Abstract
The association between blood eosinophil (EOS) counts and arterial/venous thrombosis is unclear. We aim to explore whether EOS count is a risk factor for thrombosis. We searched several databases and preprint platforms using core terms ‘eosinophil’, ‘myocardial infarction’, ‘ischemic stroke’, and ‘venous thromboembolism’ (VTE), among others. Studies comparing the odds ratios (ORs) or risk ratios (RRs) of EOSs with the abovementioned diseases were eligible. Overall, 22 studies were included. A high EOS count was associated with acute coronary artery thrombosis events (OR: 1.23, 95% CI: 1.15–1.32), short‐term cerebral infarction and mortality (RR: 2.87, 95% CI: 1.49–5.51). The short‐term risk of VTE was more common in patients with EOS‐related diseases (RR: 6.52, 95% CI: 2.42–17.54). For coronary artery disease, a high EOS count was a protective factor against 6‐month to 1‐year mortality (RR: 0.56, 95% CI: 0.45–0.69) but was associated with long‐term mortality (RR: 1.64, 95% CI: 1.25–2.14). Therefore, we conclude that for coronary artery thrombosis, EOS count is not associated with AMI events in general population. It may be associated with NSTEMI and STEMI in CAD patients, but more studies are needed to confirm this. In addition, EOS count is associated with an increased risk of both short‐ and long‐term mortality but is not predictive of the composite endpoints. For cerebral artery thrombosis, EOS count may be associated with cerebral infarction and could lead to an increased risk of poor short‐term prognosis. For VTEs, EOS count was a risk factor for some patients, especially those with acute‐phase EOS‐related diseases.
Keywords: eosinophil, ischemic stroke, myocardial infarction, pulmonary embolism, thrombosis
We searched 22 related articles from the PubMed, Embase, Cochrane Library databases and preprint platforms. Then we conducted a systematic review and meta‐analysis to assess the association between blood EOS count and thrombotic events and their associated adverse outcomes in patients with various arterial or venous thrombosis disorders.
1. INTRODUCTION
Blood eosinophilia is reported in numerous conditions, including allergic, infectious, inflammatory and neoplastic disorders. 1 In addition, recent evidence has shown that arterial/venous thrombosis disorders are associated with abnormal blood eosinophil (EOS) counts. 2 , 3 These studies emphasize the need to better understand the role of EOS counts in predicting the occurrence and severity of arterial/venous thrombosis disorders.
Coronary artery thrombosis and cerebral artery thrombosis, also called acute myocardial infarction (AMI) and ischemic stroke, respectively, are the most common arterial thrombotic diseases affecting patient health. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Recently, there has been increased clinical interest in the use of EOS counts as a predictor of coronary and cerebral arterial thrombosis disorders. However, the findings concerning EOS count and coronary or cerebral thrombosis disorders vary widely across studies. 3 , 5 , 9 , 11 Patients with venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), 3 , 12 , 13 had higher EOS counts than patients without thrombosis, 14 and persistent EOSs were the sole variable influencing the short‐term recurrence of venous thrombosis. 13 Therefore, the impact of EOS count on thrombotic events and the prognosis and severity of arterial/venous thrombosis diseases may depend on the type of arterial/venous thrombosis disorder.
Therefore, in view of the existing evidence and contradictory observations and taking into account the value of blood EOS counts in predicting the risks of thrombotic events and the poor prognosis related to such events, we considered that a systematic review and meta‐analysis assessing the association between blood EOS counts and thrombotic events and their associated adverse outcomes in patients with various arterial or venous thrombosis disorders is needed.
2. METHODS
The protocol of this study was preregistered in PROSPERO (CRD 42021284580) and adheres to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA guidelines [Table S1]). 15 Since published deidentified data were used, this study was exempt from institutional review board approval.
2.1. Systematic search
We systematically searched the MEDLINE/PubMed, Cochrane Library and Embase databases using the core terms ‘eosinophil’, ‘myocardial infarction’, ‘acute coronary syndrome’, ‘coronary heart disease’, ‘cerebral infarction’, ‘ischemic stroke’, ‘pulmonary embolism (PE)’, ‘venous thromboembolism (VTE)’ and ‘deep venous thrombosis (DVT)’ in the article titles, abstracts and keywords. We also searched articles from preprint platforms (Research Square and medRxiv) and scrutinized the reference lists of relevant articles to find additional pertinent studies. Two reviewers independently searched the databases and reviewed the full texts of the eligible articles.
2.2. Inclusion and exclusion criteria
Cohort, cross‐sectional and case–control studies comparing the odds ratios (ORs) or hazard ratios (HRs) of arterial/venous thrombosis events (AMI, ischemic stroke, cerebral infarction, VTE, PE and DVT) and clinical outcomes (including but not limited to mortality, severity classification, and major adverse cardiac events [MACEs]) related to arterial/venous disorders between patients with normal and abnormal EOS counts were eligible. The inclusion criteria were as follows: (1) studies that reported ORs, HRs, or RRs of the association between EOS count and coronary artery thrombosis and related outcomes among patients with AMI and CHD; (2) studies that reported ORs, HRs, or RRs of the association between EOS count and cerebral artery thrombosis and related outcomes among patients with cerebral infarction or ischemic stroke; (3) studies that reported ORs or HRs of the association between EOS count and VTE and related outcomes among VTE patients; and (4) studies published in English. Any studies that did not meet the abovementioned criteria and had data that were not extractable were excluded.
2.3. Data extraction
Two authors (W‐J.X. and S.W.) independently extracted information from the studies, including the authors, study design, sample size, inclusion/exclusion criteria, follow‐up terms, and results. Disagreements were resolved through consensus by discussion and inspection.
2.4. Outcomes
The primary outcomes of interest were the ORs or HRs of arterial/venous thrombosis events, severity and composite outcomes associated with EOS count. The predefined arterial/venous thrombosis events were AMI, ischemic stroke/cerebral infarction, VTE, PE and DVT. The secondary outcome was mortality.
2.5. Quality assessment
The risks of bias of the included studies were assessed with the Newcastle–Ottawa Quality Assessment Scale (NOS) for cohort and case–control studies. 16 This scale rates studies on three major domains: selection, comparability, and exposure/outcome. The NOS score represents the quality of the included study. High‐quality studies were defined as those with a score of 7 or higher, moderate‐quality studies had a score between 4 and 6, and low‐quality studies had a score less than 4. The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross‐Sectional Studies was applied for cross‐sectional studies. 17 The overall scores for each study were calculated as percentages and categorized as a quality assessment of high (80%–100%), fair (50%–79%) or low (<50%). 18
2.6. Statistical analysis
Data management, the transformation of the effect size, and the calculation of pooled prevalence were performed using Review Manager (RevMan) (Version 5.4, The Cochrane Collaboration, 2020). The results were reported using relative risks (RRs) with 95% confidence intervals (CIs) for cohort studies and ORs with 95% CIs for case–control and cross‐sectional studies using the generic inverse variance method. We applied fixed and random effect models depending on heterogeneity. The I 2 statistic was calculated to describe the heterogeneity, ranging from 0% to 100% (values of 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively). Funnel plot asymmetry was used to detect publication bias if more than 10 studies were included. 19
3. RESULTS
3.1. Search results
The initial systematic search identified 219 articles, including 209 records from the databases and 10 records from the preprint platforms, and 22 articles were qualitatively identified. Of these, 19 articles were ultimately quantitatively analyzed (Figure 1). The study characteristics of all of the included studies are summarized in Table 1. Ultimately, 22 articles were included with 805 329 subjects. Of these 22 articles, 13 were cohort studies, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 12 , 13 , 20 , 21 , 22 , 23 6 were cross‐sectional studies, 10 , 14 , 24 , 25 , 26 , 27 and 3 were case–control studies. 11 , 28 , 29 Of the included studies, 14 studies were related to cardiovascular diseases, 3 were related to cerebral thrombosis/cerebral infarction, and 6 were related to venous thrombotic events, among which the study by Anoop et al involved both cardiovascular disease and cerebral artery thrombosis. 9
FIGURE 1.
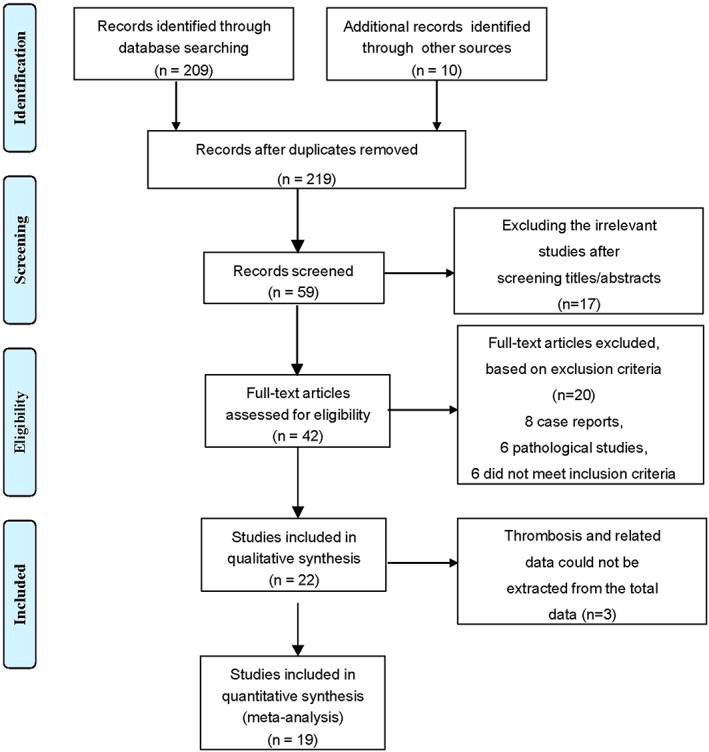
PRISMA diagram of articles screened for inclusion by disease type.
TABLE 1.
Characteristics of the included studies.
| Author and year | Design and sample size | Inclusion and exclusion criteria | Follow‐up | Endpoints | Association results |
|---|---|---|---|---|---|
| Cardiovascular diseases | |||||
| Robert Olivares 1993 20 | CoS n = 3779 | Inclusion: Male civil servants | Mean: 66.5 months | Prevalence of CAD | — |
| Exclusion: CAD history and abnormal electrocardiogram | |||||
| Iqbal S. Toor 2012 21 | CoS n = 909 | Inclusion: Elective indication or ACS (UA/NSTEMI) | Median: 54 months (IQR: 47–65) | All‐cause mortality | High EOS count was initially associated with reduced mortality but was associated with increased mortality after 6 months |
| Exclusion: STEMI; hemodynamic instability requiring angioplasty; failed PCI procedure | |||||
| Mehmet Demir 2014 27 | CSS n = 80 | Inclusion: Isolated CSF without any stenotic lesions; normal subjects | Perioperative period | CSF | High EOS count was associated with high CSF |
| Exclusion: CAD; chronic renal, liver, lung, hematological or valvular diseases; hypertension; DM; CHD; left ventricular systolic dysfunction; anemia; pregnancy; OSAS; malignancy; hyperthyroidism; infection; hypercholesterolemia, etc. | |||||
| Erhan Tenekecioglu 2015 10 | CSS n = 251 | Inclusion: NSTEMI and UA | Perioperative period | Correlation between EOS count and coronary thrombus | — |
| Exclusion: History of trauma, surgery, neoplasm, infectious disease, cardiogenic shock, etc. | |||||
| Monica Verdoia 2014 11 | CCS n = 1546 | Inclusion: Elective indication or ACS (UA/NSTEMI) | Perioperative period | Periprocedural MI; TnI ≥3 ULN or an increase by 50% | — |
| Exclusion: STEMI and hemodynamically unstable patients requiring urgent angioplasty | |||||
| Monica Verdoia 2015 28 | CCS n = 3742 | Inclusion: Elective indication and ACS | Perioperative period | Prevalence of CAD | — |
| Exclusion: STEMI or UA patients requiring urgent angiography | |||||
| Jun Wang 2016 26 | CSS n = 502 | Inclusion: UA with coronary stenosis ≥80% | Perioperative period | CCC | High EOS count was associated with high‐grade CCC |
| Exclusion: AMI | |||||
| Korkmaz Özge 2016 8 | CoS n = 251 | Inclusion: CAD patients undergoing isolated on‐pump CABG | Median: 6.2 ± 0.8 months | Cardiovascular mortality | Low EOS count predicted mortality in CAD patients with CABG |
| Exclusion: Infections; hepatic, renal or hematological disorders; inflammatory diseases with steroid therapy; malignancy, etc. | |||||
| Anoop Dinesh Shah 2016 9 | CoS n = 775 231 | Inclusion: Age ≥30 years without CVD at baseline | Median: 3.8 years | Occurrence of ACS, sudden coronary or cardiac death, etc. | Low EOS count in the general population was associated with increased short‐term incidence of HF and coronary death |
| Exclusion: A prior history of CVD, pregnancy record within 6 months, HIV, splenectomy or dialysis | |||||
| Takao Konishi 2017 7 | CoS n = 331 | Inclusion: Patients who underwent PCI for STEMI within 24 h | 1 year | MACEs | High EOS count independently predicted MACEs |
| Exclusion: Infections, allergic diseases, or malignancy; evaluated ≥48 h after onset; or died within 24 h | |||||
| Arthur Shiyovich 2017 23 | CoS n = 2129 | Inclusion: AMI | Median: 8.1 year | All‐cause death at 1, 5, and 10 years | High EOS count independently predicted death in the long term after AMI |
| Exclusion: Cancer, chronic inflammatory diseases, systemic infection, autoimmune disease, and in‐hospital stay <3 days | |||||
| Xueyan Zhao 2019 5 | CoS n = 8943 | Inclusion: Triple‐vessel CAD | Median: 7.5 year | All‐cause death MACCEs | High EOS count independently predicted MACCEs |
| Exclusion: Lack of C‐reactive protein data | |||||
| Shanshan Gao 2019 25 | CSS n = 5287 | Inclusion: CAD patients undergoing coronary angiography | Period | NSTEMI/ASTEMI | Low EOS count remained strongly associated with severe CAD and acute coronary arterial thrombotic events |
| Exclusion: Autoimmune or allergic diseases, parasitic infections, malignancies, severe liver or kidney dysfunction, HF or shock, rheumatic or valvular heart diseases, CABG | |||||
| Mohammad Alkhalil 2019 4 | CoS n = 606 | Inclusion: STEMI with PCI intervention | Median: 3.5 year | Composite AEs (death, MI, stroke) | Low EOS count was a negative marker for AMI patients |
| Exclusion: Not residents of Northern Ireland | |||||
| Cerebral artery thrombosis | |||||
| Yusuke S. Hori 2016 22 | CoS n = 405 | Inclusion: Evaluated within 24 h after symptom onset | 2 months | Mortality | Eosinopenia was strongly associated with 2‐m mortality in cerebral infarction patients |
| Exclusion: A history of cerebral infarction, infection, hematologic or chronic inflammatory disease, and the use of corticosteroids | |||||
| Anoop Dinesh Shah 2016 9 | CoS n = 775 231 | Inclusion: Age ≥30 years without CVD at baseline in the CALIBER program | Median: 3.8 years | Occurrence of TIA, stroke, subarachnoid hemorrhage | EOS count was associated with subarachnoid hemorrhage but was not associated with the short‐term incidence of intracerebral or ischemic stroke |
| Exclusion: A prior history of CVD, pregnancy record within 6 months, HIV, splenectomy or dialysis | |||||
| Huimin Zhao 2020 6 | CoS n = 174 | Inclusion: Evaluated within 72 h after symptom onset | 90 days | Modified Rankin Scale score >3 | Eosinopenia was significantly associated with mortality |
| Exclusion: Any diseases and drugs interfering with the EOS count | |||||
| Venous thromboembolism events | |||||
| Serdar Keceog˘lu 2014 29 | CCS n = 89 | Inclusion: Persistent AF | Not mentioned | Thrombus in the LA or LA appendage | High EOS count was associated with LA thrombus in patients with nonvalvular AF |
| Exclusion: CAD; chronic renal, liver, hematological and valvular diseases; CHD; HF; left ventricular systolic dysfunction; marked LA dilatation; hyperthyroidism; pregnancy; OSAS; COPD; stroke; malignancy, etc. | |||||
| F.N.Ozdemir 2005 14 | CSS n = 141 | Inclusion: AVF | 6 months prior to thrombosis events | AVF thrombosis | High EOS count was associated with AVF thrombosis |
| Exclusion: DM, graft, fistulae, amyloidosis, and vasculitis | |||||
| Asli Kurtar Mansiroglu 2021 24 | CSS n = 243 | Inclusion: Suspected DVT | At the time of diagnosis before any treatment | Acute DVT | Low EOS count may lead to a higher probability of acute DVT rather than indeterminate and chronic DVT |
| Exclusion: Prior DVT; pregnancy; inflammatory, infectious, hematological or autoimmune diseases; neoplasm; trauma; renal or liver failure | |||||
| Yecheng Liu 2020 3 | CoS n = 63 | Inclusion: Aged 18–60 years; acute VTE; onset VTE after a chronic course of HE; Autar DVT risk assessment scale score ≤5 | 3–6 months after discharge | Recurrent VTE | HE is a potential risk factor for VTE |
| Exclusion: Thrombophilia | |||||
| Valériane Réau 2021 13 | CoS n = 54 | Inclusion: Aged ≥15 years; VTE; EOS ≥1G/L at VTE occurrence | Median: 24 [IQR: 10–62] months | Recurrence of VTE, major bleeding events, vascular relapse and death. | Persistent eosinophilia was associated with VTE relapse |
| Exclusion: History of VTE, thrombophilia, or major transient or reversible predisposing factor for VTE | |||||
| Alessandra Bettiol 2021 12 | CoS n = 573 | Inclusion: EGPA patients | Median: 1677 (IQR: 663–3137) days | First AVTE events a | High EOS count was associated with AVTE |
Abbreviations: ACS, acute coronary syndrome; AEC, absolute eosinophil count; AF, atrial fibrillation; AVF, arteriovenous fistulae; AVTE, acute arterial and venous thromboembolic event; CABG, coronary artery bypass graft; CAD, coronary artery disease; CALIBER, cardiovascular disease research using linked bespoke studies and electronic health records; CCC, coronary collateral circulation; CCS, case–control study; CHD, congenital heart disease; COPD, chronic obstructive pulmonary disease; CoS, cohort study; CSF, coronary slow flow; CSS, cross‐sectional study; CVD, cardiovascular disease; CVST, cerebral venous sinus thrombosis; DM, diabetes mellitus; DVT, deep venous thrombosis; eGPA, eosinophilic granulomatosis with polyangiitis; HE, hypereosinophilia; HF, heart failure; IQR, interquartile range; LA, left atrium; MACCE, major adverse cardiovascular and cerebrovascular event; MACE, major adverse cardiac event; MI, myocardial infarction; NSTEMI, acute myocardial infarction with non‐ST‐elevation; OSAS, obstructive sleep apnea; PCI, percutaneous coronary intervention; RR, relative risk; SVT, superficial venous thrombosis; TIA, transient ischemic attack; UA, unstable angina pectoris; VTE, venous thromboembolism.
Venous events included DVT; PE; SVT; ovarian, renal, splanchnic, jugular, and retinal vein occlusion; and CVST. Arterial events included AMI, stroke, TIA, acute ischemia of the limbs, and arterial retinal occlusion.
3.2. Methodological quality assessment
All articles were assessed for methodological quality. According to the NOS assessment, most studies showed no evidence of selection bias, with good comparability in each cohort and outcome assessment. All included cohort, case–control, and cross‐sectional studies were rated as high quality (Tables S2–S4). For the subgroup analysis, fewer than 10 studies were included, so no publication bias assessment was applied.
3.3. Main analyses
3.3.1. Cardiovascular events
In an analysis of included studies of cardiovascular events, it was found that a high EOS count was associated with acute coronary artery thrombosis events (OR: 1.23, 95% CI: 1.15–1.32, p < 0.00001, I 2 = 87%) in all populations. A pooled analysis of 2 general population studies 9 , 20 showed that an increase in EOS count was not associated with AMI with moderate heterogeneity (OR: 0.96, 95% CI: 0.83–1.12, p = 0.62, I 2 = 60%). The study by Gao et al 25 revealed that an increased EOS count was associated with an increased risk of non‐ST‐elevated myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI) in the CAD population, with an OR of 1.37 (95% CI: 1.26–1.49, p < 0.00001). However, Verdoia et al 11 did not find an association between an increased EOS count and NSTEMI in the CAD population (OR: 1.04, 95% CI: 0.86–1.26, p = 0.69) (Figure 2A). In the study by Tenekecioglua et al, 10 an increased EOS count was found to be an independent predictor of coronary thrombus in patients with non‐ST segment elevation acute coronary syndrome (NST‐ACS). However, this study was not included in the pooled analysis because of the converted data.
FIGURE 2.
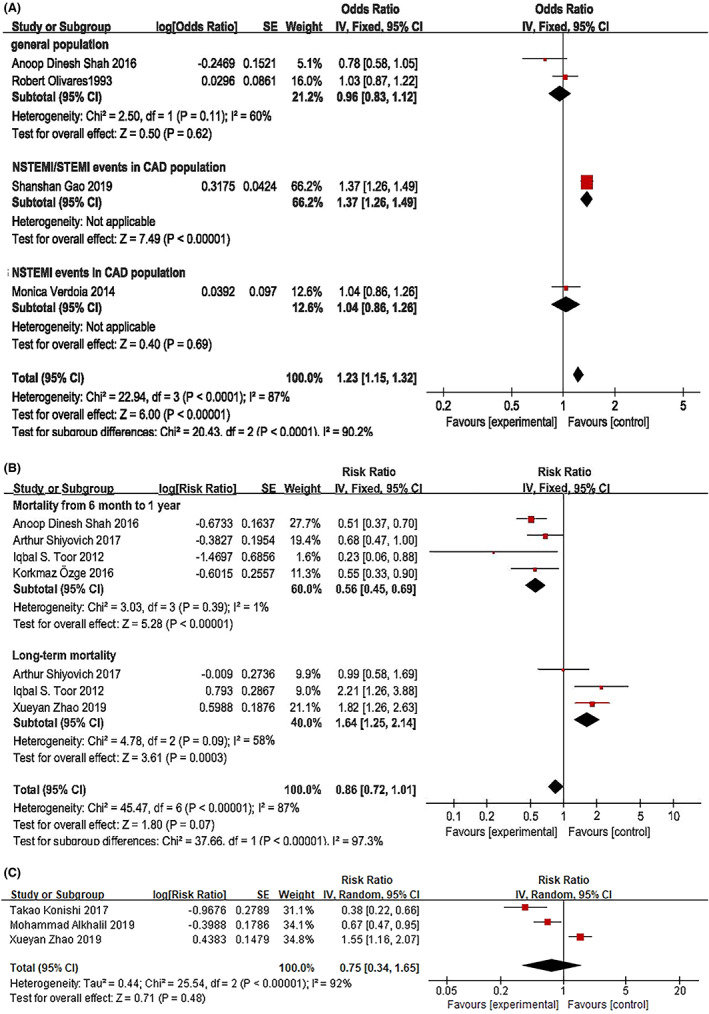
The association of EOS count with the acute phase of coronary artery thrombosis (A), the risk ratio of EOS count for mortality associated with CAD (B) and the risk ratio of EOS count for the composite endpoints of CAD (C).
With regard to mortality associated with CAD, including but not limited to AMI and isolated on‐pump coronary artery bypass, the RR of mortality was 0.56‐fold higher for patients with low EOS counts than for those with high EOS counts within 6 months to 1 year of an AMI diagnosis (95% CI: 0.45–0.69, p < 0.00001, I 2 = 1%). 8 , 9 , 21 , 23 Interestingly, a pooled analysis of 3 studies 5 , 21 , 23 showed that a high EOS count was associated with an increased risk of long‐term mortality in a median follow‐up period of 5–7.5 years with moderate heterogeneity (RR: 1.64, 95% CI: 1.25–2.14, p = 0.0003, I 2 = 58%) (Figure 2B).
For noncoronary artery thrombus and nonfatal cardiovascular events, high EOS counts were not found to be associated with CAD severity (OR: 0.99, 95% CI: 0.89–1.1, p = 0.9). 28 A high EOS count was a predictor of high‐grade coronary collateral circulation (CCC) (OR: 1.969, 95% CI: 1.210–3.321, p = 0.006), 26 and coronary slow flow (CSF) patients were more likely to have a high EOS count (OR: 1.02, 95% CI: 1.006–1.018, p = 0.076). 27 A cross‐sectional study revealed 5 that a low EOS count was associated with severe CAD (OR: 0.897, 95% CI: 0.850–0.946, p < 0.0001) and NSTEMI/anterior ST‐elevation myocardial infarction (ASTEMI) (OR: 0.728, 95% CI: 0.670–0.791, p < 0.0001). Nevertheless, pooled analysis of 3 cohort studies 4 , 5 , 7 revealed that EOS count was not predictive of the composite endpoints, such as MACEs and major adverse cardiovascular and cerebrovascular events (MACCEs) (Figure 2C).
3.3.2. Cerebral artery thrombosis
For acute ischemic stroke, only one study revealed that EOS count was independently associated with larger cerebral infarction risk, increased by 4.05‐fold (RR: 4.05, 95% CI: 1.31–12.51, p = 0.015). Only the study by Hori et al described the long‐term effect of EOS count on ischemic stroke 9 ; there was no strong association between a low EOS count and ischemic stroke in the general population, either in the first 6 months or over a median follow‐up of 3.8 years (RR: 1.17, 95% CI: 0.87–1.56; RR: 1.02, 95% CI: 0.88–1.19, both p > 0.05, respectively). For the secondary outcome, in 2 studies evaluating mortality, 6 , 22 an increased EOS count led to an increased risk for poor short‐term prognosis, with an RR of 2.87 without heterogeneity in the short‐term follow‐up (95% CI: 1.49–5.51, p = 0.002, I 2 = 0) (Figure 3A).
FIGURE 3.
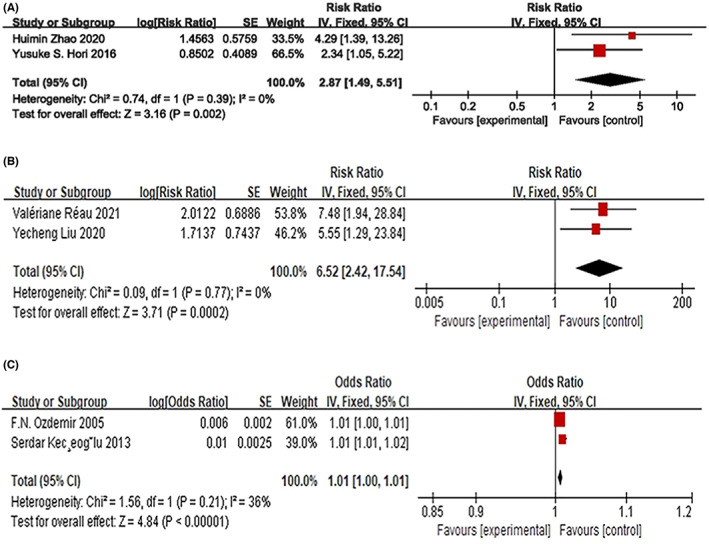
The association of EOS count with short‐term cerebral infarction and mortality associated with such events (A). The association of EOS count with venous thromboembolism events in patients with EOS‐related diseases (B) and in the general population (C).
3.3.3. VTEs
In patients with EOS‐related diseases, hypereosinophilia is a potential risk factor for VTE. 3 , 13 In pooled analysis, hypereosinophilia increased the short‐term risk of VTE over a period of 6 months without heterogeneity (HR: 6.52, 95% CI: 2.42–17.54, p = 0.0002, I 2 = 0) (Figure 3B). Only the study by Bettiol et al 12 described the long‐term VTE risk associated with high EOS counts in EOS‐related diseases and found that EOS count (≥500 U/mmc) was not associated with long‐term VTE during a median follow‐up of 1677 days (IQR: 663–3137 days) (HR: 0.70, 95% CI: 0.29–1.67, p > 0.05). In addition, Liu et al 3 found that PE in patients with IHE/HES and DVT is associated with a higher EOS count. In the general population, a high EOS count was also a risk factor for venous thrombosis in the acute phase of venous thrombosis with mild heterogeneity (OR: 1.01, 95% CI: 1.00–1.01, p < 0.00001, I 2 = 36%) 14 , 29 (Figure 3C). However, in the study by Mansiroglu et al, 24 a low EOS count was associated with DVT in the acute phase of DVT (OR: 0.035, 95% CI: 0.007–0.189, P < 0.001).
4. DISCUSSION
Our study found that there were significant differences in the risk of arteriovenous thrombosis in different populations and diseases.
4.1. EOS count and coronary artery thrombosis
In the general population, we found that EOS count was not associated with AMI events. In patients with CAD, Gao et al found that the RR of AMI patients with an increased percentage of EOSs was higher than that of patients with decreased EOS levels. However, Monica et al found that EOS levels were not associated with the occurrence of periprocedural myocardial infarction (PMI) or myonecrosis in CAD patients undergoing nonurgent percutaneous coronary intervention. 11 We did not perform a pooled analysis of Gao and Verdoia's studies because of the high heterogeneity, 11 , 25 which may be due to the different populations of NSTEMI/ASTEMI and unstable angina (UA)/NSTEMI patients. Moreover, Gao's study confirmed that there were differences in the EOSs and the percentages of eosinophils in leukocytes (PELs) between NSTEMI patients and ASTEMI patients, and they found that the PELs and leukocyte counts had a strong negative correlation in groups of PEL tertiles and that low PELs were closely related to AMI. 25 Therefore, we believe that the change in the PELs may be attributed to the change in EOS count, which is related to thrombosis events.
The role of EOSs in coronary thrombosis events usually follows two mechanisms: atherosclerotic plaque and thrombosis. On the one hand, EOSs can interact with IL‐5, interferon, interferon‐α and collagen, thereby involving them in the production of monocytes and endothelial cells and, ultimately, the development of atherosclerosis. 11 , 30 , 31 On the other hand, studies have shown that in the acute phase of coronary artery thrombosis EOSs enter coronary atherosclerotic plaques due to a stress response and the influence of treatment with anticoagulants and chemokines, 21 , 32 which leads to a decrease in the EOS count. Meanwhile, EOSs entering the plaque can not only interact with tissue factors to activate the endogenous coagulation pathway but can also release platelet‐activating factors, major basic protein (MBP), eosinophil cationic proteins (ECPs) and inflammatory substances, ultimately leading to thrombosis. 3
Interestingly, we found that patients with lower EOS counts had a high risk of short‐term (6–12 months) mortality, while patients with higher EOS counts had a high risk of long‐term mortality. The causes of the former may be emergency and stress responses, a large number of peripheral EOSs entering the thrombus site, low immunity or immunosuppression. 9 , 32 The reason for the latter may be related to the roles of EOSs, including infiltrating the stent implantation site, releasing mediators that increase platelet aggregation after PCI and promoting the formation of atherosclerotic plaques and thrombosis. 33 In the early stage of disease treatment, dual antiplatelet therapy can offset this risk. 21 As the follow‐up time is prolonged, patients with high EOS counts may release more sufficient EOSs into the lesion site and induce immunosuppression by inhibiting T‐cell activation and reducing their activity, resulting in an increase in long‐term mortality in these patients.
There are several causes of the heterogeneity in MACCEs. First, the proportion of patients who died varied among studies. Second, different follow‐up durations were noted among the studies. In three studies, the follow‐up durations were 1 year, a median of 3.5 years, and a median of 7.5 years. Third, different definitions of MACCEs were applied. The endpoints in Zhao's study 5 did not include revascularization. Alkhalil's study showed that there was a significant difference in unplanned revascularization among patients with lower or normal EOS counts, which led to a significant difference in the MACCE results. 4
Whether the change in EOS count is the cause or result of coronary thrombosis is still unknown. In Demir's study, an increase in EOS count was related to CSF, which can lead to coronary thrombotic events. 27 Gyurko's study showed that the increase in EOS count is secondary to other diseases and represents the inflammatory state caused by vascular injury; thus, increases in the EOS count are the result of atherosclerosis. Wang's study found that AMI patients establish a high level of CCC through the autocrine and paracrine regulation of EOSs and the regulation of EOSs on lymphocytes, which is conducive to a decrease in the incidence of MACCEs26. This also proves that the change in EOS count is the result of thrombotic events. We need to further study the impact of EOS counts on different MACCE endpoints in different periods.
4.2. EOS count and cerebral artery thrombosis
In this meta‐analysis, no studies on the relationship between EOS count and acute phase cerebral infarction were found, indicating the need for further attention in the future. Our meta‐analysis showed that a decreased EOS count (e.g. eosinopenia) predicted a poor short‐term risk. The causes of eosinopenia are as follows: first, the human body stimulates the release of adrenal glucocorticoids and epinephrine due to acute stress, resulting in a reduction in EOS count 34 ; second, chemokines such as complex 5A and fibrin fragments induce EOSs to enter the target organ or tissue, that is, the interior of the thrombus, resulting in a decrease in EOS count; and third, EOS degranulation causes cytotoxic protein‐mediated thrombosis and endothelial injury, resulting in EOSs that cannot be recognized in the circulation. 26 The decrease in EOSs in the peripheral blood circulation leads to a decline in immunity, called ‘stroke‐induced immunosuppression’, making patients more susceptible to infection and increasing mortality. 22 Inevitably, massive infarction and tissue ischemia will lead to poor prognosis.
4.3. EOS count and VTE
The eosinophilia‐related diseases in this study included clonal, reactive, overlapping and idiopathic eosinophilia, such as hypereosinophilic syndromes (HESs), eosinophilic granulomatosis with polyangiitis (EGPA) and parasitic diseases. In addition, EOSs can lead to venous, arterial and mixed thrombosis. 13 The RR of venous thrombosis in eosinophilia‐related disease patients with persistent eosinophilia was 6.52 times higher than that in patients with nonpersistent eosinophilia.
EOSs can play a role in the internal pathway of coagulation by producing tissue factors. 35 In addition, EOSs entering the thrombus are activated by platelets and release ECP, MBP and platelet‐activating factor, which have a positive effect on the process of coagulation activation. EOSs can also initiate and regulate local inflammation 21 and damage endothelial cells, leading to thrombosis, which is consistent with the findings of Reau et al. 13
This study also showed that patients with EOS‐related diseases with continuous increases in EOS count are more likely to develop venous thrombosis. According to our pooling analysis, we believe that increasing EOS counts are associated with thrombotic events. We speculate that EOS‐related diseases may have other mechanisms that can lead to thrombosis. In any case, we believe that thrombotic events can be prevented by reducing EOS levels. In addition, we found that coronary artery thrombosis, cerebral artery thrombosis, and VTE had different correlations with EOSs, which we thought might be related to the composition of the thrombus in addition to the location of arterial or venous thrombosis. An arterial thrombus is generally a white thrombus, while a venous thrombus is generally a red thrombus. This difference in composition may be related to EOSs.
By studying the correlation between eosinophilia and thrombus, we are able to assess the risk of coronary artery thrombosis, cerebral artery thrombosis, and DVT in the population, which can help us diagnose high‐risk patients as early as possible and improve prognosis. However, some of our findings are different from those of other articles, which we consider to be mainly related to the nature of systematic reviews, which include the results of multiple studies, and the small number of studies included.
5. LIMITATIONS
The main limitation of this study was the small sample size included in the analysis because there are few articles on the relationship between EOS count and arteriovenous thrombosis risk, which also highlights the importance of this review and meta‐analysis in providing evidence. Second, due to the small number of studies included, we did not perform a subgroup analysis of the impact of EOS count on the risk of long‐term ischemic stroke and the long‐term VTE risk in EOS‐related diseases. Third, thrombus may occur in familial EOS patients, and EOS mechanistically promotes thrombus generation. However, in all the studies we included, we could not extract relevant data to explore the relationship between familial EOS and thrombosis, and we hope to conduct more basic studies on familial EOS and thrombosis in the future. Fourth, we did not perform a sensitivity analysis. These factors may produce high heterogeneity and affect the reliability of the statistical results. In the future, multicenter randomized controlled trials are needed to further verify the impact of EOS counts on arterial and venous thromboembolism risk. Fifth, selection bias cannot be completely excluded from our meta‐analysis, and publication bias could not be assessed because of the insufficient number of research articles.
6. Clinical implications and future directions
Our analysis suggests that the levels of EOSs in different diseases may be related to the risk of arterial and venous thrombosis. For the general population, disorders of EOS levels have little influence on the occurrence of coronary and cerebral artery thrombosis events. However, for patients with cardiovascular disease, a high EOS count is associated with opposite effects on short‐term and long‐term mortality, which needs to be verified by further studies to determine whether different treatments should be used in different periods of disease for these patients. For patients with cerebral artery thrombosis, a high level of EOSs may predict a higher short‐term mortality, suggesting that we should pay more attention to these patients in the early diagnosis period and determine the treatment therapy in a timely manner. At the same time, for patients with hypereosinophilia, higher EOS levels may be associated with a short‐term risk of VTE, and more attention should be given to these patients to prevent the occurrence of VTE.
7. CONCLUSION
For coronary artery thrombosis, EOS count is not associated with AMI events in the general population. This finding may be associated with NSTEMI and STEMI in the CAD population, which needs more studies to be confirmed. In addition, it is associated with an increased risk for mortality in both the short and long term but is not predictive of the composite endpoints. For cerebral artery thrombosis, EOS count may be associated with cerebral infarction and could lead to an increased risk of poor short‐term prognosis. In terms of VTEs, EOS count was also a risk factor for some people, especially those with EOS‐related diseases in the acute phase. These findings are important for individual treatment. However, more clinical trials are needed to assess the ability of thrombotic screening and explore the optimal strategy for patients with abnormal EOS counts and a variety of different arterial/venous diseases.
AUTHOR CONTRIBUTIONS
W‐J.X. and R.J. conceived and designed the study. W‐J.X. and S.W. analyzed the data. L.W., P.Y., W‐J.X. and R.J. wrote the paper. J‐X.H. and R.J. edited the paper. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS APPROVAL STATEMENT
This article does not contain any studies with human or animal subjects.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
None.
Xu W‐J, Wang S, Yuan P, Wang L, Huang J‐X, Jiang R. Arterial and venous thromboembolism risk associated with blood eosinophils: A systematic review and meta‐analysis. Anim Models Exp Med. 2022;5:470‐481. doi: 10.1002/ame2.12277
Wei‐Jie Xu and Shang Wang contributed equally to the article.
Contributor Information
Jun‐Xia Huang, Email: huangjx075@hotmail.com.
Rong Jiang, Email: listening39@tongji.edu.cn, Email: listening39@163.com.
DATA AVAILABILITY STATEMENT
The data and materials are available from the corresponding author upon request.
REFERENCES
- 1. Valent P, Degenfeld‐Schonburg L, Sadovnik I, et al. Eosinophils and eosinophil‐associated disorders: immunological, clinical, and molecular complexity. Semin Immunopathol. 2021;43(3):423‐438. doi: 10.1007/s00281-021-00863-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ames PR, Aloj G, Gentile F. Eosinophilia and thrombosis in parasitic diseases: an overview. Clin Appl Thromb Hemost. 2011;17(1):33‐38. doi: 10.1177/1076029609348314 [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Meng X, Feng J, Zhou X, Zhu H. Hypereosinophilia with Concurrent Venous Thromboembolism: Clinical Features, Potential Risk Factors, and Short‐term Outcomes in a Chinese Cohort. Sci Rep. 2020;10(1):8359. doi: 10.1038/s41598-020-65128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alkhalil M, Kearney A, Hegarty M, et al. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am J Med. 2019;132(12):e827‐e834. doi: 10.1016/j.amjmed.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 5. Zhao X, Jiang L, Xu L, et al. Predictive value of in‐hospital white blood cell count in Chinese patients with triple‐vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872‐882. doi: 10.1177/2047487319826398 [DOI] [PubMed] [Google Scholar]
- 6. Zhao H, Xu Q, Feng H, Hou X, Wen Z, Cheng Q. Dynamic change of eosinophil and acute ischemic stroke. Research square. 2020;1‐13. doi: 10.21203/rs.3.rs-38803/v1 [DOI] [Google Scholar]
- 7. Konishi T, Funayama N, Yamamoto T, et al. Prognostic value of eosinophil to leukocyte ratio in patients with ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Atheroscler Thromb. 2017;24(8):827‐840. doi: 10.5551/jat.37937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korkmaz O, Kaya H, Beton O, et al. Is preoperative eosinopenia an independent predictor of early mortality for coronary artery bypass surgery? Heart Surg Forum. 2016;19(2):E088‐E093. doi: 10.1532/hsf.1450 [DOI] [PubMed] [Google Scholar]
- 9. Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. 2016;3(2):e000477. doi: 10.1136/openhrt-2016-000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenekecioglu E, Yilmaz M, Bekler A, Demir S. Eosinophil count is related with coronary thrombus in non ST‐elevated acute coronary syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(2):266‐271. doi: 10.5507/bp.2014.039 [DOI] [PubMed] [Google Scholar]
- 11. Verdoia M, Schaffer A, Barbieri L, et al. Eosinophils count and periprocedural myocardial infarction in patients undergoing percutaneous coronary interventions. Atherosclerosis. 2014;236(1):169‐174. doi: 10.1016/j.atherosclerosis.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 12. Bettiol A, Sinico RA, Schiavon F, et al. Risk of acute arterial and venous thromboembolic events in eosinophilic granulomatosis with polyangiitis (Churg‐Strauss syndrome). Eur Respir J. 2021;57(5):2004158. doi: 10.1183/13993003.04158-2020 [DOI] [PubMed] [Google Scholar]
- 13. Reau V, Vallee A, Terrier B, et al. Venous thrombosis and predictors of relapse in eosinophil‐related diseases. Sci Rep. 2021;11(1):6388. doi: 10.1038/s41598-021-85852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozdemir FN, Akcay A, Bilgic A, Akgul A, Arat Z, Haberal M. Effects of smoking and blood eosinophil count on the development of arteriovenous fistulae thrombosis in hemodialysis patients. Transplant Proc. 2005;37(7):2918‐2921. doi: 10.1016/j.transproceed.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17. Moola S, Munn Z, Sears K, et al. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute's approach. Int J Evid Based Healthc. 2015;13(3):163‐169. doi: 10.1097/XEB.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 18. Poudel P, Griffiths R, Wong VW, et al. Oral health knowledge, attitudes and care practices of people with diabetes: a systematic review. BMC Public Health. 2018;18(1):577. doi: 10.1186/s12889-018-5485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137(1):49‐53. doi: 10.1093/oxfordjournals.aje.a116601 [DOI] [PubMed] [Google Scholar]
- 21. Toor IS, Jaumdally R, Lip GY, Millane T, Varma C. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb Res Oct 2012;130(4):607–11. doi: 10.1016/j.thromres.2012.05.033 [DOI] [PubMed] [Google Scholar]
- 22. Hori YS, Kodera S, Sato Y, Shiojiri T. Eosinopenia as a predictive factor of the short‐term risk of mortality and infection after acute cerebral infarction. J Stroke Cerebrovasc Dis. 2016;25(6):1307‐1312. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 23. Shiyovich A, Gilutz H, Plakht Y. White blood cell subtypes are associated with a greater long‐term risk of death after acute myocardial infarction. Tex Heart Inst J. 2017;44(3):176‐188. doi: 10.14503/THIJ-16-5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mansiroglu AK, Sincer I, Cosgun M, Gunes Y. Dating thrombus organization with eosinophil counts in deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2021;9(4):874‐880. doi: 10.1016/j.jvsv.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 25. Gao S, Deng Y, Wu J, et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis. 2019;7(286):128‐134. doi: 10.1016/j.atherosclerosis.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Li Q, Li SJ, Wang DZ, Chen BX. Relationship of coronary collateral circulation with eosinophils in patients with unstable angina pectoris. Clin Interv Aging. 2016;11:105‐110. doi: 10.2147/CIA.S95363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demir M, Cosar S, Melek M. Evaluation of plasma eosinophil count and mean platelet volume in patients with coronary slow flow. Clinics (Sao Paulo). 2014;69(5):323‐326. doi: 10.6061/clinics/2014(05)05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verdoia M, Schaffer A, Cassetti E, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis. 2015;39(4):459‐466. doi: 10.1007/s11239-014-1120-3 [DOI] [PubMed] [Google Scholar]
- 29. Kecoglu S, Demir M, Uyan U, Melek M. The effects of eosinophil on the left atrial thrombus in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2014;20(3):285‐289. doi: 10.1177/1076029613483208 [DOI] [PubMed] [Google Scholar]
- 30. Zhao W, Lei T, Li H, et al. Macrophage‐specific overexpression of interleukin‐5 attenuates atherosclerosis in LDL receptor‐deficient mice. Gene Ther. 2015;22(8):645‐652. doi: 10.1038/gt.2015.33 [DOI] [PubMed] [Google Scholar]
- 31. Niccoli G, Montone RA, Sabato V, Crea F. Role of allergic inflammatory cells in coronary artery disease. Circulation. 2018;138(16):1736‐1748. doi: 10.1161/CIRCULATIONAHA.118.035400 [DOI] [PubMed] [Google Scholar]
- 32. Levinson AT, Casserly BP, Levy MM. Reducing mortality in severe sepsis and septic shock. Semin Respir Crit Care Med. 2011;32(2):195‐205. doi: 10.1055/s-0031-1275532 [DOI] [PubMed] [Google Scholar]
- 33. Marx C, Novotny J, Salbeck D, et al. Eosinophil‐platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134(21):1859‐1872. doi: 10.1182/blood.2019000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient‐level meta‐analysis. Lancet Respir Med. 2016;4(9):731‐741. doi: 10.1016/S2213-2600(16)30148-5 [DOI] [PubMed] [Google Scholar]
- 35. Moosbauer C, Morgenstern E, Cuvelier SL, et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109(3):995‐1002. doi: 10.1182/blood-2006-02-004945 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The data and materials are available from the corresponding author upon request.


