Abstract
Most studies of health-related quality of life (HRQoL) and atopic dermatitis are based on data from dermatology clinics. The aim of this study was to determine whether atopic dermatitis affects HRQoL in adolescence and young adulthood, based on data from the population-based cohort BAMSE (Children, Allergy, Environmental, Stockholm, Epidemiology). A further aim was to determine if the use of topical corticosteroids and healthcare contacts affect HRQoL. Participants with data from birth to young adulthood (n=3,064) were included. Two generic instruments were used to measure HRQoL:General Health at age 12, 16 and 24 years and EQ-5D-3L, including EQ-visual analogue scale (EQ-VAS) at age 24 years. In addition, the disease-specific Dermatology Quality Life Index (DLQI) was used at 24 years. Healthcare consultations for atopic dermatitis were obtained from Stockholm Regional Healthcare Data Warehouse (n = 1,944). Participants with atopic dermatitis had an increased odds ratio (OR) of not feeling completely healthy (adjusted OR 1.50; 95% confidence interval (95% CI): 1.30–1.73). Participants with persistent atopic dermatitis, fulfilling atopic dermatitis criteria in the 12- and/or 16- and 24-year follow-ups reported worse EQ-VAS value 70.0 (95% CI 67.3–72.7) in the 25th percentile, than peers without atopic dermatitis. Over an 8-year period, contact with healthcare was limited (mean number 0.96). In conclusion, atopic dermatitis had a negative impact on HRQoL in young adults from adolescence to adulthood and healthcare consultations were few.
Key words: atopic dermatitis, disease burden, eczema, epidemiology, health-related quality of life
It is commonly believed that most children outgrow their atopic dermatitis (AD) (1), but a review including several birth cohort studies has shown that the prevalence of AD is similar from childhood to adulthood (2). Most individuals with AD have mild disease (3, 4), while others have more persistent symptoms, which are associated with early onset, allergic rhinitis and hand eczema (5). However, regardless of severity, the chronic relapsing nature of the disease often requires daily, usually time-consuming, treatment (6, 7). Emollients and topical corticosteroids (TCS) are the first-line treatments (8, 9). Treatment adherence can be challenging, especially in adolescence; many are undertreated or completely untreated (10).
SIGNIFICANCE
Studies on atopic dermatitis and health-related quality of life are mostly based on data from dermatology clinics. Data from this population-based cohort showed that atopic dermatitis has a negative impact on health-related quality of life in young adults from adolescence to adulthood. Moreover, self-reported use of topical corticosteroids and healthcare contacts are limited in young adults with atopic dermatitis. Undertreatment of atopic dermatitis leads to symptoms, such as itching and pain, which, in turn, affect health-related quality of life. To reduce these negative effects, support from healthcare, with easy access, regular consultations and adequate therapy, is essential to increase self-care ability and to achieve control in atopic dermatitis.
Taking responsibility for treatment requires knowledge, which, in turn, requires patient education and support from healthcare providers (9). Most patients with AD are treated in primary care (11, 12). Data from our population-based cohort have shown that young adults with AD since childhood had never felt completely asymptomatic and had experienced difficulties in getting help from healthcare (13). A European cross-sectional register study in adults showed that patients with uncontrolled AD had a greater disease burden than those with controlled AD (14).
Severe AD impairs health-related quality of life (HRQoL) in both children and adults (15, 16). Even mild AD has been shown to impair general health (17). Both generic and dermatology-specific instruments have been used to measure HRQoL in patients with various severity of AD, but rarely in combination (18). Longitudinal data from population-based cohorts covering the full spectrum of HRQoL among individuals with AD are limited. In addition, most studies have been based on data from dermatology clinics (18, 19), thus excluding individuals with AD managed in primary care. Therefore, the aim of this study was to determine whether AD affects HRQoL in adolescents and young adulthood in a population-based setting. A further aim was to determine if the use of TCS and healthcare contacts affect HRQoL.
MATERIALS AND METHODS
Study design and population
This was a longitudinal study of the Swedish population-based birth cohort BAMSE (Children, Allergy, Environmental, Stockholm, Epidemiology), which includes 4,089 participants born between 1994 and 1996, who have been followed up regularly since birth (20, 21). Background characteristics were obtained through a parental questionnaire at baseline. When the children were 1, 2, 4, 8, 12 and 16 years of age, their parents answered questions about AD, asthma, and rhinitis symptoms and treatment during the preceding 12 months. The participants completed questionnaires at the 12-, 16- and 24-year follow-ups. All participants were invited to take part in clinical examinations at 4, 8, 16 and 24 years. The present study population consisted of individuals who answered the 24-year follow-up questionnaire (n = 3,064), mean age 22.5 years. Clinical examinations (n = 2,270) were performed by a clinical nurse and included assessment of HRQoL and ongoing AD (Fig. 1).
Fig. 1.
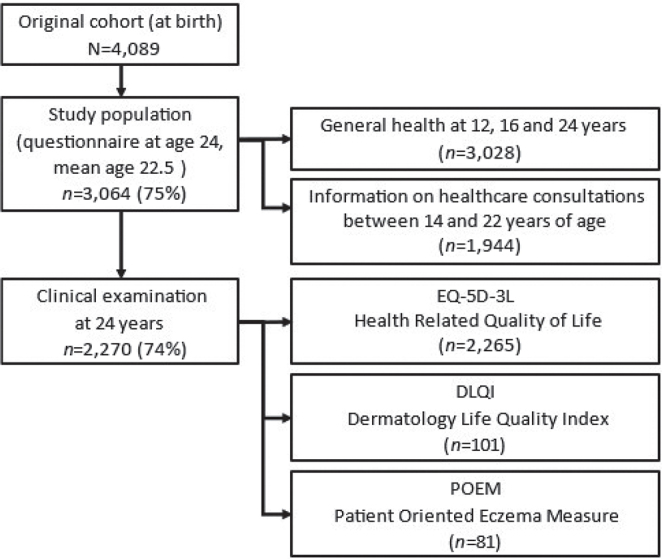
Flowchart of data collection.
To study healthcare consultations for AD, register data over an 8-year period was obtained for each participant (from 14 to 22 years of age). The analysis was restricted to participants living in the Stockholm region at both the 16- and the 24-year follow-up (n = 1,944).
This study was approved by the Regional Ethics Review Board in Stockholm, Sweden. All participants gave informed consent.
Atopic dermatitis variables
Definitions based on questionnaire data
AD at 12 years. Dry skin and itchy rash of age-typical localization (arm/leg flexures or wrists/ankles or neck/throat) in the preceding 12 months, and/or doctor’s diagnosis of eczema after the age of 10 years and up to the day of the 12-year questionnaire, reported by parents.
AD at 16 years. Dry skin and itchy rash of age-typical localization (arm/leg flexures or wrists/ankles or neck/throat) in the preceding 12 months, self-reported.
AD at 24 years. Modified William’s criteria, itchy rash in the preceding 12 months and 3 or more of the following 4 criteria: dry skin in the preceding year, history of flexural involvement, history of asthma and/or rhinitis, and onset before 2 years of age (22, 23). Symptoms at age 1–12 years were reported by parents, and symptoms at 16 and 24 years were self-reported.
Persistent AD. Fulfilling AD criteria in the 12- and/or 16-year follow-up(s) and, in addition, at the 24-year follow-up.
AD severity. A question on disturbed night sleep was used as a proxy for severity at the 24-year follow-up: “Has your sleep been disturbed by itching during the past 12 months?” (No/Rarely/Sometimes/Often/Always). This was dichotomized as mild (no/rarely) or moderate-to-severe AD (sometimes/often/always).
-
Treatment with topical corticosteroids (TCS). Data on this were self-reported and obtained through the following questions:
-
⚬
At the 12-year follow-up: “Has your child applied a cortisone cream in the past 12 months?” (Never/Rarely/Now and then/Frequently/Always).
-
⚬
At the 16-year follow-up: “Have you used cortisone cream because of eczema in the past 12 months?” (No/Yes, for less than 1 month/Yes, for 1–6 months/Yes, for more than 6 months).
-
⚬
At the 24-year follow-up: “Have you applied cortisone to your skin the last 12 months?” (No/Less than 1 month/1–6 months/Longer than 6 months).
-
⚬
Clinical data
Ongoing AD at 24 years required fulfilling the questionnaire-based AD definition and having visible flexural dermatitis at the clinical examination.
Severity of ongoing AD in the preceding week was self-assessed using the Patient-Oriented Eczema Measure (POEM) (24).
Definition of outcomes
Health-related quality of life. HRQoL was measured with 2 generic instruments and 1 disease-specific instrument:
General health (25) was measured at age 12, 16 and 24 years with the question “How healthy do you consider yourself to be?” (Completely healthy/Fairly healthy/Not very healthy). A dichotomous variable was constructed (Completely healthy vs Fairly healthy/Not very healthy).
The generic instrument EQ-5D-3L to capture health status on the day of the clinical examination (26). The EQ-5D-3L encompasses 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The severity of each dimension is rated at 1 of 3 levels (no/some/extreme problems). Participants also rated their health on a graded visual analogue scale (EQ-VAS) with labelled endpoints: 0, the worst imaginable health state, and 100, the best imaginable health state.
The disease-specific instrument Dermatology Life Quality Index (27) was used to assess HRQoL in the preceding week among participants fulfilling the criteria for ongoing AD at 24 years. The Dermatology Life Quality Index (DLQI) includes 10 questions, each scored 0–3, giving a maximum of 30. The higher the score, the greater the negative impact on HRQoL. In this study, DLQI scores were divided into 4 categories (0–1=no effect, 2–5=small effect on HRQoL, 6–10=moderate effect, and 11–20=large effect). Since no participant scored over 21, the category “very large effect” was excluded.
Healthcare consultations
Data on healthcare consultations were obtained from the Stockholm Regional Healthcare Data Warehouse (28). To identify primary and secondary diagnoses of AD, the International Classification of Diseases version 10 codes L.20 (AD) and L.30 (other dermatitis) were used, including all medical consultations (primary/specialist/inpatient care, physically or by telephone).
Statistical analysis
Dichotomous and categorical variables were analysed with the χ2 test and Fischer’s exact test. The mean value of continuous variables was analysed with the 2-sample t-test. A p-value < 0.05 was considered statistically significant.
Generalized estimating equations (GEE) with an unstructured correlation matrix were used to assess the longitudinally overall and age-specific associations between AD as an updated exposure at 12, 16 and 24 years and general health as an updated outcome at the same ages. The GEE model calculates average risks for a population, taking the correlation within individuals into account (29). To analyse age-specific associations and evaluate the effect over time, the model incorporated an interaction between time and exposure.
A priori-identified potential confounders (sex, parental socioeconomic status, comorbidity of asthma and/or rhinitis) were included in model I. Disturbed night sleep was added to model II and treatment with TCS > 6 months to model III. The model was also adjusted for healthcare consultations with the diagnosis code L.20 at any time between age 14 and 22 years. The results are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs).
The EQ-5D-3L data were stratified in relation to background characteristics: sex (male/female), family history of allergic disease and/or AD (yes/no), and parental socioeconomic status (blue-collar/white-collar workers). Since the data were skewed, median EQ-VAS was analysed with logistic quantile regression at the 25th and 75th percentile and expressed as OR with 95% CI.
The association between persistent AD and HRQoL was analysed with logistic quantile regression in the 25th percentile. The model was adjusted for a priori-identified confounders (sex, family history of allergic disease and/or AD, co-morbidity of asthma and/or rhinitis and parental socioeconomic status), presented as β-coefficients with 95% CIs.
RESULTS
The study population consisted of 3,064 participants, 75% of the original cohort (Fig. 1). The background characteristics in the original cohort and the study population were comparable, with slightly more females (52.8% vs 47.1%) and parents with higher socioeconomic status (84.5% vs 82.7%) in the study population (Table I).
Table I.
Background characteristics among participants in the original cohort and the study population
| Characteristics | Original cohort n = 4,089 (100%) | Study population n = 3,064 (75%) |
|---|---|---|
| n (%) | n (%), 95% CI | |
| Sex (male) | 2,065 (50.5) | 1,445 (47.1), 45.4–49.0 |
| Foreign-born, any parenta | 543 (16.0) | 422 (15.6), 14.3–17.0 |
| Smoking, any parentb | 855 (21.0) | 620 (20.4), 8.9–21.8 |
| Breastfeeding >4 monthsc | 3,116 (79.5) | 2,390 (80.4), 78.9–81.8 |
| Family history of allergic diseased | 1,746 (43.2) | 1,337 (44.0), 42.2–45.8 |
| Socioeconomic status of parentse | 3,323 (82.7) | 2,545 (84.5), 83.1–85.7 |
Any parent born outside of Scandinavia.
Any of the parents smoked at least 1 cigarette/day at the time of questionnaire 0.
Exclusively breastfed for 4 months or more.
Mother and/or father with doctor’s diagnosis of asthma and/or doctor’s diagnosis of hay fever in combination with allergy to furred pets and/or pollen and/or doctor’s diagnosis of atopic dermatitis at the time of questionnaire 0.
White-collar workers (including professional practician with university graduate jobs) at the time of questionnaire 0.
The 12-month prevalence of AD at the 24-year follow-up was 17.7% (n = 542), higher in females than in males (20.5% vs 14.8%, p ≤ 0.001). In the group with AD, 22% reported disturbed night sleep due to itching (occasionally to always) in the preceding 12 months, more often in females than in males (26.1% vs 15.5%, p ≤ 0.01). The proportions reporting TCS use for more than 6 months were 17% and 19%, at 12 and 16 years, respectively among those with AD. At 24 years, the proportion had decreased to 13% (Fig. S1).
Health-related quality of life measured as general health
Among the participants in the 12-, 16- and 24-year follow-ups, 27.6% (n = 2,323), 19.7% (n = 2,626) and 37.6% (2,908), respectively, stated that they did not consider themselves completely healthy. Participants with AD at age 12–24 years (Fig. 2) had an overall increased OR of not considering themselves completely healthy (adjusted OR 1.50; 95% CI 1.30–1.73). The age-specific analyses showed similar results at all ages. Adjusting the model for disturbed night sleep (as a proxy for AD severity) and treatment with TCS > 6 months, which may affect disease control or reflect a more severe disease, resulted in a minor change (adjusted OR 1.34; 95% CI 1.14–1.58).
Fig. 2.

Association between atopic dermatitis in adolescents/young adults and general health over time (Fairly healthy/Not very healthy. Reference: Completely healthy). *Odds ratios (ORs) were calculated using generalized estimating equations (GEE). Crude: Unadjusted association between atopic dermatitis in adolescents/young adults and general health over time. Model I: Adjusted for male, socioeconomic status and, comorbidity of asthma and/or rhinitis. Model II: Adjusted for male, socioeconomic status, comorbidity of asthma and/or rhinitis and, disturbed night sleep (sometimes, always, or often). Model III: Adjusted for male, socioeconomic status, comorbidity of asthma and/or rhinitis, disturbed night sleep (sometimes, always, or often), and treatment with corticosteroids for >6 months.
Health-related quality of life measured with EQ-5D-3L
In total, 74% of the study population (n = 2,270) participated in the clinical examination at age 24 years. Almost all of them (99.7%) answered the EQ-5D-3L. Young females reported significantly more problems with pain/discomfort and anxiety/depression than males (Table II).
Table II.
Health-related quality of life measured with EQ-5D-3L in relation to background characteristics at the 24-year clinical examination (n = 2,265), mean age 22.5 years
| Dimension | Total n = 2,265 | Sex | p-value | Family history of allergic disease and/or AD | p-value | Higher education/university | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| F n = 1,263 | M n = 1,002 | No n = 1,249 | Yes n = 997 | No n = 339 | Yes n = 1,886 | |||||
| Mobility | 984 (98.2) | |||||||||
| No problems | 2,218 (97.9) | 1,234 (97.7) | 17 (1.7) | 1,225 (98.1) | 947 (97.7) | 333 (98.2) | 1,845 (97.8) | |||
| Some problems | 46 (2.03) | 29 (2.3) | 1 (0.1) | 0.29 | 24 (1.9) | 22 (2.2) | 0.50 | 6 (1.8) | 40 (2.1) | 0.86 |
| Extreme problems | 1 (0.04) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||||
| Self-care | ||||||||||
| No problems | 2,251 (99.4) | 1,257 (99.5) | 994 (99.3) | 1,241 (99.4) | 991 (99.5) | 337 (99.4) | 1,874 (99.4) | |||
| Some problems | 10 (0.4) | 4 (0.32) | 6 (0.6) | 0.58 | 7 (0.6) | 3 (0.3) | 0.52 | 2 (0.6) | 8 (0.4) | 0.79 |
| Extreme problems | 3 (0.1) | 2 (0.16) | 1 (0.1) | 1 (0.1) | 2 (0.2) | 0 (0.0) | 3 (0.7) | |||
| Usual activities | ||||||||||
| No problems | 2,107 (93.0) | 1,178 (93.3) | 929 (92.7) | 1,153 (92.3) | 937 (93.9) | 311 (91.7) | 1,759 (93.3) | |||
| Some problems | 148 (6.5) | 78 (6.2) | 70 (6.9) | 0.51 | 88 (7.0) | 58 (5.8) | 0.15 | 26 (7.7) | 119 (6.3) | 0.50 |
| Extreme problems | 10 (0.4) | 7 (0.5) | 3 (0.3) | 8 (0.6) | 2 (0.2) | 2 (0.6) | 8 (0.4) | |||
| Pain/discomfort | ||||||||||
| No problems | 1,638 (72.4) | 895 (70.8) | 743 (74.2) | 927 (74.3) | 697 (69.9) | 225 (66.8) | 1,385 (73.8) | |||
| Some problems | 614 (27.1) | 358 (28.3) | 256 (25.6) | 0.047 | 313 (25.1) | 296 (29.7) | 0.042 | 112 (33.0) | 490 (25.9) | 0.021 |
| Extreme problems | 12 (0.5) | 10 (0.8) | 2 (0.2) | 8 (0.6) | 4 (0.4) | 2 (0.6) | 10 (0.5) | |||
| Anxiety/depression | ||||||||||
| No problems | 1,391 (61.4) | 728 (57.6) | 663 (66.2) | 754 (60.4) | 624 (62.6) | 199 (58.7) | 1,169 (61.9) | |||
| Some problems | 819 (36.2) | 497 (39.3) | 322 (32.1) | <0.001 | 458 (36.7) | 355 (35.6) | 0.16 | 131 (38.6) | 672 (35.6) | 0.50 |
| Extreme problems | 55 (2.4) | 38 (3.0) | 17 (1.7) | 37 (3.0) | 18 (1.8) | 9 (2.6) | 45 (2.4) | |||
| EQ VAS, mean | 78.65 | 79.52 | 0.13 | 78.42 | 79.68 | 0.03 | 78.70 | 79.19 | 0.53 | |
| EQ VAS, median (95% CI) | 80 (79.6–80.4) | 80 (79.5–80.5) | 1.00 | 80 (79.6–80.4) | 80 (79.5–80.5) | 1.00 | 80 (79.0–81.0) | 80 (79.6–80.4) | 1.00 | |
| EQ VAS 25th percentile (95% CI) | 70 (68.8–71.2) | 74 (72.7–75.3) | <0.001 | 70 (68.8–71.2) | 73 (71.7–74.3) | 0.001 | 70 (67.8–72.2) | 70 (69.1–70.9) | 1.00 | |
| EQ VAS 75th percentile (95% CI) | 90 (89.3–90.7) | 90 (89.2–90.8) | 1.00 | 90 (89.3–90.7) | 90 (89.2–90.8) | 1.00 | 90 (88.7–91.3) | 90 (89.5–90.5) | 1.00 | |
Missing data 5 participants. Analysed with Fischer’s exact test. Bold numbers are statistically significant.
EQ-5D-3L: descriptive system for health-related quality of life, with 5 dimension and 3 severity levels; EQ VAS: visual analogue scale (VAS) of current health-related quality of life, the best imaginable health to the worst imaginable health (0–100); 95% CI: 95% confidence interval; M: male; F: female.
Among those who participated in the clinical examination, 18.5% (420 of 2,270) fulfilled the criteria for AD. Young adults with AD reported more pain/discomfort (37.1% vs 24.8%, p < 0.001) than did peers without AD (Table III). Participants with AD had the same median EQ-VAS score as their peers (median 80). However, in the 25th percentile, the participants with AD reported their HRQoL as more impaired (70 (95% CI 68.4–71.6)), than those without AD (73 (95% CI 72.2–73.8)). In the group fulfilling the criteria for persistent AD (n = 180), a higher proportion reported problems with pain/discomfort (36.1% vs 22.7%, p < 0.001) and anxiety/depression (41.7% vs 34.3%, p-value = 0.02), than those without AD. In the 25th percentiles of EQ-VAS, the reported HRQoL for those with persistent AD was 70 (95% CI 67.3–72.7). The negative effects associated with AD on HRQoL in the 25th percentile remained, –5 (95% CI –8.07 to –1.93) after adjusting for sex, family history of allergic disease and/or AD, comorbidity of asthma/rhinitis and parental socioeconomic status (Table SI).
Table III.
Distribution of health-related quality of life with EQ-5D-3L dimension in relation to atopic dermatitis (AD) at the 24-year clinical examination (n = 2,270a) and persistent AD
| Dimensions | No AD | ADb | p-value | No ADc | Persistent ADd | p-value |
|---|---|---|---|---|---|---|
| (n = 1,839) | (n = 420) | (n = 1,375) | (n = 180) | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Mobility | ||||||
| No problems | 1,796 (97.7) | 416 (99.1) | 1,352 (98.3) | 178 (98.9) | ||
| Some problems | 42 (2.3) | 4 (1.0) | 0.23 | 22 (1.6) | 2 (1.1) | 1.0 |
| Extreme problems | 1 (0.05) | 0 (0.0) | 1 (0.07 ) | 0 (0.0) | ||
| Self-care | ||||||
| No problems | 1,827 (99.4) | 418 (99.8) | 1,367 (99.4) | 180 (100.0) | ||
| Some problems | 9 (0.5) | 1 (0.2) | 0.84 | 6 (0.4) | 0 (0.0) | 1.0 |
| Extreme problems | 3 (0.2) | 0 (0.0) | 2 (0.2) | 0 (0.0) | ||
| Usual activities | ||||||
| No problems | 1,713 (93.2) | 389 (92.6) | 1,292 (94.0) | 169 (93.9) | ||
| Some problems | 119 (6.5) | 28 (6.7) | 0.57 | 82 (6.0) | 11 (6.1) | 0.88 |
| Extreme problems | 7 (0.4) | 3 (0.7) | 1 (0.07) | 0 (0.0) | ||
| Pain/discomfort | ||||||
| No problems | 1,371 (74.6) | 263 (62.6) | 1,057 (76.9) | 114 (63.3) | ||
| Some problems | 456 (24.8) | 156 (37.1) | < 0.001 | 312 (22.7) | 65 (36.1) | <0.001 |
| Extreme problems | 11 (0.6) | 1 (0.2) | 6 (0.4) | 1 (0.6) | ||
| Anxiety/depression | ||||||
| No problems | 1,147 (62.4) | 240 (57.1) | 877 (63.8) | 98 (54.4) | ||
| Some problems | 650 (35.4) | 167 (39.8) | 0.11 | 472 (34.3) | 75 (41.7) | 0.02 |
| Extreme problems | 42 (2.3) | 13 (3.1) | 26 (1.9) | 7 (3.9) | ||
| EQ VAS mean | 79.4 | 77.6 | 0.01 | 79.8 | 76.0 | < 0.001 |
| EQ VAS median (95% CI) | 80 (79.6–80.4) | 80 (79.2–80.8) | 1.00 | 80 (79.2–80.8) | 80 (77.8–82.2) | 1.00 |
| EQ VAS 25th percentile (95% CI) | 73 (72.2–73.8) | 70 (68.4–71.6) | < 0.001 | 75 (74.0–76.0) | 70 (67.3–72.7) | < 0.001 |
| EQ VAS 75th percentile (95% CI) | 90 (89.5–90.5) | 87 (86.0–88.0) | < 0.001 | 90 (89.5–90.4) | 85 (83.7–86.3) | < 0.001 |
EQ-5D-3L: descriptive system for health-related quality of life, with 5 dimension and 3 severity levels; EQ VAS: visual analogue scale (VAS) of current health-related quality of life, the best imaginable health to the worst imaginable health (0–100) (analysed with Fischer’s exact test). Bold numbers are statistically significant.
95% CI: 95% confidence interval.
Missing 11.
Atopic dermatitis at 24 years defined as: itchy rash and dry skin in the preceding 12 months, history of flexural AD, asthma and/or rhinitis, onset before 2 years of age.
Participants without atopic dermatitis at 12-, 16- or 24-years.
Atopic dermatitis at 12 and/or 16 and 24 years of age, defined as: dry skin in combination with itchy rash of typical localization
Health-related quality of life measured with Dermatology Life Quality Index
Among participants with ongoing (visible) AD at the clinical examination, 70% (81/115) completed DLQI and POEM (Table IV). In total, 45.7% (n = 37) classified their AD as almost clear or mild and 54.3% (n = 44) as moderate-to-very severe. Participants with the latter rated HRQoL as more impaired than those with clear/mild AD, overall mean 4.60 (95% CI 3.80–5.38) vs 2.14 (95% CI 1.64–2.63).
Table IV.
Disease-specific Dermatology Life Quality Index (DLQI) and severity of ongoing atopic dermatitis (AD)a assessed with Patient-Oriented Eczema Measure (POEM) (n = 81)
| Severity of ongoing AD based on POEM (n = 81) |
|||
|---|---|---|---|
| Clear/mildb (n = 37) | Moderate/very severec (n = 44) | ||
| Men, n (%) | 38 (46.9) | 19 (51.3) | 19 (43.2) |
| Women, n (%) | 43 (53.1) | 18 (48.7) | 25 (56.8) |
| DLQI 0–1 no effect, n (%) | 17 (21.0) | 16 (19.8) | 1 (1.2) |
| DLQI 2–5 small effect, n (%) | 50 (61.3) | 20 (24.7 ) | 30 (37.0) |
| DLQI 6–10 moderate effect, n (%) | 13 (16.1) | 1 (1.2) | 12 (14.8) |
| DLQI 11–20 very large effect, n (%) | 1 (1.2) | 0 (0.0) | 1 (1.2) |
Fulfilling the questionnaire-based AD definition and having visible flexural dermatitis at the clinical examination.
Almost clear AD 0–2, mild AD 3–7.
Moderate AD 8–16, severe to very severe atopic dermatitis 17–28.
Healthcare consultations and atopic dermatitis
Among participants with data on healthcare consultations (n = 1,944), 17.6% fulfilled (n = 343) the definition of AD at 24 years. In total, 18.7% of them had at least 1 healthcare consultation (mean ± standard deviation (SD) number of consultations 0.57 ± 2.02) with the diagnosis code L.20 (atopic dermatitis) during the 8-year period (Table SII). When including the diagnosis code L.30 (unspecific dermatitis), the proportion with at least 1 healthcare consultation during the same period increased to 23.6%, mean ± SD number of consultations 0.69 ± 2.13. Regardless of diagnosis codes, there were no significant differences between the sexes. After restricting the criteria to young adults with persistent AD (n = 160), 29% (n = 47) had at least 1 healthcare consultation with the diagnosis code L.20, mean ± SD number of consultations 0.96 ± 2.73 during the 8-year period. The corresponding proportion for persistent AD and L.30 was 34.4%, mean ± SD number of consultations 1.09 ± 2.82. Since regular consultation with healthcare would be beneficial in management of AD, the association of AD and general health was examined with the GEE model and adjusted for healthcare contact, which resulted in almost unchanged results; overall OR for not feeling completely healthy (adjusted OR 1.42; 95% CI 1.19–1.71).
DISCUSSION
This longitudinal, birth cohort-based study assessing the impact of AD showed that young adults with AD rated their general health worse from early adolescence to adulthood than peers without AD. Participants with persistent AD reported more pain/discomfort and anxiety/depression than participants without AD. Moreover, young adults with AD have limited contact with healthcare, and healthcare consultations did not affect the association between AD and general health.
Generic health-related quality of life
It has been shown that a single-item, self-rated, general health questionnaire has good reliability (25) with strong association with both physical and mental health (30). This single-item question is sensitive, valid, and reliable in detecting physical health in longitudinal research among participants with various mental health disorders (31). Our population-based data showed that young adults with AD experienced poorer health than their peers. Self-reported treatment with TCS did not alter the effect. Furthermore, the participants with AD perceived greater negative impact of pain/discomfort. These results correspond to those of studies showing that pain in patients with AD is common and often associated with disease severity, poor sleep, uncontrolled AD, impact on HRQoL, and more healthcare consultations (32, 33). Pain is also described in patients with no or mild signs of scratching (34).
The negative impact of AD on HRQoL has been correlated with depression and anxiety among adolescents (35). Adults with AD have also been shown to have a heightened risk of developing depression and anxiety, increasing with disease severity (36). In the current study, participants with persistent AD reported more negative impact on anxiety/depression than those without AD. In comparison, the young adults with AD in the present study reported lower EQ-VAS than participants aged 18–34 years with hand eczema in a population-based study (37). As our data from EQ-VAS were skewed, the median was used to present participants’ self-reported health, which makes it difficult to compare these results with other studies, as it is common to present the mean. However, in a Taiwanese study including patients from a medical centre and hospitals, the reported mean HRQoL according to EQ-VAS was 79.5 for mild, 72.4 for moderate, and 64.2 for severe AD (38). In comparison, in our study the mean value of HRQoL according to EQ-VAS was 77.6 among participants with AD.
Dermatology Life Quality Index and severity of atopic dermatitis
More than half of the participants with ongoing AD reported moderate-to-very severe AD on POEM and this group rated their HRQoL as more impaired compared with those with mild AD. However, the difference was small and might not be considered clinically relevant (39). One explanation may be that young adults have grown accustomed to living with AD, as shown in a qualitative study from our group (13) and a qualitative study from England, including young people (40).
Healthcare consultations and treatment
In the current study’s population-based cohort, healthcare consultations due to AD were limited. The results are in accordance with a study from primary care in Stockholm (41), where the diagnosis code L.20 was not included among the 30 most common diagnoses between 2009 and 2011. Furthermore, the current study participants with AD reported more disturbed night sleep at 24 years of age than in earlier follow-ups, which may be a consequence of undertreated AD. In addition, self-reported treatment with TCS had decreased at the age of 24 years. Compared with previous follow-ups, approximately 1 in 10 had used TCS at the age of 24 years.
Strengths and limitations
The strengths of this study include the population-based design, with a well-defined study sample, and the use of longitudinal information on general health. In addition, both generic and disease-specific instruments were used for assessment of HRQoL, providing broader knowledge of the impact of AD.
Another strength was the use of register data on healthcare consumption from a regional database, which covers more than 90% of specialized ambulatory care and 85% of primary care (28). However, register data may underestimate the proportion of healthcare contacts due to inaccurate diagnosis codes. Since the current data identified few healthcare visits with the diagnosis code L.20, we performed a sensitivity analysis and included the diagnosis code L.30. This increased the number of care consultations slightly, but not as much as might be expected for a chronic recurrent illness that requires regular treatment and prescriptions.
A possible limitation of the current study was that information on healthcare consultations extended only to the age of 22 years, while the mean age of the participants was 22.5 years at the time of the questionnaire. This could theoretically underestimate the number of healthcare consultations. Another limitation was the proportion of missing data when measuring AD severity at the clinical examination.
Conclusion
This population-based birth cohort-based study found that young adults with AD rated their HRQoL worse than did peers without AD, from adolescence to adulthood. Participants with persistent AD reported more pain/discomfort and anxiety/depression. Young adults with AD have limited contact with healthcare, and healthcare consultations did not affect the association between AD and general health. One way to decrease the burden of AD is to provide easy access to healthcare, regular consultations, adequate therapy, and support for self-management.
ACKNOWLEDGEMENTS
The authors thank all the participants in the BAMSE cohort, and all the staff involved through the years.
The BAMSE study was supported by grants from the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, Formas, the Swedish Heart-Lung Foundation, the European Research Council (TRIBAL, grant agreement 757919), the Swedish Asthma and Allergy research foundation and Region Stockholm (ALF project, and for cohort and database maintenance).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Irvine AD, Mina-Osorio P. Disease trajectories in childhood atopic dermatitis: an update and practitioner’s guide. Br J Dermatol 2019; 181: 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2018; 73: 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 4.Ballardini N, Kull I, Soderhall C, Lilja G, Wickman M, Wahlgren CF. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol 2013; 168: 588–594. [DOI] [PubMed] [Google Scholar]
- 5.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015; 70: 836–845. [DOI] [PubMed] [Google Scholar]
- 6.Balieva FN, Finlay AY, Kupfer J, Aragones LT, Lien L, Gieler U, et al. The role of therapy in impairing quality of life in dermatological patients: a multinational study. Acta Derm Venereol 2018; 98: 563–569. [DOI] [PubMed] [Google Scholar]
- 7.Santer M, Burgess H, Yardley L, Ersser SJ, Lewis-Jones S, Muller I, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs 2013; 69: 2493–2501. [DOI] [PubMed] [Google Scholar]
- 8.van Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2017; 177: 1256–1271. [DOI] [PubMed] [Google Scholar]
- 9.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 10.Lundin S, Wahlgren CF, Bergstrom A, Johansson EK, Dahlen E, Andersson N, et al. Use of emollients and topical glucocorticoids among adolescents with eczema – datafrom the population-based birth cohort BAMSE. Br J Dermatol 2018; 179: 709–716. [DOI] [PubMed] [Google Scholar]
- 11.de Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. Patterns and trends in eczema management in UK primary care (2009–2018): A population-based cohort study. Clin Exp Allergy 2021; 51: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh P, Silverberg JI. Outpatient utilization patterns for atopic dermatitis in the United States. J Am Acad Dermatol 2019; 19: 30435–30439. [DOI] [PubMed] [Google Scholar]
- 13.Lundin S, Jonsson M, Wahlgren CF, Johansson E, Bergstrom A, Kull I. Young adults’ perceptions of living with atopic dermatitis in relation to the concept of self-management: a qualitative study. BMJ Open 2021; 11: e044777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert L, Gupta S, Gadkari A, Mahajan P, Gelfand JM. Burden of illness in adults with atopic dermatitis: analysis of National Health and Wellness Survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol 2019; 81: 187–195. [DOI] [PubMed] [Google Scholar]
- 15.Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 2006; 155: 145–151. [DOI] [PubMed] [Google Scholar]
- 16.Ring J, Zink A, Arents BWM, Seitz IA, Mensing U, Schielein MC, et al. Atopic eczema: burden of disease and individual suffering – results from a large EU study in adults. J Eur Acad Dermatol Venereol 2019; 33: 1331–1340. [DOI] [PubMed] [Google Scholar]
- 17.Hebert AA, Stingl G, Ho LK, Lynde C, Cappelleri JC, Tallman AM, et al. Patient impact and economic burden of mild-to-moderate atopic dermatitis. Curr Med Res Opin 2018; 34: 2177–2185. [DOI] [PubMed] [Google Scholar]
- 18.Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol 2020; 59: e75–e91. [DOI] [PubMed] [Google Scholar]
- 19.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2016; 137: 26–30. [DOI] [PubMed] [Google Scholar]
- 20.Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol 2002; 13: 11–13. [DOI] [PubMed] [Google Scholar]
- 21.Melén E, Bergström A, Kull I, Almqvist C, Andersson N, Asarnoj A, et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy 2020; 10: s13601-13020-00319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams HC. Diagnostic criteria for atopic dermatitis. Lancet 1996; 348: 1391–1392. [DOI] [PubMed] [Google Scholar]
- 23.Williams HC, Burney PG, Pembroke AC, Hay RJ. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. U.K. Diagnostic Criteria for Atopic Dermatitis Working Party. Br J Dermatol 1996; 135: 12–17. [PubMed] [Google Scholar]
- 24.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg O, Manderbacka K. Assessing reliability of a measure of self-rated health. Scand J Soc Med 1996; 24: 218–224. [DOI] [PubMed] [Google Scholar]
- 26.EQ-5D-3L User Guide , 2018. [Accessed 2021-06-15] Available from: https://euroqol.org/publications/user-guides.
- 27.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson AC, Wändell P, Ösby U, Zarrinkoub R, Wettermark B, Ljunggren G. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden – a challenge for public health. BMC Public Health 2013; 13: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 30.Baćak V, Ólafsdóttir S. Gender and validity of self-rated health in nineteen European countries. Scand J Public Health 2017; 45: 647–653. [DOI] [PubMed] [Google Scholar]
- 31.Macias C, Gold PB, Öngür D, Cohen BM, Panch T. Are single-item global ratings useful for assessing health status? J Clin Psychol Med Settings 2015; 22: 251–264. [DOI] [PubMed] [Google Scholar]
- 32.Simpson EL, Guttman-Yassky E, Margolis DJ, Feldman SR, Qureshi A, Hata T, et al. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol 2018; 154: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huet F, Shourick J, Séité S, Taïeb C, Misery L. Pain in atopic dermatitis: an online population-based survey. Acta Derm Venereol 2020; 100: adv00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol 2017; 119: 548–552.e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hon KL, Pong NH, Poon TC, Chan DF, Leung TF, Lai KY, et al. Quality of life and psychosocial issues are important outcome measures in eczema treatment. J Dermatolog Treat 2015; 26: 83–89. [DOI] [PubMed] [Google Scholar]
- 36.Schonmann Y, Mansfield KE, Hayes JF, Abuabara K, Roberts A, Smeeth L, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract 2020; 8: 248–257.e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moberg C, Alderling M, Meding B. Hand eczema and quality of life: a population-based study. Br J Dermatol 2009; 161: 397–403. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh BJ, Shen D, Hsu CJ, Chan TC, Cho YT, Tang CH, et al. The impact of atopic dermatitis on health-related quality of life in Taiwan. J Formos Med Assoc 2021; 121: 269–277. [DOI] [PubMed] [Google Scholar]
- 39.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33. [DOI] [PubMed] [Google Scholar]
- 40.Ghio D, Muller I, Greenwell K, Roberts A, McNiven A, Langan SM, et al. ‘It’s like the bad guy in a movie who just doesn’t die’: a qualitative exploration of young people’s adaptation to eczema and implications for self-care. Br J Dermatol 2020; 182: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wändell P, Carlsson AC, Wettermark B, Lord G, Cars T, Ljunggren G. Most common diseases diagnosed in primary care in Stockholm, Sweden, in 2011. Fam Pract 2013; 30: 506–513. [DOI] [PubMed] [Google Scholar]


