Abstract
Background:
Renal cell carcinoma (RCC) represents 1% of all cancers and its brain metastases amount to 8.1% of all metastatic tumors. Late brain metastases are defined as tumors that appear 10 years after diagnosis of the primary lesion. The objective of this work is to discuss which biological pathways are responsible for the late appearance of these metastases analyzing eight cases.
Case Description:
We report here eight cases of late brain metastases of RCC treated between 2018 and 2021. Patients consulted for different clinical complaints. Brain magnetic resonance imaging and computed tomography scan were performed on all patients. They were treated by complete surgical resection plus radiosurgery or by radiosurgery alone. The histology of most metastases showed clear cell RCC.
Conclusion:
In the presence of a patient with an intracranial tumor and a history of RCC with more than 10 years of evolution, the presence of late metastasis should always be considered. There are many theories described in the literature that try to explain the late appearance of brain metastases from RCC (low mitotic index, impaired immune system, cross talk, self-seeding, and among others).
Keywords: Brain, Late metastasis, Metastases, Pathways, Renal cell carcinoma

INTRODUCTION
Renal cell carcinoma (RCC) is the most common kidney cancer with an incidence estimated of 7.5/100,000 people.[3,29] It represents approximately 1% of all cancers in adults. The organs to which it most frequently metastasizes are: lung (45.2%), bone (29.5%), lymph nodes (21.8%), liver (20, 3%), adrenal gland (8.9%), and brain (8.1%).[3] Brain metastases are usually hypervascular and prone to bleed, and when appear 10 years after nephrectomy, they are called late metastases.[14,16,20] If the primary tumor is under control and the patient clinical status allows it, the recommended treatment is of surgical resection and/or stereotaxic radiosurgery, depending on the number and location of the lesions. In some cases, holocranial radiotherapy is indicated.[4,5,10,14,19,23,25]
Many cases of late brain metastases from RCC have been described in the literature;[1,2,6,9,11,12,14,15,16,20,21,25,27] however, few of them analyze the pathophysiology of the appearance of these metastases. The objective of this work is to discuss which biological pathways are responsible for the late appearance of these metastases analyzing eight cases.
CASE REPORT
Eight patients with a 10 years history at least of nephrectomy for RCC were studied. As background, non-robotic-assisted open radical nephrectomy was performed, without adjuvant treatment (radiotherapy or antineoplastic drugs). The patients were free of disease until new symptoms appeared. The symptomatology was related to the affected brain area and due lesion mass effect (headaches, nausea, vomiting, gait disturbance, etc.). Every patient had a brain magnetic resonance imaging (MRI) with and without contrast plus total body tomography.
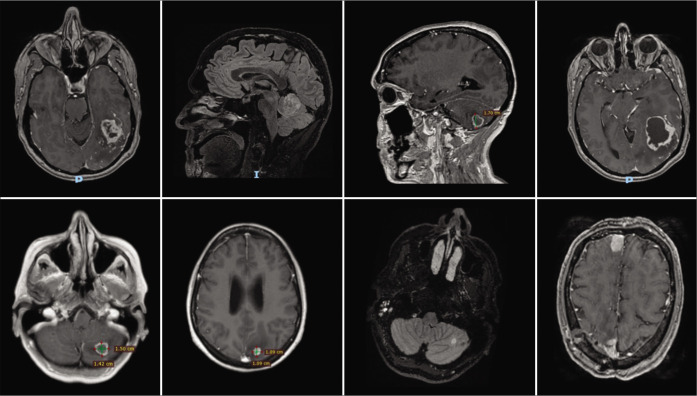
All MRI images were enhanced following contrast administration. Tomography showed no lesions in the rest of the body in the eight patients. Five patients had a single metastasis and three patients suffer from multiple metastases. The areas more affected were the cerebellum, parietal, frontal, and occipital lobes [Figure 1].
Figure 1:
Presurgical images of the presented patients.
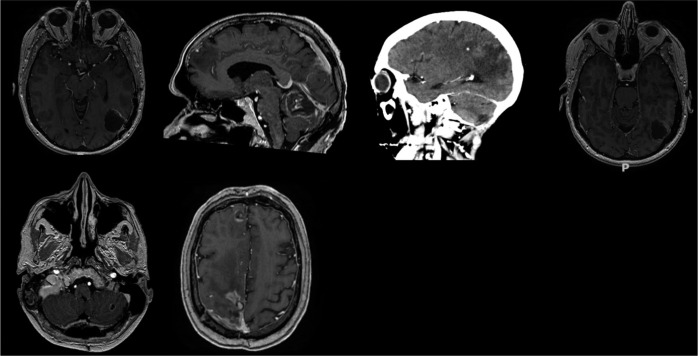
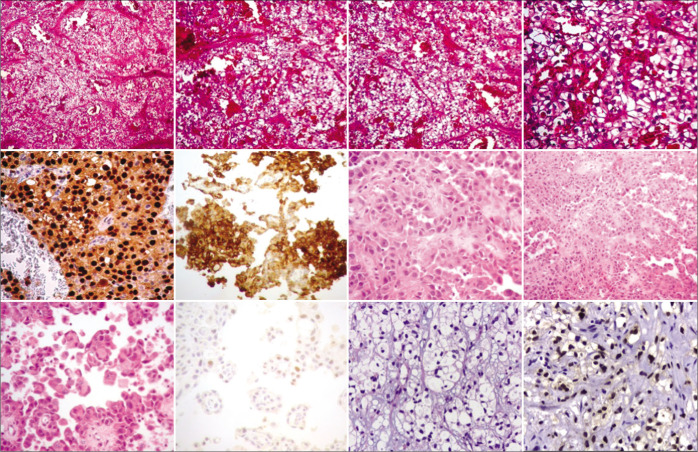
Surgical removal of metastases was carried out in seven patients, whereas in the remaining patient, stereotactic biopsy was performed [Figure 2]. Histopathologic study of the metastases showed CCR clear cells in seven patients and a chromophobe cells in one patient [Figure 3]. Following surgery, all patients were transferred to the oncology department for further treatment.
Figure 2:
Postsurgical images of the presented patients.
Figure 3:
Histopathology images of the presented patients.
Up to now, none of the eight patients had evidence of recurrence.
DISCUSSION
RCC is characterized by slow growth and resistance to cytostatics. This tumor derives from the cells of the proximal convoluted tubule and suffers almost entirely a conducting mutation in the VHL gene, which provides two distinct features such as great vascularization and growth dependent on the balance with the immune system and with angiogenesis. These characteristics determine that the renal cell tumor follows a different development than other solid tumors, because it depends on molecules such as transforming growth factor alpha, vascular endothelial growth factor, and platelet-derived growth factor receptor that allow or stimulate growth.[24] The three most common histological variants in RCC include: clear cell RCC, papillary RCC, and chromophobic cell RCC, accounting for 75–85%, 10–15%, and 5–10% of all RCCs, respectively.
Brain metastases from RCC can appear early or late in the course of the disease. Late recurrence is defined as that developing patients who had a disease-free interval of 10 years after the initial surgery.[14,16,20] Nakano et al. reported that 2 (4.3%) of 43 patients without evidence of disease for 10 years after the nephrectomy had a late recurrence;[22] while McNichols et al.[18] reported that in his series, the incidence of late recurrence was 11%. On the other hand, Miyao et al.[20] published series of 470 patients stated that the late recurrence rate was calculated as 10.5% and 21.6% at 15 and 20 years, respectively, after nephrectomy. Although the study of Miyao et al. did not fully clarify the clinical and pathological features of the late recurrences, it showed that lymph node metastases were the only independent predictor of a late recurrence. Several studies have attempted to describe the pathophysiology of the appearance of late metastases in RCC. Karasawa et al.[11] stated that the slow onset is related to the low mitotic index of most RCCs (MIB-1 cell proliferation marker <1%). Sadatomo et al.,[28] in turn, reported the presence of a MIB-1 proliferation marker <7% and indicated that the metastatic lesion could grow faster when the patient’s immunity decreases.
The eight cases in the present report had late metastases. In all these patients, nephrectomy was the initial treatment. This sequence of events was central to the understanding of the pathophysiology of this process, since there is a clear relationship between the immune system and RCC. It would be possible that the tumor can suppress the immune system or make it its partner, as suggested by Vuong et al.[30] There would be a crossed-talk between the primary tumor and the metastases resulting in the production of new metastatic sites. Once this is reached, the crossed-talk between the primary tumor and the metastases would continue. Other lines of evidence showed that removal of the primary tumor[7] and the regression of the metastases following treatment with IL-2, both prolonged the rate of survival, can be taken to indicate that the immunity plays a significant role in the control of the disease.[26]
The preceding observations are lead us to suggest that the slow growth of metastases following the resection of the primary tumor may have been caused by the suppression of the crossed-talk between the primary tumor and its metastases. Another view is that of a self-seeding, consisting of infiltration of aggressive circulating tumor cells into the primary tumor.[13] This mechanism would imply that cells originating in metastases can feed on the primary tumor, mutate, and generate new foci with the ability to metastasize again. Such metastases would be inhibited by the resection of the primary tumor. In addition to the preceding theory, basic research models refer to clones that although having metastatic capacity, they do not show an aggressive behavior, allowing them to grow in a single territory of the organism (solitary metastasis).[8]
These pathways would support the hypothesis proposed here, that these patients presented synchronous metastases, and it would be the elimination of the primitive tumor that generates orphan metastases and a state of quiescence exceeding 10 years.[17] It is worth noting that during asymptomatic stage of patients with kidney tumors, tests of brain imaging are not commonly prescribed. Under these conditions, the possible presence of synchronous brain metastases could be missed. This circumstance might obstruct an early diagnosis of synchronous brain metastases.
CONCLUSION
In a patient with an intracranial tumor and a history of RCC of more than 10-year duration, the possible presence of late metastasis should be considered. There are many theories described in the literature that try to explain the late appearance of brain metastases from RCC (low mitotic index, impaired immune system, cross-talk, self-seeding, and among others). More cases of late brain metastases in RCC need to be investigated to recognize the biological and genetic pathways underlying the presence of metastases after a long period of time. The series reported in the literature showed that the proper management can control the disease, improve the quality of life, and prolong survival.
Footnotes
How to cite this article: Minghinelli FE, Recalde RJ, Prost DM, Cutuli HJ, Giovannini SJ, Zaninovich RS. Which biological pathways are responsible for the late appearance of brain metastases in renal cell carcinoma? Analysis of eight cases. Surg Neurol Int 2022;13:466.
Contributor Information
Federico E. Minghinelli, Email: minghinelli.f@gmail.com.
Rodolfo José Recalde, Email: rodorecalde@gmail.com.
Diego Martín Prost, Email: diepros@gmail.com.
Hernán Javier Cutuli, Email: hjcutuli2020@gmail.com.
Sebastián Juan María Giovannini, Email: sjmgiova@gmail.com.
Roberto Steven Zaninovich, Email: rs@zaninovich.com.ar.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ammirati M, Samii M, Skaf G, Sephernia A. Solitary brain metastasis 13 years after removal of renal adenocarcinoma. J Neurooncol. 1993;15:87–90. doi: 10.1007/BF01050268. [DOI] [PubMed] [Google Scholar]
- 2.Bademci G, Bozdogan O, Berdan F, Evliyaoglu C. Extremely delayed renal cell carcinoma metastasis mimicking convexity meningioma. Neurocirugia (Astur) 2008;19:562–4. [PubMed] [Google Scholar]
- 3.Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol. 2012;23:973–80. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 4.Cannady SB, Cavanaugh KA, Lee SY, Bukowski RM, Olencki TE, Stevens GH, et al. Results of whole brain radiotherapy and recursive partitioning analysis in patients with brain metastases from renal cell carcinoma: A retrospective study. Int J Radiat Oncol Biol Phys. 2004;58:253–8. doi: 10.1016/s0360-3016(03)00818-6. [DOI] [PubMed] [Google Scholar]
- 5.Cervoni L, Salvati M, Delfini R. Late solitary cerebral metastasis from renal carcinoma. J Neurosurg Sci. 1993;37:247–9. [PubMed] [Google Scholar]
- 6.Choi WH, Koh YC, Song SW, Roh HG, Lim SD. Extremely delayed brain metastasis from renal cell carcinoma. Brain Tumor Res Treat. 2013;1:99–102. doi: 10.14791/btrt.2013.1.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima Y, Yoshikawa G, Takasago M, Shimizu S, Tsutsumi K. Extremely delayed multiple brain metastases from renal cell carcinoma: Remission achieved with total surgical removal: Case report and literature review. World Neurosurg. 2016;92:583.e13–7. doi: 10.1016/j.wneu.2016.05.065. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa J, Umezu K, Yamashita H, Maeda S. Solitary brain metastasis from renal cell carcinoma 14 years after nephrectomy: A case report. Hinyokika Kiyo. 1990;36:1439–41. [PubMed] [Google Scholar]
- 10.Jubelirer SJ. Late solitary cerebral metastasis from renal cell carcinoma: A case report and review of the literature. W V Med J. 1996;92:26–7. [PubMed] [Google Scholar]
- 11.Karasawa H, Naito H, Sugiyama K, Ueno J, Kin H, Nagayama T, et al. A brain metastasis developed eleven years after a renal cell carcinoma removal: A case report. No Shinkei Geka J. 1994;3:446–50. [Google Scholar]
- 12.Killebrew K, Krigman M, Mahaley MS, Jr, Scatliff JH. Metastatic renal cell carcinoma mimicking a meningioma. Neurosurgery. 1983;13:430–4. doi: 10.1227/00006123-198310000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YH, Kim JW, Chung HT, Paek SH, Kim DG, Jung HW. Brain metastasis from renal cell carcinoma. Prog Neurol Surg. 2012;25:163–75. doi: 10.1159/000331190. [DOI] [PubMed] [Google Scholar]
- 15.Kolsi F, Mechergui H, Kammoun B, Mellouli M, Khrifech M, Boudawara MZ. Delayed brain metastasis from renal cell carcinoma. Urol Case Rep. 2019;22:54–6. doi: 10.1016/j.eucr.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroki K, Taguchi H, Sumida M, Daimaru Y, Onda J. Cerebral metastasis from a renal cell carcinoma more than 10 years after nephrectomy: Report of two cases. No Shinkei Geka. 1999;27:89–93. [PubMed] [Google Scholar]
- 17.McIntosh AG, Ristau BT, Ruth K, Jennings R, Ross E, Smaldone MC, et al. Active surveillance for localized renal masses: Tumor growth, delayed intervention rates, and >5-yr clinical outcomes. Eur Urol. 2018;74:157–64. doi: 10.1016/j.eururo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 18.McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: Long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 19.Middleton R. Surgery for metastatatic renal cell carcinomas. J Urol. 1967;97:973–7. doi: 10.1016/S0022-5347(17)63157-4. [DOI] [PubMed] [Google Scholar]
- 20.Miyao N, Naito S, Ozono S, Shinohara N, Masumori N, Igarashi T, et al. Late recurrence of renal cell carcinoma: Retrospective and collaborative study of the Japanese society of renal cancer. Urology. 2011;77:379–84. doi: 10.1016/j.urology.2010.07.462. [DOI] [PubMed] [Google Scholar]
- 21.Montano N, Puca A, Pierconti F, Larocca LM. Extremely delayed falx metastasis from renal cell carcinoma. Neurology. 2007;68:1541–2. doi: 10.1212/01.wnl.0000261253.45209.97. [DOI] [PubMed] [Google Scholar]
- 22.Nakano E, Fujioka H, Matsuda M, Osafune M, Takaha M, Sonoda T. Late recurrence of renal cell carcinoma after nephrectomy. Eur Urol. 1984;10:347–9. doi: 10.1159/000463826. [DOI] [PubMed] [Google Scholar]
- 23.Nieder C, Spanne O, Nordøy T, Dalhaug A. Treatment of brain metastases from renal cell cancer. Urol Oncol. 2011;29:405–10. doi: 10.1016/j.urolonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Pulido EG, Centeno AM, Rey PM, Narbón ES. Molecular biology of the clear cell renal cell carcinoma: Principles for a selective treatment. Actas Urol Esp. 2007;31:233–43. doi: 10.1016/s0210-4806(07)73628-8. [DOI] [PubMed] [Google Scholar]
- 25.Radley MG, McDonald JV, Pilcher WH, Wilbur DC. Late solitary cerebral metastases from renal cell carcinoma: Report of two cases. Surg Neurol. 1993;39:230–4. doi: 10.1016/0090-3019(93)90189-8. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161:1169–88. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roser F, Rosahl SK, Samii M. Single cerebral metastasis 3 and 19 years after primary renal cell carcinoma: Case report and review of the literature. J Neurol Neurosurg Psychiatry. 2002;72:257–8. doi: 10.1136/jnnp.72.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Solitary brain metastasis from renal cell carcinoma 15 years after nephrectomy: Case report. Neurol Med Chir (Tokyo) 2005;45:423–7. doi: 10.2176/nmc.45.423. [DOI] [PubMed] [Google Scholar]
- 29.Satomi Y. A clinical study of the prognosis of renal carcinoma with reference to factors on the part of host. Nippon Hinyokika Gakkai Zasshi. 1973;64:195–216. doi: 10.5980/jpnjurol1928.64.3_195. [DOI] [PubMed] [Google Scholar]
- 30.Vuong L, Kotecha RR, Voss MH, Hakimi AA. Tumor microenvironment dynamics in clear-cell renal cell carcinoma. Cancer Discov. 2019;9:1349–57. doi: 10.1158/2159-8290.CD-19-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]