Abstract
The prevalence of celiac disease (CD) in Sweden is about 4 cases per 1,000 people. Screening for CD with serological tests indicates similar high prevalences in many other countries. Between 1 November 1992 and 30 April 1995, 133 children (9 months to 16.7 years of age) with suspected CD were studied. The predictive value (PV) of immunoglobulin A antigliadin antibodies (IgA-AGA) in the serum as assayed with two new commercial automated immunoassays—the Pharmacia CAP System Gliadin IgA FEIA (CAP) and the UNICAP-100 (UNICAP)—and with three “in-house” methods was evaluated using assessment of the small intestinal mucosa morphology as the “gold standard.” All serum samples were analyzed for total serum IgA. At presentation the diagnostic sensitivities and specificities of the different tests varied from 0.72 to 0.88 and 0.67 to 0.87, respectively. All methods showed a higher sensitivity for CD in younger children. The area under each assay's receiver operating characteristic curve was calculated and varied between 0.82 and 0.89. The positive and negative PVs for the CAP and UNICAP, which were assays with a high sensitivity and a high specificity, respectively, were estimated. In the clinically selected population (prevalence of CD, 1 in 3) the positive PV was about 55%, and in the general population (prevalence, 1 in 250) it was about 1%. The negative PVs for both CAP and UNICAP were close to 100%; thus, when the AGA test was negative, the risk for CD was small. Interestingly, five children had serum IgA levels below the detection limit (<0.07 g/liter) when on a gluten-free diet, whereas they had normal levels at the time of the first biopsy. In conclusion, the automated immunoassays—based on ImmunoCAP technology—for analysis of IgA-AGA had a reliability comparable to that of the in-house methods.
In many European countries celiac disease (CD) has become a public health problem. The prevalence of CD in Sweden is about 4 per 1,000 in both children and adults, although the majority of the adults are undiagnosed (5, 12, 18; K. Borch, E. Grodzinsky, F. Petersson, K. Å. Jönsson, S. Mårdh, and T. Valdimarsson, submitted for publication). Screening by serological tests for CD indicates similar prevalences in Italy, Northern Ireland, Denmark, and the United States (4, 19, 24, 31).
In childhood the most common symptoms are gastrointestinal, with the consequences of malabsorption, but the symptoms can be vague, e.g., short stature (3). The prevalence of CD is also high among first-degree relatives (22), and there is a strong association with the HLA-DQα1∗0501, β1∗0201 heterodimer (28). Furthermore CD is strongly associated with insulin-dependent diabetes mellitus (IDDM) (27), Down's syndrome (7), and selective immunoglobulin A (IgA) deficiency (17).
CD is caused by a lifelong intolerance to wheat gliadin (gluten) and related proteins in barely, rye, and possibly also oats (8). The mucosal lesion is characterized by villous atrophy, epithelial cell disarray, crypt hyperplasia, and lymphocytic infiltration of the epithelium and lamina propria (1). CD diagnosis in childhood is based on the small intestinal mucosa morphology, according to the criteria of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN); structurally abnormal jejunal mucosa on a gluten-containing diet, clear improvement of villous structure on a gluten-free diet (GFD), and deterioration of the mucosa upon gluten challenge (23). ESPGHAN has, however, revised the diagnostic criteria—reducing the number of small intestinal biopsies—which will increase the need for reliable serological tests (30). Many attempts have been made to develop reliable screening tests for CD to be used for diagnostic purposes and to monitor dietary compliance during the follow-up period. The most widely used serological marker is serum antibodies against gliadin (AGA), where the IgA isotype is considered to be the most specific (29). Two other serological tests frequently used are antibodies against reticulin (ARA) (26) and endomysium (EMA) (6), where the latter is generally accepted as highly specific for CD. Both ARA and EMA tests are based on immunofluorescence techniques and are thus more time-consuming and require more experience than the enzyme-linked immunosorbent assay (ELISA) method usually used for analyses of AGA. Recently tissue transglutaminase (tTG) was suggested as the target for endomysium antibodies in CD (9). Thus, ELISA methods for analyses of antibodies against tTG (AtTG) are promising as screening tests for CD (10), but so far experience is limited.
A problem is that the sensitivities and specificities of these different tests vary considerably (25), probably due to differences in the methodologies used and how the cutoff value is defined. The population studied is also important, since that will define the prevalence of the disease, which in turn affects the predictive value of the test (11).
Today laboratory medicine faces growing pressures for economic accountability and cost cutting, and thus, when possible, simplified technology should be used. Specific IgE antibodies to different antigens can be measured by the large Pharmacia CAP System, and a smaller automated test system is also available. Thus, it may be feasible to use this equipment for analyses of serological markers for CD also, and an evaluation is therefore warranted.
The aim of the present study was to evaluate the use of serum IgA-AGA as assayed with these two new commercial automated immunoassays, the Pharmacia CAP System Gliadin IgA FEIA and UNICAP-100, compared to three “in-house” methods, for prediction of CD, using the assessment of the small intestinal mucosa morphology as the “gold standard.”
MATERIALS AND METHODS
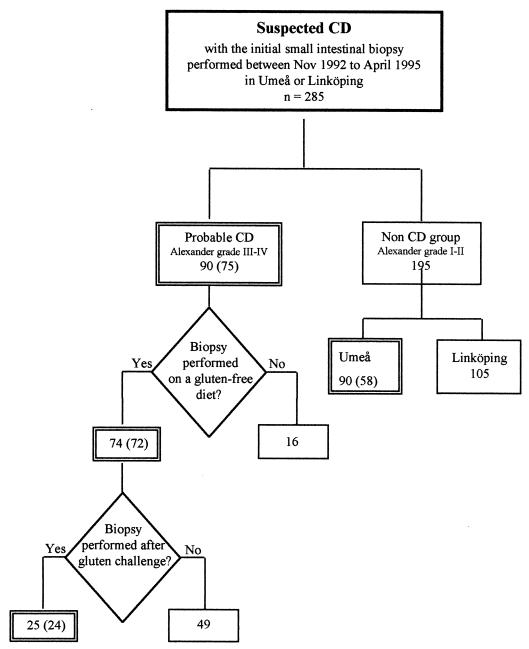
During the period between 1 November 1992 and 30 April 1995, 285 children aged 9 months to 16.7 years with suspected CD underwent small intestinal biopsy at the University Hospital in Umeå or in Linköping. Of these, 133 children for whom serum samples, taken within 1 week from the time of biopsy, were available, were included in the study (Fig. 1).
FIG. 1.
Flow chart of the procedure for examining children with suspected CD. Double frames indicate groups included in the study, and number of children from whom serum samples were available is given in parentheses.
The CD group encompassed children who underwent a first jejunal biopsy with a histology suggestive of CD at the University Hospital in Umeå (n = 37) or in Linköping (n = 38). Their ages ranged from 9 months to 12.3 years (median, 1.7 years). Children were included in the follow-up if they underwent a second biopsy while on a GFD (n = 72) and a third biopsy after gluten challenge (n = 24) by 30 April 1996.
The non-CD group encompassed children who underwent a first jejunal biopsy with a histology classified as non-CD at the University Hospital in Umeå (n = 58). Their ages ranged from 9 months to 16.7 years (median, 2.6 years).
Venous blood samples for measuring AGA and total serum IgA were stored at −20°C prior to analyses.
Small intestinal biopsy.
A small intestinal mucosal biopsy specimen was taken at, or distally to, the ligament of Treitz with a Watson or Storz pediatric capsule under fluoroscopic control. The specimens were examined by the local pathologists, and then the mucosal findings were classified by one experienced pathologist according to the system of Alexander, where grade I is normal and grades II through IV represent increasing damage to the mucosa (1). This pathologist had no information on clinical symptoms or AGA results. In principal, criteria for verified CD were Alexander grades III through IV both at presentation and after gluten challenge, and a clear improvement to Alexander grades I and II on GFD. A mucosal finding from the first intestinal biopsy classified as Alexander grades I and II rendered the diagnosis non-CD.
AGA.
All serum samples were analyzed in duplicate except for the CAP assay in Örebro, which was performed as single tests. The results were interpreted according to the cutoff value set for each assay by the manufacturer or the laboratory, respectively. The assays and the laboratories performing them are listed below.
Both the Pharmacia CAP System Gliadin IgA FEIA (CAP) and the UNICAP-100 (UNICAP) are based on the same ImmunoCAP technology (Pharmacia & Upjohn Diagnostics, Uppsala, Sweden).
Briefly, the principal element in the assay is a solid phase consisting of a flexible hydrophilic carrier polymer encased in a capsule, ImmunoCAP. The carrier consists of a CNBr-activated cellulose derivative with a large surface for binding protein, i.e., gliadin (catalog no. G-3375, Sigma, St. Louis, Mo.). The procedures for the two assays differ in the respect that all incubations in CAP are performed at room temperature while in UNICAP they are performed at 37°C. As a reference, a diluted standard curve calibrated against the World Health Organization (WHO) international reference preparation 67/86 was used. The cutoff value for a positive outcome (3mgA/liter) was based on celiac patients (n = 82; age, 0 to 65 years) and controls (n = 72; age, 0 to 65 years) with gastrointestinal symptoms unrelated to CD. As internal quality controls, one negative, one low-positive, and one high-positive serum sample were used according to the manufacturer. The interassay coefficient of variation (CV) given by the manufacturer was 5 to 7% for CAP and 4 to 11% for UNICAP.
(i) CAP.
All serum samples were analyzed independently with CAP at two of the participating laboratories: the Department of Clinical Sciences, Pediatrics, Umeå, and the Department of Clinical Microbiology and Immunology, Örebro. Each laboratory had its own instrument, and the procedure was performed in accordance with the instructions given by the manufacturer. In short, serum diluted 1/100 was incubated with immunoCAP for 30 min at room temperature and then automatically washed. Anti-IgA antibody, labeled with β-galactosidase generating a fluorescent cleavage product, was added and incubated for 150 min at room temperature before being washed as above.
(ii) UNICAP.
UNICAP analyses were performed at the Department of Clinical Immunology, Linköping, in accordance with the instructions given by the manufacturer. Briefly, serum diluted 1/100 was incubated with immunoCAP for 24 min at 37°C and then automatically washed. Anti-IgA antibodies, labeled with β-galactosidase generating a fluorescent cleavage product, were added and incubated for 30 min at 37°C before being washed as above.
Three in-house methods. (i) Umeå ELISA.
An ELISA developed at the Department of Clinical Microbiology, Virology, Umeå, was used (20). MaxiSorp microtiter plates (Nunc, Århus, Denmark) were coated with 50 μg of gliadin (catalog no. G-3375; Sigma)/ml dissolved in 70% ethanol, which was allowed to evaporate. Controls' and patients' serum samples were diluted 1/200 in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 0.05% Tween 20 (Pharmacia, Uppsala, Sweden) and incubated on the plates for 60 min at 37°C. After plates were washed in deionized water, alkaline phosphatase-conjugated swine anti-human IgA (Orion, Espoo, Finland), diluted 1/300, was added, and plates were incubated for 90 min at 37°C and then washed as above. Five milligrams of the substrate p-nitrophenylacetate (catalog no. P-6001; Sigma), dissolved in 5 ml of diethanolamine buffer, pH 9.2, was incubated for 30 min at 37°C, and the reaction was stopped with 3 M NaOH. The absorbance was read at 405 nm in a Titertek multiscan spectrophotometer. The cutoff value for a positive outcome (1 U) was defined as the mean of the optical densities (OD) of six negative sera multiplied by 2.1, to equal an OD of at least 0.1. The antibody activity in each sample was defined as the OD divided by the cutoff value. A low- and a high-positive serum sample were used as internal quality controls. The interassay CVs for the low-positive control and the high-positive control were 19.8 and 13.6%, respectively.
(ii) Örebro DIG-ELISA.
A diffusion in gel (DIG) ELISA was set up at the Department of Clinical Microbiology and Immunology, Örebro, and was used as previously described (21). Briefly, petri dishes were coated with gliadin (catalog no. G-3375; Sigma), and the bottoms were covered with a layer of agarose gel (Noble Agar, Difco, Detroit, Mich.). Undiluted patient serum samples (20 μl) and appropriate control sera were added and incubated for 48 h at room temperature. The agar was removed, and the dishes were washed five times. Peroxidase-conjugated rabbit anti-human IgA (catalog no. P216; Dakopatts; Glostrup, Denmark), diluted 1/100 in PBS with 0.05% Tween 20 was applied for 2 h at room temperature. The dish was washed as above and a layer of agarose gel containing the substrate p-phenylene diamine (catalog no. P-6001; Sigma) was added. Brown areas appear with a diameter proportional to the concentration of AGA. The cutoff value for a positive outcome was defined as a zone diameter of 11 mm according to an evaluation using sera from apparently healthy blood donors. Serum from an untreated celiac patient was used as a positive internal control (mean zone diameter, 17.7 mm) with an interassay CV of <15%.
(iii) Linköping ELISA.
An ELISA developed at the Division of Clinical Immunology, Linköping, was used (13). Medium binding microtiter plates (Dynatech, Alexandria, Va.) were coated with 50 μg of gliadin (catalog no. G-3375; Sigma)/ml dissolved in 70% ethanol and allowed to evaporate for 3 to 4 days. Patient sera, diluted 1/10, in PBS containing 0.5% human serum albumin (HSA), were incubated for 60 min at room temperature, and washed three times with PBS containing 0.05% Tween 20. Peroxidase-conjugated rabbit anti-human IgA (catalog no. P-216; Dakopatts) was diluted 1/2,000 and the reaction mixture was incubated for 60 min at room temperature and washed as above. o-Phenylene diamine (catalog no. S-2045, Dakopatts) was used as the substrate, and the reaction was stopped by adding 1 M H2SO4. The absorbance was read spectrophotometrically in a Dynatech minireader II. A serially diluted positive serum sample was used as reference curve. The OD of the reference serum was converted to units by a program (Dynatech) for semilog fit. The cutoff for a positive outcome (30 U) was defined as approximately the 95th percentile for a blood donor population (n = 1,866) and for healthy children of different ages (1.5 years [n = 47], 7 years [n = 58], and 12 years [n = 100]). One negative, one low-positive, and one high-positive serum sample served as internal quality controls. The interassay CV was below 15% for both the low- and the high-positive control.
Serum IgA.
All serum samples were analyzed for total serum IgA. Sera appropriately diluted in PBS and mixed with anti-IgA serum (Beckman Instruments Inc., Fullerton, Calif.) were analyzed by a nephelometric immunoassay according to the manufacturer's instructions (Beckman Instruments, Palo Alto, Calif.) with a detection level of 0.07 g/liter. Reference values were set according to age against the international reference preparation CRM 470 (32).
Statistics.
The CV was calculated by the standard deviation divided by the average of a sample of values. The Pearson correlation coefficient was calculated for the CAP system analyses of the same sera at two different laboratories, and in a comparison with UNICAP. The sensitivity and specificity of serological tests were calculated with 95% confidence intervals (CI) using the exact binomial method, and also compared with McNemar's chi-square test. A chi-square test was used in the comparison of the total-IgA levels between groups. Receiver operating characteristic (ROC) analyses were used to compare the predictive abilities of IgA-AGA measurements by the different methods (16). The model plots sensitivity versus 1 − specificity for each possible value of the test. The area under the ROC curve shows the ability of a test to discriminate between disease and no disease.
Definitions.
Terms used are defined as follows: sensitivity, the probability that the test will be positive when disease is present; specificity, the probability that the test will be negative when disease is not present; positive predictive value, the probability that the disease is present when the test is positive; negative predictive value, the probability that the disease is not present when the test is negative; cutoff value, the arbitrary value used to separate positive from negative results for any given test; internal quality control, the operational techniques and activities needed to fulfil the quality requirements.
Ethical considerations.
This study was approved by the Research Ethics committees of the medical faculties at the Universities of Umeå and Linköping.
RESULTS
Reproducibility of ImmunoCAP technology.
The manufacturer gave the interassay CVs for the different internal controls for both the CAP and the UNICAP as shown above. For the UNICAP the low control was always below 1.0 mg/liter, and for the controls with medium and high levels the CVs were 12.9 and 13.3%, respectively (n = 9). The interassay CV could not be calculated for CAP in Umeå or Örebro due to too few measurements of the internal controls.
The correlations between values obtained in the two different laboratories, which used two different CAP instruments, were 0.96 and 0.98 for the non-CD group and the CD group, respectively. The correlations between the values for UNICAP and CAP (Umeå) were 0.99 and 0.96 for the two groups, respectively.
Sensitivity and specificity for IgA-AGA at presentation (first biopsy).
The sensitivity was calculated for the CD group, i.e., children with mucosal biopsy specimens at presentation classified as Alexander grades III and IV, and the specificity was calculated for the non-CD group, i.e., children with mucosal biopsy specimens classified as Alexander grades I and II (Table 1).
TABLE 1.
Diagnostic sensitivity and specificity of IgA-AGA for the different assays and laboratories in relation to mucosal morphology at presentation with symptoms suggestive of CD
| Assay (laboratory) | Result for CD groupa at:
|
Result for non-CD groupb (all ages)
|
||||
|---|---|---|---|---|---|---|
| <2 yr of age
|
All ages
|
|||||
| No. of positive tests/no. of patients | Sensitivityc (95% CI) | No. of positive tests/no. of patients | Sensitivityc (95% CI) | No. of negative tests/no. of patients | Specificityc (95% CI) | |
| CAP (Umeå) | 46/48 | 96 (86–99) | 66/75 | 88 (78–94) | 40/58 | 69 (55–80) |
| CAP (Örebro) | 44/48 | 92 (80–98) | 61/75 | 81 (71–89) | 43/58 | 74 (61–85) |
| UNICAP (Linköping) | 42/47 | 89 (77–96) | 53/74 | 72 (60–81) | 47/54 | 87 (75–95) |
| ELISA (Umeå) | 44/48 | 92 (80–98) | 66/75 | 87 (78–94) | 44/58 | 76 (63–86) |
| ELISA (Linköping) | 45/48 | 94 (83–99) | 65/75 | 88 (77–93) | 39/58 | 67 (54–79) |
| DIG-ELISA (Örebro) | 42/48 | 88 (75–95) | 57/75 | 76 (65–85) | 39/58 | 67 (54–79) |
Small intestinal morphology according to Alexander grades III and IV, i.e., villous atrophy.
Small intestinal morphology according to Alexander grades I and II, i.e., normal mucosa.
Expressed as a percentage.
The sensitivities of the different tests varied from 0.72 to 0.88, and the specificities varied from 0.67 to 0.87 (Table 1). For the CAP assay, the sensitivity in Umeå was higher (0.88) than in Örebro (0.81), whereas the specificities were 0.69 and 0.74, respectively (P < 0.02). For UNICAP the opposite was observed, i.e., a higher specificity (0.87) than sensitivity (0.72), which differed significantly from all the other assays (P < 0.001). The CAP in Umeå had a better performance than the DIG-ELISA (P < 0.05), but besides this no significant differences were found when comparing CAP in Umeå and Örebro with the in-house methods. No significant differences were found among the in-house methods.
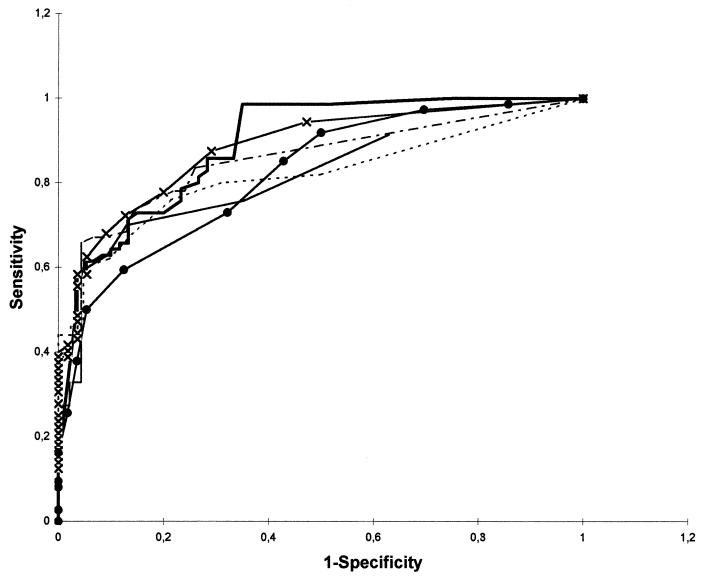
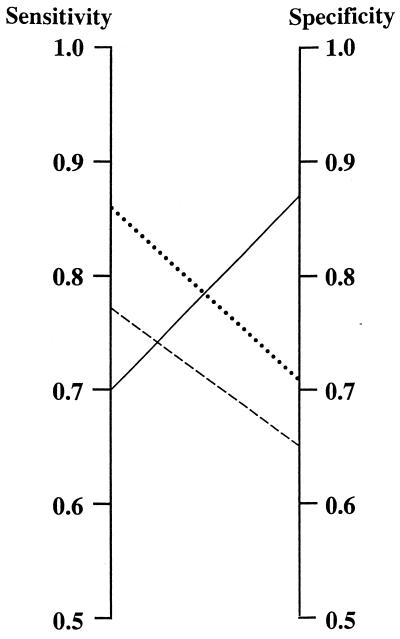
The diagnostic performances of the different assays were, however, comparable, as illustrated by the ROC curves in Fig. 2. The area under the curve varied only from 0.82, i.e., the UNICAP and CAP (Örebro), to 0.89, i.e., the ELISA in Linköping. The differences in sensitivity and specificity described above were thus to a large extent due to choice of cutoff levels. For the ImmunoCAP assays we used the cutoff value of 3 mg/liter recommended by the manufacturer. If priority were given to a high sensitivity, and comparable results for the CAP and UNICAP, the cutoff value for UNICAP should have been changed to 2 mg/liter (Fig. 3).
FIG. 2.
ROC curves for all methods used in the study. Symbols: ×—×, CAP performed in Umeå; –––, CAP performed in Örebro; ——, UNICAP (Linköping); –·–, ELISA performed in Umeå; ——, ELISA performed in Linköping; ●——●, DIG-ELISA performed in Örebro.
FIG. 3.
Sensitivity and specificity for the ImmunoCAP technology calculated with the cutoff value given by the manufacturer, 3 mg/liter, and an adjusted cutoff value to give the CAP and UNICAP comparable sensitivity and specificity. Solid line, UNICAP with a cutoff of 3 mg/liter; dashed line, UNICAP with a cutoff of 2 mg/liter; dotted line, CAP performed at Umeå (cutoff, 3 mg/liter).
PPV and NPV for IgA-AGA.
We estimated the positive predictive value (PPV) and negative predictive value (NPV) for the CAP and UNICAP, as these assays had a high sensitivity (0.88) and a high specificity (0.87), respectively, using the prevalence of 1 in 3 representing a clinically selected population and 1 in 250 representing the general population. At a prevalence of 1 in 3, the PPV was 58.3% for CAP and 55.6% for UNICAP, whereas the NPVs were 50 and 87%, respectively. At a prevalence of 1 in 250, the PPV decreased to 1.1% for CAP and 1.0% for UNICAP, whereas the NPV increased close to 100% for both.
IgA-AGA levels at presentation in relation to age.
For all methods the sensitivity was higher in children below the age of 2 years, compared to that for the whole group (Table 1), while the opposite was seen with regard to specificity (data not shown).
IgA-AGA levels after GFD (second biopsy).
Serum samples were available for 72 of the children who underwent a second small intestinal biopsy after a GFD. Forty-six of them had a histological specimen classified as Alexander grade I. All but one of these had negative results irrespective of the method used. This child had an AGA level at the cutoff limit (index 1.0) as measured with the ELISA in Umeå. Twenty-five children had a specimen classified as Alexander grade II. All except one had a negative outcome on the AGA tests. This child had an AGA level at the cutoff limit as measured with the ELISA in Umeå (index 1.0) and the DIG-ELISA in Örebro (11 mm). One child had mucosal lesions classified as Alexander grade III, and the only method indicating this was the ELISA in Linköping with a level just above the cutoff value (33 U). According to his parents, this child's diet was not strictly gluten free but the symptoms had disappeared.
IgA-AGA levels after gluten challenge (third biopsy).
Serum samples were available for 24 of the children who underwent a biopsy after gluten challenge. Twenty of them had a mucosal lesion classified as Alexander grade III or IV. Of these, 13 had a positive outcome and 2 had a negative outcome irrespective of the method used, whereas for 5 the outcomes were unsystematically different with different methods. Two children with mucosa classified as grade I had a negative AGA outcome by all methods used. During 2 years of further follow-up, repeated antibody analyses were negative and no clinical symptoms reappeared. Two children had mucosae classified as Alexander grade II, and for both, symptoms had reappeared during gluten challenge. One of them had a positive outcome on all assays and the other had a positive outcome by all methods except UNICAP and one of the CAP analyzes (Örebro).
Distribution of serum IgA.
Total-IgA levels were measured in the CD group at presentation (median, 1.0 g/liter; range, 0.2 to 3.4 g/liter), after GFD (median, 0.6 g/liter; range, <0.07 to 1.6 g/liter), and after gluten challenge (median, 0.9 g/liter; range, 0.2 to 2.4 g/liter). In the non-CD group, the median serum IgA level was 0.8 g/liter (range, <0.07 to 5.0 g/liter). The CD group had a significantly lower median serum IgA level on a GFD compared to the results both at presentation (P < 0.001) and after gluten challenge (P = 0.004), and also compared to the non-CD group (P < 0.001).
Six serum samples had total IgA levels below 0.07 g/liter; five of these were from children in the CD group after GFD, and one was from the non-CD group. It is noteworthy that at presentation the five children in the CD group all had normal serum IgA levels.
DISCUSSION
Screening tests predicting the small bowel mucosal lesion in CD—or its absence—are needed to detect patients with vague symptoms and avoid unnecessary small intestinal biopsies. Furthermore, such methods are needed for screening of risk groups, such as first-degree relatives of CD patients (22), children with unexplained short stature (3), diabetes mellitus (27), and Down's syndrome (7). Access to assays detecting AGA, ARA, and EMA has contributed to identification of more CD cases (4, 12, 18, 19, 24, 31; Borch et al., submitted). These tests can also be helpful to monitor adherence to a GFD and to select a suitable time point for biopsy during gluten challenge (2, 15). AtTG seem to be a possible screening test for CD (10), but so far the experience is limited.
Circulating AGA, the first serological marker for CD to be described, have been extensively investigated and the techniques for detection have varied (29). Thus the reported sensitivity and specificity of these antibodies have varied considerably, which is a problem in comparing results of different studies. Even seemingly identical techniques may yield different results, as gliadin is a complex mixture of proteins and the antigen is poorly characterized.
In the present study we measured IgA-AGA in sera from children with suspected CD, and when possible also on GFD and gluten challenge, respectively. We used two new commercial automated immunoassays, CAP and UNICAP, and compared these with three in-house methods. The main principles of the assays used in the present study are similar, and the gliadin was from the same supplier. Nevertheless, at presentation with symptoms compatible with CD, the sensitivities of the assays varied between 72 and 88%, and the specificities varied between 67 and 87%. A higher sensitivity for CD in children below the age of 2 years was found for all methods in this study, which is in accordance with earlier reports (2, 15).
The results of the CAP and UNICAP IgA-AGA tests differed, although they are both based on the same immunoCAP technology, which has been quantitatively calibrated against the WHO international reference preparation. Furthermore, the same cutoff value was used for both assays. The sensitivity for the CAP in Umeå was higher (88%) than in Örebro (81%), whereas UNICAP with a sensitivity of 72% had the highest specificity (87%). Part of the explanation could be a difference in processing temperature for the CAP and UNICAP, i.e., in the CAP the antibody was allowed to react at room temperature whereas in the UNICAP the incubation temperature was 37°C. Our results illustrate, however, that the type of immunoCAP technology used must also be taken into account when sensitivity and specificity are determined. Further, the sensitivity and specificity varied significantly between the two CAP instruments. A possible explanation for this might be differences between the laboratories with regard to different batches of standards, autoCAPs, and/or secondary antibodies used. This illustrates the importance of analyzing one's reference material (i.e., samples from patients and individuals without disease) before using a new method routinely, as is also recommended by many manufacturers.
Comparison of the results of the different assays after GFD and upon gluten challenge found no major differences. Thus, all the AGA methods can be helpful in monitoring compliance to a GFD and for detecting a relapse of the mucosal lesion after gluten challenge; however, they can not replace a follow-up with assessment of the small intestinal mucosa.
Our results clearly illustrate that the prevalence of a disease in a population studied has a more dramatic influence on the PPV than the sensitivity and specificity of an assay. When a clinical population with a CD prevalence of 1 in 3 (15) and a general population with a prevalence of 1 in 250 (5) were compared, the PPVs for both the CAP and UNICAP shifted from about 58% to 1%, even though these tests have a high sensitivity and specificity, respectively. Thus, when an AGA test is used for screening of CD in the general population, a serial testing approach is needed, i.e., analyses of AGA should be performed for all subjects, and positive results should be followed up with a confirmatory test, e.g., EMA, to avoid unnecessary biopsies (4, 14, 18, 24). It is important that in such a screening the NPV for both the CAP and UNICAP would be close to 100%; thus, when the AGA test is negative, the risk for CD is small.
Since selective IgA deficiency is associated with increased risk for CD, we also measured total serum IgA (17). Interestingly we found that five children had serum IgA levels below the detection limit (<0.07 g/liter) when on GFD, whereas they had normal levels at the time of the first biopsy. This illustrates a difficulty in diagnosing IgA deficiency in children.
Laboratory medicine faces growing pressures for economic accountability and cost cutting. Thus, it is an advantage if the same electronic instrument can be used for several types of analyses, and further if simplified technology can be used. The Pharmacia ImmunoCAP technology can be used for analyses of specific IgE antibodies. Our conclusion from this study is that it also can replace the in-house methods we presently use for routine analyses of IgA-AGA.
ACKNOWLEDGMENTS
We gratefully acknowledge the medical laboratory technologists C. Lagerqvist, C. Andersson, and A.-K. Åberg for excellent technical assistance; R. Stenling for diagnostic support; M. Eriksson for the ROC curve analyses; and A.-B. Wiréhn for statistical support.
This study was supported by grants from the Swedish Foundation for Health Care Sciences and Allergy Research and by Pharmacia & Upjohn Diagnostics.
REFERENCES
- 1.Alexander J O. The small intestine in dermatitis herpetiformis. In: Alexander J O, editor. Major problems in pathology. IV. Dermatitis herpetiformis. London, United Kingdom: W. B. Saunders; 1975. pp. 236–280. [Google Scholar]
- 2.Ascher H, Hahn-Zoric M, Hanson L, Kilander K F, Nilsson L, Tlaskalová H. Value of serologic markers for clinical diagnosis and population studies of coeliac disease. Scand J Gastroenterol. 1996;31:61–67. doi: 10.3109/00365529609031628. [DOI] [PubMed] [Google Scholar]
- 3.Cacciari E, Salardi S, Volta U, Biasco G, Lazzari R, Corazza G R, Feliciani M, Cicognani A, Partesotti S, Azzaroni D, et al. Can antigliadin antibody detect symptomless coeliac disease in children with short stature. Lancet. 1985;i:1469–1471. doi: 10.1016/s0140-6736(85)92251-2. [DOI] [PubMed] [Google Scholar]
- 4.Catassi C, Fabiani E, Rätsch I M, Coppa G V, Giorgi P L, Pierdomenico R, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 5.Cavell B, Stenhammar L, Ascher H, Danielsson L, Dannaeus A, Lindberg T, Lindquist B. Increasing incidence of childhood coeliac disease in Sweden. Results of a national study. Acta Paediatr. 1992;81:589–592. doi: 10.1111/j.1651-2227.1992.tb12306.x. [DOI] [PubMed] [Google Scholar]
- 6.Chorzelski T P, Sulej J, Tchorzewska H, Jablonska S, Beutner E H, Kumar V. IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease. Ann N Y Acad Sci. 1983;420:325–334. doi: 10.1111/j.1749-6632.1983.tb22220.x. [DOI] [PubMed] [Google Scholar]
- 7.Dias J A, Walker-Smith J A. Down's syndrome and coeliac disease. J Pediatr Gastroenterol Nutr. 1990;10:41–43. [PubMed] [Google Scholar]
- 8.Dicke W K. Coeliakie. M.D. thesis. Utrecht, The Netherlands: University of Utrecht; 1950. [Google Scholar]
- 9.Dietrich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E O, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich W, Laag E, Schöpper H, Volta U, Ferguson A, Gillet H, Rieken E O, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 11.Galen R S, Gambino S R. Beyond normality: the predictive value and efficiency of medical diagnoses. New York, N.Y: John Wiley & Sons; 1975. [Google Scholar]
- 12.Grodzinsky E, Franzén L, Hed J, Ström M. High prevalence of celiac disease in healthy adults revealed by antigliadin antibodies. Ann Allergy. 1992;69:66–70. [PubMed] [Google Scholar]
- 13.Grodzinsky E, Hed J, Lieden G, Sjögren F, Ström M. Presence of IgA and IgG antigliadin antibodies in healthy adults as measured by micro-ELISA. Int Arch Allergy Appl Immunol. 1990;92:119–123. doi: 10.1159/000235201. [DOI] [PubMed] [Google Scholar]
- 14.Grodzinsky E, Hed J, Skogh T. IgA antiendomysium antibodies have a high positive predictive value for celiac disease in asymptomatic patients. Allergy. 1994;49:593–597. doi: 10.1111/j.1398-9995.1994.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 15.Grodzinsky E, Jansson G, Skogh T, Stenhammar L, Fälth-Magnusson K. Anti-endomysium and anti-gliadin antibodies as serological markers for coeliac disease in childhood: a clinical study to develop a practical routine. Acta Paediatr. 1995;84:294–298. doi: 10.1111/j.1651-2227.1995.tb13631.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanley J A, McNeil B J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 17.Heneghan M A, Stevens F M, Cryan E M, Warner R H, McCarthy C F. Celiac sprue and immunodeficiency states: a 25-year review. J Clin Gastroenterol. 1997;25:421–425. doi: 10.1097/00004836-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Ivarsson A, Persson L, Juto P, Peltonen M, Suhr O, Hernell O. High prevalence of undiagnosed coeliac disease in adults—a Swedish population-based study. J Intern Med. 1999;245:63–68. doi: 10.1046/j.1365-2796.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnston S D, Watson R G P, McMillan S A, Sloan J, Love A H G. Prevalence of coeliac disease in Northern Ireland. Lancet. 1997;350:1370. doi: 10.1016/s0140-6736(05)65142-2. [DOI] [PubMed] [Google Scholar]
- 20.Juto P, Fredrikzon B, Hernell O. Gliadin-specific serum immunoglobulins A, E, G, and M in childhood: relation to small intestine mucosal morphology. J Pediatr Gastroenterol Nutr. 1985;4:723–729. doi: 10.1097/00005176-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kilander A F, Dotevall G, Fällström S P, Gillberg R E, Nilsson L, Tarkowski A. Evaluation of gliadin antibodies for detection of coeliac disease. Scand J Gastroenterol. 1983;18:377–383. doi: 10.3109/00365528309181610. [DOI] [PubMed] [Google Scholar]
- 22.Mäki M, Holm K, Lipsanen V, Hallström O, Viander M, Collin P, Savilahti E, Koskimies S. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet. 1991;338:1350–1353. doi: 10.1016/0140-6736(91)92234-s. [DOI] [PubMed] [Google Scholar]
- 23.Meuwisse G W. Diagnostic criteria in coeliac disease. Acta Paediatr. 1970;59:461–463. [Google Scholar]
- 24.Not T, Horvath K, Hill I D, Partanen J, Hammed A, Magazzu G, Fasano A. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494–498. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 25.Rossi T M, Tjota A. Serologic indicators of celiac disease. J Pediatr Gastroenterol Nutr. 1998;26:205–210. doi: 10.1097/00005176-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Seah P P, Fry L, Rossiter M A, Hoffbrand A V, Holborow E J. Anti-reticulin antibodies in childhood coeliac disease. Lancet. 1971;ii:681–682. doi: 10.1016/s0140-6736(71)92248-3. [DOI] [PubMed] [Google Scholar]
- 27.Sigurs N, Johansson C, Elfstrand P O, Viander M, Lanner Å. Prevalence of coeliac disease in diabetic children and adolescents in Sweden. Acta Paediatr. 1993;82:748–751. doi: 10.1111/j.1651-2227.1993.tb12551.x. [DOI] [PubMed] [Google Scholar]
- 28.Sollid L M, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 29.Troncone R, Ferguson A. Anti-gliadin antibodies. J Pediatr Gastroenterol Nutr. 1991;12:150–158. doi: 10.1097/00005176-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Walker-Smith J A, Guandalini S, Schmitz J, Schmerling D H. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weile B, Grodzinsky E, Skogh T, Jordal R, Cavell B, Krasilnikoff P A. Screening Danish blood donors for antigliadin and antiendomysium antibodies. Acta Paediatr Suppl. 1996;412:46. doi: 10.1111/j.1651-2227.1996.tb14248.x. [DOI] [PubMed] [Google Scholar]
- 32.Whicher J T, Ritchie R F, Johnson A M, Baudner S, Bienvenu J, Blirup-Jensen S, Carlstrom A, Dati F, Ward A M, Svendsen P J. New international reference preparation for proteins in human serum (RPPHS) Clin Chem. 1994;40:934–938. [PubMed] [Google Scholar]