable Claudication. Ann. Vasc. Surg. 2018;50:38–45. doi: 10.1016/j.avsg.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey A.S., Coresh J., Greene T., Stevens L.A., Zhang Y.L., Hendriksen S., Kusek J.W., Van Lente F., Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 29.Drudi L.M., Schaller M.S., Hiramoto J., Gasper W., Harris W.S., Hills N.K., Grenon S.M. Predictors of change in omega-3 index with fish oil supplementation in peripheral artery disease. J. Surg. Res. 2017;210:124–131. doi: 10.1016/j.jss.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris W.S., Pottala J.V., Lacey S.M., Vasan R.S., Larson M.G., Robins S.J. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012;225:425–431. doi: 10.1016/j.atherosclerosis.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thygesen K., Alpert J.S., White H.D., Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 33.Fabsitz R.R., Sidawy A.N., Go O., Lee E.T., Welty T.K., Devereux R.B., Howard B.V. Prevalence of peripheral arterial disease and associated risk factors in American Indians: The Strong Heart Study. Am. J. Epidemiol. 1999;149:330–338. doi: 10.1093/oxfordjournals.aje.a009817. [DOI] [PubMed] [Google Scholar]
- 34.Curb J.D., Masaki K., Rodriguez B.L., Abbott R.D., Burchfiel C.M., Chen R., Petrovitch H., Sharp D., Yano K. Peripheral artery disease and cardiovascular risk factors in the elderly. The Honolulu Heart Program. Arterioscler. Thromb. Vasc. Biol. 1996;16:1495–1500. doi: 10.1161/01.ATV.16.12.1495. [DOI] [PubMed] [Google Scholar]
- 35.Murabito J.M., Evans J.C., Larson M.G., Nieto K., Levy D., Wilson P.W.F. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: The Framingham Study. Arch. Intern. Med. 2003;163:1939–1942. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 36.Newman A.B., Shemanski L., Manolio T.A., Cushman M., Mittelmark M., Polak J.F., Powe N.R., Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler. Thromb. Vasc. Biol. 1999;19:538–545. doi: 10.1161/01.ATV.19.3.538. [DOI] [PubMed] [Google Scholar]
- 37.Vogt M.T., Cauley J.A., Newman A.B., Kuller L.H., Hulley S.B. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–469. doi: 10.1001/jama.1993.03510040069031. [DOI] [PubMed] [Google Scholar]
- 38.Vogt M.T., McKenna M., Anderson S.J., Wolfson S.K., Kuller L.H. The relationship between ankle-arm index and mortality in older men and women. J. Am. Geriatr. Soc. 1993;41:523–530. doi: 10.1111/j.1532-5415.1993.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 39.McDermott M.M., Ferrucci L., Simonsick E.M., Balfour J., Fried L., Ling S., Gibson D., Guralnik J.M. The ankle brachial index and change in lower extremity functioning over time: The Women’s Health and Aging Study. J. Am. Geriatr. Soc. 2002;50:238–246. doi: 10.1046/j.1532-5415.2002.50054.x. [DOI] [PubMed] [Google Scholar]
- 40.Criqui M.H., Langer R.D., Fronek A., Feigelson H.S., Klauber M.R., McCann T.J., Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 41.McDermott M.M., Liu K., Criqui M.H., Ruth K., Goff D., Saad M.F., Wu C., Homma S., Sharrett A.R. Ankle-brachial index and subclinical cardiac and carotid disease: The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 42.Diehm C., Schuster A., Allenberg J.R., Darius H., Haberl R., Lange S., Pittrow D., von Stritzky B., Tepohl G., Trampisch H.-J. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/S0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 43.Leng G.C., Lee A.J., Fowkers F.G.R., Whiteman M., Dunbar J., Housley E., Ruckley C.V. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int. J. Epidemiol. 1996;25:1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 44.Leng G.C., Fowkes F.G.R., Lee A.J., Dunbar J., Housley E., Ruckley C.V. Use of ankle brachial pressure index to predict cardiovascular events and death: A cohort study. BMJ. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDermott M.M., Feinglass J., Slavensky R., Pearce W.H. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J. Gen. Intern. Med. 1994;9:445–449. doi: 10.1007/BF02599061. [DOI] [PubMed] [Google Scholar]
- 46.McKenna M., Wolfson S., Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-T. [DOI] [PubMed] [Google Scholar]
- 47.Paeslack N., Mimmler M., Becker S., Gao Z., Khuu M.P., Mann A., Malinarich F., Regen T., Reinhardt C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids. 2022:1–18. doi: 10.1007/s00726-022-03161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Fang X., Zhou J., Chen Z., Zhao B., Xiao L., Liu A., Li Y.-S.J., Shyy J.Y.-J., Guan Y., et al. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc. Natl. Acad. Sci. USA. 2013;110:13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagedorn K.A., Cooke C.L., Falck J.R., Mitchell B.F., Davidge S.T. Regulation of vascular tone during pregnancy: A novel role for the pregnane X receptor. Hypertension. 2007;49:328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 50.Venu V.K.P., Saifeddine M., Mihara K., Tsai Y.C., Nieves K., Alston L., Mani S., McCoy K.D., Hollenberg M.D., Hirota S.A. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am. J. Physiol. Endocrinol. Metab. 2019;317:E350–E361. doi: 10.1152/ajpendo.00572.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Mello V.D., Paananen J., Lindström J., Lankinen M.A., Shi L., Kuusisto J., Pihlajamäki J., Auriola S., Lehtonen M., Rolandsson O., et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017;7:46337. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuomainen M., Lindström J., Lehtonen M., Auriola S., Pihlajamäki J., Peltonen M., Tuomilehto J., Uusitupa M., De Mello V.D., Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes. 2018;8:35. doi: 10.1038/s41387-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue H., Chen X., Yu C., Deng Y., Zhang Y., Chen S., Chen X., Chen K., Yang Y., Ling W. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ. Res. 2022;131:404–420. doi: 10.1161/CIRCRESAHA.122.321253. [DOI] [PubMed] [Google Scholar]
- 54.Shivanna S., Kolandaivelu K., Shashar M., Belghasim M., Al-Rabadi L., Balcells M., Zhang A., Weinberg J., Francis J., Pollastri M., et al. The Aryl Hydrocarbon Receptor is a Critical Regulator of Tissue Factor Stability and an Antithrombotic Target in Uremia. J. Am. Soc. Nephrol. 2016;27:189–201. doi: 10.1681/ASN.2014121241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chitalia V.C., Shivanna S., Martorell J., Balcells M., Bosch I., Kolandaivelu K., Edelman E. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376. doi: 10.1161/CIRCULATIONAHA.112.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka S., Watanabe H., Nakano T., Imafuku T., Kato H., Tokumaru K., Arimura N., Enoki Y., Maeda H., Tanaka M., et al. Indoxyl Sulfate Contributes to Adipose Tissue Inflammation through the Activation of NADPH Oxidase. Toxins. 2020;12:502. doi: 10.3390/toxins12080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A., European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin T.-Y., Chou H.-H., Huang H.-L., Hung S.-C. Indoxyl Sulfate and Incident Peripheral Artery Disease in Hemodialysis Patients. Toxins. 2020;12:696. doi: 10.3390/toxins12110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Alexeev E.E., Lanis J.M., Kao D.J., Campbell E.L., Kelly C.J., Battista K.D., Gerich M.E., Jenkins B.R., Walk S.T., Kominsky D.J., et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018;188:1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swimm A., Giver C.R., DeFilipp Z., Rangaraju S., Sharma A., Antonova A.U., Sonowal R., Capaldo C., Powell D., Qayed M., et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132:2506–2519. doi: 10.1182/blood-2018-03-838193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langan D., Perkins D., Vogel S., Moudgil K. Microbiota-Derived Metabolites, Indole-3-aldehyde and Indole-3-acetic Acid, Differentially Modulate Innate Cytokines and Stromal Remodeling Processes Associated with Autoimmune Arthritis. Int. J. Mol. Sci. 2021;22:2017. doi: 10.3390/ijms22042017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc. Natl. Acad. Sci. USA. 1981;78:129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munn D.H., Mellor A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedersen E.R., Tuseth N., Eussen S.J., Ueland P.M., Strand E., Svingen G.F.T., Midttun Ø., Meyer K., Mellgren G., Ulvik A., et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler. Thromb. Vasc. Biol. 2015;35:455–462. doi: 10.1161/ATVBAHA.114.304674. [DOI] [PubMed] [Google Scholar]
- 66.Berg M., Polyzos K.A., Agardh H., Baumgartner R., Forteza M.J., Kareinen I., Gisterå A., Bottcher G., Hurt-Camejo E., Hansson G.K., et al. 3-Hydroxyanthralinic acid metabolism controls the hepatic SREBP/lipoprotein axis, inhibits inflammasome activation in macrophages, and decreases atherosclerosis in Ldlr-/- mice. Cardiovasc. Res. 2020;116:1948–1957. doi: 10.1093/cvr/cvz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lees H.J., Swann J.R., Wilson I.D., Nicholson J.K., Holmes E. Hippurate: The natural history of a mammalian-microbial cometabolite. J. Proteome Res. 2013;12:1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F., Jia Z., Gao P., Kong H., Li X., Chen J., Yang Q., Yin P., Wang J., Lu X., et al. Metabonomics study of atherosclerosis rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Talanta. 2009;79:836–844. doi: 10.1016/j.talanta.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Calvani R., Miccheli A., Capuani G., Miccheli A.T., Puccetti C., Delfini M., Iaconelli A., Nanni G., Mingrone G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int. J. Obes. 2010;34:1095–1098. doi: 10.1038/ijo.2010.44. [DOI] [PubMed] [Google Scholar]
- 70.Mulder T.P., Rietveld A.G., van Amelsvoort J.M. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am. J. Clin. Nutr. 2005;81((Suppl. 1)):256S–260S. doi: 10.1093/ajcn/81.1.256S. [DOI] [PubMed] [Google Scholar]
- 71.McCullough M.L., Peterson J.J., Patel R., Jacques P.F., Shah R., Dwyer J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X., Ouyang Y.Y., Liu J., Zhao G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014;111:1–11. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- 73.Peterson J.J., Dwyer J.T., Jacques P.F., McCullough M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012;70:491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmes E., Loo R.L., Stamler J., Bictash M., Yap I.K.S., Chan Q., Ebbels T., De Iorio M., Brown I.J., Veselkov K.A., et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akira K., Masu S., Imachi M., Mitome H., Hashimoto M., Hashimoto T. 1H NMR-based metabonomic analysis of urine from young spontaneously hypertensive rats. J. Pharm. Biomed. Anal. 2008;46:550–556. doi: 10.1016/j.jpba.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Lee T.-S., Lu T.-M., Chen C.-H., Guo B., Hsu C.-P. Hyperuricemia induces endothelial dysfunction and accelerates atherosclerosis by disturbing the asymmetric dimethylarginine/dimethylarginine dimethylaminotransferase 2 pathway. Redox Biol. 2021;46:102108. doi: 10.1016/j.redox.2021.102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jalkanen J., Maksimow M., Hollmen M., Jalkanen S., Hakovirta H. Compared to Intermittant Claudication Critical Limb Ischemia Is Associated with Elevated Levels of Cytokines. PLoS ONE. 2016;11:e0162353. doi: 10.1371/journal.pone.0162353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gremmels H., Teraa M., De Jager S.C.A., Pasterkamp G., De Borst G.J., Verhaar M.C. A Pro-Inflammatory Biomarker-Profile Predicts Amputation-Free Survival in Patients with Severe Limb Ischemia. Sci. Rep. 2019;9:10740. doi: 10.1038/s41598-019-47217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sartipy F., Sigvant B., Lundin F., Wahlberg E. Ten Year Mortality in Different Peripheral Arterial Disease Stages: A Population Based Observational Study on Outcome. Eur. J. Vasc. Endovasc. Surg. 2018;55:529–536. doi: 10.1016/j.ejvs.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 80.Kumakura H., Kanai H., Hojo Y., Iwasaki T., Ichikawa S. Long-term survival and fate of the leg in de novo intermittent claudication. Eur. Heart J. Qual. Care Clin. Outcomes. 2017;3:208–215. doi: 10.1093/ehjqcco/qcw057. [DOI] [PubMed] [Google Scholar]
- 81.Brown J.M., Hazen S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018;16:171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sonnenburg J.L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willing B.P., Russell S.L., Finlay B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 84.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.