Abstract
Antibody responses to SARS-CoV-2 vaccines vary for reasons that remain poorly understood. A range of sociodemographic, behavioural, clinical, pharmacologic and nutritional factors could explain these differences. To investigate this hypothesis, we tested for presence of combined IgG, IgA and IgM (IgGAM) anti-Spike antibodies before and after 2 doses of ChAdOx1 nCoV-19 (ChAdOx1, AstraZeneca) or BNT162b2 (Pfizer-BioNTech) in UK adults participating in a population-based longitudinal study who received their first dose of vaccine between December 2020 and July 2021. Information on sixty-six potential sociodemographic, behavioural, clinical, pharmacologic and nutritional determinants of serological response to vaccination was captured using serial online questionnaires. We used logistic regression to estimate multivariable-adjusted odds ratios (aORs) for associations between independent variables and risk of seronegativity following two vaccine doses. Additionally, percentage differences in antibody titres between groups were estimated in the sub-set of participants who were seropositive post-vaccination using linear regression. Anti-spike antibodies were undetectable in 378/9101 (4.2%) participants at a median of 8.6 weeks post second vaccine dose. Increased risk of post-vaccination seronegativity associated with administration of ChAdOx1 vs. BNT162b2 (adjusted odds ratio (aOR) 6.6, 95% CI 4.2–10.4), shorter interval between vaccine doses (aOR 1.6, 1.2–2.1, 6–10 vs. >10 weeks), poor vs. excellent general health (aOR 3.1, 1.4–7.0), immunodeficiency (aOR 6.5, 2.5–16.6) and immunosuppressant use (aOR 3.7, 2.4–5.7). Odds of seronegativity were lower for participants who were SARS-CoV-2 seropositive pre-vaccination (aOR 0.2, 0.0–0.6) and for those taking vitamin D supplements (aOR 0.7, 0.5–0.9). Serologic responses to vaccination did not associate with time of day of vaccine administration, lifestyle factors including tobacco smoking, alcohol intake and sleep, or use of anti-pyretics for management of reactive symptoms after vaccination. In a sub-set of 8727 individuals who were seropositive post-vaccination, lower antibody titres associated with administration of ChAdOx1 vs. BNT162b2 (43.4% lower, 41.8–44.8), longer duration between second vaccine dose and sampling (12.7% lower, 8.2–16.9, for 9–16 weeks vs. 2–4 weeks), shorter interval between vaccine doses (10.4% lower, 3.7–16.7, for <6 weeks vs. >10 weeks), receiving a second vaccine dose in October–December vs. April–June (47.7% lower, 11.4–69.1), older age (3.3% lower per 10-year increase in age, 2.1–4.6), and hypertension (4.1% lower, 1.1–6.9). Higher antibody titres associated with South Asian ethnicity (16.2% higher, 3.0–31.1, vs. White ethnicity) or Mixed/Multiple/Other ethnicity (11.8% higher, 2.9–21.6, vs. White ethnicity), higher body mass index (BMI; 2.9% higher, 0.2–5.7, for BMI 25–30 vs. <25 kg/m2) and pre-vaccination seropositivity for SARS-CoV-2 (105.1% higher, 94.1–116.6, for those seropositive and experienced COVID-19 symptoms vs. those who were seronegative pre-vaccination). In conclusion, we identify multiple determinants of antibody responses to SARS-CoV-2 vaccines, many of which are modifiable.
Keywords: SARS-CoV-2 vaccines, antibody responses, serology, immunology, epidemiology
1. Introduction
Vaccination against SARS-CoV-2 represents a key tool for COVID-19 control. However, existing vaccines offer imperfect protection against disease, reflecting heterogeneity in immunogenicity [1,2]. Improved understanding of factors influencing these responses to SARS-CoV-2 vaccines could lead to discovery of effect-modifiers that could be harnessed to augment vaccine immunogenicity, and identify groups of poor responders who might benefit from more intensive vaccine dosing regimens or implementation of other protective measures [3].
Existing studies investigating determinants of vaccine immunogenicity have reported that lower antibody responses following SARS-CoV-2 vaccination associate with administration of viral vector vs. messenger RNA (mRNA) vaccines, older age, poorer general health, immunosuppression and shorter inter-dose intervals [4,5,6,7,8,9,10,11]. Higher post-vaccination antibody titres are seen in those with evidence of SARS-CoV-2 infection before vaccination [9,12,13]. However, these studies are limited in several respects: many are conducted in specific populations such as health care workers [5,9,12,13] or in individuals with a particular demographic or clinical characteristic that may influence vaccine immunogenicity [6,10,11], which may constrain generalisability of their results. Where population-based studies have been done [4,7,8], these did not compare the effect of two doses of viral vector vs. mRNA vaccines on host response; neither did they systematically ascertain participants’ pre-vaccination SARS-CoV-2 serostatus, which is an important potential confounder of associations reported. Moreover, these studies did not investigate effects of modifiable factors that have been posited to influence responses to vaccination, such as time of day of inoculation [14], nutrition [15], sleep [16], alcohol use [17], tobacco smoking [18] and peri-vaccination use of anti-pyretic analgesics [19].
We sought to address these limitations by investigating a comprehensive range of potential sociodemographic, behavioural, clinical, pharmacologic and nutritional determinants of antibody responses in a population-based cohort of UK adults (COVIDENCE UK) [20] following administration of two doses of either BNT162b2 (Pfizer-BioNTech) or ChAdOx1 nCoV-19 (Oxford-AstraZeneca; hereafter ChAdOx1) SARS-CoV-2 vaccines. We utilised a semi-quantitative assay with proven sensitivity for detection of combined IgG, IgA and IgM antibodies to the SARS-CoV-2 spike antigen [21] that has been validated as a correlate of protection against breakthrough COVID-19 in two populations [22,23].
2. Materials and Methods
2.1. Study Design and Participants
COVIDENCE UK is a prospective, longitudinal, population-based observational study of COVID-19 in the UK population (www.qmul.ac.uk/covidence accessed on 20 September 2022) [20]. Inclusion criteria were age ≥ 16 years and UK residence at enrolment, with no exclusion criteria. Participants were invited via a national media campaign to complete an online baseline questionnaire to capture information on potential symptoms of COVID-19 experienced since 1 February 2020; results of any COVID-19 tests; and details of a wide range of potential risk factors for COVID-19 and determinants of vaccine response (Table S1, Online Data Supplement). Online monthly follow-up questionnaires captured incident test-confirmed COVID-19 and vaccination details (Table S2, Online Data Supplement), and a pre-vaccination serology study was conducted to evaluate risk factors for SARS-CoV-2 infection [24]. The study was launched on 1 May 2020, and closed to enrolment on 6 October 2021. Participants in the cohort were invited via email to participate in post-vaccination antibody studies and to give additional consent for these. No incentives were offered to participants. The primary analysis presented here includes data from all participants for whom a serology result was available following administration of two doses of ChAdOx1 or BNT162b2.
COVIDENCE UK was sponsored by Queen Mary University of London and approved by Leicester South Research Ethics Committee (ref 20/EM/0117). It is registered with ClinicalTrials.gov (NCT04330599).
2.2. Statistical Analysis
Full details of statistical analyses, study procedures, outcomes and sample size calculation are provided in the Online Data Supplement. Briefly, logistic regression models were used to estimate adjusted ORs, 95% (CIs) and associated pairwise p-values for potential determinants of post-vaccination seronegativity in all participants who had not reported a SARS-CoV-2 infection prior to their 2nd vaccine dosing date. Linear regression models were used to estimate beta-coefficients, 95% CIs and associated pairwise p-values for potential determinants of log-transformed antibody titres in the subset of seropositive participants. For ease of interpretation, log-transformed estimates of antibody titres were exponentiated and a percentage increase or decrease was calculated for every one-unit increase in the potential determinant. We first estimated ORs and beta-coefficients in minimally adjusted models, and included factors independently associated with each outcome at the 10% significance level in fully adjusted models.
In a sensitivity analysis, we excluded participants from the analysis of antibody titres after two vaccine doses who reported a positive RT-PCR or lateral flow test for SARS-CoV-2 between the date of their second dose of vaccine and the date on which they provided their dried blood spot sample. We also stratified the analysis of antibody titres following two vaccine doses according to the type of vaccine received to explore whether determinants of antibody responses to two vaccine doses were consistent for ChAdOx1 vs. BNT162b2. Given reports that peri-vaccination use of antipyretic analgesics may attenuate vaccine immunogenicity [25], we also conducted an exploratory analysis to determine the influence of taking paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs) to treat post-vaccination symptoms on post-vaccination antibody titres.
3. Results
A total of 15,527 participants were invited to participate in the study of antibody responses following two vaccine doses, of whom 13,005 consented to take part. Serology results were available for 9244 participants, of whom 9101 received two doses of ChAdOx1 (n = 5770) or BNT162b2 (n = 3331) for their primary vaccination course and were included in this analysis (Figure S1, Online Data Supplement). Average participant outcome data completeness was 99.5%. Participant characteristics are presented in Table 1: median age was 64.2 years at enrolment (IQR 57.1, 69.9), 71.1% were female, and 96.5% were of White ethnic origin. Post-vaccination dried blood spot samples were provided from 12 January to 18 September 2021 by participants who received their first dose of vaccine from 15 December 2020 to 10 July 2021 and their second dose from 5 January 2021 to 4 September 2021. Median time from the date of participants’ second vaccine dose to the date on which post-vaccination dried blood spot samples were provided was 8.6 weeks (IQR 6.4–10.7 weeks) and 374 of 9101 participants (4.1%) were seronegative following two doses of vaccine.
Table 1.
Participant characteristics, by vaccine type and overall (n = 9101).
| Characteristic | ChAdOx1 (n = 5770) |
BNT162b2 (n = 3331) |
Overall (n = 9101) |
|
|---|---|---|---|---|
| Age | Median age, years (IQR) | 63.5 (57.0–68.9) | 65.5 (57.4–71.6) | 64.2 (57.1–69.9) |
| Age range, years | 17.4 89.4 | 16.6 90.5 | 16.6 90.5 | |
| Sex, n (%) | Male | 1671 (29.0) | 956 (28.7) | 2627 (28.9) |
| Female | 4099 (71.0) | 2375 (71.3) | 6474 (71.1) | |
| Ethnicity, n (%) 1 | White | 5565 (96.4) | 3222 (96.7) | 8787 (96.5) |
| South Asian | 119 (2.1) | 60 (1.8) | 179 (2) | |
| Black/African/Caribbean/Black British | 62 (1.1) | 38 (1.1) | 100 (1.1) | |
| Mixed/Multiple/Other | 24 (0.4) | 10 (0.3) | 34 (0.4) | |
| Nation of residence 2 | England | 5177 (89.8) | 2906 (87.3) | 8083 (88.8) |
| Northern Ireland | 39 (0.7) | 78 (2.3) | 117 (1.3) | |
| Scotland | 358 (6.2) | 199 (6.0) | 557 (6.1) | |
| Wales | 194 (3.4) | 147 (4.4) | 341 (3.8) | |
| Body mass index, kg/m2, n (%) 3 | <25 | 2845 (49.3) | 1596 (47.9) | 4441 (48.8) |
| 25–30 | 1881 (32.6) | 1097 (32.9) | 2978 (32.7) | |
| >30 | 1034 (17.9) | 636 (19.1) | 1670 (18.3) | |
| Highest educational level attained, n (%) 4 | Primary/Secondary | 636 (11) | 401 (12) | 1037 (11.4) |
| Higher/Further (A levels) | 857 (14.9) | 441 (13.2) | 1298 (14.3) | |
| College | 2540 (44) | 1500 (45) | 4040 (44.4) | |
| Post-graduate | 1736 (30.1) | 984 (29.5) | 2720 (29.9) | |
| Quantiles of IMD rank, n (%) 5 | Q1 (most deprived) | 1172 (20.3) | 786 (23.6) | 1958 (21.5) |
| Q2 | 1417 (24.6) | 782 (23.5) | 2199 (24.2) | |
| Q3 | 1537 (26.6) | 868 (26.1) | 2405 (26.4) | |
| Q4 (least deprived) | 1639 (28.4) | 893 (26.8) | 2532 (27.8) | |
| Tobacco smoking, n (%) | Non-current/never smoker | 5546 (96.1) | 3194 (95.9) | 8740 (96) |
| Current smoker | 224 (3.9) | 137 (4.1) | 361 (4) | |
| Alcohol consumption/week, units, n (%) | None | 1505 (26.1) | 896 (26.9) | 2401 (26.4) |
| 1–7 | 2018 (35) | 1212 (36.4) | 3230 (35.5) | |
| 8–14 | 1186 (20.6) | 695 (20.9) | 1881 (20.7) | |
| 15–21 | 593 (10.3) | 310 (9.3) | 903 (9.9) | |
| 22–28 | 263 (4.6) | 127 (3.8) | 390 (4.3) | |
| >28 | 205 (3.6) | 91 (2.7) | 296 (3.3) | |
| Self-assessed general health | Excellent | 1183 (20.5) | 654 (19.6) | 1837 (20.2) |
| Very good | 2336 (40.5) | 1315 (39.5) | 3651 (40.1) | |
| Good | 1441 (25.0) | 925 (27.8) | 2366 (26.0) | |
| Fair | 637 (11.0) | 344 (10.3) | 981 (10.8) | |
| Poor | 173 (3.0) | 93 (2.8) | 266 (2.9) | |
| Pre-vaccination anti-spike IgG/A/M serostatus | Negative | 3789 (65.7) | 1770 (53.1) | 5559 (61.1) |
| Positive | 696 (12.1) | 325 (9.8) | 1021 (11.2) | |
| Unknown | 1285 (22.3) | 1236 (37.1) | 2521 (27.7) | |
| Post-vaccination anti-spike IgG/A/M serostatus | Negative | 334 (5.8) | 40 (1.2) | 374 (4.1) |
| Positive | 5436 (94.2) | 3291 (98.8) | 8727 (95.9) | |
| Median inter-dose interval, weeks (IQR) | 11.0 (10.0–11.2) | 10.7 (9.5–11.1) | 11.0 (9.8–11.1) | |
| Median time from date of second vaccine dose to date of sampling, weeks (IQR) | 7.6 (5.7–9.6) | 10.1 (8.3–13.1) | 8.6 (6.4–10.7) | |
Abbreviations: IQR, inter-quartile range; s.d., standard deviation; IMD, index of multiple deprivation; Ig, Immunoglobulin. (1) Ethnicity not reported for n = 1 who received BNT162b2 vaccine. (2) Nation of residence not reported for n = 1 who received BNT162b2 vaccine and n = 2 who received ChAdOx1 vaccine. (3) BMI not reported for n = 10 who received BNT162b2 vaccine and n = 2 who received ChAdOx1 vaccine. (4) Level of education not reported for n = 5 who received BNT162b2 vaccine and n = 1 who received ChAdOx1 vaccine. (5) IMD rank not reported for n = 2 who received BNT162b2 vaccine and n = 5 who received ChAdOx1 vaccine.
3.1. Determinants of Seronegativity following a Primary Course of SARS-CoV-2 Vaccination
Table 2 presents results of minimally adjusted and multivariable analyses to identify factors that were independently associated with the absence of detectable anti-spike IgG/A/M following two doses of ChAdOx1 or BNT162b2. After adjusting for age and sex, 32 independent variables associated with post-vaccination serostatus with p < 0.10 and were fitted in a fully adjusted model. Nine factors in the fully adjusted model remained independently associated with post-vaccination serostatus (pairwise p-value below the multiple comparison test (MCT) threshold of 0.024, and/or p for trend <0.05). Seven of these were associated with higher risk of post-vaccination seronegativity: receiving ChAdOx1 vs. BNT162b2 vaccine (aOR 6.62, 95% CI 4.21–10.42); shorter interval between first and second vaccine doses (aOR 1.60, 1.20–2.14, for 6–10 weeks vs. >10 weeks, and aOR 2.56, 1.22–5.37, for <6 weeks vs. >10 weeks); receiving the second vaccine dose during the first quarter (Q1), third quarter (Q3) or the final quarter (Q4) of the year (aOR 1.08, 0.61–1.92, for Q1 vs. Q2, aOR 12.64, 2.21–72.27, for Q3 vs. Q2, and aOR 21.83, 1.74–273.33, for Q4 vs. Q2; older age (aOR per 10-year increase in age 1.68, 1.42–2.00), poorer self-assessed general health (aOR per level of declining health, 1.26, 1.09–1.45); immunodeficiency disorder (aOR 6.48, 2.53–16.59), and use of systemic immunosuppressants (aOR 3.71, 2.41–5.69). Two factors were associated with lower risk of post-vaccination seronegativity: pre-vaccination seropositivity for SARS-CoV-2 (aOR 0.53, 0.32–0.89, for individuals who were seropositive without COVID-19 symptoms pre-vaccination vs. those who were seronegative pre-vaccination, and aOR 0.16, 0.04–0.56, for individuals who were seropositive with COVID-19 symptoms pre-vaccination vs. those who were seronegative pre-vaccination) and regular consumption of vitamin D supplements (aOR 0.66, 0.51–0.87). No associations were seen for other independent variables investigated, including time of day of inoculation, nutrition, sleep, alcohol use, tobacco smoking or socioeconomic deprivation.
Table 2.
Determinants of post-vaccination seronegativity, all participants (n = 9101).
| Predictor | n Seronegative (%) | Minimally Adjusted Odds Ratio (95% CI) 1 | Pairwise p Value | Fully Adjusted Odds Ratio (95% CI) 2 | Pairwise p Value | P for Trend | |
|---|---|---|---|---|---|---|---|
| Vaccine type and timing | |||||||
| Vaccine type | ChAdOx1 | 334/5770 (5.8) | 5.49 (3.94, 7.66) | <0.001 | 6.62 (4.21, 10.42) | <0.001 * | - |
| BNT162b2 | 40/3331 (1.2) | Referent | |||||
| Time from date of second vaccine dose to date of sampling, weeks (IQR) | <2 | 3/34 (8.8) | 2.85 (0.80, 10.20) | 0.11 | |||
| 2–4 | 19/593 (3.2) | Referent | |||||
| 5–8 | 127/3878 (3.3) | 0.87 (0.53, 1.42) | 0.57 | ||||
| 9–16 | 213/4155 (5.1) | 1.27 (0.78, 2.07) | 0.33 | ||||
| >16 | 12/441 (2.7) | 0.68 (0.33, 1.43) | 0.31 | ||||
| Inter-dose interval, weeks | <6 | 25/474 (5.3) | 1.52 (0.99, 2.34) | 0.054 | 2.56 (1.22, 5.37) | 0.013 * | <0.001 |
| 6–10 | 142/2959 (4.8) | 1.47 (1.17, 1.83) | 0.001 | 1.60 (1.20, 2.14) | 0.001 * | ||
| >10 | 207/5668 (3.7) | Referent | |||||
| Time of second vaccine dose | Before 12 p.m. | 148/3692 (4.0) | Referent | ||||
| 12 p.m.–2 p.m. | 74/1561 (4.7) | 1.21 (0.91, 1.61) | 0.19 | ||||
| 2 p.m.–5 p.m. | 103/2573 (4.0) | 1.01 (0.78, 1.31) | 0.92 | ||||
| After 5 p.m. | 30/941 (3.2) | 0.84 (0.57, 1.26) | 0.41 | ||||
| Quarter of second vaccine dose | Q1 | 56/1318 (4.2) | 1.02 (0.76, 1.37) | 0.87 | 1.08 (0.61, 1.92) | 0.79 | 0.005 † |
| Q2 | 313/7755 (4.0) | Referent | |||||
| Q3 | 2/13 (15.4) | 4.10 (0.89, 18.77) | 0.07 | 12.64 (2.21, 72.27) | 0.004 * | ||
| Q4 | 3/15 (20.0) | 5.82 (1.60, 21.17) | 0.007 | 21.83 (1.74, 273.33) | 0.017 * | ||
| Socio-demographic factors | |||||||
| Age, years | <30 | 3/83 (3.6) | Referent | <0.001 | |||
| 30–39.99 | 6/192 (3.1) | 0.87 (0.21, 3.57) | 0.85 | 0.96 (0.09, 10.65) | 0.97 | ||
| 40–49.99 | 11/595 (1.8) | 0.51 (0.14, 1.85) | 0.30 | 0.53 (0.06, 4.97) | 0.58 | ||
| 50–59.99 | 62/2266 (2.7) | 0.75 (0.23, 2.44) | 0.63 | 0.88 (0.10, 7.41) | 0.90 | ||
| 60–69.99 | 152/3706 (4.1) | 1.11 (0.35, 3.55) | 0.86 | 1.64 (0.20, 13.67) | 0.65 | ||
| 70–79.99 | 131/2077 (6.3) | 1.69 (0.52, 5.42) | 0.38 | 2.86 (0.34, 24.18) | 0.33 | ||
| ≥80 | 9/182 (4.9) | 1.25 (0.33, 4.76) | 0.74 | 3.38 (0.25, 46.30) | 0.36 | ||
| Sex | Female | 234/6474 (3.6) | Referent | ||||
| Male | 140/2627 (5.3) | 1.33 (1.07, 1.66) | 0.01 | 1.32 (0.98, 1.77) | 0.06 | - | |
| Ethnicity | White | 368/8787 (4.2) | Referent | ||||
| Mixed/Multiple/Other | 4/179 (2.2) | 0.59 (0.22, 1.59) | 0.30 | ||||
| South Asian | 1/100 (1.0) | 0.27 (0.04, 1.94) | 0.19 | ||||
| Black/African/Caribbean/ Black British |
1/34 (2.9) | 0.85 (0.12, 6.28) | 0.88 | ||||
| BMI, kg/m2 | <25 | 164/4441 (3.7) | Referent | 0.71 | |||
| 25–30 | 135/2978 (4.5) | 1.20 (0.95, 1.52) | 0.13 | 1.15 (0.85, 1.55) | 0.37 | ||
| >30 | 74/1670 (4.4) | 1.30 (0.98, 1.72) | 0.07 | 1.05 (0.70, 1.56) | 0.82 | ||
| Highest educational level attained | Primary/Secondary | 54/1036 (5.2) | 1.12 (0.80, 1.57) | 0.50 | |||
| Higher/further (A levels) | 56/1298 (4.3) | 0.99 (0.72, 1.38) | 0.97 | ||||
| College | 150/4041 (3.7) | 0.86 (0.67, 1.10) | 0.23 | ||||
| Post-graduate | 114/2720 (4.2) | Referent | |||||
| Quantiles of IMD rank | Q1 (most deprived) | 85/1958 (4.3) | 1.20 (0.89, 1.61) | 0.24 | |||
| Q2 | 94/2199 (4.3) | 1.12 (0.84, 1.49) | 0.45 | ||||
| Q3 | 96/2405 (4.0) | 1.01 (0.76, 1.35) | 0.92 | ||||
| Q4 (least deprived) | 99/2532 (3.9) | Referent | |||||
| Lifestyle factors | |||||||
| Tobacco smoking | No | 363/8740 (4.2) | Referent | ||||
| Yes | 11/361 (3.0) | 0.77 (0.42, 1.42) | 0.40 | ||||
| Vaping | No | 364/8881 (4.1) | Referent | ||||
| Yes | 9/196 (4.6) | 1.16 (0.59, 2.29) | 0.67 | ||||
| Alcohol, units/week | None | 118/2401 (4.9) | Referent | 0.81 | |||
| 1–7 | 103/3230 (3.2) | 0.63 (0.48, 0.82) | 0.001 | 0.71 (0.50, 1.01) | 0.06 | ||
| 8–14 | 84/1880 (4.5) | 0.85 (0.64, 1.14) | 0.29 | 1.10 (0.76, 1.60) | 0.61 | ||
| 15–21 | 40/903 (4.4) | 0.83 (0.57, 1.20) | 0.31 | 1.00 (0.63, 1.60) | 0.99 | ||
| 22–28 | 17/391 (4.3) | 0.78 (0.46, 1.32) | 0.35 | 1.15 (0.61, 2.17) | 0.68 | ||
| >28 | 12/296 (4.1) | 0.71 (0.38, 1.30) | 0.27 | 0.60 (0.25, 1.46) | 0.26 | ||
| Light exercise, hours/week | 0–4 | 140/2824 (5.0) | 1.52 (1.18, 1.96) | 0.001 | 1.19 (0.86, 1.67) | 0.30 | 0.31 |
| 5–9 | 120/2987 (4.0) | 1.20 (0.93, 1.56) | 0.17 | 1.09 (0.79, 1.50) | 0.61 | ||
| ≥10 | 114/3268 (3.5) | Referent | |||||
| Vigorous exercise, hours/week | 0 | 155/3458 (4.5) | 1.19 (0.91, 1.56) | 0.20 | |||
| 1–3 | 132/3387 (3.9) | 1.04 (0.79, 1.37) | 0.79 | ||||
| ≥4 | 87/2230 (3.9) | Referent | |||||
| Sleep, hours/night | ≤5 | 43/797 (5.4) | 1.26 (0.87, 1.81) | 0.22 | 1.29 (0.81, 2.05) | 0.29 | 0.54 |
| 6 | 93/2215 (4.2) | 0.93 (0.70, 1.23) | 0.60 | 0.91 (0.63, 1.32) | 0.62 | ||
| 7 | 133/3750 (3.6) | 0.77 (0.59, 1.00) | 0.05 | 0.84 (0.60, 1.17) | 0.29 | ||
| ≥8 | 105/2336 (4.5) | Referent | |||||
| Self-assessed general health | Excellent | 52/1837 (2.8) | Referent | 0.002 | |||
| Very good | 136/3652 (3.7) | 1.31 (0.95, 1.82) | 0.10 | 1.57 (1.03, 2.40) | 0.036 | ||
| Good | 100/2365 (4.2) | 1.54 (1.09, 2.16) | 0.01 | 1.87 (1.19, 2.95) | 0.007 * | ||
| Fair | 60/981 (6.1) | 2.35 (1.60, 3.44) | <0.001 | 2.00 (1.15, 3.47) | 0.014 * | ||
| Poor | 26/266 (9.8) | 3.85 (2.35, 6.28) | <0.001 | 3.12 (1.39, 6.96) | 0.006 * | ||
| Anxiety or depression | No | 288/6910 (4.2) | Referent | ||||
| Yes | 86/2187 (3.9) | 1.00 (0.78, 1.29) | 0.98 | ||||
| Food choice | None | 362/8607 (4.2) | Referent | ||||
| Vegetarian | 9/380 (2.4) | 0.59 (0.30, 1.15) | 0.12 | ||||
| Vegan | 3/114 (2.6) | 0.63 (0.20, 2.01) | 0.44 | ||||
| Medical conditions | |||||||
| Heart disease 3 | No | 351/8707 (4.0) | Referent | ||||
| Yes | 23/394 (5.8) | 1.26 (0.81, 1.97) | 0.30 | ||||
| Arterial disease 4 | No | 340/8572 (4.0) | Referent | ||||
| Yes | 34/529 (6.4) | 1.45 (1.00, 2.11) | 0.05 | 0.50 (0.23, 1.09) | 0.08 | - | |
| Hypertension | No | 246/6902 (3.6) | Referent | ||||
| Yes | 128/2199 (5.8) | 1.56 (1.25, 1.95) | <0.001 | 0.89 (0.58, 1.37) | 0.61 | - | |
| Immunodeficiency disorder 5 | No | 364/9042 (4.0) | Referent | ||||
| Yes | 10/59 (16.9) | 4.62 (2.32, 9.23) | <0.001 | 6.48 (2.53, 16.59) | <0.001 * | - | |
| Major neurological condition 6 | No | 350/8833 (4.0) | Referent | ||||
| Yes | 24/268 (9.0) | 2.23 (1.44, 3.44) | <0.001 | 1.79 (0.82, 3.91) | 0.15 | - | |
| Cancer | Never | 330/8128 (4.1) | Referent | ||||
| Past (cured or in remission) | 40/888 (4.5) | 1.04 (0.74, 1.47) | 0.80 | ||||
| Present (active) | 4/85 (4.7) | 1.00 (0.36, 2.77) | 0.99 | ||||
| Asthma | No | 303/7652 (4.0) | Referent | ||||
| Yes | 71/1449 (4.9) | 1.30 (1.00, 1.70) | 0.05 | 0.99 (0.69, 1.41) | 0.94 | - | |
| COPD | No | 364/8905 (4.1) | Referent | ||||
| Yes | 10/196 (5.1) | 1.19 (0.62, 2.27) | 0.60 | ||||
| Diabetic status | No diabetes | 317/8334 (3.8) | Referent | 0.06 | |||
| Pre-diabetes | 16/296 (5.4) | 1.38 (0.82, 2.31) | 0.23 | 1.12 (0.57, 2.22) | 0.74 | ||
| Type 1 diabetes | 4/69 (5.8) | 1.59 (0.57, 4.41) | 0.37 | 1.87 (0.52, 6.75) | 0.34 | ||
| Type 2 diabetes | 34/385 (8.8) | 2.26 (1.56, 3.28) | <0.001 | 2.02 (0.94, 4.33) | 0.07 | ||
| Atopy 7 | No | 281/6794 (4.1) | Referent | ||||
| Yes | 93/2307 (4.0) | 1.01 (0.80, 1.29) | 0.92 | ||||
| Pre-vaccination SARS-CoV-2 status | Seronegative | 226/5422 (4.2) | Referent | <0.001 | |||
| Seropositive, asymptomatic | 18/722 (2.5) | 0.58 (0.35, 0.94) | 0.03 | 0.53 (0.32, 0.89) | 0.015 * | ||
| Seropositive, symptomatic | 2/264 (0.8) | 0.20 (0.05, 0.81) | 0.02 | 0.16 (0.04, 0.56) | 0.004 * | ||
| Nutritional supplements | |||||||
| Multivitamin | No | 299/7200 (4.2) | Referent | ||||
| Yes | 75/1901 (3.9) | 0.98 (0.75, 1.26) | 0.86 | ||||
| Vitamin A | No | 371/9053 (4.1) | Referent | ||||
| Yes | 3/48 (6.3) | 1.56 (0.48, 5.04) | 0.46 | ||||
| Vitamin C | No | 346/8192 (4.2) | Referent | ||||
| Yes | 28/909 (3.1) | 0.73 (0.49, 1.08) | 0.11 | ||||
| Vitamin D | No | 204/4455 (4.6) | Referent | ||||
| Yes | 170/4646 (3.7) | 0.80 (0.65, 0.98) | 0.03 | 0.66 (0.51, 0.87) | 0.003 * | - | |
| Zinc | No | 357/8664 (4.1) | Referent | ||||
| Yes | 17/437 (3.9) | 0.94 (0.57, 1.55) | 0.82 | ||||
| Selenium | No | 372/9003 (4.1) | Referent | ||||
| Yes | 2/98 (2.0) | 0.49 (0.12, 2.01) | 0.32 | ||||
| Iron | No | 368/8816 (4.2) | Referent | ||||
| Yes | 6/285 (2.1) | 0.53 (0.23, 1.19) | 0.12 | ||||
| Probiotics | No | 359/8528 (4.2) | Referent | ||||
| Yes | 15/573 (2.6) | 0.64 (0.38, 1.09) | 0.10 | ||||
| Omega-3 fatty acids | No | 336/7975 (4.2) | Referent | ||||
| Yes | 38/1126 (3.4) | 0.80 (0.57, 1.12) | 0.19 | ||||
| Cod liver oil | No | 341/8279 (4.1) | Referent | ||||
| Yes | 33/822 (4.0) | 0.93 (0.64, 1.34) | 0.68 | ||||
| Garlic | No | 364/8907 (4.1) | Referent | ||||
| Yes | 10/194 (5.2) | 1.20 (0.63, 2.30) | 0.57 | ||||
| Medications | |||||||
| Beta-2 adrenergic agonists | No | 334/8275 (4.0) | Referent | ||||
| Yes | 40/826 (4.8) | 1.23 (0.87, 1.72) | 0.24 | ||||
| Beta blockers | No | 334/8398 (4.0) | Referent | ||||
| Yes | 40/703 (5.7) | 1.35 (0.96, 1.89) | 0.09 | 1.01 (0.62, 1.65) | 0.96 | - | |
| Statins | No | 277/7337 (3.8) | Referent | ||||
| Yes | 97/1764 (5.5) | 1.31 (1.02, 1.68) | 0.03 | 0.92 (0.64, 1.32) | 0.65 | - | |
| ACE inhibitors | No | 320/8122 (3.9) | Referent | ||||
| Yes | 54/979 (5.5) | 1.30 (0.96, 1.76) | 0.09 | 1.12 (0.70, 1.80) | 0.64 | - | |
| Proton pump inhibitors | No | 287/7723 (3.7) | Referent | ||||
| Yes | 87/1378 (6.3) | 1.69 (1.32, 2.16) | <0.001 | 0.91 (0.63, 1.33) | 0.63 | - | |
| H2-receptor antagonists | No | 373/9040 (4.1) | Referent | ||||
| Yes | 1/61 (1.6) | 0.39 (0.05, 2.80) | 0.35 | ||||
| Inhaled corticosteroids | No | 349/8505 (4.1) | Referent | ||||
| Yes | 25/596 (4.2) | 1.01 (0.66, 1.53) | 0.98 | ||||
| Bronchodilators | No | 333/8240 (4.0) | Referent | ||||
| Yes | 41/861 (4.8) | 1.20 (0.85, 1.67) | 0.30 | ||||
| Systemic Immunosuppressants | No | 327/8630 (3.8) | Referent | ||||
| Yes | 47/471 (10.0) | 2.86 (2.08, 3.95) | <0.001 | 3.71 (2.41, 5.69) | <0.001 * | - | |
| Angiotensin receptor blockers | No | 331/8493 (3.9) | Referent | ||||
| Yes | 43/608 (7.1) | 1.75 (1.25, 2.43) | <0.001 | 0.98 (0.58, 1.68) | 0.95 | - | |
| SSRI antidepressants | No | 350/8533 (4.1) | Referent | ||||
| Yes | 24/568 (4.2) | 1.11 (0.73, 1.70) | 0.62 | ||||
| Non-SSRIs antidepressants | No | 352/8726 (4.0) | Referent | ||||
| Yes | 22/375 (5.9) | 1.57 (1.01, 2.46) | 0.05 | 0.73 (0.35, 1.50) | 0.39 | - | |
| Calcium channel blockers | No | 314/8100 (3.9) | Referent | ||||
| Yes | 60/1001 (6.0) | 1.45 (1.09, 1.94) | 0.01 | 1.00 (0.65, 1.55) | 0.99 | - | |
| Thiazide diuretics | No | 359/8765 (4.1) | Referent | ||||
| Yes | 15/336 (4.5) | 1.04 (0.61, 1.76) | 0.89 | ||||
| Vitamin K antagonists | No | 370/9027 (4.1) | Referent | ||||
| Yes | 4/74 (5.4) | 1.17 (0.42, 3.23) | 0.76 | ||||
| SGLT-2 inhibitors | No | 369/9054 (4.1) | Referent | ||||
| Yes | 5/47 (10.6) | 2.56 (1.01, 6.54) | 0.05 | 2.99 (0.92, 9.65) | 0.07 | - | |
| Anticholinergics | No | 354/8656 (4.1) | Referent | ||||
| Yes | 20/445 (4.5) | 1.08 (0.67, 1.72) | 0.76 | ||||
| Metformin | No | 353/8827 (4.0) | Referent | ||||
| Yes | 21/274 (7.7) | 1.83 (1.16, 2.90) | 0.01 | 0.61 (0.24, 1.55) | 0.30 | - | |
| Bisphosphonates | No | 362/8912 (4.1) | Referent | ||||
| Yes | 12/189 (6.3) | 1.72 (0.94, 3.12) | 0.08 | 0.77 (0.31, 1.89) | 0.57 | - | |
| Anti-platelet drugs | No | 328/8453 (3.9) | Referent | ||||
| Yes | 46/648 (7.1) | 1.67 (1.21, 2.32) | 0.002 | 2.79 (1.06, 7.38) | 0.038 | - | |
| Sex hormone therapy | No | 347/8376 (4.1) | Referent | ||||
| Yes | 27/725 (3.7) | 1.04 (0.69, 1.56) | 0.87 | ||||
| Aspirin 8 | No | 341/8593 (4.0) | Referent | ||||
| Yes | 33/508 (6.5) | 1.47 (1.01, 2.14) | 0.04 | 0.37 (0.14, 1.01) | 0.05 | - | |
| Paracetamol 8 | No | 345/8677 (4.0) | Referent | ||||
| Yes | 29/424 (6.8) | 1.77 (1.19, 2.62) | 0.005 | 0.84 (0.47, 1.51) | 0.56 | - | |
| BCG vaccinated | No | 44/1075 (4.1) | Referent | ||||
| Yes | 280/7166 (3.9) | 0.99 (0.71, 1.37) | 0.13 | ||||
Abbreviations: CI, confidence interval; BMI, body mass index; IMD, index of multiple deprivation; COPD, chronic obstructive pulmonary disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; ACE, angiotensin-converting enzyme; H2, histamine 2; SSRI, selective serotonin reuptake inhibitor; BCG, Bacille Calmette-Guérin; SGLT2, sodium-glucose cotransporter-2. (1) Adjusted for age and sex only. (2) Adjusted for age, sex, vaccine type, inter-dose interval, quarter of second vaccination, BMI, alcohol intake, light exercise, sleep, self-assessed general health, arterial disease, hypertension, immunodeficiency disorder, major neurological condition, asthma, diabetic status, pre-vaccination SARS-CoV-2 status and pre-vaccination use of vitamin D supplements, beta blockers, statins, ACE inhibitors, proton pump inhibitors, systemic immunosuppressants, angiotensin receptor blockers, non-SSRI antidepressants, calcium channel blockers, SGLT2 inhibitors, metformin, bisphosphonates, anti-platelet drugs use, aspirin and paracetamol. (3) Heart disease defined as coronary artery disease or heart failure. (4) Arterial disease defined as ischaemic heart disease, peripheral vascular disease or cerebrovascular disease. (5) Immunodeficiency defined as HIV, primary immune deficiency or other immunodeficiency. (6) Major neurological conditions defined as stroke, transient ischaemic attack, dementia, Parkinson’s disease, multiple sclerosis or motor neurone disease. (7) Atopy defined as atopic eczema/dermatitis and/or hay fever/allergic rhinitis. (8) Chronic use prior to vaccination (i.e., distinct from acute post-vaccination use for treatment of reactogenic symptoms). † Global p value presented for this non-scalar categorical variable. * Below multiple comparisons testing critical p-value threshold of 0.024.
3.2. Determinants of Post-Vaccination Antibody Titres in Subset of Individuals Who Were Seropositive following a Primary Course of SARS-CoV-2 Vaccination
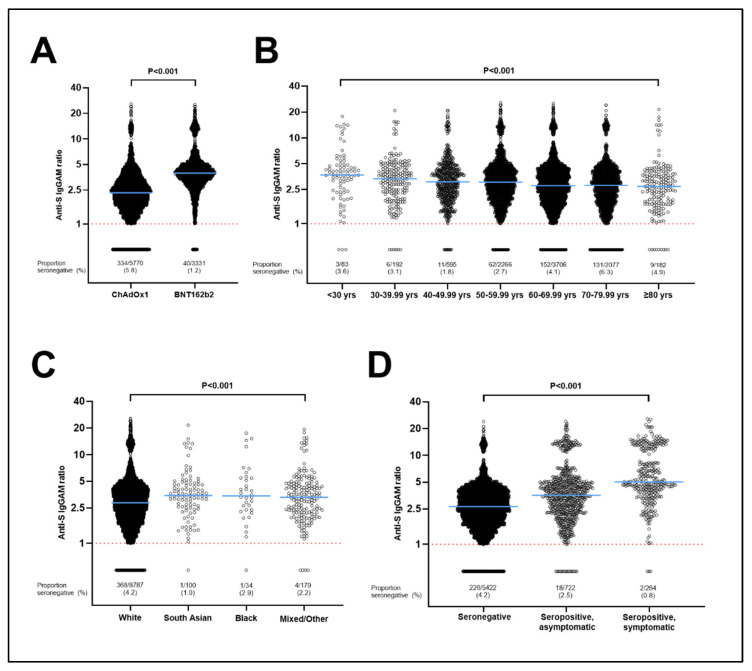
To identify factors influencing the magnitude of antibody responses to COVID-19 vaccination, we investigated determinants of SARS-CoV-2 antibody titres in the subset of 8727 participants who were seropositive following their second COVID-19 vaccine dose. Results of this analysis are presented in Table 3. A total of 22 factors associated with antibody titres after adjusting for age and sex with p < 0.10 and were fitted in a fully adjusted model. Eight factors remained independently associated with antibody titres in the fully adjusted model (pairwise p-values below the MCT threshold of 0.025, and/or P for trend < 0.05). Six of these independently associated with lower antibody titres: receipt of ChAdOx1 vs. BNT162b2 vaccine (43.4% lower titres, 95% CI 41.8–44.8, Figure 1); longer duration between date of second vaccine dose and date of sampling (7.4% lower, 3.0–11.6, for 5–8 weeks vs. 2–4 weeks; 12.7% lower, 8.2–16.9, for 9–16 weeks vs. 2–4 weeks, and 7.8% lower, 1.3–16.1, for >16 weeks vs. 2–4 weeks); shorter interval between first and second vaccine doses (10.4% lower, 3.7–16.7, for <6 weeks vs. >10 weeks; 5.9% lower, 3.3–8.3, for 6–10 weeks vs. >10 weeks); receiving the second vaccine dose during the first quarter (Q1) or the final quarter (Q4) of the year (8.1% lower, 3.2–12.7, for Q1 vs. Q2, and 47.7% lower, 11.4–69.1, for Q4 vs. Q2); older age (3.3% lower per 10-year increase in age, 2.1–4.6), and presence of hypertension (4.1% lower, 1.1–6.9). Two factors independently associated with higher post-vaccination antibody titres: South Asian ethnicity (16.2% higher, 3.0–31.1, vs. White ethnicity) or Mixed/Multiple/Other ethnicity (11.8% higher, 2.9–21.6, vs. White ethnicity), and pre-vaccination seropositivity for SARS-CoV-2 (39.8% higher, 34.7–45.0, for individuals who were seropositive without COVID-19 symptoms pre-vaccination vs. those who were seronegative pre-vaccination, and 105.1% higher, 94.1–116.6, for individuals who were seropositive with COVID-19 symptoms pre-vaccination vs. those who were seronegative pre-vaccination).
Table 3.
Determinants of post-vaccination antibody titres, subset of seropositive participants (n = 8727).
| Predictor | Median IgGAM Ratio (IQR) | Minimally Adjusted % Difference (95% CI) 1 | p Value | Fully Adjusted % Difference (95% CI) 2 | Pairwise p Value | P for Trend | |
|---|---|---|---|---|---|---|---|
| Vaccine type | ChAdOx1 | 2.39 (1.76, 3.30) | −39.35 (−40.65, −38.02) | <0.001 | −43.31 (−44.8, −41.78) | <0.001 * | - |
| BNT162b2 | 3.96 (3.15, 4.86) | Referent | |||||
| Time from second vaccine dose to sampling, weeks | <2 | 2.96 (2.23, 13.62) | 36.68 (11.82, 67.05) | 0.002 | 1.98 (−17.97, 26.79) | 0.86 | <0.001 |
| 2–4 | 2.86 (2.06, 4.05) | Referent | |||||
| 5–8 | 2.81 (1.95, 3.99) | −0.59 (−5.37, 4.43) | 0.81 | −7.41 (−11.62, −3.00) | 0.001 * | ||
| 9–16 | 3.13 (2.10, 4.24) | 7.54 (2.33, 13.01) | 0.004 | −12.68 (−16.94, −8.20) | <0.001 * | ||
| >16 | 2.93 (2.03, 4.43) | 7.14 (−0.08, 14.89) | 0.05 | −7.83 (−16.12, 1.28) | 0.09 | ||
| Inter−dose interval, weeks | <6 | 2.91 (1.94, 4.12) | −1.23 (−6.38, 4.21) | 0.65 | −10.43 (−16.69, −3.70) | 0.003 * | <0.001 |
| 6–10 | 2.89 (2.00, 4.01) | −5.12 (−7.51, −2.66) | <0.001 | −5.85 (−8.3, −3.34) | <0.001 * | ||
| >10 | 2.99 (2.06, 4.21) | Referent | |||||
| Time of second vaccine dose | Before 12 p.m. | 2.92 (2.02, 4.10) | Referent | 0.96 † | |||
| 12 p.m.–2 p.m. | 2.91 (2.00, 4.07) | −1.12 (−4.39, 2.26) | 0.51 | −2.87 (−6.00, 0.36) | 0.08 | ||
| 2 p.m.–5 p.m. | 3.03 (2.06, 4.21) | 2.88 (−0.01, 5.86) | 0.05 | 1.15 (−1.61, 3.99) | 0.42 | ||
| After 5 p.m. | 2.98 (2.07, 4.07) | 0.54 (−3.45, 4.70) | 0.79 | −2.39 (−6.2, 1.58) | 0.24 | ||
| Quarter of second vaccine dose | Q1 | 3.44 (2.42, 4.43) | 15.46 (11.70, 19.34) | <0.001 | −8.07 (−12.69, −3.22) | 0.001 * | 0.004 † |
| Q2 | 2.88 (1.99, 4.09) | Referent | |||||
| Q3 | 3.17 (1.36, 4.47) | −3.37 (−30.38, 34.12) | 0.84 | 31.63 (−22.10, 122.40) | 0.30 | ||
| Q4 | 1.93 (1.28, 3.24) | −24.08 (−44.54, 3.92) | 0.09 | −47.69 (−69.11, −11.39) | 0.016 * | ||
| Age, years | <30 | 3.79 (2.48, 4.85) | Referent | <0.001 | |||
| 30–39.99 | 3.43 (2.34, 4.55) | −12.71 (−24.56, 1.00) | 0.07 | −5.94 (−20.45, 11.21) | 0.47 | ||
| 40–49.99 | 3.13 (2.16, 4.31) | −16.59 (−26.77, −5.01) | 0.006 | −4.12 (−17.60, 11.58) | 0.59 | ||
| 50–59.99 | 3.10 (2.14, 4.30) | −16.58 (−26.32, −5.55) | 0.004 | 0.70 (−13.04, 16.61) | 0.93 | ||
| 60–69.99 | 2.85 (1.98, 4.05) | −22.79 (−31.75, −12.65) | <0.001 | −5.39 (−18.27, 9.53) | 0.46 | ||
| 70–79.99 | 2.92 (1.96, 4.03) | −23.08 (−32.09, −12.87) | <0.001 | −10.54 (−22.81, 3.69) | 0.14 | ||
| ≥80 | 2.80 (1.84, 3.78) | −24.71 (−35.06, −12.72) | <0.001 | −19.62 (−34.77, −0.95) | 0.040 | ||
| Sex | Female | 3.01 (2.07, 4.19) | Referent | ||||
| Male | 2.87 (1.95, 4.04) | −3.17 (−5.68, −0.58) | 0.02 | −2.48 (−5.05, 0.17) | 0.07 | - | |
| Ethnicity | White | 2.94 (2.02, 4.11) | Referent | <0.001 † | |||
| Mixed/Multiple/Other | 3.35 (2.24, 4.73) | 12.78 (3.75, 22.59) | 0.005 | 11.83 (2.85, 21.59) | 0.009 * | ||
| South Asian | 3.46 (2.58, 4.64) | 16.26 (4.10, 29.85) | 0.008 | 16.21 (3.02, 31.10) | 0.015 * | ||
| Black/African/ Caribbean/Black British |
3.54 (2.39, 5.46) | 26.76 (4.79, 53.33) | 0.015 | 12.31 (−6.94, 35.54) | 0.23 | ||
| BMI, kg/m2 | <25 | 2.90 (2.03, 4.03) | Referent | 0.038 | |||
| 25–30 | 2.96 (2.04, 4.15) | 3.62 (0.91, 6.40) | 0.009 | 2.89 (0.18, 5.67) | 0.037 | ||
| >30 | 3.17 (2.02, 4.36) | 4.87 (1.56, 8.29) | 0.004 | 2.62 (−0.85, 6.21) | 0.14 | ||
| Highest educational level attained | Primary/Secondary | 3.02 (1.93, 4.29) | 1.05 (1.01, 1.10) | 0.018 | 1.73 (−2.36, 5.99) | 0.41 | 0.37 |
| Higher/further (A levels) | 2.98 (2.07, 4.14) | 1.03 (1.00, 1.07) | 0.07 | 1.59 (−2.14, 5.45) | 0.41 | ||
| College | 2.99 (2.07, 4.11) | 1.04 (1.01, 1.07) | 0.009 | 3.31 (0.52, 6.17) | 0.020 * | ||
| Post-graduate | 2.89 (1.98, 4.10) | Referent | |||||
| Quantiles of IMD rank | Q1 (most deprived) | 3.06 (2.12, 4.24) | 2.28 (−1.10, 5.79) | 0.19 | |||
| Q2 | 2.97 (1.98, 4.19) | 0.53 (−2.68, 3.85) | 0.75 | ||||
| Q3 | 2.90 (2.04, 4.01) | −1.34 (−4.42, 1.84) | 0.41 | ||||
| Q4 (least deprived) | 2.95 (2.01, 4.14) | Referent | |||||
| Tobacco smoking | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.20 (2.24, 4.20) | 1.22 (−4.65, 7.46) | 0.69 | ||||
| Vaping | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.37 (2.18, 4.37) | 5.47 (−2.73, 14.37) | 0.20 | ||||
| Alcohol, units/week | None | 2.97 (2.05, 4.20) | Referent | 0.71 | |||
| 1–7 | 3.03 (2.06, 4.14) | −0.68 (−3.63, 2.35) | 0.66 | 1.36 (−1.66, 4.48) | 0.38 | ||
| 8–14 | 2.94 (2.02, 4.16) | −1.87 (−5.21, 1.58) | 0.28 | 0.9 (−2.57, 4.48) | 0.62 | ||
| 15–21 | 2.91 (1.98, 4.01) | −3.31 (−7.46, 1.03) | 0.13 | 0.3 (−3.97, 4.76) | 0.89 | ||
| 22–28 | 2.65 (1.91, 3.94) | −6.32 (−11.90, −0.39) | 0.04 | −4.21 (−9.79, 1.71) | 0.16 | ||
| >28 | 2.84 (2.03, 4.18) | −3.06 (−9.56, 3.90) | 0.38 | 0.01 (−6.59, 7.07) | 1.00 | ||
| Light exercise, hours/week | 0–4 | 3.07 (2.10, 4.26) | 5.22 (2.21, 8.31) | 0.001 | 0.18 (−2.81, 3.26) | 0.91 | 0.92 |
| 5–9 | 2.95 (2.02, 4.13) | 1.29 (−1.54, 4.20) | 0.38 | −1.02 (−3.77, 1.82) | 0.48 | ||
| ≥10 | 2.88 (1.99, 4.06) | Referent | |||||
| Vigorous exercise, hours/week | 0 | 3.02 (2.05, 4.21) | 4.97 (1.82, 8.21) | 0.002 | 1.34 (−1.82, 4.6) | 0.41 | 0.45 |
| 1–3 | 2.98 (2.05, 4.12) | 3.09 (−0.01, 6.28) | 0.05 | 0.01 (−2.96, 3.06) | 1.00 | ||
| ≥4 | 2.86 (1.99, 4.06) | Referent | |||||
| Sleep, hours/night | ≤5 | 3.07 (2.13, 4.29) | 5.03 (0.31, 9.98) | 0.04 | 0.64 (−3.96, 5.45) | 0.79 | 0.94 |
| 6 | 3.00 (2.04, 4.19) | 1.12 (−2.18, 4.52) | 0.51 | −1.20 (−4.41, 2.11) | 0.47 | ||
| 7 | 2.92 (2.00, 4.08) | −1.82 (−4.66, 1.11) | 0.22 | −2.48 (−5.28, 0.39) | 0.39 | ||
| ≥8 | 2.96 (2.05, 4.13) | Referent | |||||
| Self−assessed general health | Excellent | 2.92 (2.05, 4.07) | Referent | 0.27 | |||
| Very good | 2.92 (2.01, 4.09) | −0.20 (−3.32, 3.02) | 0.90 | −2.35 (−5.36, 0.75) | 0.14 | ||
| Good | 3.03 (2.06, 4.23) | 4.73 (1.17, 8.42) | 0.009 | −0.86 (−4.34, 2.74) | 0.63 | ||
| Fair | 3.07 (2.02, 4.15) | 2.38 (−2.07, 7.03) | 0.30 | −1.96 (−6.45, 2.74) | 0.41 | ||
| Poor | 2.95 (2.04, 4.39) | 5.60 (−2.04, 13.84) | 0.16 | −6.73 (−14.02, 1.17) | 0.09 | ||
| Anxiety or depression | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 2.99 (2.03, 4.17) | 0.68 (−2.06, 3.50) | 0.63 | ||||
| Food choice | None | 2.96 (2.03, 4.13) | Referent | ||||
| Vegetarian | 2.92 (2.04, 4.13) | −2.98 (−8.46, 2.83) | 0.31 | ||||
| Vegan | 2.61 (2.00, 4.33) | −2.04 (−11.76, 8.74) | 0.70 | ||||
| Heart disease 3 | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.76 (1.93, 4.17) | 1.33 (−4.46, 7.48) | 0.66 | ||||
| Arterial disease 4 | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.96 (1.94, 4.14) | 2.02 (−3.09, 7.40) | 0.45 | ||||
| Hypertension | No | 2.98 (2.06, 4.16) | Referent | ||||
| Yes | 2.89 (1.92, 4.08) | −2.62 (−5.32, 0.14) | 0.06 | −4.09 (−6.94, −1.14) | 0.007 * | - | |
| Immunodeficiency disorder 5 | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.85 (1.98, 3.96) | −0.17 (−14.65, 16.77) | 0.98 | ||||
| Major neurological condition 6 | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.90 (1.84, 4.10) | −0.74 (−7.55, 6.57) | 0.84 | ||||
| Cancer | Never | 2.95 (2.04, 4.13) | Referent | ||||
| Past (cured or in remission) | 2.99 (2.00, 4.15) | 1.24 (−2.72, 5.35) | 0.55 | ||||
| Present (active) | 2.93 (1.89, 3.82) | −5.33 (−16.23, 7.00) | 0.38 | ||||
| Asthma | No | 2.97 (2.03, 4.14) | Referent | ||||
| Yes | 2.91 (2.06, 4.10) | −0.74 (−3.88, 2.51) | 0.65 | ||||
| COPD | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (1.98, 4.12) | 2.97 (−5.06, 11.67) | 0.48 | ||||
| Diabetic status | No diabetes | 2.97 (2.04, 4.14) | Referent | ||||
| Pre-diabetes | 2.68 (1.87, 3.93) | −4.13 (−10.31, 2.46) | 0.21 | ||||
| Type 1 diabetes | 3.13 (1.95, 4.10) | −1.81 (−14.31, 12.52) | 0.79 | ||||
| Type 2 diabetes | 2.95 (1.88, 4.30) | 0.77 (−5.09, 6.98) | 0.80 | ||||
| Atopy 7 | No | 2.95 (2.02, 4.14) | Referent | ||||
| Yes | 2.97 (2.05, 4.11) | −0.14 (−2.80, 2.59) | 0.92 | ||||
| Pre−vaccination SARS−COV−2 status | Seronegative | 2.74 (1.92, 3.80) | Referent | <0.001 | |||
| Seropositive, asymptomatic | 3.62 (2.40, 5.01) | 37.37 (31.83, 43.13) | <0.001 | 39.77 (34.73, 45.00) | <0.001 * | ||
| Seropositive, symptomatic | 5.50 (4.17, 12.62 | 126.30 (112.07, 141.5) | <0.001 | 105.06 (94.13, 116.60) | <0.001 * | ||
| Multivitamin | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.91 (2.05, 4.17) | −0.64 (−3.46, 2.26) | 0.66 | ||||
| Vitamin A | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.90 (1.94, 3.93) | −4.11 (−18.56, 12.91) | 0.61 | ||||
| Vitamin C | No | 2.95 (2.02, 4.11) | Referent | ||||
| Yes | 3.13 (2.10, 4.26) | 4.07 (0.10, 8.19) | 0.04 | 1.47 (−2.42, 5.52) | 0.46 | - | |
| Vitamin D | No | 2.98 (2.05, 4.12) | Referent | ||||
| Yes | 2.94 (2.02, 4.15) | −0.87 (−3.16, 1.49) | 0.47 | ||||
| Zinc | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.91 (2.07, 4.18) | 1.48 (−3.92, 7.18) | 0.60 | ||||
| Selenium | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.57 (1.85, 4.06) | −6.21 (−16.16, 4.92) | 0.26 | ||||
| Iron | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.80 (1.88, 4.10) | −3.18 (−9.42, 3.49) | 0.34 | ||||
| Probiotics | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.87 (2.08, 4.27) | 0.52 (−4.18, 5.45) | 0.83 | ||||
| Omega−3 fatty acids | No | 2.95 (2.02, 4.13) | Referent | ||||
| Yes | 3.01 (2.12, 4.21) | 3.08 (−0.51, 6.80) | 0.09 | 2.09 (−1.48, 5.79) | 0.26 | - | |
| Cod liver oil | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.97 (2.00, 4.12) | 1.15 (−2.90, 5.38) | 0.58 | ||||
| Garlic | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (2.26, 4.42) | 6.51 (−1.83, 15.55) | 0.13 | ||||
| Beta−2 adrenergic agonists | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.94 (2.05, 4.13) | 0.83 (−3.21, 5.04) | 0.69 | ||||
| Beta blockers | No | 2.97 (2.04, 4.13) | Referent | ||||
| Yes | 2.87 (1.94, 4.11) | −1.17 (−5.47, 3.32) | 0.60 | ||||
| Statins | No | 2.98 (2.06, 4.17) | Referent | ||||
| Yes | 2.86 (1.91, 4.01) | −2.52 (−5.51, 0.57) | 0.11 | ||||
| ACE inhibitors | No | 2.97 (2.05, 4.14) | Referent | ||||
| Yes | 2.89 (1.85, 4.09) | −1.85 (−5.55, 2.00) | 0.34 | ||||
| Proton pump inhibitors | No | 2.97 (2.05, 4.13) | Referent | ||||
| Yes | 2.91 (1.92, 4.16) | 0.26 (−3.00, 3.63) | 0.88 | ||||
| H2−receptor antagonists | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.02 (2.09, 4.20) | 1.57 (−11.84, 17.01) | 0.83 | ||||
| Inhaled corticosteroids | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.88 (2.03, 4.11) | −0.44 (−5.04, 4.39) | 0.86 | ||||
| Bronchodilators | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.97 (2.08, 4.18) | 2.06 (−1.95, 6.24) | 0.32 | ||||
| Systemic immunosuppressants | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.80 (1.99, 3.91) | −4.33 (−9.40, 1.02) | 0.11 | ||||
| Angiotensin receptor blockers | No | 2.96 (2.04, 4.14) | Referent | ||||
| Yes | 2.94 (1.93, 4.03) | −2.33 (−6.89, 2.45) | 0.33 | ||||
| SSRI antidepressants | No | 2.95 (2.03, 4.11) | Referent | ||||
| Yes | 3.17 (2.08, 4.33) | 5.15 (0.17, 10.39) | 0.04 | 0.18 (−4.69, 5.29) | 0.94 | - | |
| Non−SSRIs antidepressants | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 2.98 (2.01, 4.18) | 2.24 (−3.66, 8.51) | 0.46 | ||||
| Calcium channel blockers | No | 2.96 (2.05, 4.14) | Referent | ||||
| Yes | 2.94 (1.92, 4.11) | −1.54 (−5.23, 2.29) | 0.43 | ||||
| Thiazide diuretics | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.75 (1.84, 3.95) | −5.56 (−11.27, 0.52) | 0.07 | −2.77 (−8.88, 3.76) | 0.40 | - | |
| Vitamin K antagonists | No | 2.96 (2.03, 4.14) | Referent | ||||
| Yes | 2.94 (1.89, 3.81) | −5.97 (−17.55, 7.23) | 0.36 | ||||
| SGLT2 inhibitors | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.93 (2.44, 4.86) | 10.26 (−6.90, 30.57) | 0.26 | ||||
| Anticholinergics | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.04 (2.11, 4.18) | 3.08 (−2.38, 8.84) | 0.27 | ||||
| Metformin | No | 2.95 (2.03, 4.12) | Referent | ||||
| Yes | 3.20 (2.02, 4.46) | 6.77 (−0.44, 14.50) | 0.07 | 0.36 (−6.45, 7.66) | 0.92 | - | |
| Bisphosphonates | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.73 (1.99, 4.16) | −1.09 (−8.99, 7.50) | 0.80 | ||||
| Anti−platelet drugs | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.87 (1.88, 4.05) | 0.49 (−4.12, 5.32) | 0.84 | ||||
| Sex hormone therapy | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (2.08, 4.18) | 0.53 (−3.77, 5.03) | 0.81 | ||||
| Aspirin 8 | No | 2.97 (2.04, 4.13) | Referent | ||||
| Yes | 2.84 (1.89, 4.11) | 0.01 (−5.09, 5.39) | 1.00 | ||||
| Paracetamol 8 | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.03 (1.99, 4.23) | 2.81 (−2.82, 8.76) | 0.34 | ||||
| BCG vaccinated | No | 2.86 (2.05, 4.07) | Referent | ||||
| Yes | 3.00 (2.05, 4.17) | 2.77 (−0.92, 6.59) | 0.65 | ||||
Abbreviations: IQR, inter-quartile range; CI, confidence interval; BMI, body mass index; Ig, immunoglobulin; IMD, index of multiple deprivation; COPD, chronic obstructive pulmonary disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; ACE, angiotensin-converting enzyme; H2, histamine 2; SSRI, selective serotonin reuptake inhibitor; BCG, Bacille Calmette-Guérin; SGLT2, sodium-glucose cotransporter-2. (1) Adjusted for age and sex only. (2) Adjusted for age, sex, vaccine type, time from second dose to sampling, inter-dose interval, time of second vaccination, quarter of second vaccination, ethnicity, BMI, educational level, alcohol intake, light exercise, vigorous exercise, sleep, self-assessed general health, hypertension, pre-vaccination SARS-CoV-2 status and pre-vaccination use of vitamin C supplements, omega-3 fatty acid supplements, SSRI antidepressants, thiazide diuretics and metformin. (3) Heart disease defined as coronary artery disease or heart failure. (4) Arterial disease defined as ischaemic heart disease, peripheral vascular disease or cerebrovascular disease. (5) Immunodeficiency defined as HIV, primary immune deficiency or other immunodeficiency. (6) Major neurological conditions defined as stroke, transient ischaemic attack, dementia, Parkinson’s disease, multiple sclerosis or motor neurone disease. (7) Atopy defined as atopic eczema/dermatitis and/or hay fever/allergic rhinitis. (8) Chronic use prior to vaccination (i.e., distinct from acute post-vaccination use for treatment of reactogenic symptoms). † Global p value presented for this non-scalar categorical variable. * Below multiple comparisons testing critical p-value threshold of 0.025.
Figure 1.
Antibody titres following two doses of vaccine by type of vaccine (A), age (B), ethnicity (C) and pre-vaccination SARS-CoV-2 status (D). For C, South Asian indicates people who self-identified their ethnic origin as Indian, Pakistani, or Bangladeshi, and Black indicates people who self-identified their ethnic origin as Black, African, Caribbean or Black British. p values from Mann–Whitney test (A) and Kruskal–Wallis tests (B–D). Anti-S, anti-spike; IgGAM, immunoglobulin G, A or M. Dotted line = limit of detection.
All the above determinants of post-vaccination antibody titres remained significant when we performed a sensitivity analysis excluding 23 individuals who reported RT-PCR- or lateral flow test-confirmed SARS-CoV-2 infection after their second vaccine dose, but before their serology sampling date (data not shown).
3.3. Stratification of Antibody Responses by Vaccine Type
To explore whether determinants of antibody responses to vaccination were consistent for ChAdOx1 vs. BNT162b2, we stratified the analysis of post-vaccination antibody titres according to type of vaccine received. Tables S3 and S4 (Online Data Supplement) present the results of these analyses. Seven factors associated independently with post-vaccination antibody titres in ChAdOx1 recipients, but not in BNT162b2 recipients: lower titres in this group were associated with active cancer, consumption of selenium supplements and use of statins, Angiotensin Converting Enzyme (ACE) inhibitors and proton pump inhibitors (PPI), while South Asian and Mixed/Multiple/Other ethnic origin associated with higher titres. Conversely, three factors associated independently with lower post-vaccination antibody titres in BNT162b2 recipients, but not in ChAdOx1 recipients: older age, hypertension and use of systemic immunosuppressant medication. To test whether vaccine type modified the effects of these independent variables on post-vaccination antibody titres, we fitted all significant factors in our main multivariable model and included an interaction term for vaccine type. Three factors showed a small but statistically significant interaction: hypertension (Pinteraction = 0.035), regular consumption of selenium supplements (Pinteraction = 0.021) and use of systemic immunosuppressant medication (Pinteraction = 0.046; Figure S2, Online Data Supplement).
3.4. Influence of Post-Vaccination Paracetamol/NSAIDs on Antibody Response to Primary Course of Vaccination
Results of an exploratory analysis to determine the influence of taking paracetamol or NSAIDs to treat post-vaccination symptoms are presented in Table S5. After fitting post-vaccination paracetamol/NSAID use into the main multivariable model we found a significant positive association between this factor and post-vaccination antibody titres. We then reasoned that this association could be confounded by an association between presence or severity of post-vaccination symptoms and higher post-vaccination antibody titres. After additionally correcting for report of post-vaccination symptoms (i.e., headache, fever, local soreness), the association between post-vaccination paracetamol/NSAID use and post-vaccination titres was rendered statistically non-significant.
4. Discussion
We present results of a large population-based study examining a comprehensive range of potential determinants of combined IgG, IgA and IgM responses to two very widely used SARS-CoV-2 vaccines. A major finding was that 5.8% of participants did not have detectable IgG/A/M anti-spike antibodies following two doses of ChAdOx1, as compared with 1.2% of those who had received two doses of BNT162b2 (aOR 6.62, 95% CI 4.21 to 10.42). Other risk factors for post-vaccination seronegativity included shorter inter-dose interval, administration during colder months, older age, poorer general health, immunodeficiency disorder and use of systemic immunosuppressive medication. By contrast, pre-vaccination SARS-CoV-2 seropositivity and regular use of vitamin D supplements at the time of vaccination were associated with lower risk of post-vaccination seronegativity. Post-vaccination antibody titres were higher in participants of South Asian and mixed/other ethnic origin vs. White participants, and in those who were overweight vs. normal weight; these associations were independent of pre-vaccination SARS-CoV-2 serostatus. Post-vaccination use of paracetamol or NSAIDs was not associated with lower antibody responses to vaccination, after adjustment for post-vaccination symptoms, and determinants of post-vaccination anti-spike titres were not substantively different for participants who received two doses of ChAdOx1 vs. BNT162b2.
Our findings confirm previous reports of higher antibody responses to BNT162b2 vs. ChAdOx1 [4,13,26]. Pre-existing immunity to adenovirus vectors may be one of the factors underlying this difference [27], and experiments to compare the prevalence of adenovirus neutralising antibody in pre-vaccination serum of participants who did vs. did not develop anti-spike antibodies in response to ChAdOx1 are planned.
Our results with respect to the adverse influence of older age, shorter inter-dose interval, poorer general health, immunodeficiency and immunosuppressants on post-vaccination antibody responses are also broadly consistent with those of others [4,5,6,7,8,9]–as is the finding that pre-vaccination SARS-CoV-2 infection associates with higher antibody titres after vaccination [9,12,13]. However, we also report a number of novel findings. The independent association between regular use of vitamin D supplements and reduced risk of post-vaccination seronegativity echoes results of a previous study where we showed that high-dose vitamin D replacement enhances antigen-specific cellular responses following vaccination against varicella zoster virus [28]; an intervention study nested within a randomised controlled trial of vitamin D supplementation for prevention of COVID-19 (ClinicalTrials.gov identifier NCT04579640) is currently on-going to explore this finding further. With respect to season, we found that anti-S titres were significantly higher for participants who received their second vaccine dose in Q1 vs. Q2. Seasonal variations in human immune responses are well recognised, with studies in Europeans showing a more pro-inflammatory transcriptomic profile in winter vs. summer [29,30]. Linder and colleagues reported a stronger immune response to rubella vaccine in children vaccinated in winter vs. summer in Israel [31], while Martins and colleagues found that children in Guinea-Bissau who were vaccinated against measles during the rainy season had higher antibody levels than those vaccinated in the dry season [32]. However, other investigators have found no differences in antibody responses according to season of vaccination against tetanus, diphtheria or measles or rubella [33,34]. Further research is needed to investigate the mechanisms by which season of inoculation may influence vaccine immunogenicity.
We also found that White ethnicity and lower BMI both associated with lower post-vaccination antibody titres, independently of pre-vaccination SARS-CoV-2 serostatus. Ethnic variation in SARS-CoV-2 vaccine immunogenicity has not been widely studied to date: one study has reported lower antibody responses to vaccination in Jewish vs. non-Jewish health care workers in Israel [9], while a population-based UK study reported higher antibody titres in ‘non-Whites’ vs. Whites. However, pre-vaccination serostatus was not available for all participants in these studies; this omission is potentially important, because unvaccinated people of South Asian ethnic origin were at higher risk of SARS-CoV-2 infection in the pre-vaccination era [20,24]. We demonstrate for the first time that higher anti-spike titres among vaccinated people of South Asian origin are not attributable to the higher rates of pre-vaccination SARS-CoV-2 infection that we have previously reported [20,24]. The reasons for this phenomenon require further investigation: ethnic variation in recognition of SARS-CoV-2 T cell epitopes is recognised [35], and it may be that analogous variation in B cell epitopes underlies the ethnic differences in antibody responses to vaccination seen here. By contrast with ethnicity, several studies have investigated the impact of BMI on post-vaccination antibody titres. Their results are inconsistent, with one reporting lower antibody responses in those with BMI > 30 kg/m2 [4], another reporting higher responses in those with higher BMI [36] and two reporting no association [37,38]. Of note, studies investigating antibody responses to influenza vaccination report higher titres initially in obese participants, but a greater decline thereafter [39]. This finding highlights the importance of longer term follow-up to elucidate the influence of BMI on humoral responses to vaccination.
We also report some important null results. The lack of association between time of day of vaccination and degree of antibody response conflicts with findings of studies which variously report higher anti-spike responses in those vaccinated earlier [40] or later [41] in the day. We also show no association between pre- or post-vaccination use of paracetamol or NSAIDs and antibody responses, after adjustment for incidence of post-vaccination symptoms. This provides reassurance that use of these medications to manage reactive post-vaccination symptoms does not compromise SARS-CoV-2 vaccine immunogenicity. We also show no association between higher titres and socioeconomic deprivation, as reported by others [4,7]; this may reflect the fact that other studies did not adjust for pre-vaccination serostatus, which is more likely to be positive in socioeconomically deprived populations. There was no evidence of association for lifestyle factors hypothesised to influence vaccine responses including dietary factors, use of micronutrient supplements other than vitamin D, sleep, alcohol use and tobacco smoking.
Our study has several strengths. We utilised a CE-marked semi-quantitative assay with high sensitivity and specificity that targets three different types of antibody [21], and that we have validated as a correlate of protection against breakthrough disease [22,23]. Its large size affords power to detect determinants of having undetectable anti-spike antibodies after SARS-CoV-2 vaccination–a relatively rare, but potentially clinically important outcome. The population-based nature of this study, and the fact that we investigated effects of two major vaccines utilising differing technology (one live vector, one mRNA) enhances the generalisability of our findings. Very detailed characterisation of participants allowed us to investigate a wide range of potential determinants of vaccine immunogenicity, and to adjust for multiple confounders, including pre-vaccination serostatus.
Our study also has some limitations. We did not study cellular responses to vaccination, and these may be discordant with humoral responses [10,26,42,43,44]. Methods to detect SARS-CoV-2-specific T cell responses have been used by other groups [45,46] We also studied humoral responses at an early time point only; a high early peak in antibody titres following vaccination is not necessarily an indicator of durable response [39]. As with any observational study, we cannot exclude the possibility that some of the associations we report might be explained by residual or unmeasured confounding. However, we have minimised the risk of this by adjusting for a comprehensive list of putative determinants of vaccine immunogenicity. COVIDENCE UK is also a self-selected cohort, and thus certain demographics—such as people < 30 years old, people of lower socioeconomic status, and non-White ethnic groups—are under-represented. This may have limited our power to investigate antibody responses in some sub-groups, such as participants of Black ethnicity (who are at higher risk of SARS-CoV-2 infection [47,48,49] and adverse outcomes [50] than White people).
In conclusion, this large population-based study reports on the influence of a comprehensive range of potential sociodemographic, behavioural, clinical, pharmacologic and nutritional determinants on antibody responses to two major SARS-CoV-2 vaccines.
Acknowledgments
We thank all participants of COVIDENCE UK, and the following organisations who supported study recruitment: Asthma UK, the British Heart Foundation, the British Lung Foundation, the British Obesity Society, Cancer Research UK, Diabetes UK, Future Publishing, Kidney Care UK, Kidney Wales, Mumsnet, the National Kidney Federation, the National Rheumatoid Arthritis Society, the North West London Health Research Register (DISCOVER), Primary Immunodeficiency UK, the Race Equality Foundation, SWM Health, the Terence Higgins Trust, and Vasculitis UK.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10101601/s1. Table S1. Baseline questions; Table S2. Monthly follow-up questions; Table S3. Determinants of antibody titres following administration of two doses of ChAdOx1 nCoV-19 vaccine; Table S4. Determinants of antibody titres following administration of two doses of BNT162b2 vaccine; Table S5. Influence of post-vaccination use of paracetamol/NSAIDs on antibody titres; Figure S1. Study profile; Figure S2. Post-vaccination SARS-CoV-2 antibody titres by vaccine type vs. diagnosis of hypertension (A), prescribed immunosuppressant medications (B) or consumption of selenium supplements (C) [51,52].
Author Contributions
A.R.M. wrote the study protocol, with input from D.A.J., M.T. and S.O.S.; H.H., M.T., J.S., G.A.D., R.A.L., C.J.G., F.K., A.S. and A.R.M. contributed to questionnaire development and design; D.A.J. managed the study, with input from A.R.M., N.P., H.H. and S.M.; H.H., J.S., A.R.M. and S.O.S. supported recruitment; S.E.F. and A.G.R. developed, validated, and performed laboratory assays; D.A.J., M.T., H.H., M.G., G.V. and F.T. contributed to data management; Statistical analyses were done by D.A.J., with input from A.R.M.; D.A.J. and A.R.M. wrote the first draft of the report. All authors critically revised it before submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
COVIDENCE UK was sponsored by Queen Mary University of London and approved by Leicester South Research Ethics Committee (ref 20/EM/0117). It is registered with ClinicalTrials.gov (NCT04330599).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
De-identified participant data will be made available upon reasonable request to the corresponding author, subject to terms of Research Ethics Committee approval and Sponsor requirements.
Conflicts of Interest
J.S. declares receipt of payments from Reach plc for news stories written about recruitment to, and findings of, the COVIDENCE UK study. A.S. is a member of various UK and Scottish Government COVID-19 Advisory Groups, was an unremunerated member of Astra-Zeneca’s Thrombotic Thrombocytopenic Task Force, and holds COVID-19 research grants from UKRI (MRC), HDRUK, National Core Studies, CSO, GSK and NIHR. A.R.M. declares receipt of funding in the last 36 months to support vitamin D research from the following companies who manufacture or sell vitamin D supplements: Pharma Nord Ltd., DSM Nutritional Products Ltd., Thornton & Ross Ltd. and Hyphens Pharma Ltd. A.R.M. also declares support for attending meetings from the following companies who manufacture or sell vitamin D supplements: Pharma Nord Ltd and Abiogen Pharma Ltd. ARM also declares participation on the Data and Safety Monitoring Board for the Chair, DSMB, VITALITY trial (Vitamin D for Adolescents with HIV to reduce musculoskeletal morbidity and immunopathology). A.R.M. also declares unpaid work as a Programme Committee member for the Vitamin D Workshop. A.R.M. also declares receipt of vitamin D capsules for clinical trial use from Pharma Nord Ltd., Synergy Biologics Ltd. and Cytoplan Ltd. All other authors declare no competing interests. R.A.L. and A.S. are members of the Welsh Government COVID-19 Technical Advisory Groups and holds COVID-19 research grants from UKRI (MRC), HDRUK, National Core Studies, and HCRW.
Funding Statement
This work was supported by Barts Charity (refs MGU0466, MGU0459 and MGU0570), Rosetrees Trust and The Bloom Foundation (ref M771), The Fischer Family Trust, The Exilarch’s Foundation, UK National Institute for Health and Care Research Clinical Research Network (refs 52255 and 52257), and the UK Research and Innovation Industrial Strategy Challenge Fund (ref MC_PC_19004).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Monte S.M., Long C., Szczepanski N., Griffin C., Fitzgerald A., Chapin K. Heterogeneous Longitudinal Antibody Responses to COVID-19 mRNA Vaccination. Clin. Pathol. 2021;14:2632010X211049255. doi: 10.1177/2632010X211049255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann P., Curtis N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019;32:e00084-18. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., Bell J.I., Newton J.N., Farrar J., Diamond I., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021;6:1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne R.P., Longet S., Austin J.A., Skelly D.T., Dejnirattisai W., Adele S., Meardon N., Faustini S., Al-Taei S., Moore S.C., et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirthalingam G., Bernal J.L., Andrews N.J., Whitaker H., Gower C., Stowe J., Tessier E., Subbarao S., Ireland G., Baawuah F., et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat. Commun. 2021;12:7217. doi: 10.1038/s41467-021-27410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kertes J., Gez S.B., Saciuk Y., Supino-Rosin L., Stein N.S., Mizrahi-Reuveni M., Zohar A.E. Effectiveness of mRNA BNT162b2 Vaccine 6 Months after Vaccination among Patients in Large Health Maintenance Organization, Israel. Emerg. Infect. Dis. 2021;28:338–346. doi: 10.3201/eid2802.211834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoli R., De Sarro C., Palleria C., Gallelli L., Pileggi C., De Sarro G. Serological Response to SARS-CoV-2 Messenger RNA Vaccine: Real-World Evidence from Italian Adult Population. Vaccines. 2021;9:1494. doi: 10.3390/vaccines9121494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Ruiz M., Almendro-Vazquez P., Carretero O., Ruiz-Merlo T., Laguna-Goya R., San Juan R., Lopez-Medrano F., Garcia-Rios E., Mas V., Moreno-Batenero M., et al. Discordance Between SARS-CoV-2-specific Cell-mediated and Antibody Responses Elicited by mRNA-1273 Vaccine in Kidney and Liver Transplant Recipients. Transplant. Direct. 2021;7:e794. doi: 10.1097/TXD.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parry H., McIlroy G., Bruton R., Ali M., Stephens C., Damery S., Otter A., McSkeane T., Rolfe H., Faustini S., et al. Antibody responses after first and second COVID-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11:136. doi: 10.1038/s41408-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manisty C., Otter A.D., Treibel T.A., McKnight A., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre D.W., Lumley S.F., Wei J., Cox S., James T., Justice A., Jesuthasan G., O’Donnell D., Howarth A., Hatch S.B., et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021;27:1516.e7–1516.e14. doi: 10.1016/j.cmi.2021.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long J.E., Drayson M.T., Taylor A.E., Toellner K.M., Lord J.M., Phillips A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine. 2016;34:2679–2685. doi: 10.1016/j.vaccine.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayman M.P., Calder P.C. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br. J. Nutr. 2021;126:1919–1920. doi: 10.1017/S0007114521000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict C., Cedernaes J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir. Med. 2021;9:447–448. doi: 10.1016/S2213-2600(21)00126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calina D., Hartung T., Mardare I., Mitroi M., Poulas K., Tsatsakis A., Rogoveanu I., Docea A.O. COVID-19 pandemic and alcohol consumption: Impacts and interconnections. Toxicol. Rep. 2021;8:529–535. doi: 10.1016/j.toxrep.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara P., Ponticelli D., Aguero F., Caci G., Vitale A., Borrelli M., Schiavone B., Antonazzo I.C., Mantovani L.G., Tomaselli V., et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health. 2022;203:97–99. doi: 10.1016/j.puhe.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prymula R., Siegrist C.A., Chlibek R., Zemlickova H., Vackova M., Smetana J., Lommel P., Kaliskova E., Borys D., Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: Two open-label, randomised controlled trials. Lancet. 2009;374:1339–1350. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 20.Holt H., Talaei M., Greenig M., Zenner D., Symons J., Relton C., Young K.S., Davies M.R., Thompson K.N., Ashman J., et al. Risk factors for developing COVID-19: A population-based longitudinal study (COVIDENCE UK) Thorax. 2021;77:900–912. doi: 10.1136/thoraxjnl-2021-217487. [DOI] [PubMed] [Google Scholar]
- 21.Morley G.L., Taylor S., Jossi S., Perez-Toledo M., Faustini S.E., Marcial-Juarez E., Shields A.M., Goodall M., Allen J.D., Watanabe Y., et al. Sensitive Detection of SARS-CoV-2-Specific Antibodies in Dried Blood Spot Samples. Emerg. Infect. Dis. 2020;26:2970–2973. doi: 10.3201/eid2612.203309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields A.M., Faustini S.E., Kristunas C.A., Cook A.M., Backhouse C., Dunbar L., Ebanks D., Emmanuel B., Crouch E., Kroger A., et al. COVID-19: Seroprevalence and Vaccine Responses in UK Dental Care Professionals. J. Dent. Res. 2021;100:1220–1227. doi: 10.1177/00220345211020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivaldi G., Jolliffe D.A., Faustini S.E., Holt H., Perdek N., Talaei M., Tydeman F., Chambers E.S., Cai W., Li W., et al. Correlation between post-vaccination titres of IgG, IgA and IgM anti-Spike antibodies and protection against breakthrough SARS-CoV-2 infection: A population-based longitudinal study (COVIDENCE UK) medRxiv. 2022 doi: 10.1101/2022.02.11.22270667. [DOI] [Google Scholar]
- 24.Talaei M., Faustini S.E., Holt H., Jolliffe D.A., Vivaldi G., Greenig M., Perdek N., Maltby S., Bigogno C.M., Symons J., et al. Determinants of pre-vaccination antibody responses to SARS-CoV-2: A population-based longitudinal study (COVIDENCE UK) BMC Med. 2022;20:87. doi: 10.1186/s12916-022-02286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh E., Moody M.A., Walter E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccin. Immunother. 2016;12:2391–2402. doi: 10.1080/21645515.2016.1183077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Otter A., Hallis B., Zuo J., Moss P. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun. Ageing. 2021;18:34. doi: 10.1186/s12979-021-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fausther-Bovendo H., Kobinger G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014;10:2875–2884. doi: 10.4161/hv.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers E.S., Vukmanovic-Stejic M., Turner C.T., Shih B.B., Trahair H., Pollara G., Tsaliki E., Rustin M., Freeman T.C., Mabbott N.A., et al. Vitamin D3 replacement enhances antigen-specific immunity in older adults. Immunother. Adv. 2020;1:ltaa008. doi: 10.1093/immadv/ltaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dopico X.C., Evangelou M., Ferreira R.C., Guo H., Pekalski M.L., Smyth D.J., Cooper N., Burren O.S., Fulford A.J., Hennig B.J., et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyse C., O’Malley G., Coogan A.N., McConkey S., Smith D.J. Seasonal and daytime variation in multiple immune parameters in humans: Evidence from 329,261 participants of the UK Biobank cohort. iScience. 2021;24:102255. doi: 10.1016/j.isci.2021.102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linder N., Abudi Y., Abdalla W., Badir M., Amitai Y., Samuels J., Mendelson E., Levy I. Effect of season of inoculation on immune response to rubella vaccine in children. J. Trop. Pediatr. 2011;57:299–302. doi: 10.1093/tropej/fmp104. [DOI] [PubMed] [Google Scholar]
- 32.Martins C., Garly M.L., Bale C., Rodrigues A., Njie-Jobe J., Benn C.S., Whittle H., Aaby P. Measles virus antibody responses in children randomly assigned to receive standard-titer edmonston-zagreb measles vaccine at 4.5 and 9 months of age, 9 months of age, or 9 and 18 months of age. J. Infect. Dis. 2014;210:693–700. doi: 10.1093/infdis/jiu117. [DOI] [PubMed] [Google Scholar]
- 33.Abreu T.C., Boshuizen H., Mollema L., Berbers G.A.M., Korthals A.H. Association between season of vaccination and antibody levels against infectious diseases. Epidemiol. Infect. 2020;148:e276. doi: 10.1017/S0950268820002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore S.E., Collinson A.C., Fulford A.J., Jalil F., Siegrist C.A., Goldblatt D., Hanson L.Å., Prentice A.M. Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop. Med. Int. Health. 2006;11:1529–1541. doi: 10.1111/j.1365-3156.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 35.Bose T., Pant N., Pinna N.K., Bhar S., Dutta A., Mande S.S. Does immune recognition of SARS-CoV2 epitopes vary between different ethnic groups? Virus Res. 2021;305:198579. doi: 10.1016/j.virusres.2021.198579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra S.K., Pradhan S.K., Pati S., Sahu S., Nanda R.K. Waning of Anti-spike Antibodies in AZD1222 (ChAdOx1) Vaccinated Healthcare Providers: A Prospective Longitudinal Study. Cureus. 2021;13:e19879. doi: 10.7759/cureus.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uysal E.B., Gumus S., Bektore B., Bozkurt H., Gozalan A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2021;94:1060–1066. doi: 10.1002/jmv.27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S.W., Moon J.Y., Lee S.K., Lee H., Moon S., Chung S.J., Yeo Y., Park T.S., Park D.W., Kim T.H., et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021;12:779212. doi: 10.3389/fimmu.2021.779212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheridan P.A., Paich H.A., Handy J., Karlsson E.A., Hudgens M.G., Sammon A.B., Holland L.A., Weir S., Noah T.L., Beck M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Liu Y., Liu D., Zeng Q., Li L., Zhou Q., Li M., Mei J., Yang N., Mo S., et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 2021;31:1215–1217. doi: 10.1038/s41422-021-00541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W., Balfe P., Eyre D.W., Lumley S.F., O’Donnell D., Warren F., Crook D.W., Jeffery K., Matthews P.C., Klerman E.B., et al. Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers. J. Biol. Rhythms. 2022;37:124–129. doi: 10.1177/07487304211059315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picchianti-Diamanti A., Aiello A., Lagana B., Agrati C., Castilletti C., Meschi S., Farroni C., Lapa D., Najafi Fard S., Cuzzi G., et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 mRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021;12:740249. doi: 10.3389/fimmu.2021.740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farroni C., Picchianti-Diamanti A., Aiello A., Nicastri E., Lagana B., Agrati C., Castilletti C., Meschi S., Colavita F., Cuzzi G., et al. Kinetics of the B- and T-Cell Immune Responses After 6 Months From SARS-CoV-2 mRNA Vaccination in Patients With Rheumatoid Arthritis. Front. Immunol. 2022;13:846753. doi: 10.3389/fimmu.2022.846753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tortorella C., Aiello A., Gasperini C., Agrati C., Castilletti C., Ruggieri S., Meschi S., Matusali G., Colavita F., Farroni C., et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrone L., Petruccioli E., Vanini V., Cuzzi G., Najafi Fard S., Alonzi T., Castilletti C., Palmieri F., Gualano G., Vittozzi P., et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2021;27:286-e7. doi: 10.1016/j.cmi.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petruccioli E., Najafi Fard S., Navarra A., Petrone L., Vanini V., Cuzzi G., Gualano G., Pierelli L., Bertoletti A., Nicastri E., et al. Exploratory analysis to identify the best antigen and the best immune biomarkers to study SARS-CoV-2 infection. J. Transl. Med. 2021;19:272. doi: 10.1186/s12967-021-02938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward H., Atchison C., Whitaker M., Ainslie K.E.C., Elliott J., Okell L., Redd R., Ashby D., Donnelly C.A., Barclay W., et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat. Commun. 2021;12:905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chadeau-Hyam M., Bodinier B., Elliott J., Whitaker M.D., Tzoulaki I., Vermeulen R., Kelly-Irving M., Delpierre C., Elliott P. Risk factors for positive and negative COVID-19 tests: A cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020;49:1454–1467. doi: 10.1093/ije/dyaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozenfeld Y., Beam J., Maier H., Haggerson W., Boudreau K., Carlson J., Medows R. A model of disparities: Risk factors associated with COVID-19 infection. Int. J. Equity Health. 2020;19:126. doi: 10.1186/s12939-020-01242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathur R., Rentsch C.T., Morton C.E., Hulme W.J., Schultze A., MacKenna B., Eggo R.M., Bhaskaran K., Wong A.Y.S., Williamson E.J., et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: An observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook A.M., Faustini S.E., Williams L.J., Cunningham A.F., Drayson M.T., Shields A.M., Kay D., Taylor L., Plant T., Huissoon A., et al. Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients. J. Immunol. Methods. 2021;494:113046. doi: 10.1016/j.jim.2021.113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data will be made available upon reasonable request to the corresponding author, subject to terms of Research Ethics Committee approval and Sponsor requirements.