Abstract
Cholestatic itch is a disabling symptom that may be secondary to liver or biliary diseases. Management of cholestatic pruritus is complex. A systematic review and meta-analysis on the efficacy of treatments for cholestatic pruritus were performed. PubMed and Cochrane Library were searched using the algorithm “(hepatitis OR cholestatic OR liver) AND (pruritus OR itch) AND (management OR treatment OR treatments)” for 1975–2019. Of the 2,264 articles identified, 93 were included in a systematic review and 15 in a meta-analysis (studies evaluating pruritus with a visual analogue scale). Some treatments act by reducing levels of pruritogens in the enterohepatic cycle, others modify the metabolism or secretion of these pruritogens, or act on pruritus pathways. A further possible treatment is albumin dialysis. However, due to many heterogeneities in the reviewed studies it is difficult to identify and recommend an optimum treatment. Only 15 studies were included in the meta-analysis, due to the small number of randomized studies using a visual analogue scale.
Key words: cholestatic pruritus, itch, meta-analysis, systematic review, rifampicin, ursodeoxycholic acid
Pruritus is defined as an unpleasant sensory sensation, which leads to the urge to scratch (1). The International Forum for the Study of Itch (IFSI) has defined 3 groups of chronic pruritus: pruritus on diseased skin, pruritus on non-diseased skin, and chronic scratch lesions. Chronic pruritus can also be classified into 6 different categories according to the underlying origin (dermatological, systemic, neurological, psychological/psychosomatic, mixed, and other) (2). Cholestatic pruritus is considered a systemic pruritus. The exact physiopathology of cholestatic pruritus is unknown, and is probably multifactorial. It has been suggested that circulating and accumulating pruritogens (such as bile salts, steroid hormones, endogenous opioids, histamine, and serotonin) can be the cause of cholestatic pruritus (3). Recently, autotaxin, a lysophosphatidic acid-forming enzyme, was found to be significantly higher in the serum of patients with cholestatic pruritus (4–6). The characteristics of cholestatic pruritus are well described. The itching often starts at the distal part of the limbs, especially the palms and soles, before it spreads over the rest of the body, and it has a circadian rhythm (7). Pruritus appears more frequently in intrahepatic disorders, such as primary biliary cholangitis (PBC), viral hepatitis, autoimmune hepatitis (AIH), cholestasis of pregnancy (ICP) and Alagille syndrome (AS). Pruritus caused by extrahepatic cholestasis includes primary sclerosing cholangitis (PSC) or cancer of the head of the pancreas (8). Pruritus is often the most burdensome symptom in patients with liver diseases, having a major impact on their quality of life (9). The management of cholestatic itch remains difficult, due to insufficient patient information and the wide variety of underlying causes, making it difficult to reach a standard consensus on treatment (10). Some guidelines concerning its treatment have been proposed by the IFSI and the European Association for the Study of the Liver (EASL) (11, 12). However, the treatment is still debated (7).
SIGNIFICANCE
Cholestatic itch is a disabling symptom that may be secondary to liver or biliary diseases. This systemic review on the treatment of cholestatic pruritus highlighted that the data are of very low quality and that there is a great heterogeneity in treatment approaches. The high diversity in pathological entities makes it difficult to analyse the treatment of cholestatic pruritus, because the treatment must be adapted to the aetiology. Guidelines suggest a stepwise approach to cholestatic itch, but evidence of the effectiveness of many treatments is lacking. New treatments, such as fibrates and ileal bile acid transporter inhibitors, are promising future options.
The aim of this study was to conduct a systematic review of the literature and a meta-analysis to evaluate the effect of systemic treatments on cholestatic pruritus.
MATERIALS AND METHODS
A systematic literature search was performed in January 2020 to find studies about systemic treatments prescribed for cholestatic pruritus in humans. Only articles in English were selected. A systemic literature search was performed in the databases of Medline and the Cochrane Library, from 1975 until December 2019. The algorithm used was ((hepatitis OR cholestatic OR liver) AND (pruritus OR itch) AND (management OR treatment OR treatments)). Clinical trial and open-label studies (prospective or retrospective), which evaluated the efficacy of the treatment of pruritus with an objective scale (visual analogue score or semi-quantitative scale) were selected. Case reports, book sections, fundamental studies and publications about topical treatment were not considered. Articles that did not address the question, were not sufficiently detailed, or were of too poor quality were excluded. After removing duplicates, 2 independent authors (CD and NB) reviewed all titles and abstracts and then reviewed the full text of the potentially relevant articles. Disagreements were resolved by consensus or by a third party (EB, LM). In the second stage, a meta-analysis was performed. The primary outcome was to evaluate the effects of systemic treatments on cholestatic itch based on the improvement in itch score. Only randomized controlled trial evaluating itch with a visual analogue scale (VAS) 0–100 mm or 0–10 cm were included. The meta-analysis was performed using Review Manager software (version 5.3; Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) with an inverse variance model. Heterogeneity was evaluated with Cochran’s Q test and the I2 value. In cases of I2 values higher than 20%, a random effect model was used. p-values < 0.05 were considered significant. This work was registered in Prospero data (ID CRD42020166025).
RESULTS
Among the 2,264 articles identified, 2,037 were excluded after reading the title or abstract, 134 were excluded after reading the article, and 93 were selected for the systematic review. The flow chart is presented in Fig. 1. For the meta-analysis, 14 randomized studies comparing treatments vs placebo were included. The results of studies are presented according to the mechanism of action of the treatment.
Fig. 1.
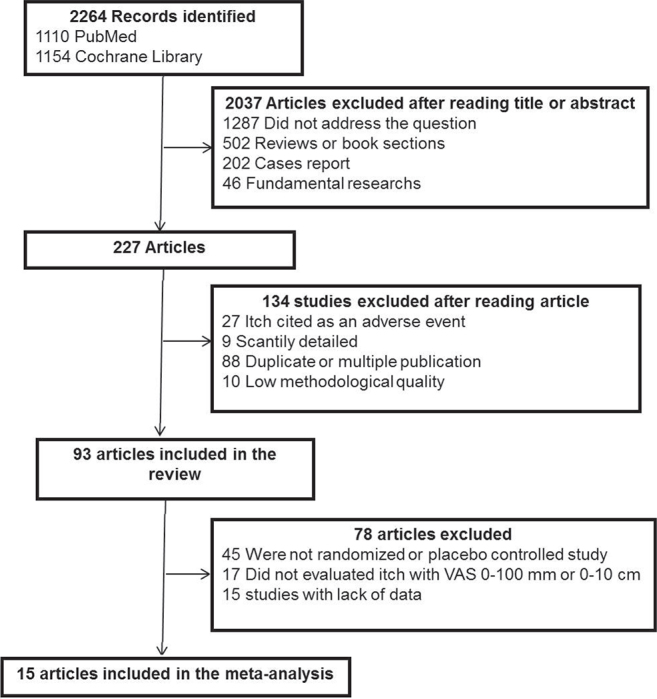
Flow chart of the bibliographic search strategy. VAS: visual analogue scale.
Removal of pruritogens from the enterohepatic cycle
Cholestyramine (3 studies). Kondrackiene et al. (13) evaluated the effect of cholestyramine vs ursodeoxycholic acid (UDCA) in 84 women with ICP. Cholestyramine (8 g/day) reduced itch by 50% in 19% of patients, compared with 66.7% in the UDCA group. The reduction in itch was also slower in the cholestyramine group (7–10 days vs 3–4 days with UDCA). Both results were statistically significant. In a placebo-controlled trial, Padova et al. (14) (5 patients in each group, cholestyramine 3×3 g/day) reported a reduction in pruritus in the cholestyramine group (VAS 0–100 mm: –55.7% at week 2, –63.6% at week 4), whereas placebo increased itch (+8.7% and +24.7%, respectively). Yokomori et al. (15) compared 2 groups (UDCA 600 mg/day and colestilan 6.42 g/day vs UDCA alone) in a randomized open-label study including 11 patients. The combined treatment improved the pruritus score by 70% after 8 weeks and was statistically more effective than UDCA alone.
Colesevelam (1 study). In a double-blind placebo-controlled study (35 patients included), Kuiper et al. (16) reported that colesevelam (1,875 mg twice a day) was effective in 36% of patients, with a mean VAS (0–10 cm) reduction of 40%. Placebo led to a decrease in 35% of patients, and the difference was not significant.
Ileal bile acid transporter (IBAT) (4 studies). In a phase II multicentre double-blind placebo-controlled crossover trial, 21 patients with PBC suffering from pruritus received IBAT (45 to 90 mg twice a day), which was named “GSK2330672” (linerixibat currently) (17). A significant decrease in itch score (VAS 0–10 cm) of 57% was obtained, compared with 23% with the placebo (p < 0.0001). Mayo et al. (18) compared maralixibat (2 groups: 10 and 20 mg/day) vs placebo in 66 patients with PBC. Both treatments resulted in a decrease in itch score: 58% in the maralixibat group and 45% in the placebo group (p = 0.48). In a double-blind placebo-controlled randomized trial, Shneider et al. (19) used maralixibat at 3 different dosages in 37 patients with cholestatic pruritus secondary to AS. Statistically significant decreases in itch scores were observed with doses of 70 and 140 μg/kg/day, but not 280 μg/kg/day or when considering all dosage groups combined. Al-Dury et al. (20) tested another IBAT, named A4250 (odevixibat), at 0.75–3 mg orally in 10 patients. An improvement in itch of 60–80% (VAS 0–100 mm) was noted after 2 days of treatment, and itch disappeared in 2 patients. A relapse of pruritus was observed for all of them after discontinuation of A4250.
Surgical biliary drainage (10 studies). Surgery has been evaluated mainly in children with genetic disorders that induce chronic cholestasis, such as AS or progressive familial intrahepatic cholestasis (PFIC). Four techniques are principally reported: ileocaecal bypass (creating terminal ileal exclusion by conducting ileocolonic anastomosis), partial internal biliary diversion (PIBD, a jejunal conduit connecting the gallbladder to the colon for bile drainage), partial external biliary diversion (PEBD, a jejunal segment anastomosed to the side of the gallbladder end-to-side and terminated as an end stoma), and endoscopic stenting. All these techniques had the same objective: to increase the elimination of bile components. Hollands et al. (21) conducted an interventional study in 5 patients with PFIC. Approximately 80% of patients experienced improvement in their pruritus (decrease in itch score of 77%), and itch completely disappeared for 2 patients. Modi et al. (22) treated 3 boys with AS with ileal exclusion. The mean pruritus score improved by 90%, and only one patient had mild residual pruritus during the follow-up. Jankowska et al. (23) used the same technique in 9 patients with PFIC: 8 patients reported an improvement in pruritus score (mean decrease of 40% in a semiquantitative scale from 0 to 4). The efficacy was persistent at 6 months. Van Vaisberg et al. (24) analysed 11 children who were treated with ileocaecal bypass in a retrospective study. For 8 patients, pruritus remained clinically controlled postoperatively (72.7%). In the study by Ramachandran et al. (25), 12 children with cholestatic liver diseases and intractable pruritus were candidates for PIBD. Although pruritus is difficult to evaluate in young children, the authors concluded that pruritus significantly improved in 75% of patients. Khan et al. (26) performed PIBD by cholecystojejunocolic anastomosis in 4 children with PFIC. At 4 months of follow-up, 3 children were free of pruritus, and 1 patient had an itch score of 1 out of 4. Whitington et al. (27) treated 5 children (3 with PFIC and 2 with arteriohepatic dysplasia (AHD)) with PEBD. The patients with PIHC had complete clinical remission of their pruritus, and the 2 patients with AHD had incomplete remission. Clinical improvement occurred gradually over 2 weeks in these 2 patients, but both had mild persistent itching. In a retrospective study, Ng et al. (28) included 8 patients with chronic cholestatic pruritus who underwent PEBD: 1 patient was lost to follow-up, and 6/7 patients reported a complete resolution of pruritus. Ponsioen et al. (29) reported, in a retrospective study, a good efficacy of endoscopic stenting in patients with PSC. Of the 15 patients, 14 had an improvement in itch score (semiquantitative scale from 0 to 4; –72%; p < 0.01). Hegade et al. (30) performed a nasobiliary drainage procedure in 27 patients with pruritus who had not responded to classical medications. After 7 days, itch improved for 89.6% of patients, with a mean decrease of 94% (VAS 0–10 cm; p < 0.0001). Complete resolution was observed in 12 patients.
Altered metabolism and/or secretion of potential pruritogens in the liver or intestine
Ursodeoxycholic acid (UDCA) and metabolites (15 studies). Battezzati et al. (31) conducted a double-blind placebo-controlled trial in 88 patients with PBC. At 6 months, UDCA (500 mg/day) decreased pruritus by 39% and placebo by 31.2%, but the difference was not significant. Calmus et al. (32) administered UDCA (13–15 mg/kg/day) or placebo in 146 patients with PBC. Among the 73 patients who received UDCA, 40% reported an improvement in itch, vs 19% in the placebo group. Parés et al. (33) conducted a 2-year placebo-controlled trial with UDCA (14–16 mg/kg/day) in 192 PBC patients (pruritus was a secondary endpoint). At the end of the study, pruritus improved by 25% in the UDCA group and 15% in the placebo group. Diaferia et al. (34) compared the efficacy of UDCA (600 mg/day) vs placebo in 16 women with ICP. Both UDCA (–54%, p < 0.001) and placebo (–26%, p < 0.01) significantly improved pruritus in all patients. Floreani et al. (35) compared 2 treatments, UDCA (450 mg/day) and oral S-adenosylmethionine (SAMe, 1,000 mg/day), in 20 women with ICP. In the 10 patients treated with UDCA, pruritus completely disappeared within 3 days. Pruritus persisted in the 10 patients treated with SAMe. Joutsiniemi et al. (36) conducted a randomized double-blind placebo-controlled trial in 20 women. In the UDCA group (450 mg/day), the pruritus score decreased by 81% on the VAS compared with 12% with the placebo (p = 0.007), although the VAS in the placebo group was also significantly improved over baseline (5.4 vs 8.3, respectively). Kondrackiene et al. (13) included 84 women with ICP who were randomized to receive UDCA (8–10 mg/kg/day) or cholestyramine. The primary endpoint was a reduction in pruritus by more than 50% after 14 days of treatment, which was obtained in 66.6% of the UDCA group vs 19% of the cholestyramine group (p < 0.005). Zhang et al. (37) conducted a randomized controlled study with 3 groups (group 1: oral UDCA 4×250 mg; group 2: intravenous SAMe 1,000 mg daily; group 3: combination of the 2 drugs at the same dosages) in 41 women with ICP. Pruritus score improved in groups 1 and 3 (–58% and –55%, respectively), with no statistically significant difference between the groups. In a controlled study, Chappell et al. (38) used UDCA (500 mg twice a day) in 125 women with ICP. Thirty-three percent of patients receiving UDCA experienced a reduction in worst itching, compared with 16% in the placebo group (p = 0.11). Roncaglia et al. (39) conducted a study in 46 patients with ICP to compare the efficacy of UDCA vs SAMe. Both treatments similarly improved the pruritus score, with 58% of women showing a reduction in itch score at the last visit before delivery. In an open-label randomized study, Jain et al. (40) observed a decrease in itch (defined as a VAS reduction ≥ 3) in 52% of patients in the UDCA group (300 mg/day; 25 patients). In a randomized open-label study, UDCA alone (600 mg/day; 6 patients) improved the pruritus score by 19% at 8 weeks (15). Wunsch et al. (41) evaluated the effect of withdrawal of UDCA after 1 year of treatment (10–15 mg/kg/day) in 26 patients with PSC. The mean NRS was 0.5 ± 1.2 points at the beginning of the study and 0.9 ± 2.1 points (33% increase) at the end of the study (p = 0.2). In 10 women with PBC, Matsuzaki et al. (42) reported a disappearance of itching in 6 of the 7 patients who complained of pruritus after 1 month of UDCA (3×600 mg/day). Tauroursodeoxycholic acid (TUDCA) is a metabolite of UDCA that is more hydrophilic. Floreani et al. (43) used it at 500 mg daily orally for 6 months in 40 patients. Thirty-three patients with HCV-related cirrhosis finished the study. After 6 months, pruritus decreased significantly in the TUDCA group (–30%) compared with an increase in the placebo group (+50%) (p < 0.05). Pruritus at baseline was significantly lower in the control group (baseline 0.13 ± 0.35 and 1.18 ± 1.24, respectively).
Rifampicin (8 studies). In a double-blind cross-over randomized trial, Ghent & Carruthers tested rifampicin in 9 patients with PBC (44). Rifampicin (300–450 mg/day) significantly reduced pruritus score compared with placebo (VAS 0–100 mm, p < 0.002). Cynamon et al. (45) used rifampicin (10 mg/kg/day) in 5 children with chronic cholestasis in a cross-over study with placebo. Rifampicin significantly improved pruritus (–55%; p < 0.001) compared with placebo (–11%; p = 0.78). Podesta et al. (46) tried a higher dose of rifampicin (300 mg twice day) in 14 patients with PBC in a cross-over study with placebo. Itch disappeared in 11 patients, whereas placebo was partially relieved itch in 2 patients. Ataei et al. compared the effect of rifampicin vs sertraline (47). Pruritus score decreased similarly under both treatments (VAS 0–10 cm, –43% rifampicin and –46% sertraline) (p = 0.740). In a double-blind randomized cross-over trial including 12 patients, there was no significant difference between placebo and rifampicin (48). In a cross-over study, Bachs et al. (49) compared the efficacy of rifampicin vs phenobarbitone in 22 women with PBC. Pruritus improved in 19 patients treated with rifampicin and in 8 patients treated with phenobarbitone. Improvement was greater with rifampicin (–66% and –22%, respectively; p < 0.001). In a second study, rifampicin was tested at 10 mg/kg/day in 16 women with PBC (50). Pruritus decreased by 74% within 14 days and completely disappeared in 10 patients after 1 year. In a retrospective study with 33 children with various cholestatic diseases, rifampicin was effective in 52% of children, with complete relief in 15% of patients (51).
Vancomycin (1 study). In a placebo-controlled trial, Rahimpour et al. (52) evaluated the safety and efficacy of vancomycin (125 mg/day) in 29 patients with PSC. Six of the 18 patients in the vancomycin group and 7/11 patients in the placebo group experienced pruritus. Pruritus improved in 5/6 patients in the vancomycin group and 6/7 patients in the placebo group, with no significant difference between the 2 groups.
S-adenosylmethionine (SAMe) (7 studies). Frezza et al. (53) conducted a double-blind placebo-controlled trial in 220 patients with chronic hepatitis who complained of itch. A response was defined as a decrease of half in the pruritus. Seventy-seven percent of the patients in the treatment group (SAMe 1,600 mg/day) fulfilled the primary criteria, compared with 33% of patients in the placebo group (p < 0.01). In a randomized clinical trial, Frezza et al. (54) compared 2 concentrations of SAMe in 20 women with ICP. The 200 mg dosage and the placebo worsened the pruritus score (+7.7% and + 20%, respectively), whereas the 800 mg dosage reduced itch (–64%; p < 0.001), with no side-effects. The study of Zhang et al. (37) comparing UDCA and SAMe is detailed in the paragraph on UDCA. Wunsch et al. used SAMe (1,200 mg/day) in 24 patients with PBC already treated with UDCA. Eighteen patients completed the study and reported a mean improvement in pruritus score of 26.4% (p = 0.006) (55). In a randomized controlled trial, 46 women with ICP were included, and both UDCA (300 mg twice daily) and SAMe (500 mg twice daily) alleviated pruritus, with no significant differences between the 2 groups (39). Floreani et al. (35) included 20 patients with ICP; 10 patients treated with UDCA had their pruritus disappear within 3 days, whereas no patient treated with SAMe had a complete regression of pruritus. Fiorelli et al. (56) analysed the effects of intramuscular (500 mg) or intravenous (800 mg) injections of SAMe in 359 patients with intrahepatic cholestasis. With the 500 mg dose, 74% of treated patients reported an improvement in itch compared with 69% in the 800 mg group.
Fibrates (3 studies). In a double-blind placebo-controlled randomized trial, Corpechot et al. (57) used bezafibrate (400 mg/day) in patients with PBC (50 patients in each group). The difference in itch intensity score (VAS 0–10 cm) from baseline to 24 months between the bezafibrate and placebo groups was –95% [–241%; 50%]. Reig et al. (58) conducted a prospective study in 26 patients with PBC. Bezafibrate 400 mg decreased VAS score by 73% (p < 0.001). Lemoinne et al. (59) conducted a retrospective study in 20 patients with PSC treated with fenofibrate (200 mg/day) or bezafibrate (400 mg/day) after an inadequate response to UDCA. Only 8/20 patients had pruritus at baseline. During treatment with fibrate, the intensity of pruritus significantly decreased (p = 0.021). Seven patients reported relief of itch (88%), and 3 patients had complete remission.
Modulation of itch and pain pathways
Nalfurafine (5 studies). Kumada et al. (60) tested 2 doses (nalfurafine 2.5 or 5 µg) in a double-blinded randomized placebo-controlled trial. The change in VAS at week 4 was significantly greater in the nalfurafine groups (–36.9% in the 2.5 μg group; –35.5% in the 5 μg groups) than in the placebo group (–24.9%). All results were significant. Akuta et al. (61) evaluated the recurrence rate of pruritus after stopping nalfurafine in a prospective study. The rate was 100% in the discontinuation group (5 patients), and 3 patients had relief when retreated with nalfurafine. Kamimura et al. (62) evaluated nalfurafine in 18 patients with cholestatic itch. All patients described relief of pruritus, and itch completely disappeared in 7 patients. Yagi et al. (63) introduced nalfurafine to 44 patients with pruritus secondary to PBC. Both scores used to evaluate pruritus intensity (PBC-40 itch domain Q8–Q10 and VAS 0–100 mm) decreased (–10.8% and –31.7, respectively). In a retrospective study, Akuta et al. (64) evaluated the efficacy of nalfurafine (2.5 µg) in 138 Japanese patients, and 67.4% of patients reached the primary objective (decrease in VAS of 50 mm).
Naltrexone (4 studies). In a double-blind placebo-controlled trial, Wolfhagen et al. (65) used naltrexone (50 mg/day) in 16 patients with generalized cholestatic pruritus. Daytime VAS decreased by 54% in the naltrexone group, compared with 8% in the placebo group (p < 0.001). The results at night were similar (night-time VAS decrease of 44% vs 7%, p = 0.003). Mansour-Ghanaei et al. (66) performed a similar trial in 34 patients. Naltrexone was more effective than placebo in improving itch during the day (–41% vs –9.6%, p < 0.001) and night (–39% vs –9.2%, p < 0.001). Terg et al. (67) conducted a randomized double-blind cross-over trial using naltrexone (50 mg/day) in 20 patients. Naltrexone induced a relief of itch (VAS 0–10 cm; –43.5%; p = 0.0003), whereas placebo showed no significant improvement (–14.7%; p = 0.007). Jain et al. (40) conducted an open-label trial with 3 arms (UDCA 300 mg/day vs naltrexone 50 mg/day vs ondansetron 4 mg/day). Naltrexone was the most effective, decreasing VAS score by a mean of 4.5 cm in 88% of patients.
Naloxone (3 studies). In a single-blinded placebo-controlled cross-over trial, Bergasa et al. (68) treated 8 women with naloxone (intravenous bolus of 0.4 mg, then 0.2 µg/kg/min for 24 h). There was no difference between the mean values on the VAS during naloxone infusions and the corresponding values during placebo infusions. Bergasa et al. (69) conducted a study in 29 patients with cholestatic pruritus with the same protocol. The mean VAS (0–10 cm) was significantly lower (p < 0.01) with naloxone (2.24) than placebo (2.86). More recently, Joshi et al. (70) used naloxone (0.4 mg intravenously every 8 h) in 22 patients with pruritus secondary to acute hepatitis. After 48 h of treatment, (81.8%) patients had a significant reduction in VAS score (mean reduction 52%; p < 0.0001).
Nalmefene (2 studies). Bergasa et al. (71) evaluated nalmefene in a double-blind placebo-controlled cross-over study. The 8 patients included received nalmefene (dose gradually increased from 2 to 20 mg twice a day). All of them reported an improvement in their pruritus (mean decrease 77%; p < 0.01). Bergasa et al. (72) gave nalmefene (progressively increasing to a final dose between 30 and 120 mg twice daily) to 14 patients in an open-label study: 93% of patients indicated an improvement in itch, with a mild relief of 53% (p = 0.002). Adverse events were frequent (3 withdrawal reactions and 2 psychotic symptoms).
Gabapentin (1 study). Bergasa et al. (73) gave gabapentin to 16 women with PBC in a double-blind randomized placebo-controlled trial. A significant decrease in VAS was observed in the placebo group (–22%), whereas gabapentin increased itch (+44%).
Lidocaine (1 study). Villamil et al (74) used lidocaine (100 mg intravenous) in a randomized double-blind placebo-controlled study. Lidocaine significantly decreased itch (–45%), whereas placebo did not change the VAS (p < 0.005) in 11/12 patients who received the treatment. The effect was temporary, as patients returned to their baseline pruritus within 7 days.
Propofol (1 study). Borgeat et al. (75) used a subhypnotic dose of propofol (15 mg) in a cohort of 10 patients with refractory cholestatic pruritus. In this double-blind placebo-controlled randomized cross-over trial, propofol induced a decrease in itch score by at least 4 points (VAS 0–10) in 85% of patients within 5–10 min, vs an improvement of 10% with placebo (p < 0.001). The effect lasted approximately 1 h. Propofol also induced sedation as a side-effect.
Ondansetron (5 studies). Jones et al. (76) conducted a double-blind placebo-controlled cross-over study in 14 patients with cholestatic pruritus secondary to chronic liver diseases. Five patients out of 13 reported that the itch score was lower with ondansetron (3×8 mg/day) than with placebo. In the 8 remaining patients, the pruritus score was similar to that under placebo or ondansetron. In a double-blind placebo-controlled trial in 19 patients, ondansetron (8 mg/day) and placebo were compared (77). The 2 groups had no significant difference in the reduction in the mean pruritus burden over the 5-day treatment period compared with baseline (–21% in the ondansetron group and –22% in the placebo group). Schwörer et al. (78), in a single-blind cross-over placebo-controlled trial in 10 patients with cholestatic pruritus, showed that intravenous injection of 8 mg of ondansetron reduced itch by 50% within 30 min. The best outcome was obtained after 2 h, when there was a mean decrease in VAS score of 90% (p < 0.05), while placebo had almost no effect (–1.3%). The effect lasted up to 6 h. In the Müller et al. study (79), the use of ondansetron in 14 patients reduced pruritus by 27% on the VAS, compared with 5% for the placebo (p = 0.033). In Jain et al.’s study (40), ondansetron and UDCA both decreased pruritus (defined as a VAS reduction ≥ 3) in 52% of patients.
Sertraline (4 studies). Ataei et al. (47) compared the efficacy of sertraline and rifampicin. The results are presented in the paragraph about rifampicin. Mayo et al. (80) showed a dose-response relationship by using progressively increased concentrations of sertraline (25, 50 and 75 mg) in 21 patients with various cholestatic liver diseases. The 25 mg dose did not significantly change the VAS (p = 0.11), whereas pruritus improved significantly (p = 0.002) with the dosage of 50 mg. Better results were obtained with a mean dose of 1.52 mg/kg/day, corresponding to 75–100 mg daily (decrease in VAS score of 35%, p < 0.0001). The placebo increased itch (+8%, p = 0.009). Thébaut et al. (81) used sertraline in a prospective study including 20 children. At 3 months, a median decrease in VAS score was observed (–37.5%) in 70% of the patients. In a retrospective study, sertraline (dose range 50–100 mg/day) improved itch in 6 of the 7 patients, with a possible dose-response relationship (82).
Removal of potential pruritogens from the body system
Molecular Absorbent Recirculating System (MARS) (10 studies). After MARS therapy, Doria et al. (83) observed a decrease of 70%, 90%, and 100% in pruritus intensity in 3 patients, respectively. At 6 months after MARS treatment, the reductions were still 40%, 50%, and 25%. Bellman et al. (84) conducted an interventional study in 7 patients: 86% of patients reported an improvement in itch, with a mean decrease on the VAS of 62%. Montero et al. (85) tested MARS in 4 adult patients with invalidating intractable pruritus due to liver disease. All patients felt less itchy, with a decrease in pruritus score of 50% (semiquantitative scale from 1 to 5). Moreover, discontinuation of treatment led to relapse of pruritus, which was cured with another cycle of MARS. Novelli et al. (86) successfully relieved pruritus in 9 patients with resistant itch and terminal liver failure awaiting liver transplantation. In this interventional trial, VAS (0–10 cm) score decreased by 92.5%, and pruritus completely disappeared in 3 patients (33%). Good results were also obtained by Rifai et al. (87), who reported a mean decrease in VAS score of 66.7% (p < 0.001) in 7 patients. Pares et al. (88) used MARS therapy in 4 patients with PBC. Pruritus completely disappeared in 2 patients and decreased markedly in the 2 others (VAS 0–100 mm; mean decrease of 60% after 2 sessions). Improvement of pruritus was maintained at 1 month. In 20 patients with advanced liver diseases, VAS score decreased by 72% immediately after the treatment (p < 0.001) (89). One month later, VAS score remained at 51% of the baseline. Leckie et al. (90) referred to 15 adults with intractable pruritus for albumin dialysis using MARS. Thirteen patients responded with regard to itching, with an overall reduction of 4 points on the VAS (p < 0.001). The Itch Severity Scale (ISS) score was strongly correlated with VAS score. In Schaefer et al.’s (91) retrospective study, the authors described the effects of MARS in 3 children with severe cholestatic pruritus and indication for liver transplantation. A mean decrease in VAS of 51% was found (p < 0.001) in the whole cohort. Cisneros-Garza et al. (92) analysed 70 patients with hepatitis cholestasis who were treated with MARS. Among the 17 patients with pruritus, a marked improvement in itch was observed, with a decrease on the VAS (0–100 mm) of 96.6%.
Phototherapy (2 studies). Bergasa et al. (93) exposed 8 patients to a bright light of 10,000 lux for 1 h twice a day for 8 weeks. The mean VAS (0–10 cm) score decreased by 42% (p = 0.05), and 6 of 8 patients felt relief of pruritus. In the Decock et al. (94) study, 13 patients were exposed to total-body ultraviolet B (UVB) irradiation 3 times a week until the VAS score did not improve further or 80% improvement in pruritus was obtained. Thereafter, the intensity of itch decreased by 73.6% (VAS 0–10 cm; p < 0.001).
Plasmapheresis (2 studies). In an interventional trial, Cohen et al. (95) used plasmapheresis (3 times a week) in 5 patients with PBC. All of them reported an improvement in itch, with a mean decrease in pruritus score of 80% (semiquantitative scale from 0 to 4). Krawczyk et al. (96) performed plasmapheresis in 17 patients with PBC, and the mean VAS decrease observed was 63%. All patients reported relief of itch. Interestingly, no significant increase in pruritus was observed after 1 month. A relapse of itch was observed at 3 months, but pruritus remained significantly (p < 0.0001) lower than at the beginning.
Charcoal haemoperfusion (1 study). Kittanamongkolchai et al. (97) retrospectively analysed the effects of charcoal haemoperfusion, which consists of an extracorporeal technique similar to MARS. Charcoal was effective in eliminating protein-bound substances that may have accumulated during cholestasis. The procedure was carried out on 13 patients with cholestatic pruritus, and 69% reported a relief of pruritus (mean decrease in VAS score of 44%).
Other treatments
Methotrexate (MTX) (3 studies). Kaplan et al. (98) conducted a randomized double-blind trial to assess the efficacy of methotrexate (15 mg/week) vs colchicine (0.6 mg twice a day) in 85 patients with PBC. Both treatments led to a significant decrease in itch score (–83%; p = 0.0001 for MTX and –51%; p = 0.04 for colchicine), with no significant difference between the 2 treatments. Hendrickse et al. (99) compared MTX (7.5 mg a week) vs placebo in 60 patients. MTX improved itch score (–23%) compared with placebo (+15%), but the difference was not significant (p = 0.08). Babatin et al. (100) gave MTX (13.75 mg/week) to 8 women for a mean period of 49 months. Pruritus disappeared in 6 of the 7 patients who completed the study.
Colchicine (2 studies). Almasio et al. (101) conducted a double-blind randomized trial in 90 patients. Two groups were compared: group 1 was treated with UDCA (500 mg/day), and group 2 was treated with UDCA (500 mg/day) + colchicine (1 mg/day). An improvement in pruritus was noted in both groups. Pruritus had resolved in 45% of group 1 and 50% of group 2 at the time of randomization to UDCA + colchicine or UDCA + placebo, and these rates increased to 65% and 70%, respectively, at the end of the treatment, with no significant difference between the 2 groups. Kaplan et al. (98) showed no significant difference between methotrexate and colchicine in improving pruritus secondary to PBC in 85 patients.
Corticosteroids (1 study). Van Hoogstraten et al. (102) tested corticosteroids in a randomized blind study in PSC patients. Three groups of 6 patients were composed: prednisone 10 mg, budesonide 3 mg, and budesonide 9 mg. The median difference between baseline and 8-week VAS scores (0–10 cm) was +0.15 points for the 3 mg group, –0.1 for the 9 mg group, and –1.1 for the 10 mg group. Pruritus decreased significantly more in the prednisone group than in both other groups (p < 0.05), but the improvement in itch was moderate, and the numbers of patients with pruritus were small (4 patients in the prednisone group, 2 in the 3 mg group, and 1 in the 9 mg group).
Carbon (1 study). Sherker et al. (103) tested AST-120 in 47 cirrhosis patients through a single-blind randomized placebo-controlled trial. After 4 weeks of treatment, pruritus improved by 53%, whereas placebo did not modify VAS score (p = 0.005).
Antioxidant vitamins (1 study). Watson et al. (104) used a combination of antioxidant vitamins with or without Bio-Quinone Q10® in 24 patients with PBC. Only 13 of them had pruritus. They described a significant improvement in VAS (0–10 cm) score in the group taking Bio-Quinone Q10® (–83%), while no significant improvement (p < 0.005) was observed in the control group (–24%).
Flumecinol (1 study). Turner et al. (105) conducted a randomized double-blind placebo-controlled parallel study evaluating 2 different doses of flumecinol (600 mg weekly and 300 mg a day). Initially, 50 patients who complained of itch related to cholestatic disorders were randomized to receive flumecinol 600 mg or placebo. Pruritus improved in 13/24 patients treated with flumecinol 600 mg/weekly and in 10/26 patients treated with placebo, and the mean difference was not significant. Secondly, 19 patients were randomized to receive flumecinol 300 mg/day or placebo. Pruritus improved in 7/10 on flumecinol and 1/9 on placebo. The difference in the median VAS score was significant, in favour of the treatment group (median VAS improvement was 19.8 mm; p < 0.02).
Meta-analysis
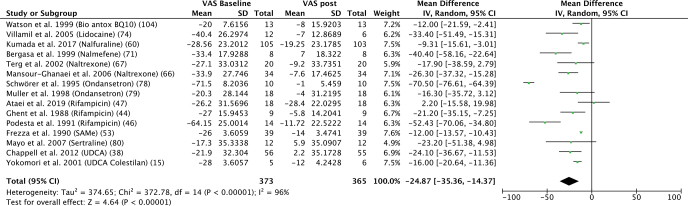
The meta-analysis is summarized in Fig. 2, and the characteristics of the studies are listed in Table I and discussed below. Meta-analysis according to some molecules (rifampicin, odansetron, naltrexone) are presented in Figs. S1–S3.
Fig. 2.
Results of the meta-analysis. SD: standard deviation; VAS: visual analogue scale; 95% CI: 95% confidence interval.
Table I.
Summary of the characteristics of the studies included in the meta-analysis
| Reference | Drug tested | Dosage | Study design | Patients n | Pruritus scale | Results |
|---|---|---|---|---|---|---|
| Ataei, et al. (47) | Sertraline vs rifampin | 100 mg/day vs 300 mg/day | Randomized trial | 36 | VAS 0–10 cm | No significant differences between the 2 groups (p = 0.740). |
| Bergasa, et al. (70) | Nalmefene | 2 mg × 2 on day 1, 5 mg × 2 on day 2, l0 mg × 2 on day 3, and 20 mg × 2 on day 4 | Cross-over trial | 8 | VAS 0–10 cm | Overall mean decrease with nalmefene of 77% (p < 0.01) |
| Chappell, et al. (37) | UDCA | 500 mg × 2. Dose was increased to a maximum of 2 g/day if no response | Randomized trial | 125 | VAS 0–100 mm | UDCA failed to reduce itch pre-specified by the clinicians and women as clinically meaningful |
| Frezza, et al. (52) | SAMe | 1600 mg/day | Randomized trial | 220 | VAS 0–10 cm | Significant decrease in itch compared with placebo (p < 0.01). |
| Ghent & Carruthers (43) | Rifampin | 300–450 mg/day | Cross-over trial | 9 | VAS 0–100 mm | Significant decrease in itch compared with placebo (p < 0.002). |
| Kumada, et al. (59) | Nalfurafine | 2.5 pg or 5 pg once a day | Randomized trial | 317 | VAS 0–100 mm | Significant decrease in itch compared with placebo (p = 0.0022 for 2.5 pg group and 0.0056 for 5 pg group). |
| Mansour-Ghanaei, et al. (66) | Naltrexone | 50 mg daily | Cross-over trial | 34 | VAS 0–10 cm | Significant decrease in itch compared with placebo (p < 0.001). |
| Mayo, et al. (80) | Sertraline | 25 mg once daily increased every 4 week (25/50/75/100 mg) | Cross-over trial | 21 | VAS 0–10 cm | Significant decrease in itch compared with placebo (p = 0.009). Median optimal dose of 1.52 mg/kg/daily (75–100 mg daily). |
| Müller, et al. (79) | Ondansetron | 8 mg/day | Cross-over trial | 18 | VAS 0–10 cm | Significant decrease in itch compared with placebo (p = 0.033). |
| Podesta, et al. (46) | Rifampicin | 300 mg twice daily | Cross-over trial | 14 | VAS 0–100 mm | Significant decrease in itch compared with placebo (p < 0.001). |
| Schwörer, et al. (78) | Ondansetron | 8 mg/day | Cross-over trial | 10 | VAS 0–10 cm | Significant decrease in itch compared with placebo (p < 0.005). |
| Terg, et al. (67) | Naltrexone | 50 mg/day | Cross-over trial | 20 | VAS 0–10 cm | Greater decrease in VAS with naltrexone (p = 0.0003) than placebo (p = 0.07). |
| Villamil, et al. (74) | Lidocaine | 100 mg intravenous | Cross-over trial | 18 | VAS 0–100 mm | Significant decrease in itch compared with placebo (p < 0.005). |
| Watson, et al. (104) | Antioxidant vitamin preparation | Bio-Antox (4 tablet/day) +/– 100 mg Bio-Quinone Q10(BQ10) | Randomized trial | 24 | VAS 0–10 cm | Significant VAS decrease in the BQ10 group (p < 0.05). No difference in the control group (Bio-Antox alone). |
| Yokomori, et al. (15) | UDCA or UDCA + colestilan | UDCA 600 mg/day +/– colestilan 6.42 g/day | Open-label trial | 11 | VAS 0–10 cm | Significant decrease in itch compared with UDCA alone (p <0.05). |
UDCA: ursodeoxycholic acid; SAMe: S-adenosylmethionine; VAS: visual analogue scale.
DISCUSSION
Pruritus is often described as the worst symptom of liver diseases, having a major impact on the quality of life of affected patients (9). Since the aetiologies of liver diseases leading to pruritus vary, different treatments can be initiated. Progress in understanding the underlying mechanisms of cholestatic pruritus led to the development of therapies that act on the different pathways involved in cholestatic pruritus. Recent guidelines, with a step-by-step management of itch secondary to cholestatic diseases, were proposed (106, 107).
Cholestyramine is currently the first-line therapy for cholestatic pruritus, although studies evaluating its efficacy are limited. As an anion exchange resin, cholestyramine alleviates symptoms by binding and sequestering systemic bile salts. Its effect is modest, and it has poor compliance due to side-effects (constipation and bloating) and its unpalatable taste (108). Rifampicin is an antibiotic that is thought to function as a pregnane X receptor agonist (44, 109). Its efficacy was highlighted in 2 meta-analyses, and it is currently considered the second-line treatment for cholestatic itch (108, 110). The side-effects are the main limitations to its use (hepatotoxicity, haemolysis, and drug interactions) (44, 50, 111). The third line is represented by naltrexone, an opioid receptor blocker. Other opioid receptors can be used in cholestatic itch. Naloxone has a short half-life and low bioavailability, limiting its use. A possible use of naloxone could be introducing it during hospitalization before switching to oral naltrexone (112). Nalmefene is an interesting alternative due to its long plasma half-life, oral bioavailability and good results in reducing itch. Nalfurafine is currently not available in Europe. The risk-benefit balance of these therapeutics is impaired by opioid withdrawal-like syndrome that they can induce and adverse events such as dizziness, abdominal pain and nausea (65, 67, 69, 72). Low doses are required at the start of treatment. The use of sertraline is interesting because of its possible double action in the serotoninergic pathway, which could be involved in cholestatic pruritus and in treating depressive disorders that are frequently associated with itch (9, 113). It is considered the fourth-line treatment.
The management of cholestatic pruritus is experiencing increased interest with the development of new therapies, such as IBAT (17–20) and fibrates (agonists of peroxisome proliferator-activated receptors) (57–59). Two studies of linerixibat in cholestatic itch secondary to PBC (114, 115) are ongoing. A multicentre double-blind randomized placebo-controlled trial is in progress evaluating bezafibrate 400 mg daily in cholestatic pruritus (116). Inclusion is underway in Europe, and depending on the results, a second phase would consist of establishing a comparison of bezafibrate with rifampicin. The results could change the order of the different lines actually available (106). The use of UDCA and SAMe could be useful, particularly in PBC and ICP patients (117). Ondansetron, a serotonin 5-HT3 receptor subtype antagonist, has a modest anti-pruritic effect despite its good tolerance. The major inconvenience is its limited effect in time, maximum 6 h after its injection (78). The other treatments reported in this review were evaluated by few studies, so there are not enough scientific data recommending them for cholestatic pruritus. The use of these drugs can be discussed in patients with refractory pruritus who do not experience relief with previous treatments and do not want an invasive procedure.
If these different lines fail to improve pruritus, experimental treatment (such as MARS therapy, plasmapheresis, phototherapy and surgery) should be discussed. MARS often improves cholestatic itch with impressive results, but the difficulty of conducting randomized placebo-controlled studies with these methods has kept MARS out of standard guidelines. In addition, the system is difficult to set up and requires significant resources. The surgical techniques have the same limitation, as they have only been evaluated in retrospective studies or studies conducted on small cohorts. Interestingly, no study has assessed the efficacy of liver transplantation for pruritus. Intractable pruritus can be an indication for liver transplantation, even in the absence of liver failure (107). Pruritus seems to be rapidly controlled after surgery, with a major improvement in quality of life (118). This indication should be carefully discussed regarding cholestatic itch because it raises issues of organ allocation priority, in addition to the surgical risks of the procedure, in patients who would not necessarily require transplantation (119). Vloo & Nevens proposed a new treatment flowchart that includes recent advancements in cholestatic itch (3).
The current study had some limitations. Six articles were published the same year as the EASL guidelines, and 12 articles were published after 2017, which were not taken into account in the writing of the recommendations. In addition, many articles were relatively old, and they lacked some data, especially VAS scores, associated with a low methodological quality. In 25 studies of our systematic review (mostly studies that evaluated UDCA and SAMe), itch was evaluated as a secondary objective and not a primary objective. Among our 93 selected studies, 46 articles were open-label or retrospective studies, resulting in a lower level of evidence for the treatments evaluated in these studies. Another limitation is the sample size of some studies: 21 studies included fewer than 10 patients, particularly the MARS and surgery trials. Finally, evaluation of itch in children is less reliable because patients are frequently too young to describe their own pruritus, which is a potential bias in these studies (120), particularly in the studies on surgical procedures.
Fourteen studies that compared a treatment to placebo were included in the meta-analysis of the heterogeneity in the evaluation method of itch. Different methods were used to evaluate pruritus in the clinical trials. Most publications used the validated VAS (from 0 to 10 cm or 0–100 mm) filled in by patients, which is only quantitative, or various semiquantitative scales (from 0 to 2, 0 to 3, 0 to 4, and 0 to 5), which included the quantitative and qualitative characteristics of itch (121). The timing and frequency of evaluation of itch are critical due to its fluctuating intensity (65, 66). The measurement of scratching activity was more objective than other itch scales and was, most of the time, correlated with VAS score, with the exception of Bergasa et al. (68). Important variations in itch evaluation make it difficult to compare the different studies. The placebo effect should not be neglected in the treatment of pruritus. Van Laarhoven et al. (122) concluded in their meta-analysis that itch can be considerably reduced by the placebo effect (overall reduction of 24%), leading to the much more important challenge of showing an improvement in pruritus in randomized trials and making non-randomized trials difficult to analyse. All these factors led to an important level of heterogeneity in our meta-analysis, and thus, it was difficult to show a clear trend or superiority in the treatment of cholestatic pruritus. Further randomized clinical trials with large sample sizes are required to evaluate the efficacy of treatment for cholestatic pruritus.
In conclusion, this systemic review on the treatment of cholestatic pruritus highlighted that data are of very low quality (many open-label studies and few clinical trials) and that there is a great heterogeneity in treatment approaches. The high diversity in pathological entities makes it difficult to analyse the treatment of cholestatic pruritus, because the treatment must be adapted to the aetiology. Guidelines suggest a stepwise approach to cholestatic itch, but evidence of the effectiveness of many treatments is lacking. New treatments, such as fibrates and IBAT inhibitors, are promising future options in the therapeutic approach to cholestatic pruritus.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Misery L, Ständer S, editors. Pruritus. Cham: Springer International Publishing; 2016. [accessed 13 Apr 2020]. Avalable from: http://link.springer.com/10.1007/978-3-319-33142-3. [Google Scholar]
- 2.Ständer S. Classification of itch. Curr Probl Dermatol 2016; 50: 1–4. [DOI] [PubMed] [Google Scholar]
- 3.De Vloo C, Nevens F. Cholestatic pruritus: an update. Acta Gastro-Enterol Belg 2019; 82: 75–82. [PubMed] [Google Scholar]
- 4.Sun Y, Zhang W, Evans JF, Floreani A, Zou Z, Nishio Y, et al. Autotaxin, pruritus and primary biliary cholangitis (PBC). Autoimmun Revt 2016; 15: 795–800. [DOI] [PubMed] [Google Scholar]
- 5.Kremer AE, Gonzales E, Schaap FG, Oude Elferink RPJ, Jacquemin E, Beuers U. Serum autotaxin activity correlates with pruritus in pediatric cholestatic disorders. J Pediatr Gastroenterol Nutr 2016; 62: 530–535. [DOI] [PubMed] [Google Scholar]
- 6.Kremer AE, Martens JJWW, Kulik W, Ruëff F, Kuiper EMM, van Buuren HR, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010; 139: 1008–1018. [DOI] [PubMed] [Google Scholar]
- 7.Düll MM, Kremer AE. Management of chronic hepatic itch. Dermatol Clin 2018; 36: 293–300. [DOI] [PubMed] [Google Scholar]
- 8.Bhalerao A, Mannu GS. Management of pruritus in chronic liver disease. Dermatol Res Pract 2015; 2015: 295891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc Taiwan Yi Zhi 2016; 115: 689–702. [DOI] [PubMed] [Google Scholar]
- 10.Hegade VS, Mells GF, Fisher H, Kendrick S, DiBello J, Gilchrist K, et al. Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin Gastroenterol Hepatol 2019; 17: 1379–1387.e3. [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017; 67: 145–172. [DOI] [PubMed] [Google Scholar]
- 12.Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Giménez-Arnau AM, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol 2019; 99: 469–506. [DOI] [PubMed] [Google Scholar]
- 13.Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology 2005; 129: 894–901. [DOI] [PubMed] [Google Scholar]
- 14.Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra- and extra-hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol 1984; 6: 773–776. [PubMed] [Google Scholar]
- 15.Yokomori H. Effects of ursodeoxycholic acid and colestilan versus ursodeoxycholic acid alone on serum bile acids and pruritus: a randomized, open-label study. 2001. [accessed Dec 15 2019]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00442075/full.
- 16.Kuiper EMM, van Erpecum KJ, Beuers U, Hansen BE, Thio HB, de Man RA, et al. The potent bile acid sequestrant cole-sevelam is not effective in cholestatic pruritus: results of a double-blind, randomized, placebo-controlled trial. Hepatol Baltim Md 2010; 52: 1334–1340. [DOI] [PubMed] [Google Scholar]
- 17.Hegade VS, Kendrick SFW, Dobbins RL, Miller SR, Thompson D, Richards D, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet Lond Engl 2017; 389: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 18.Mayo MJ, Pockros PJ, Jones D, Bowlus CL, Levy C, Patanwala I, et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol Commun 2019; 3: 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo-controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in alagille syndrome. Hepatol Commun 2018; 2: 1184–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Dury S, Wahlström A, Wahlin S, Langedijk J, Elferink RO, Ståhlman M, et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci Rep 2018; 8: 6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollands CM, Rivera-Pedrogo FJ, Gonzalez-Vallina R, Loretde-Mola O, Nahmad M, Burnweit CA. Ileal exclusion for Byler’s disease: an alternative surgical approach with promising early results for pruritus. J Pediatr Surg 1998; 33: 220–224. [DOI] [PubMed] [Google Scholar]
- 22.Modi BP, Suh MY, Jonas MM, Lillehei C, Kim HB. Ileal exclusion for refractory symptomatic cholestasis in Alagille syndrome. J Pediatr Surg 2007; 42: 800–805. [DOI] [PubMed] [Google Scholar]
- 23.Jankowska I, Czubkowski P, Kaliciński P, Ismail H, Kowalski A, Ryżko J, et al. Ileal exclusion in children with progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 2014; 58: 92–95. [DOI] [PubMed] [Google Scholar]
- 24.Van Vaisberg V, Tannuri ACA, Lima FR, Tannuri U. Ileal exclusion for pruritus treatment in children with progressive familial intrahepatic cholestasis and other cholestatic diseases. J Pediatr Surg 2020; 55: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran P, Shanmugam NP, Sinani SA, Shanmugam V, Srinivas S, Sathiyasekaran M, et al. Outcome of partial internal biliary diversion for intractable pruritus in children with cholestatic liver disease. Pediatr Surg Int 2014; 30: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 26.Khan I, Qureshi MA, Karim F, Shaukat M. Surgical treatment for intractable pruritus in progressive familial intrahepatic cholestasis. JPMA J Pak Med Assoc 2018; 68: 953–955. [PubMed] [Google Scholar]
- 27.Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology 1988; 95: 130–136. [DOI] [PubMed] [Google Scholar]
- 28.Ng VL, Ryckman FC, Porta G, Miura IK, de Carvalho E, Servidoni MF, et al. Long-term outcome after partial external biliary diversion for intractable pruritus in patients with intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 2000; 30: 152–156. [DOI] [PubMed] [Google Scholar]
- 29.Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. Am J Gastroenterol 1999; 94: 2403–2407. [DOI] [PubMed] [Google Scholar]
- 30.Hegade VS, Krawczyk M, Kremer AE, Kuczka J, Gaouar F, Kuiper EMM, et al. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: a multicentre European study. Aliment Pharmacol Ther 2016; 43: 294–302. [DOI] [PubMed] [Google Scholar]
- 31.Battezzati PM, Podda M, Bianchi FB, Naccarato R, Orlandi F, Surrenti C, et al. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis: preliminary analysis of a double-blind multicenter trial. J Hepatol 1993; 17: 332–338. [DOI] [PubMed] [Google Scholar]
- 32.Calmus Y, Poupon R. Ursodeoxycholic acid (UDCA) in the treatment of chronic cholestatic diseases. Biochimie 1991; 73: 1335–1338. [DOI] [PubMed] [Google Scholar]
- 33.Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza Aet al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis results of a double-blind controlled muticentric trial. J Hepatol 2000; 32: 561–566 [DOI] [PubMed] [Google Scholar]
- 34.Diaferia A, Nicastri PL, Tartagni M, Loizzi P, Iacovizzi C, Di Leo A. Ursodeoxycholic acid therapy in pregnant women with cholestasis. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 1996; 52: 133–140. [DOI] [PubMed] [Google Scholar]
- 35.Floreani A, Paternoster D, Melis A, Grella PV. S-adenosylmethionine versus ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: preliminary results of a controlled trial. Eur J Obstet Gynecol Reprod Biol 1996; 67: 109–113. [DOI] [PubMed] [Google Scholar]
- 36.Joutsiniemi T, Timonen S, Leino R, Palo P, Ekblad U. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: a randomized controlled trial. Arch Gynecol Obstet 2014; 289: 541–547. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Liu X-H, Qi H-B, Li Z, Fu X-D, Chen L, et al. Ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy: a multi-centered randomized controlled trial. Eur Rev Med Pharmacol Sci 2015; 19: 3770–3776. [PubMed] [Google Scholar]
- 38.Chappell LC, Gurung V, Seed P, Chambers J, Williamson C, Thornton J. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ 2012; 344: e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roncaglia N, Locatelli A, Arreghini A, Assi F, Cameroni I, Pezzullo JC, et al. A randomised controlled trial of ursodeoxycholic acid and S-adenosyl-l-methionine in the treatment of gestational cholestasis. BJOG Int J Obstet Gynaecol 2004; 111: 17–21. [DOI] [PubMed] [Google Scholar]
- 40.Jain AK, Waghmare C, Adkar S, Jain M, Sircar C, Chahwala F. The comparison of efficacy of ursodeoxycholic acid, ondandsetron and natrexone in the prupitus or acute cholestatic viral hepatitis. J Clin Exp Hepatol 2013; 3: S47. [Google Scholar]
- 41.Wunsch E, Trottier J, Milkiewicz M, Raszeja-Wyszomirska J, Hirschfield GM, Barbier O, et al. Prospective evaluation of ursodeoxycholic acid withdrawal in patients with primary sclerosing cholangitis. Hepatol Baltim Md 2014; 60: 931–940. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki Y, Tanaka N, Osuga T, Aikawa T, Shoda J, Doi M, et al. Improvement of biliary enzyme levels and itching as a result of long-term administration of ursodeoxycholic acid in primary biliary cirrhosis. Am J Gastroenterol 1990; 85: 15–23. [PubMed] [Google Scholar]
- 43.Floreani A, Mioni D, Chiaramonte M, Naccarato R. Double-blind, controlled study of tauroursodeoxycholic acid in elderly patients with hepatitis C virus-related cirrhosis. Curr Ther Res 1999; 60: 550–557. [Google Scholar]
- 44.Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology 1988; 94: 488–493. [DOI] [PubMed] [Google Scholar]
- 45.Cynamon HA, Andres JM, Iafrate RP. Rifampin relieves pruritus in children with cholestatic liver disease. Gastroenterology 1990; 98: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 46.Podesta A, Lopez P, Terg R, Villamil F, Flores D, Mastai R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci 1991; 36: 216–220. [DOI] [PubMed] [Google Scholar]
- 47.Ataei S, Kord L, Larki A, Yasrebifar F, Mehrpooya M, Seyedtabib M, et al. Comparison of sertraline with rifampin in the treatment of cholestatic pruritus: a randomized clinical trial. Rev Recent Clin Trials 2019; 14: 217–223. [DOI] [PubMed] [Google Scholar]
- 48.Woolf GM, Reynolds TB. Failure of rifampin to relieve pruritus in chronic liver disease. J Clin Gastroenterol 1990; 12: 174–177. [DOI] [PubMed] [Google Scholar]
- 49.Bachs L. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. 1989. [accessed 15 Dec 2019]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00058452/full. [DOI] [PubMed]
- 50.Bachs L, Parés A, Elena M, Piera C, Rodés J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology 1992; 102: 2077–2080. [DOI] [PubMed] [Google Scholar]
- 51.Gregorio GV, Ball CS, Mowat AP, Mieli-Vergani G. Effect of rifampicin in the treatment of pruritus in hepatic cholestasis. Arch Dis Child 1993; 69: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahimpour S. Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A triple blinded, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of oral vancomycin in primary sclerosing cholangitis: a pilot study. J Gastrointestin Liver Dis 2016; 25: 457–464 [DOI] [PubMed] [Google Scholar]
- 53.Frezza M, Surrenti C, Manzillo G, Fiaccadori F, Bortolini M, Di Padova C. Oral S-adenosylmethionine in the symptomatic treatment of intrahepatic cholestasis. A double-blind, placebo-controlled study. Gastroenterology 1990; 99: 211–215. [DOI] [PubMed] [Google Scholar]
- 54.Frezza M, Pozzato G, Chiesa L, Stramentinoli G, di Padova C. Reversal of intrahepatic cholestasis of pregnancy in women after high dose S-adenosyl-L-methionine administration. Hepatol Baltim Md 1984; 4: 274–278. [DOI] [PubMed] [Google Scholar]
- 55.Wunsch E, Raszeja-Wyszomirska J, Barbier O, Milkiewicz M, Krawczyk M, Milkiewicz P. Effect of S-adenosyl-L-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J Gastrointest Liver Dis 2018; 27: 273–279. [DOI] [PubMed] [Google Scholar]
- 56.Fiorelli G. S-Adenosylmethionine in the treatment of intrahepatic cholestasis of chronic liver disease: A field trial. Curr Ther Res 1999; 60: 335–348. [Google Scholar]
- 57.Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018; 378: 2171–2181. [DOI] [PubMed] [Google Scholar]
- 58.Reig A, Sesé P, Parés A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol 2018; 113: 49–55. [DOI] [PubMed] [Google Scholar]
- 59.Lemoinne S, Pares A, Reig A, Ben Belkacem K, Kemgang Fankem AD, Gaouar F, et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French-Spanish experience. Clin Res Hepatol Gastroenterol 2018; 42: 521–528. [DOI] [PubMed] [Google Scholar]
- 60.Kumada H, Miyakawa H, Muramatsu T, Ando N, Oh T, Takamori K, et al. Efficacy of nalfurafine hydrochloride in patients with chronic liver disease with refractory pruritus: a randomized, double-blind trial. Hepatol Res Off J Jpn Soc Hepatol 2017; 47: 972–982. [DOI] [PubMed] [Google Scholar]
- 61.Akuta N, Kumada H, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, et al. Recurrence rates of pruritus after the stop of nalfurafine hydrochloride in chronic liver disease: preliminary prospective confirmatory trial. Hepatol Res Off J Jpn Soc Hepatol 2018; 48: 810–813. [DOI] [PubMed] [Google Scholar]
- 62.Kamimura K, Yokoo T, Kamimura H, Sakamaki A, Abe S, Tsuchiya A, et al. Long-term efficacy and safety of nalfurafine hydrochloride on pruritus in chronic liver disease patients: patient-reported outcome based analyses. PloS One 2017; 12: e0178991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yagi M, Tanaka A, Namisaki T, Takahashi A, Abe M, Honda A, et al. Is patient-reported outcome improved by nalfurafine hydrochloride in patients with primary biliary cholangitis and refractory pruritus? A post-marketing, single-arm, prospective study. J Gastroenterol 2018; 53: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 64.Akuta N, Kumada H, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, et al. Predictors of pruritus in patients with chronic liver disease and usefulness of nalfurafine hydrochloride. Hepatol Res Off J Jpn Soc Hepatol 2018; 48: 45–50. [DOI] [PubMed] [Google Scholar]
- 65.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology 1997; 113: 1264–1269. [DOI] [PubMed] [Google Scholar]
- 66.Mansour-Ghanaei F, Taheri A, Froutan H, Ghofrani H, Nasiri-Toosi M, Bagherzadeh A-H, et al. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol WJG 2006; 12: 1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terg R, Coronel E, Sordá J, Muñoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol 2002; 37: 717–722. [DOI] [PubMed] [Google Scholar]
- 68.Bergasa NV, Talbot TL, Alling DW, Schmitt JM, Walker EC, Baker BL, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992; 102: 544–549. [DOI] [PubMed] [Google Scholar]
- 69.Bergasa NV, Alling DW, Talbot TL, Swain MG, Yurdaydin C, Turner ML, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med 1995; 123: 161–167. [DOI] [PubMed] [Google Scholar]
- 70.Joshi GG, Thakur BS, Sircar S, Namdeo A, Jain AK. Role of intravenous naloxone in severe pruritus of acute cholestasis. Indian J Gastroenterol Off J Indian Soc Gastroenterol 2009; 28: 180–182. [DOI] [PubMed] [Google Scholar]
- 71.Bergasa NV, Alling DW, Talbot TL, Wells MC, Jones EA. Oral nalmefene therapy reduces scratching activity due to the pruritus of cholestasis: a controlled study. J Am Acad Dermatol 1999; 41: 431–434. [DOI] [PubMed] [Google Scholar]
- 72.Bergasa NV, Schmitt JM, Talbot TL, Alling DW, Swain MG, Turner ML, et al. Open-label trial of oral nalmefene therapy for the pruritus of cholestasis. Hepatol Baltim Md 1998; 27: 679–684. [DOI] [PubMed] [Google Scholar]
- 73.Bergasa NV, McGee M, Ginsburg IH, Engler D. Gabapentin in patients with the pruritus of cholestasis: a double-blind, randomized, placebo-controlled trial. Hepatol Baltim Md 2006; 44: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 74.Villamil AG, Bandi JC, Galdame OA, Gerona S, Gadano AC. Efficacy of lidocaine in the treatment of pruritus in patients with chronic cholestatic liver diseases. Am J Med 2005; 118: 1160–1163. [DOI] [PubMed] [Google Scholar]
- 75.Borgeat A, Wilder-Smith OH, Mentha G. Subhypnotic doses of propofol relieve pruritus associated with liver disease. Gastroenterology 1993; 104: 244–247. [DOI] [PubMed] [Google Scholar]
- 76.Jones EA, Molenaar HAJ, Oosting J. Ondansetron and pruritus in chronic liver disease: a controlled study. Hepatogastroenterology 2007; 54: 1196–1199. [PubMed] [Google Scholar]
- 77.O’Donohue JW, Pereira SP, Ashdown AC, Haigh CG; Wilkison JR, Williams R. A controlled trial of ondansetron in the pruritus of cholestasis. Aliment Pharmacol Ther 2005; 21: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 78.Schwörer H, Hartmann H, Ramadori G. Relief of cholestatic pruritus by a novel class of drugs: 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists: effectiveness of ondansetron. Pain 1995; 61: 33–37. [DOI] [PubMed] [Google Scholar]
- 79.Müller C, Pongratz S, Pidlich J, Penner E, Kaider A, Schemper M, et al. Treatment of pruritus in chronic liver disease with the 5-hydroxytryptamine receptor type 3 antagonist ondansetron: a randomized, placebo-controlled, double-blind crossover trial. Eur J Gastroenterol Hepatol 1998; 10: 865–870. [DOI] [PubMed] [Google Scholar]
- 80.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatol Baltim Md 2007; 45: 666–674. [DOI] [PubMed] [Google Scholar]
- 81.Thébaut A, Habes D, Gottrand F, Rivet C, Cohen J, Debray D, et al. Sertraline as an additional treatment for cholestatic pruritus in children. J Pediatr Gastroenterol Nutr 2017; 64: 431–435. [DOI] [PubMed] [Google Scholar]
- 82.Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol 2003; 98: 2736–2741. [DOI] [PubMed] [Google Scholar]
- 83.Doria C, Mandalá L, Smith J, Vitale CH, Lauro A, Gruttadauria S, et al. Effect of molecular adsorbent recirculating system in hepatitis C virus-related intractable pruritus. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2003; 9: 437–443. [DOI] [PubMed] [Google Scholar]
- 84.Bellmann R, Graziadei IW, Feistritzer C, Schwaighofer H, Stellaard F, Sturm E, et al. Treatment of refractory cholestatic pruritus after liver transplantation with albumin dialysis. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2004; 10: 107–114. [DOI] [PubMed] [Google Scholar]
- 85.Montero JL, Pozo JC, Barrera P, Fraga E, Costán G, Domínguez JL, et al. Treatment of refractory cholestatic pruritus with molecular adsorbent recirculating system (MARS). Transplant Proc 2006; 38: 2511–2513. [DOI] [PubMed] [Google Scholar]
- 86.Novelli G, Rossi M, Poli L, Predagostini R, Iappelli M, Morabito V, et al. Intractable pruritus in patients with hepatitis C virus. Transplant Proc 2006; 38: 1089–1091. [DOI] [PubMed] [Google Scholar]
- 87.Rifai K, Hafer C, Rosenau J, Athmann C, Haller H, Peter Manns M, et al. Treatment of severe refractory pruritus with fractionated plasma separation and adsorption (Prometheus). Scand J Gastroenterol 2006; 41: 1212–1217. [DOI] [PubMed] [Google Scholar]
- 88.Parés A, Cisneros L, Salmerón JM, Caballería L, Mas A, Torras A, et al. Extracorporeal albumin dialysis: a procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol 2004; 99: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 89.Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol 2010; 53: 307–312. [DOI] [PubMed] [Google Scholar]
- 90.Leckie P, Tritto G, Mookerjee R, Davies N, Jones D, Jalan R. «Out-patient» albumin dialysis for cholestatic patients with intractable pruritus. Aliment Pharmacol Ther 2012; 35: 696–704. [DOI] [PubMed] [Google Scholar]
- 91.Schaefer B, Schaefer F, Wittmer D, Engelmann G, Wenning D, Schmitt CP. Molecular adsorbents recirculating system dialysis in children with cholestatic pruritus. Pediatr Nephrol Berl Ger 2012; 27: 829–834. [DOI] [PubMed] [Google Scholar]
- 92.Cisneros-Garza LE, Muñoz-Ramírez M del R, Muñoz-Espinoza LE, Ruiz Velasco JAV, Moreno-Alcántar R, Marín-López E, et al. The molecular adsorbent recirculating system as a liver support system: summary of Mexican experience. Ann Hepatol 2014; 13: 240–247. [PubMed] [Google Scholar]
- 93.Bergasa NV, Link MJ, Keogh M, Yaroslavsky G, Rosenthal RN, McGee M. Pilot study of bright-light therapy reflected toward the eyes for the pruritus of chronic liver disease. Am J Gastroenterol 2001; 96: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 94.Decock S, Roelandts R, Steenbergen WV, Laleman W, Cassiman D, Verslype C, et al. Cholestasis-induced pruritus treated with ultraviolet B phototherapy: an observational case series study. J Hepatol 2012; 57: 637–641. [DOI] [PubMed] [Google Scholar]
- 95.Cohen LB, Ambinder EP, Wolke AM, Field SP, Schaffner F. Role of plasmapheresis in primary biliary cirrhosis. Gut 1985; 26: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krawczyk M, Liebe R, Wasilewicz M, Wunsch E, Raszeja-Wyszomirska J, Milkiewicz P. Plasmapheresis exerts a longlasting antipruritic effect in severe cholestatic itch. Liver Int Off J Int Assoc Study Liver 2017; 37: 743–747. [DOI] [PubMed] [Google Scholar]
- 97.Kittanamongkolchai W, El-Zoghby ZM, Eileen Hay J, Wiesner RH, Kamath PS, LaRusso NF, et al. Charcoal hemoperfusion in the treatment of medically refractory pruritus in cholestatic liver disease. Hepatol Int 2017; 11: 384–389. [DOI] [PubMed] [Google Scholar]
- 98.Kaplan MM, Schmid C, Provenzale D, Sharma A, Dickstein G, McKusick A. A prospective trial of colchicine and methotrexate in the treatment of primary biliary cirrhosis. Gastroenterology 1999; 117: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 99.Hendrickse MT, Rigney E, Giaffer MH, Soomro I, Triger DR, Underwood JCE, et al. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial. Gastroenterology 1999; 117: 400–407. [DOI] [PubMed] [Google Scholar]
- 100.Babatin MA, Sanai FM, Swain MG. Methotrexate therapy for the symptomatic treatment of primary biliary cirrhosis patients, who are biochemical incomplete responders to ursodeoxycholic acid therapy. Aliment Pharmacol Ther 2006; 24: 813–820. [DOI] [PubMed] [Google Scholar]
- 101.Almasio PL, Floreani A, Chiaramonte M, Provenzano G, Battezzati P, Crosignani A, et al. Multicentre randomized placebo-controlled trial of ursodeoxycholic acid with or without colchicine in symptomatic primary biliary cirrhosis. Aliment Pharmacol Ther 2000; 14: 1645–1652. [DOI] [PubMed] [Google Scholar]
- 102.van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, et al. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol 2000; 95: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 103.Sherker AH, Vierling JM, Pockros P, Battish R, LaPlaca C, Resler M, et al. Oral Ast-120 (spherical carbon adsorbent) improves pruritus and lowers serum bile acidsin patients with cirrhosis of various etiologies. Hepatology 2009; 50: 462A–463A.19444874 [Google Scholar]
- 104.Watson JP, Jones DE, James OF, Cann PA, Bramble MG. Case report: oral antioxidant therapy for the treatment of primary biliary cirrhosis: a pilot study. J Gastroenterol Hepatol 1999; 14: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 105.Turner IB, Rawlins MD, Wood P, James OF. Flumecinol for the treatment of pruritus associated with primary biliary cirrhosis. Aliment Pharmacol Ther 1994; 8: 337–342. [DOI] [PubMed] [Google Scholar]
- 106.Düll MM, Kremer AE. Treatment of pruritus secondary to liver disease. Curr Gastroenterol Rep 2019; 21: 48. [DOI] [PubMed] [Google Scholar]
- 107.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009; 51: 237–267. [DOI] [PubMed] [Google Scholar]
- 108.Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol 2007; 102: 1528–1536. [DOI] [PubMed] [Google Scholar]
- 109.Hoensch HP, Balzer K, Dylewizc P, Kirch W, Goebell H, Ohnhaus EE. Effect of rifampicin treatment on hepatic drug metabolism and serum bile acids in patients with primary biliary cirrhosis. Eur J Clin Pharmacol 1985; 28: 475–477. [DOI] [PubMed] [Google Scholar]
- 110.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int 2006; 26: 943–948. [DOI] [PubMed] [Google Scholar]
- 111.Prince MI, Burt AD, Jones DEJ. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut 2002; 50: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kremer AE, Beuers U, Oude-Elferink RPJ, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs 2008; 68: 2163–2182. [DOI] [PubMed] [Google Scholar]
- 113.Cheung AC, Patel H, Meza-Cardona J, Cino M, Sockalingam S, Hirschfield GM. Factors that influence health-related quality of life in patients with primary sclerosing cholangitis. Dig Dis Sci 2016; 61: 1692–1699. [DOI] [PubMed] [Google Scholar]
- 114.Kline GlaxoSmith. A Randomized, Double-blind, Multidose, Placebo-controlled study to evaluate the efficacy, safety and tolerability of GSK2330672 administration for the treatment of pruritus in patients with primary biliary cholangitis (GLIMMER: GSK2330672 triaL of IBAT inhibition with multidose measurement for evaluation of response). clinicaltrials.gov; 2020 Apr [accessed 19 May 2020]. Report No.: NCT02966834. Available from: https://clinicaltrials.gov/ct2/show/NCT02966834
- 115.Kline GlaxoSmith. Long-term safety and tolerability study of linerixibat for the treatment of cholestatic pruritus in participants with primary biliary cholangitis. clinicaltrials.gov; 2020 avr [accessed 19 May 2020]. Report No.: NCT04167358. Available from: https://clinicaltrials.gov/ct2/show/NCT04167358.
- 116.Bolier R, de Vries ES, Parés A, Helder J, Kemper EM, Zwinderman K, et al. Fibrates for the treatment of cholestatic itch (FITCH): study protocol for a randomized controlled trial. Trials 2017; 18: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghosh S, Chaudhuri S. Intra-hepatic cholestasis of pregnancy: a comprehensive review. Indian J Dermatol 2013; 58: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gross CR, Malinchoc M, Kim WR, Evans RW, Wiesner RH, Petz JL, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatol Baltim Md 1999; 29: 356–364. [DOI] [PubMed] [Google Scholar]
- 119.Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med 2018; 16: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jagadisan B, Srivastava A. Child with jaundice and pruritus: how to evaluate? Indian J Pediatr 2016; 83: 1311–1320. [DOI] [PubMed] [Google Scholar]
- 121.Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92: 497–501. [DOI] [PubMed] [Google Scholar]
- 122.van Laarhoven AIM, van der Sman-Mauriks IM, Donders ART, Pronk MC, van de Kerkhof PCM, Evers AWM. Placebo effects on itch: a meta-analysis of clinical trials of patients with dermatological conditions. J Invest Dermatol 2015; 135: 1234–1243. [DOI] [PubMed] [Google Scholar]