Abstract
Purpose:
Data comparing moderately hypofractionated intensity-modulated radiation therapy (IMRT) and proton beam therapy (PBT) are lacking. We aim to compare late toxicity profiles of patients with early stage prostate cancer treated with moderately hypofractionated PBT and IMRT.
Materials and Methods:
This multi-institutional analysis included patients with low- or intermediate-risk biopsy-proven prostate adenocarcinoma from 7 tertiary referral centers treated from 1998 to 2018. All patients were treated with moderately hypofractionated radiation, defined as 250 – 300 cGy per daily fraction given over 4–6 weeks, and stratified by use of IMRT or PBT. Primary outcomes were late genitourinary (GU) and gastrointestinal (GI) toxicity. Adjusted toxicity rates were calculated using inverse probability of treatment weighting, accounting for race, NCCN risk group, age, pretreatment IPSS (GU only) and anti-coagulant use (GI only).
Results:
A total of 1850 patients were included, 1282 IMRT (median follow-up 80.0 months) and 568 PBT (median follow-up 43.9 months). Overall toxicity rates were low, with the majority of patients experiencing no late GU (56.6%, n=1048) or late GI (74.4%, n=1377) toxicity. No difference was seen in the rates of late toxicity between the groups, with late Grade 3+ GU toxicity of 2.0% vs 3.9%, OR 0.47 (0.17–1.28) and late Grade 2+ GI toxicity of 14.6% vs 4.7%, OR 2.69 (0.80–9.05) for the PBT and IMRT cohorts respectively. On multivariable analysis, no factors were significantly predictive of GU toxicity and only anti-coagulant use was significantly predictive of GI toxicity (OR 1.90, p=0.008).
Conclusions:
In this large, multi-institutional analysis of 1850 early-stage prostate cancer patients, treatment with moderately hypofractionated intensity-modulated radiation therapy (IMRT) and proton beam therapy (PBT) resulted in low rates of toxicity. No difference was seen in late GI and GU toxicity between the modalities over long term follow up. Both treatments are safe and well-tolerated.
Introduction
Moderately hypofractionated radiation therapy for low- and intermediate-risk prostate cancer has now been established by multiple randomized trials1–6 as an appropriate alternative to conventionally fractionated regimens. Given the pragmatic and economic advantages of a shorter radiation treatment schedule7, as well as the potential radiobiologic advantages8 of higher doses per fraction, moderate hypofractionation is being increasingly adopted9 in the U.S. as a preferred choice and the new standard for low- and intermediate-risk prostate cancer10. The clinical trials establishing the safety and efficacy of moderate hypofractionation for prostate cancer have typically employed photon-based radiation. Proton beam therapy (PBT) is an established radiation modality for prostate cancer patients11–14 and has been demonstrated to offer potential dosimetric advantages15,16 that in turn may permit dose escalation and/or hypofractionation while maintaining, if not reducing the incidence and/or severity of side effects relative to photon therapy17.
There is a growing body of literature documenting the toxicity outcomes with moderately hypofractionated regimens using photon intensity-modulated radiation therapy (IMRT)18–20, and to a lesser degree using PBT14,21. However, data directly comparing modalities for moderate hypofractionation are lacking. This multi-institutional collaborative study aims to characterize and compare late toxicity profiles between moderately hypofractionated IMRT and PBT using a propensity-score-weighted analysis. We hypothesize that a difference in late GI and GU toxicities will be seen between the PBT and IMRT groups.
Material and Methods
Patient Population
Prospectively-collected institutional databases from seven tertiary referral centers in the US were queried for patients with biopsy-proven prostate adenocarcinoma treated from 1/1/1998 to 12/31/2018. All patients had clinical documentation of low- or intermediate-risk prostate cancer per National Comprehensive Cancer Network (NCCN) risk grouping22 and were treated with definitive moderately fractionated radiation using either IMRT or PBT. Data sharing agreements were reviewed and approved by each institution’s internal review board, allowing contribution of data to the coordinating sites. De-identified data were shared in concordance with the Health Insurance Portability and Accountability Act and managed using a secure electronic data capture tool23,24. Given the retrospective nature of the study, requirement for informed consent was waived by the institutions.
Radiotherapy Treatment
All patients underwent definitive radiation to an intact prostate gland using moderate hypofractionation, defined as 250 – 300 cGy per daily fraction given over 4–6 weeks. Patients were grouped based on radiation treatment modality, either PBT or IMRT. Static IMRT and rotational arc therapy/volumetric modulated arc therapy were both included in the IMRT group. Uniform scanning and pencil beam scanning were both included in the PBT group. Modern standard of care treatment planning techniques, motion management, and CT simulation were used, and all patients underwent daily three-dimensional image guidance. For specific treatment and planning details for each institution please refer to previously published experiences2,11,14,25. Use of androgen deprivation therapy (ADT) was allowed. All patients had a minimum of 1 year follow up.
Outcomes
Primary outcomes were late Grade 3+ Genitourinary (GU) and late Grade 2+ Gastrointestinal (GI) toxicity, per Common Terminology Criteria for Adverse Events version 4.0, scored by treating institution. Patients treated in the time period before CTCAE v4.0 were retrospectively reassigned the toxicity grade. Late toxicity was defined as occurring >3 months after radiation treatment completion. Covariates of interest included age, race, NCCN risk group, tumor stage, Gleason Score, initial PSA, smoking status, alcohol use, Eastern Oncology Cooperate Group (ECOG) performance status, body mass index (BMI), diabetes, peripheral vascular disease, connective tissue disease, prior trans-urethral resection of the prostate (TURP), use of ADT, baseline International Prostate Symptom Score (IPSS), and anti-coagulant use, which included concurrent treatment with aspirin, warfarin, heparin, anti-platelet agents, or other clotting factor inhibitors.
Statistical Analysis
Unadjusted odds ratios and 95% confidence intervals were calculated using mixed-effects logistic regression models. Adjusted toxicity rates were calculated using propensity score-based inverse probability of treatment weighting, accounting for race, NCCN risk group, age, pretreatment IPSS (GU only) and anti-coagulant use (GI only)26. Covariates used in the propensity score models were pre-specified based on their clinical relevance for development of the toxicities being evaluated. Propensity scores were estimated via logistic regression, checked for overlap and outliers, and for covariate balance after weighting. Adjusted toxicity rates were calculated using weighted frequency tables, and weighted mixed-effects logistic regression models with random effects by site were used to estimate odds ratios (PS-WT). We also performed (unweighted) multivariable mixed-effects logistic regression models (MVA), controlling for the covariates described above, with random effects by site. Missing data was handled both by analyzing only those with complete data (PS-WT) and using multiple imputation via chained equations in conjunction with the MVA models27. For the imputed analyses, we calculated pooled results over 10 imputations, with variance estimated via Rubin’s rule.
Results
Patient and Treatment Characteristics
A total of 1850 patients were included, with 1282 in the IMRT cohort and 568 in the PBT cohort. Of the 7 participating institutions, 3 institutions contributed patients only to the IMRT cohort, 3 institutions contributed patients only to the PBT cohort, and 1 contributed to both the IMRT and PBT cohort. All patients were treated in 250 to 300 cGy daily fractions to a total dose of 6000 to 7250 cGy. Specific dose fractionation schemes are listed in Table 1. Median follow up was 80.0 months in the IMRT cohort and 43.9 months in the PBT cohort. Both of the cohorts were similar with respect to age (median 68 vs 67 years), BMI (median 28.3 vs 27.3), NCCN risk group (70.6% vs 72.9% intermediate risk), and tumor stage (64.4% vs 68.5% T1c). However, the IMRT group had significantly higher baseline IPSS (mean 10.9 vs 7.7, p<0.001), more anti-coagulant use (30.7% vs 11.6%, p<0.001), higher baseline PSA (6.7 vs 5.6 ng/mL, p<0.001), and more patients with Gleason 6 disease (43.1% vs 34.0%, p<0.001). More patients were treated with androgen deprivation therapy (ADT) in the IMRT cohort (38.8% vs 8.3%, p<0.001). A small minority of patients in the PBT cohort were treated with rectal balloon (n=82, 14.4% of the PBT cohort, 4.4% of the total population) or hydrogel spacer (n=21, 3.7% of the PBT cohort, 1.1% of the total population) in place. No patients in the IMRT arm had known rectal balloon or hydrogel spacer use. A full listing of patient and treatment characteristics is detailed in Table 1.
Table 1:
Patient and Treatment Characteristics
| Characteristic | IMRT (N=1282) | PBT (N=568) | Total (N=1850) | P Value | |
|---|---|---|---|---|---|
|
| |||||
| Age | Median (Range) | 68 (43–89) | 67 (41–88) | 67 (41–89) | 0.002 |
| Treatment Year | 1998–2018 | 2008–2018 | 1998–2018 | <0.001 | |
| BMI | Median (Range) | 28.3 (16.2–56.6) | 27.3 (18.3–65.0) | 28.0 (16.2–65.0) | 0.11 |
| NCCN Risk Group | Low Risk | 377 (29.4%) | 154 (27.1%) | 531 (28.7%) | 0.31 |
| Intermediate Risk | 905 (70.6%) | 414 (72.9%) | 1319 (71.3%) | ||
| Tumor Stage | T1c | 825 (64.4%) | 389 (68.5%) | 1214 (65.6%) | 0.21 |
| T2 | 444 (34.6%) | 178 (31.3%) | 622 (33.6%) | ||
| Unknown | 13 (1.0%) | 1 (0.2%) | 14 (0.8%) | ||
| Baseline PSA | Median | 6.7 | 5.6 | 6.3 | <0.001 |
| <10 ng/mL | 941 (74.4%) | 490 (87.0%) | 1431 (78.3%) | ||
| 10–20 ng/mL | 324 (25.6%) | 73 (13.0%) | 397 (21.7%) | ||
| Gleason Score | 6 or less | 552 (43.1%) | 193 (34.0%) | 745 (40.3%) | <0.001 |
| 7 | 730 (56.9%) | 375 (66.0%) | 1105 (59.7%) | ||
| Anti-Coagulant | Yes | 394 (30.7%) | 66 (11.6%) | 460 (24.9%) | <0.001 |
| No | 814 (63.5%) | 366 (64.4%) | 1180 (63.8%) | ||
| Unknown | 74 (5.8%) | 136 (23.9%) | 210 (11.4%) | ||
| Baseline IPSS | mean (SD) | 10.9 (6.8) | 7.7 (5.4) | 9.2 (6.3) | <0.001 |
| Race | Caucasian | 888 (69.3%) | 488 (85.9%) | 1376 (74.4%) | <0.001 |
| African American | 366 (28.5%) | 47 (8.3%) | 413 (22.3%) | ||
| Other/Unknown | 28 (2.2%) | 33 (5.8%) | 61 (3.3%) | ||
| Smoking Status | Current Smoker | 105 (8.2%) | 22 (3.9%) | 127 (6.9%) | <0.001 |
| Former Smoker | 450 (35.1%) | 89 (15.7%) | 539 (29.1%) | ||
| Never Smoker | 399 (31.1%) | 85 (15.0%) | 484 (26.2%) | ||
| Unknown | 328 (25.6%) | 372 (65.5%) | 700 (37.8%) | ||
| Alcohol Use | Current Use | 71 (5.5%) | 17 (3.0%) | 88 (4.8%) | <0.001 |
| Former Use | 371 (28.9%) | 125 (22.0%) | 496 (26.8%) | ||
| No Use | 429 (33.5%) | 48 (8.5%) | 477 (25.8%) | ||
| Unknown | 411 (32.1%) | 378 (66.5%) | 789 (42.6%) | ||
| Cardiac Disease | Yes | 115 (9.0%) | 48 (8.5%) | 163 (8.8%) | 0.29 |
| No | 306 (23.9%) | 155 (27.3%) | 461 (24.9%) | ||
| Unknown | 861 (67.2%) | 365 (64.3%) | 1226 (66.3%) | ||
| Diabetes | Yes | 194 (15.1%) | 32 (5.6%) | 226 (12.2%) | <0.001 |
| No | 780 (60.8%) | 308 (54.2%) | 1088 (58.8%) | ||
| Unknown | 308 (24.0%) | 228 (40.1%) | 536 (29.0%) | ||
| ADT Use | Yes | 497 (38.8%) | 47 (8.3%) | 544 (29.4%) | <0.001 |
| No | 537 (41.9%) | 343 (60.4%) | 880 (47.6%) | ||
| Unknown | 248 (19.3%) | 178 (31.3%) | 426 (23.0%) | ||
| RT Fractionation | 70 Gy in 28 fx | 905 (70.6%) | 410 (72.2%) | 1315 (71.1%) | <0.001 |
| 70.2 Gy in 26 fx | 280 (21.8%) | 49 (8.6%) | 329 (17.8%) | ||
| 60 Gy in 20 fx | 97 (7.6%) | 35 (6.1%) | 132 (7.1%) | ||
| 72.5 Gy in 29 fx | 0 (0.0%) | 74 (13.0%) | 74 (4%) | ||
| Rectal Spacer | Yes | 0 (0.0%) | 21 (3.7%) | 21 (1.1%) | 0.051 |
| No | 100 (100.0%) | 547 (96.3%) | 1829 (98.9%) | ||
| Rectal Balloon | Yes | 0 (0.0%) | 82 (14.4%) | 82 (4.4%) | <0.001 |
| No | 100 (100.0%) | 486 (85.6%) | 1768 (95.6%) | ||
| Institutions | 1 | 0 (0.0%) | 184 (32.4%) | 184 (9.9%) | N/A |
| 2 | 0 (0.0%) | 215 (37.9%) | 215 (11.6%) | ||
| 3 | 0 (0.0%) | 81 (14.3%) | 81 (4.4%) | ||
| 4 | 248 (19.3%) | 88 (15.5%) | 336 (18.2%) | ||
| 5 | 610 (47.6%) | 0 (0.0%) | 610 (33.0%) | ||
| 6 | 285 (22.2%) | 0 (0.0%) | 285 (15.4%) | ||
| 7 | 139 (10.8%) | 0 (0.0%) | 139 (7.5%) | ||
Acute Toxicity
A subset of the total patient population had available data regarding acute toxicity (901 total patients, 298 of the PBT cohort and 612 of the IMRT cohort). Of these patients, acute toxicity rates were low with an overall acute grade 3+ GU toxicity rate of 1.8% (n=18) and acute grade 2+ GI toxicity rate of 4.2% (n=38). Acute Grade 3+ GU toxicity rates were 2.7% in the IMRT group compared to 0% in the PBT group (p=0.002) and Acute Grade 2+ GI toxicity rates were 4.4% in the IMRT group compared to 3.8% in the PBT group (p=0.67).
Late GU Toxicity
The majority (56.6%, n=1048) of the total population experienced no late GU toxicity. The overall cumulative rate of late Grade 2+ GU toxicity was 18.5% (342 patients), with rates of 15.0% and 20.1%, OR 0.79 (0.29–2.18) for the PBT and IMRT cohorts respectively. Due to substantial variability in Grade 2+ GU reporting across institutions, we did not conduct further analysis of late grade 2+ GU toxicity as treatment versus institutional effects could not be easily differentiated. The overall cumulative rate of late Grade 3+ GU toxicity was 3.0% (56 patients), with rates of 1.6% and 3.7%, OR 0.45 (0.16–1.28) for the PBT and IMRT cohorts respectively. After adjustment for patient and treatment factors, there was no significant difference in late Grade 3+ GU toxicity between the PBT and IMRT cohorts both when analyzed using MVA with multiple imputation, OR 0.55 (0.15–1.99), or when analyzing only those with complete data, 2.0% vs 3.9%, OR 0.48 (0.18–1.28) (PS-WT).
Rates of higher grade GU toxicity were extremely low. In the IMRT group, one patient (0.08%) experienced a late Grade 5 GU toxicity (urosepsis, occurring at 183 months from treatment) and 4 patients (0.3%) experienced a late Grade 4 GU toxicity (cystitis/hematuria requiring urgent intervention, occurring at 20, 45, 81, and 133 months from treatment). No patients in the PBT group experienced late Grade 4 or 5 GU toxicity. The most common toxicity was urinary frequency in the PBT group and cystitis in the IMRT group. The distribution of graded toxicity by treatment modality is detailed in Figure 1, and specific toxicity rates are outlined in Table 2. On multivariable analysis (Table 3) no factors were significantly predictive of GU toxicity.
Figure 1: Maximum Graded Late Toxicity.
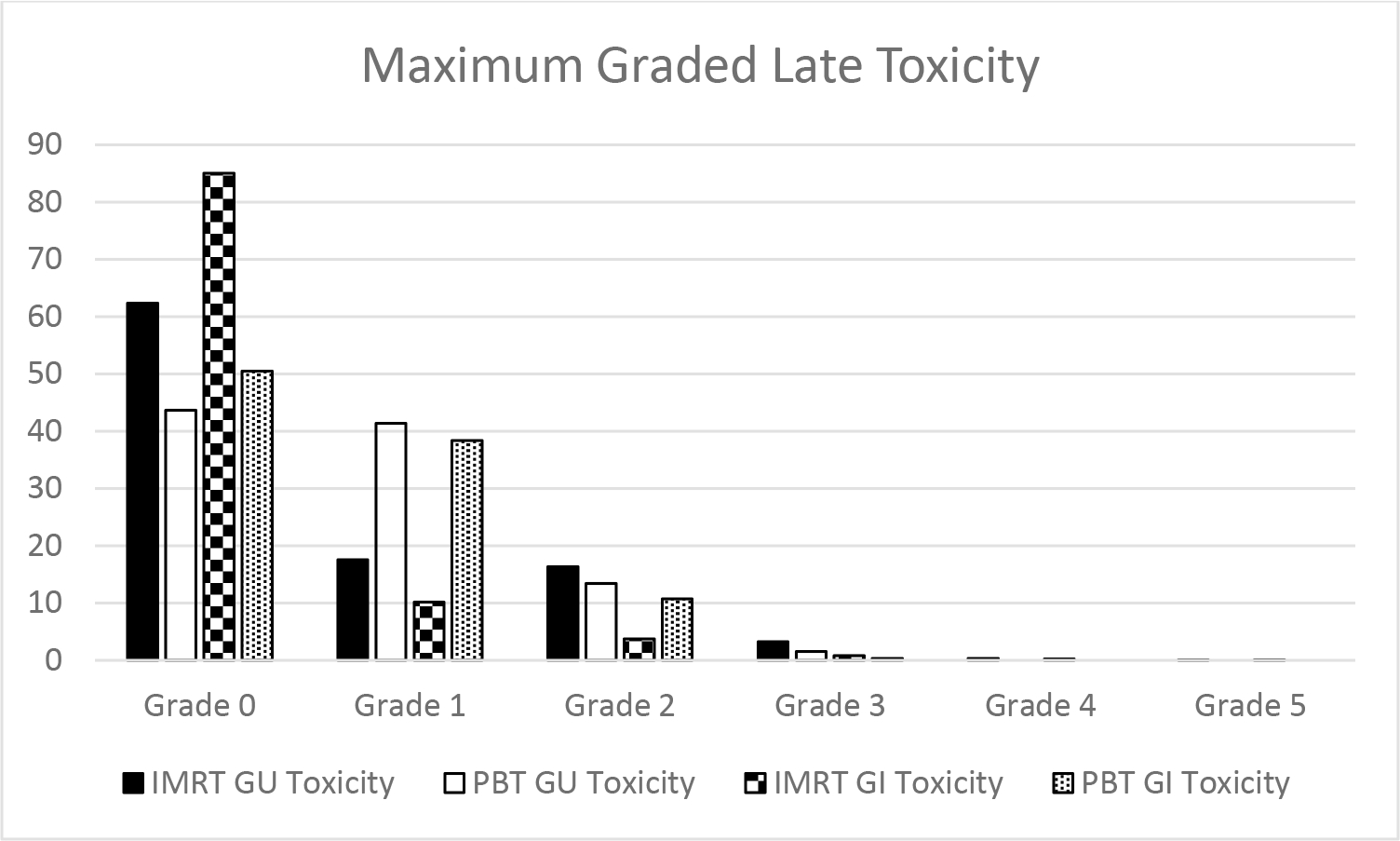
Maximum graded late GU and GI toxicity by treatment type for the IMRT and PBT cohorts
Table 2:
Toxicity Details and Differences between Treatment Cohorts
| IMRT (N=1282) | PBT (N=568) | Total (N=1850) | P Value | |
|---|---|---|---|---|
|
| ||||
| Late GU Toxicity (any grade) | ||||
| Frequency | 267 (20.8%) | 200 (35.2%) | 467 (25.2%) | <0.001 |
| Urgency | 256 (20.0%) | 187 (32.9%) | 443 (23.9%) | <0.001 |
| Obstruction | 264 (20.6%) | 103 (18.1%) | 367 (19.8%) | 0.221 |
| Dysuria | 252 (19.7%) | 141 (24.8%) | 393 (21.2%) | 0.012 |
| Stenosis | 212 (16.5%) | 87 (15.3%) | 299 (16.2%) | 0.511 |
| Urinary Incontinence | 258 (20.1%) | 126 (22.2%) | 384 (20.8%) | 0.314 |
| Cystitis | 249 (19.4%) | 97 (17.1%) | 346 (18.7%) | 0.233 |
|
| ||||
| Late GI Toxicity (any grade) | ||||
| Diarrhea | 253 (19.7%) | 104 (18.3%) | 357 (19.3%) | 0.474 |
| Proctitis | 265 (20.7%) | 132 (23.2%) | 397 (21.5%) | 0.214 |
| Rectal Bleeding | 295 (23.0%) | 177 (31.2%) | 472 (25.5%) | <0.001 |
| Bowel Incontinence | 247 (19.3%) | 96 (16.9%) | 343 (18.5%) | 0.227 |
Table 3:
Multivariable Analysis of Late GU and GI Toxicity
| Risk of Late GU Toxicity (3+) | Risk of Late GI Toxicity (2+) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | 95% CI | P value | OR | 95% CI | P value | |||
|
| ||||||||
| Treatment Type (vs IMRT) | ||||||||
| PBT | 0.55 | 0.15 | 1.99 | 0.55 | 2.68 | 0.80 | 8.98 | 0.11 |
| Race – (vs African American) | ||||||||
| Caucasian | 0.96 | 0.36 | 2.61 | 0.94 | 1.07 | 0.62 | 1.83 | 0.82 |
| Other/Unknown | 1.71 | 0.34 | 8.73 | 0.52 | 1.50 | 0.51 | 4.43 | 0.47 |
| NCCN Risk Group (vs Intermediate) | ||||||||
| Low | 0.66 | 0.24 | 1.80 | 0.41 | 0.81 | 0.51 | 1.27 | 0.35 |
| Age at Diagnosis (per year) | 0.95 | 0.91 | 1.01 | 0.09 | 1.02 | 0.99 | 1.05 | 0.22 |
| Baseline IPSS (per point) | 1.04 | 0.98 | 1.10 | 0.23 | - | - | - | - |
| Anti-Coagulant Use (vs none) | - | - | - | - | 1.90 | 1.19 | 3.04 | 0.008 |
Late GI Toxicity
The majority (74.4%, n=1377) of patients in the total population experienced no late GI toxicity. The overall cumulative rate of late Grade 2+ GI toxicity was 6.8% (125 patients). This unadjusted rate was higher in the PBT arm, with late Grade 2+ GI toxicity rates of 11.1% versus 4.8%, OR 2.71 (1.17–6.26) in the PBT and IMRT cohorts, respectively. However, after adjustment for patient and treatment factors, there was no significant difference in late Grade 2+ GI toxicity between the PBT and IMRT cohorts both when analyzed using MVA with multiple imputation, OR 2.68 (0.80–8.99), or when analyzing only those with complete data, 13.6% vs 4.7%, OR 2.40 (0.73–7.84) (PS-WT). The overall cumulative rate of late Grade 3+ GI toxicity was 0.9% (16 patients), with rates of 0.4% and 1.1%, 0.32 OR (0.07–1.49) for the PBT and IMRT cohorts respectively. Due to low event numbers, we could not conduct adjusted analysis for late Grade 3+ GI toxicity.
Rates of higher grade GI toxicity were extremely low. In the IMRT group, one patient (0.08%) experienced a late Grade 5 GI toxicity (sepsis secondary to proctitis and recto-vesicular fistula, occurring at 20 months from treatment) and 3 patients (0.23%) experienced a late Grade 4 GI toxicity (rectal bleeding, occurring at 6, 9, and 22 months from treatment). No patients in the PBT group experienced late Grade 4 or 5 GI toxicity. The most common toxicity was rectal bleeding in both cohorts. The distribution of graded toxicity by treatment modality is detailed in Figure 1 and specific toxicity rates are outlined in Table 2.
On multivariable analysis (see Table 3), only anti-coagulant use was significantly predictive of GI toxicity (OR 1.90, 95% CI 1.19–3.04, p=0.008). Among the 125 patients who experienced a late Grade 2+ GI toxicity, 34 patients (27.2%) were on anti-coagulant medication, 73 patients (58.4%) were not, and for 18 patients (14.4%) anticoagulation status was unknown.
As no statistically significant difference was seen in rates of late GI and GU toxicity, we failed to reject our hypothesis that toxicity rates between the PBT and IMRT group would differ.
Discussion
In this large, multi-institutional dataset analysis, rates of late GU and GI toxicity after moderately hypofractionated radiation with either PBT or IMRT were low. No randomized data currently exist directly comparing the late toxicity of these moderately hypofractionated treatment modalities. Trials such as COMPPARE (NCT03561220)28 and PAROS (NCT04083937)29 will attempt to answer this question via pragmatic and randomized study designs in intact and post-prostatectomy settings, respectively. Until these results are available, the strongest evidence can be found through pooled analyses of prospectively collected institutional databases. To our knowledge, the current study is the largest to pool results from a number of tertiary referral centers treating prostate cancer with moderately hypofractionated IMRT and PBT.
Our rates of late GI and GU toxicity are consistent with other published toxicity results of moderately fractionated IMRT and PBT. In the randomized controlled trials investigating moderately hypofractionated IMRT, including PROFIT6, RTOG 04152, the Fox Chase Hypofractionation Trial30, and CHHiP4, the range of late Grade 3+ GU toxicity rates were 1.5–6.0% and late Grade 3+GI toxicity rates were 2.0–4.1%. Our rates of late Grade 3+ GU toxicity at 3.7% and late Grade 3+ GI of 1.1% for the IMRT group compare favorably with these previously published results. The toxicity rates for our PBT arm also compare favorably with the literature, where rates of late Grade 3+ GU toxicity ranged from 1.0–5.4% and late Grade 3+ GI of 0–1.0% in single-institution trials of moderately hypofractionated PBT11,13,14. Other comparative series of proton and photon treatment, typically with standard fractionation, report similar patterns of toxicity. For example, a study from MD Anderson showed that among prostate cancer patients younger than 65, composite GU toxicity was lower and composite GI toxicity was higher at 2 years with PBT as compared with IMRT31.
Although both Grade 2+ and Grade 3+ late GU and GI toxicity rates were reported, only late Grade 3+ GU and late Grade 2+ GI rates were adjusted for patient and treatment factors due to size of effect and consistency among institutions and their greater clinical importance. For GU toxicity, late Grade 2+ toxicity includes the use of Alpha-1 blocking agents including tamsulosin. There can be a great deal of inconsistency in the prescribing patterns of these medications during and following treatment, and subsequently we found a wide variability in the rates of Grade 2+ GU toxicity among the institutions included in this study. Late Grade 3+ GU toxicity is thought to be a more clinically relevant measure and thus underwent further analysis. Late Grade 3+ GI toxicity rates were too low to have a statistically relevant adjustment analysis.
No significant difference was seen in late Grade 2+ or Grade 3+ GU and late Grade 3+ GI toxicity rates between the IMRT and PBT groups when looking at both unadjusted rates as well as after adjustment for covariates. Although higher rates of late Grade 2+ GI toxicity were found with PBT, this difference lost statistical significance when adjusted for covariates such as anti-coagulant use. As not all patients had information on anti-coagulant use, this loss of significance is likely due to the reduced power after adjusting for covariates and not a decrease in the effect size. To account for this difference, the adjusted analysis was done via two methods, one with multiple imputation and the other adjustment analysis including only patients with complete data. Results were similar with both analyses. The unadjusted OR was 2.71 (1.17–6.26) as compared to adjusted OR of 2.69 (0.80–9.05) with multiple imputation and adjusted OR of 2.92 (0.82–10.44) for complete data.
The most significant factor predictive of GI toxicity was the use of anti-coagulant medication. Of the 125 patients who experienced a late Grade 2+ GI toxicity, 34 (27.2%) had known anti-coagulant use. This is consistent with other published reports. The only two Grade 3+ GI events on the Mendenhall et al. PR-02 study13 were in patients with concurrent anti-coagulant use. Caution should be exercised when considering hypofractionation for patients requiring concurrent anti-coagulant use as they may be at higher bleeding risk.
Overall rates of high-grade toxicities were exceedingly low, with Grade 5 toxicity of 0.01% (n=2) and Grade 4 toxicity rate of 0.38% (n=7). All incidences of Grade 4 or 5 toxicity occurred following IMRT treatment delivered prior to the year 2006. It should be noted that the IMRT cohort is over twice size of the PBT cohort and none of the PBT patients were treated prior to 2008. Technological advancement may likely play a role in the absence of high grade toxicity in patient treated after 2006. The patient who experienced grade 5 GI toxicity developed radiation proctitis leading to a recto-vesicular fistula and sepsis which was the cause of death two years after the completion of treatment. The patient who experienced grade 5 GU toxicity had a periurethral abscess with pubic bone osteomyelitis. He died of sepsis 15 years after treatment with radiation after multiple admissions for UTI and urosepsis. Although it is not clear that these infections were directly attributable to radiation therapy, they were coded as such in order to be as thorough as possible. All of the late grade 4 toxicities required urgent interventions for bleeding (cystitis/hematuria or rectal bleeding).
Our study has significant strengths, including the diverse patient population, detailed toxicity reporting, and long duration of follow up. However, certain limitations must be considered, including patient and treatment heterogeneity. Although all patient data were recorded prospectively, the study still represents a collaboration of multiple single-institution experiences. The participating institutions may have small differences in treatment technique and patient population, as well as slight variation in the grading and reporting of toxicity. Comparison between the PBT and IMRT arms may also reflect some selection bias related to the choice of modality by the patient and/or providers. As only one institution treated patients with both modalities, it may be hard to separate treatment effects from differences among institutions. Additionally, due to the complexity of the regression models, we could not use robust standard errors with the propensity score weights. Furthermore, the present study only includes physician-scored toxic events, and evidence exists that patient reported quality of life is also a clinically important endpoint32. Additional studies from this collaboration will include further analysis of outcomes as well as dosimetric predictors of outcomes and toxicity.
Conclusions
In this multi-institutional cohort of prospectively-collected data from seven tertiary referral centers, we demonstrated that that PBT and IMRT delivered using a moderately hypofractionated schedule are both well tolerated, with very low rates of late toxicity over long-term follow up. No significant differences were seen with late Grade 2+ or Grade 3+ GU toxicity between modalities. Although PBT did show an increase in unadjusted Grade 2+ GI toxicities, this was no longer significant after adjustment for patient and treatment factors and Grade 3+ GI toxicity was not different between modalities. The only predictor of GI toxicity was use of anti-coagulant medication. Both IMRT and PBT are safe when given with a moderately hypofractionated schedule and represent appropriate definitive treatment options for low- and intermediate-risk prostate cancer.
References
- 1.Avkshtol V, Ruth KJ, Ross EA, et al. Ten-Year Update of a Randomized, Prospective Trial of Conventional Fractionated Versus Moderate Hypofractionated Radiation Therapy for Localized Prostate Cancer. J Clin Oncol 2020: Jco1901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WR, Dignam JJ, Amin MB, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol 2016; 34(20): 2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. The Lancet Oncology 2016; 17(8): 1061–9. [DOI] [PubMed] [Google Scholar]
- 4.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. The Lancet Oncology 2016; 17(8): 1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman KE, Voong KR, Levy LB, et al. Randomized Trial of Hypofractionated, Dose-Escalated, Intensity-Modulated Radiation Therapy (IMRT) Versus Conventionally Fractionated IMRT for Localized Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(29): 2943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catton CN, Lukka H, Gu CS, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol 2017; 35(17): 1884–90. [DOI] [PubMed] [Google Scholar]
- 7.Voong KR, Lal LS, Kuban DA, et al. Long-term economic value of hypofractionated prostate radiation: Secondary analysis of a randomized trial. Advances in Radiation Oncology 2017; 2(3): 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82(1): e17–24. [DOI] [PubMed] [Google Scholar]
- 9.Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: A National Cancer Database analysis. Pract Radiat Oncol 2017; 7(4): 270–8. [DOI] [PubMed] [Google Scholar]
- 10.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: An ASTRO, ASCO, and AUA Evidence-Based Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018: JCO1801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson RH, Bryant C, Hoppe BS, et al. Five-year outcomes from a prospective trial of image-guided accelerated hypofractionated proton therapy for prostate cancer. Acta Oncol 2017; 56(7): 963–70. [DOI] [PubMed] [Google Scholar]
- 12.Vargas CE, Hartsell WF, Dunn M, et al. Hypofractionated Versus Standard Fractionated Proton-beam Therapy for Low-risk Prostate Cancer. American Journal of Clinical Oncology 2015. [DOI] [PubMed] [Google Scholar]
- 13.Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 88(3): 596–602. [DOI] [PubMed] [Google Scholar]
- 14.Grewal AS, Schonewolf C, Min EJ, et al. Four-Year Outcomes From a Prospective Phase II Clinical Trial of Moderately Hypofractionated Proton Therapy for Localized Prostate Cancer. Int J Radiat Oncol Biol Phys 2019; 105(4): 713–22. [DOI] [PubMed] [Google Scholar]
- 15.Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008; 70(3): 744–51. [DOI] [PubMed] [Google Scholar]
- 16.Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys 2007; 69(2): 444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouw KW, Trofimov A, Zietman AL, Efstathiou JA. Clinical controversies: proton therapy for prostate cancer. Seminars in radiation oncology 2013; 23(2): 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman KE, Voong KR, Pugh TJ, et al. Risk of Late Toxicity in Men Receiving Dose-Escalated Hypofractionated Intensity Modulated Prostate Radiation Therapy: Results From a Randomized Trial. International Journal of Radiation Oncology*Biology*Physics 2014; 88(5): 1074–84. [DOI] [PubMed] [Google Scholar]
- 19.Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. The Lancet Oncology 2015; 16(3): 274–83. [DOI] [PubMed] [Google Scholar]
- 20.Shaikh T, Li T, Handorf EA, et al. Long-Term Patient-Reported Outcomes From a Phase 3 Randomized Prospective Trial of Conventional Versus Hypofractionated Radiation Therapy for Localized Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics 2017; 97(4): 722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vargas CE, Hartsell WF, Dunn M, et al. Image-guided hypofractionated proton beam therapy for low-risk prostate cancer: Analysis of quality of life and toxicity, PCG GU 002. Reports of Practical Oncology & Radiotherapy 2016; 21(3): 207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw 2018; 16(5s): 620–3. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009; 42(2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys 2006; 64(2): 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46(3): 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software; Vol 1, Issue 3 (2011) 2011. [Google Scholar]
- 28.A Prospective Comparative Study of Outcomes With Proton and Photon Radiation in Prostate Cancer (COMPPARE). https://clinicaltrials.gov/ct2/show/NCT03561220?term=hypofractionated+proton&cond=Prostate+Cancer&draw=2&rank=9.
- 29.Prostate Cancer Patients Treated With Alternative Radiation Oncology Strategies (PAROS). https://clinicaltrials.gov/ct2/show/NCT04083937?term=hypofractionated+proton&cond=Prostate+Cancer&draw=2&rank=10.
- 30.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013; 31(31): 3860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan HY, Jiang J, Hoffman KE, et al. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(18): 1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008; 358(12): 1250–61. [DOI] [PubMed] [Google Scholar]


