Abstract
In Great Britain an independent scientific review for the government has concluded that the development of a cattle vaccine against Mycobacterium bovis infection holds the best long-term prospect for tuberculosis control in British herds. A precondition for vaccination is the development of a complementary diagnostic test to differentiate between vaccinated animals and those infected with M. bovis so that testing and slaughter-based control strategies can continue alongside vaccination. To date bacillus Calmette-Guérin (BCG), an attenuated strain of M. bovis, is the only available vaccine for the prevention of tuberculosis. However, tests based on tuberculin purified protein derivative cannot distinguish between M. bovis infection and BCG vaccination. Therefore, specific antigens expressed by M. bovis but absent from BCG constitute prime candidates for differential diagnostic reagents. Recently, two such antigens, ESAT-6 and CFP-10, have been reported to be promising candidates as diagnostic reagents for the detection of M. bovis infection in cattle. Here we report the identification of promiscuous peptides of CFP-10 that were recognized by M. bovis-infected cattle. Five of these peptides were formulated into a peptide cocktail together with five peptides derived from ESAT-6. Using this peptide cocktail in T-cell assays, M. bovis-infected animals were detected, while BCG-vaccinated or Mycobacterium avium-sensitized animals did not respond. The sensitivity of the peptide cocktail as an antigen in a whole-blood gamma interferon assay was determined using naturally infected field reactor cattle, and the specificity was determined using blood from BCG-vaccinated and noninfected, nonvaccinated animals. The sensitivity of the assay in cattle with confirmed tuberculosis was found to be 77.9%, with a specificity of 100% in BCG-vaccinated or nonvaccinated animals. This compares favorably with the specificity of tuberculin when tested in noninfected or vaccinated animals. In summary, our results demonstrate that this peptide cocktail can discriminate between M. bovis infection and BCG vaccination with a high degree of sensitivity and specificity.
Bovine tuberculosis (BTB) is caused by Mycobacterium bovis. It is a zoonotic disease and was the cause of approximately 6% of total human deaths due to BTB in the 1930s and 1940s and of more than 50% of all cervical lymphadenitis cases in children (13, 23). The introduction of pasteurization of milk in the 1930s dramatically reduced the transmission from cattle to humans, and in 1995 only 1% of 3,200 isolates from patients with TB in Great Britain were identified as M. bovis (23). However, human TB caused by M. bovis is still a major health issue in many developing countries (12, 14, 15).
BTB has severe implications for animal welfare in both developed and developing countries, since it causes reduced productivity and premature death in cattle and since affected farms also suffer severe economic losses. A compulsory eradication program based on the slaughter of infected animals detected by the single intradermal comparative cervical tuberculin test (SICCT) began in Great Britain in 1950, and by 1960 it had been implemented in all of Great Britain. These measures resulted in a dramatic reduction of BTB in Great Britain. In 1934 approximately 40% of all cattle were infected with M. bovis, whereas in 1996 the annual incidence of confirmed herd breakdowns had been reduced to 0.41% in Great Britain (reviewed in reference 29). However, despite continued implementation of these control measures, the incidence of BTB in cattle has been steadily rising since 1988, possibly due to a wildlife reservoir of BTB. A cattle vaccine would reduce the risk of cattle infection and hence result in lower tuberculin test frequencies and significant cost savings. Recently, a panel of scientists was commissioned by the British government to conduct an independent review of this problem, and they concluded that the development of a cattle vaccine holds the best long-term prospect for BTB control in British herds (29). It was also recommended that a complementary diagnostic test to differentiate between vaccinated animals and those infected with M. bovis (differential diagnosis) should be developed in parallel with the vaccine to ensure continuation of the testing and slaughter-based control strategies (29). These recommendations have been accepted and are now being implemented by the British government (1).
Bacillus Calmette-Guérin (BCG), an attenuated strain of M. bovis, is presently the only available vaccine for the prevention of BTB. Encouraging results with BCG have been reported from experiments in New Zealand, where a significant level of protection in BCG-vaccinated cattle against experimental M. bovis infection has been demonstrated recently (7, 8). However, vaccination with BCG compromises tuberculin purified protein derivative (PPD) specificity (see, e.g., references 4, 25, and 27). Thus, cattle BCG vaccination constitutes an appropriate model to develop strategies for differential diagnosis associated with vaccines based on attenuated M. bovis strains. Theoretically, diagnostic reagents which distinguish between vaccinated and infected cattle could be developed using specific, defined antigens that are present in virulent M. bovis but absent from the vaccine strain. Genetic analysis of BCG has revealed that the genes encoding the M. bovis antigens ESAT-6, CFP-10, and MPB64 have been deleted from the Pasteur strain of BCG (5, 21, 22, 55). Phenotypic analysis of M. bovis and BCG Pasteur has also demonstrated that the M. bovis antigens MPB70 and MPB83 are highly expressed in M. bovis but expressed only at low levels in BCG Pasteur (26, 55). Using this information, it has recently been demonstrated that protein cocktails composed of ESAT-6, MPB70, and MPB59 (6) or of ESAT-6, MPB64, and MPB83 (50, 51) can be used to distinguish between BCG-vaccinated and M. bovis-infected cattle.
An alternative approach to using recombinant proteins is the application of synthetic peptides derived from antigens such as those described above. Synthetic peptides have the advantages of lower production costs and easier standardization and quality control, and they carry no risk of infection since they are fully chemically synthesized. Encouragingly, we have recently provided proof of principle that the development of peptide-based reagents is a feasible approach by demonstrating that a pool of seven peptides derived from ESAT-6, MPB70, MPB83, and MPB64 differentiates between infected and BCG-vaccinated cattle. However, further improvement to the sensitivity of this peptide-based reagent was required before the levels of sensitivity would be acceptable for the implementation of testing and slaughter-based strategies based on such reagents (50, 51).
The objective of the present study was to develop an improved peptide-based diagnostic reagent. Since MPB70 and MPB83 have shown promise as candidates for DNA-based subunit vaccination (9, 34, 52), we decided to concentrate our efforts on developing diagnostic reagents based on the antigens ESAT-6 and CFP-10. ESAT-6 is a well-studied antigen found in short-term culture filtrates of pathogenic mycobacteria of the M. tuberculosis complex. It has been shown to be able to discriminate between infected and BCG-vaccinated guinea pigs and humans (16, 28, 45, 47) and also to discriminate between M. bovis-infected cattle and cattle sensitized with environmental mycobacteria (39). CFP-10 has been also identified in the low-molecular-mass fraction of culture filtrate. The genes encoding CFP-10 and ESAT-6 are adjacent on the genome and are transcribed together. Both encode small exported proteins and share some degree of homology at the DNA level (5). Both are therefore members of the so-called ESAT-6 family of small mycobacterial proteins (5, 11, 46). Like ESAT-6, CFP-10 is recognized by M. tuberculosis-infected guinea pigs and humans but not by individuals vaccinated with BCG (2, 45, 49). In addition, it is also recognized by lymphocytes from cattle with BTB (49). In humans, the combination of recombinant CFP-10 and ESAT-6 demonstrated a high sensitivity in detecting TB patients, with a higher specificity than PPD (49).
Peptide epitopes within the sequence of CFP-10 were identified using a set of highly overlapping peptides that were tested with lymphocytes from cattle experimentally or naturally infected with M. bovis. This approach identified five promiscuously recognized peptides that were formulated together with ESAT-6-derived peptides previously identified by us (50, 51) into a peptide cocktail composed of 10 peptides. Our results demonstrated that this diagnostic cocktail composed of synthetic peptides from ESAT-6 and CFP-10 can be employed to differentiate M. bovis-infected cattle from those vaccinated with BCG with a high degree of sensitivity and specificity.
MATERIALS AND METHODS
Cattle.
For BCG vaccination and experimental M. bovis infection experiments, ca. 6-month-old calves (Friesian or Friesian crosses) were obtained from herds free of BTB and kept in the Animal Services Unit. The following groups of cattle were used in this study.
(i) M. bovis infection.
Calves were infected with an M. bovis field strain from Great Britain (AF 2122/97) by intratracheal instillation of 2 × 104 CFU as described previously (7, 8, 44). Infection was confirmed by the presence of tuberculous lesions in the lungs and lymph nodes of these animals as well as by the culture of M. bovis from tissue collected at postmortems performed ca. 20 weeks after the infection. Heparinized blood samples were obtained at least 6 weeks after infection, when strong and sustained in vitro tuberculin responses were observed. Data from a total of 23 experimentally infected cattle are presented in this study.
(ii) BCG vaccination.
Calves were vaccinated with BCG Pasteur by subcutaneous injection of 106 CFU into the side of the neck (7, 8) followed 8 weeks later by a booster injection using the same route and dose. Heparinized blood samples were taken 4 to 8 weeks after the booster vaccination. Data from 16 calves are presented in this study.
(iii) Field samples with confirmed BTB.
Heparinized blood was obtained from 76 cattle of eight different breeds and cross-breeds, from different parts of Great Britain, that had been designated tuberculin test reactors following skin testing with the SICCT (field reactors). The skin tests were performed as specified in reference 17. Blood samples were taken 10 to 20 days after the disclosing skin test, and all samples were transported by courier to our laboratory within 24 h of sampling and processed immediately upon arrival. M. bovis infection was confirmed by bacterial culture from pooled head lymph nodes and/or by histopathology on visible lesions found at necroscopy.
(iv) Negative controls.
Heparinized blood from SICCT-negative animals from herds free of BTB (30 animals) was also obtained.
Antigens and peptides. (i) Antigens.
Bovine (PPD-B) and avian (PPD-A) tuberculins were obtained from the Tuberculin Production Unit at the Veterinary Laboratories Agency-Weybridge and used in culture at 10 μg/ml.
(ii) Peptides.
An overlapping set of 44 synthetic peptides spanning the entire sequence of CFP-10 (14 residues long with a 12-residue overlap) was prepared by multi-rod peptide synthesis (pin-synthesized peptides) (35) (for sequence information, see reference 5) and used in mapping experiments at 25 μg/ml. The peptides were purchased from Chiron Mimotopes (Clayton, Australia). ESAT-6 and CFP-10 peptides, when part of the peptide cocktail, were synthesized by solid-phase peptide synthesis as described earlier (54). The purity and sequence fidelity of these peptides were confirmed by analytical reverse-phase high-pressure liquid chromatography and electron spray mass spectrometry, respectively. Peptides were used at 25 μg/ml when used individually and at 10 μg/ml each when used as components of the peptide cocktail (see Table 1 for sequences of the peptides included in this cocktail).
TABLE 1.
Composition of ESAT-6–CFP-10 peptide cocktail
| Peptidea | Sequenceb |
|---|---|
| CFP-10 derived | |
| P1.1–18 | MAEMKTDAATLAQEAGNF |
| P2.13–28 | QEAGNFERISGDLKTQ |
| P4.55–72 | VVRFQEAANKQKQELDEI |
| P7.75–92 | NIRQAGVQYSRADEEQQQ |
| P8.85–100 | RADEEQQQALSSQMGF |
| ESAT-6 derived | |
| P.1–16 | MTEQQWNFAGIEAAAS |
| P.9–24 | AGIEAAASAIQGNVTS |
| P.17–32 | AIQGNVTSIHSLLDEG |
| P.57–72 | KWDATATELNNALQNL |
| P.80–95 | GQAMASTEGNVTGMFA |
Numbers indicate positions of peptides within the protein sequences.
Amino acid residues are given in the one-letter code.
Lymphocyte transformation assay.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Histopaque-1077 (Sigma) gradient centrifugation and cultured in RPMI 1640 supplemented with 5% Controlled Process Serum Replacement type 1 (Sigma Aldrich, Poole, United Kingdom), nonessential amino acids (Sigma Aldrich), 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin per ml, and 100 μg of streptomycin sulfate per ml. PBMC (2 × 105/well in 0.2-ml aliquots) were cultured in triplicate for 6 days in flat-bottom 96-well microtiter plates in the presence of antigen, radiolabeled during the last 16 to 20 h of culture with 37 kBq of [3H]thymidine (Amersham, Little Chalfont, United Kingdom) per well, and harvested onto glass fiber filters, and radioactivity was counted in a scintillation counter (TopCount; Packard, Pangborne, United Kingdom). Positive responses are defined by a stimulation index (counts per minute with antigen/counts per minute without antigen) of ≥3 together with a signal strength of ≥1,000 cpm (24, 51, 53, 56).
IFN-γ assays.
Whole-blood cultures were performed in 96-well plates in aliquots of 0.20 ml/well by mixing 0.1 ml of heparinized blood with an equal volume of antigen-containing solution. Supernatants were harvested after 24 h of culture, and gamma interferon (IFN-γ) was determined using the BOVIGAM enzyme-linked immunosorbent assay (ELISA) kit (CSL, Melbourne, Australia) (57). Results obtained with individual peptides and diagnostic cocktails were deemed positive when the ratios of optical density at 450 nm (OD450) with antigens to OD450 without antigens (IFN-γ stimulation index) (31, 32) were ≥3.0. For comparative analysis of PPD-B versus PPD-A responses, a positive result was defined as a value for the OD450 with PPD-B minus the OD450 with PPD-A of ≥0.1 and a value for the OD450 with PPD-B minus the OD450 without stimulation of ≥0.1.
IFN-γ ELISPOT assay.
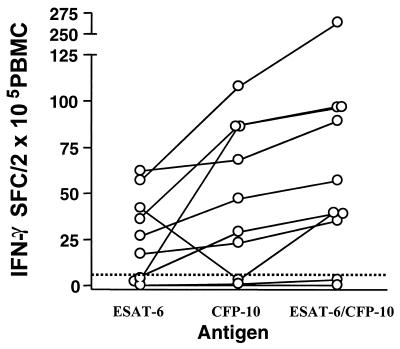
Direct enzyme-linked immunospots (ELISPOTs) were enumerated by modifying the protocol described for indirect ELISPOTs by van Drunen Littel-van den Hurk et al. (48). Briefly, ELISPOT plates (Immobilon-P polyvinylidene difluoride membranes; Millipore, Molsheim, France) were coated overnight at 4°C with the bovine IFN-γ-specific monoclonal antibody 2.2.1. Unbound antibody was removed by washing, and the wells were blocked with 10% fetal calf serum in AIM-V medium (Life Technologies, Paisley, Scotland, United Kingdom). PBMC (2 × 105/well suspended in AIM-V–2% FCS) were then added and cultured at 37°C and 5% CO2 in a humidified incubator for 24 h. Spots were developed with rabbit serum specific for IFN-γ followed by incubation with an alkaline phosphatase-conjugated monoclonal antibody specific for rabbit immunoglobulin G (Sigma Aldrich). Both the monoclonal antibody 2.2.1 and the rabbit anti-bovine IFN-γ serum were kindly supplied by D. Godson (VIDO, Saskatoon, Saskatchewan, Canada). The spots were visualized with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium substrate (Sigma Aldrich). Cutoff values for positive responses in Fig. 2 (>5 spot-forming cells [SFC]/200,000 PBMC) were calculated using the formula [mean SFC from cultures without antigen + (3.2498 × standard error)], to achieve a confidence interval of 99% (10, 36).
FIG. 2.
Combining ESAT-6- and CFP-10-derived peptides improves sensitivity. PBMC (2 × 105/well) from 10 cattle with confirmed BTB were incubated in ELISPOT plates in the presence of either the five ESAT-6-derived peptides, the five CFP-10-derived peptides, or both sets of peptides combined (ESAT-6–CFP-10 peptide cocktail) (Table 1). A positive response was defined as >5 IFN-γ-secreting SFC/2 × 105 PBMC. Black lines link results from individual animals.
RESULTS
Identification of regions within CFP-10 recognized by bovine T cells.
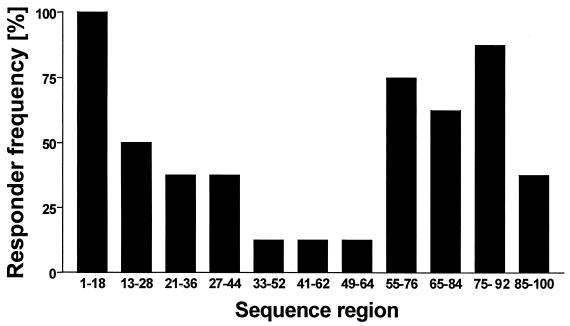
PBMC isolated from eight naturally infected, SICCT-positive field reactor cattle were incubated in the presence of a set of 44 highly overlapping pin-synthesized peptides derived from the sequence of CFP-10 (14-mer peptides overlapping by 12 amino acid residues). Peptides inducing proliferative responses were identified, and thereby regions containing T-cell-stimulating determinants were defined. The aim was to identify regions of the protein which were recognized by at least 50% of the cattle tested to identify promiscuous epitopes. Responses to CFP-10-derived peptides were detected in all eight field reactors tested, and regions containing epitopes could be identified between amino acid residues 1 and 18, 13 and 28, 21 and 36, 27 and 44, 33 and 52, 41 and 62, 49 and 64, 55 and 76, 65 and 84, 75 and 92, and 85 and 100. Epitopes within the regions defined by amino acids 1 to 18, 13 to 28, 55 to 76, 65 to 84, and 75 to 92 were recognized by lymphocytes from at least 50% of the animals tested (Fig. 1).
FIG. 1.
Identification of sequence regions within CFP-10 recognized by M. bovis-infected cattle. Proliferative responses of PBMC isolated from eight naturally infected SICCT-positive animals and incubated with 44 pin-synthesized peptides (14 residues long, overlapping by 12 residues) covering the complete sequence of CFP-10 were determined. Sequence regions recognized by PBMC from these animals were identified, and the results are expressed as the percentage of cattle recognizing peptides within these regions (responder frequency).
To confirm these results, eight peptides, covering amino acid residues 1 to 18, 13 to 28, 21 to 44, 55 to 72, 59 to 84, 65 to 84, 75 to 92, and 85 to 100, were designed and produced by conventional solid-phase synthesis, which, in contrast to pin synthesis, allows for more detailed quality control, to cover the majority of these regions containing putative promiscuous epitopes. These peptides were tested in lymphocyte transformation assays using PBMC from five animals experimentally infected with M. bovis and one naturally infected animal. The results suggested the presence of strong epitopes from CFP-10 within five of these peptides, since these peptides were prominently recognized by three or more of the cattle tested (see Table 1 for a list of these five peptides). These five peptides were also able to induce IFN-γ production in vitro (data not shown). Encouragingly, none of these five peptides were recognized by T cells isolated from BCG-vaccinated cattle, confirming the specificity of these T-cell determinants (data not shown). The peptides covering residues 59 to 84 and 65 to 84 were recognized by one and two animals, respectively; none of the tested animals recognized the peptide covering residues 21 to 44 (data not shown).
The combination of CFP-10 and ESAT-6 improves sensitivity.
Since we were encouraged by the results described above, the peptides listed in Table 1 were selected and formulated into a peptide cocktail for further evaluation together with five previously identified peptides derived from the sequence of ESAT-6 (50).The amino acid sequences of these peptides are given in Table 1. We first determined whether combining peptides from both antigens could improve the sensitivity of detection of an immune response to M. bovis infection over that with the use of single antigens. The IFN-γ responses of PBMC from 10 field reactors with confirmed BTB against the five peptides from ESAT-6 or the five peptides from CFP-10 listed in Table 1, or against the combined ESAT-6–CFP-10 peptide cocktail, were compared using the highly sensitive ELISPOT assay format to detect IFN-γ-secreting cells. As shown in Fig. 2, PBMC from 5 of 10 cattle produced IFN-γ upon in vitro challenge with both ESAT-6- and CFP-10-derived peptides, whereas T cells from one cow responded exclusively to the ESAT-6-derived peptides and those from two cows responded exclusively to the CFP-10-derived peptides. T cells from 8 of 10 animals responded to the combined ESAT-6–CFP-10 peptide cocktail (Fig. 2). Interestingly, when heparinized blood from the same animals was stimulated with the same peptide preparations and the supernatants were tested with the conventional BOVIGAM IFN-γ ELISA, only four and three animals, respectively, responded to CFP-10 or ESAT-6 peptide stimulation alone, whereas all eight identified by ELISPOT were also identified by ELISA (data not shown). This suggests that the combination of the two peptide pools increased the ELISA signal strength, resulting in sensitivity comparable to that of the IFN-γ ELISPOT.
In a second experiment with eight experimentally infected cattle, ESAT-6 induced IFN-γ ELISPOT responses in five animals whereas seven animals responded to the combined ESAT-6–CFP-10 peptide cocktail (data not shown). Taking both experiments together, a total of 10 of 18 animals responded to ESAT-6 alone, whereas 15 of 18 responded to the combined ESAT-6–CFP-10 peptide cocktail. Thus, we have provided proof of principle for a strategy of combining several antigens to improve the sensitivity of detection of the immune response to M. bovis infection in cattle over that with the use of single antigens.
The ESAT-6–CFP-10 peptide cocktail can distinguish between infected and BCG-vaccinated cattle.
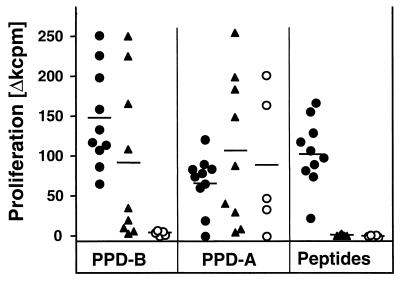
We next investigated whether the ESAT-6–CFP-10 peptide cocktails described above would be able to discriminate between M. bovis-infected animals and either BCG-vaccinated or nonvaccinated control cattle. The peptide cocktail was therefore tested in a first set of experiments with PBMC from cattle infected experimentally with M. bovis (10 animals), BCG-vaccinated cattle (9 animals), or noninfected negative controls (5 animals). The results of this experiment are shown in Fig. 3. Proliferative immune responses were induced by the peptide cocktail in all experimentally infected calves tested in this experiment. In contrast, none of the BCG-vaccinated or negative control cattle responded to the peptide cocktail. Significantly, T cells from all BCG-vaccinated animals proliferated strongly to both avian and bovine tuberculin. This confirmed the lack of specificity of tuberculin in the face of BCG vaccination. In addition, four of the noninfected control animals responded to avian tuberculin, indicating that they had been exposed to environmental mycobacteria (Fig. 3). Thus, we have demonstrated that a cocktail composed of peptides from antigens not expressed in BCG can differentiate between M. bovis-infected and BCG-vaccinated animals, whereas tuberculin cannot.
FIG. 3.
Proliferative responses induced by the ESAT-6–CFP-10 peptide cocktail. PBMC isolated from 10 M. bovis-infected cattle (●), 9 BCG-vaccinated cattle (▴), and 5 nonvaccinated controls (○) were incubated with either the protein cocktail, PPD-B, or PPD-A. Results are expressed as mean proliferative responses, and the black lines indicate response means.
Although measuring proliferation by [3H]thymidine incorporation is a reproducible, sensitive, and simple readout system for T-cell responses, its use of radioactivity and the length of the test make it not an attractive candidate for routine diagnosis. Therefore, and in order to estimate more closely the levels of sensitivity of the peptide cocktail, we next determined the IFN-γ responses in whole-blood cultures induced by the peptide cocktail. Heparinized blood samples were obtained from tuberculin-positive field reactors with confirmed BTB (68 animals), from BCG-vaccinated cattle (16 animals), and from cattle from herds free of BTB (25 animals) and were stimulated within 24 h of blood sampling with tuberculins or the peptide cocktail. IFN-γ in these supernatants was measured with the commercially available BOVIGAM IFN-γ ELISA kit. In this assay the ESAT-6–CFP-10 cocktail detected 77.9% of the confirmed reactors (Table 2). This compared well with the sensitivity achieved by the comparison of PPD-B and PPD-A responses, which detected 88.2% of confirmed reactors (Table 2).
TABLE 2.
IFN-γ responses to tuberculin and the ESAT-6–CFP-10 peptide cocktail in tuberculin skin test-positive field reactors, BCG-vaccinated cattle, and cattle from herds free of TBa
| Antigen test | Cattle with confirmed TB
|
TB-free cattle
|
BCG-vaccinated cattle
|
|||
|---|---|---|---|---|---|---|
| Responder frequency (%)b | Responsec | Responder frequency (%) | Response | Responder frequency (%) | Response | |
| Comparison of PPD-B and PPD-A | 88.2 (60/68) | 0.735 (−0.381 to 3.287) | 8 (2/25) | −0.03 (−2.417 to 0.516) | 75 (12/16) | 0.258 (−0.294 to 0.552) |
| Peptide cocktail | 77.9 (53/68) | 5.01 (1.1 to 66.04) | 0 (0/25) | 1.18 (0.81 to 2.6) | 0 (0/16) | 1.24 (0.65 to 1.98) |
Heparinized whole-blood cultures were initiated within 24 h of sampling, and IFN-γ in plasma supernatants was determined using the BOVIGAM ELISA kit. For tuberculins, a positive response was defined as a value for the OD450 with PPD-B minus the OD450 with PPD-A of ≥0.1 and a value for the OD450 with PPD-B minus the OD450 without stimulation of ≥0.1. For the ESAT-6–CFP-10 peptide cocktail, a positive response was defined as an interferon stimulation index (OD450 with antigen/OD450 for medium control) of ≥3.
Values in parentheses are number of responders/total number of animals tested.
Tuberculin-specific responses are expressed as median OD differences between PPD-B and PPD-A (medians of OD450 with PPD-B minus OD450 with PPD-A, with ranges in parentheses. Peptide-induced responses are expressed as median interferon stimulation indices, with ranges in parentheses.
The high specificity of the peptide cocktail in the whole-blood IFN-γ assay compared to tuberculin was confirmed because it did not induce IFN-γ responses in blood from BCG-vaccinated cattle or cattle from TB-free herds tested (100% specificity). In contrast, 12 of 16 of the BCG-vaccinated cattle and 2 of 25 of the TB-free cattle gave rise to positive responses when the production of IFN-γ upon stimulation with PPD-B was compared to that induced by PPD-A (25 and 92% specificity, respectively) (Table 2). The differences in ODs between PPD-B and PPD-A responses of BCG-vaccinated animals were smaller than those for cattle with confirmed BTB (medians of 0.258 for BCG-vaccinated cattle and 0.735 for cattle with BTB) (Table 2). This was due to the induction of high PPD-A responses in the BCG-vaccinated animals concomitant with the development of PPD-B responses rather than to significantly lower PPD-B responses per se in BCG-vaccinated animals (median ODs with PPD-B in animals with BTB and in BCG-vaccinated cattle, 1.119 and 0.804, respectively).
DISCUSSION
The development of diagnostic reagents capable of differentiating between infection and vaccination is necessary for the development of a vaccine against TB in cattle so that existing test and slaughter control strategies can continue alongside vaccination. BCG compromises the specificity of the tuberculin skin test and is therefore not used as a control measure for BTB in cattle. Vaccination of cattle with BCG therefore provides a challenging model system of an attenuated live mycobacterial vaccine to develop strategies and reagents for differential diagnosis. The application of recombinant ESAT-6 as a diagnostic antigen to differentiate between infected and BCG-vaccinated humans has been widely tested, and responder frequencies of between 60 and 95% have been reported (see, e.g., references 28, 38, 43, and 49). Comparable data on the application of recombinant CFP-10 are available from several reports, which indicated responder frequencies of between ca. 50 and 90% of the TB patients (2, 45, 49). Studies in cattle indicated that about the same percentage of M. bovis-infected cattle as human TB patients recognized ESAT-6 (6, 39, 42, 49, 51) and that it could be applied to the differential diagnosis of infected and vaccinated cattle (6, 51). It has also been reported recently that CFP-10 is recognized by a large proportion of infected cattle (49).
Only a subset of infected animals will respond to a single antigen (18, 19, 42, 49, 51), which means that it is unrealistic to expect that any single antigen will be able to detect the proportion of infected animals required of a successful diagnostic reagent. Therefore, cocktails of antigens will be required to achieve broad population coverage. Such cocktails, composed of, e.g., ESAT-6, MPB64, MPB83, MPB70, and CFP-10 in different combinations, have been described recently by us and other groups as useful in the detection of TB in cattle or humans (6, 49–51). An additional advantage to this approach is that higher signal strengths are often obtained with reagent pools than with their individual components (51). Furthermore, antigen cocktails reduce the possibility of selecting escape mutants, i.e., strains of bacilli lacking the diagnostic antigen. Such natural mutants have been described. For example, strains of M. tuberculosis which do not express the 19-kDa lipoprotein have been identified (30).
The approach described in the present report exploits the use of synthetic peptides derived from the sequences of these proteins as diagnostic reagents to detect TB in cattle. For a long time this approach has been deemed impractical. It has been argued that due to the genetic diversity of the major histocompatibility complex (MHC) system in mammalian species, a large pool of epitopes would be required to achieve sufficient population coverage to be a practical alternative to recombinant protein antigens. However, it has now been widely accepted that a significant proportion of MHC class II-restricted peptides are recognized by T cells in the context of multiple MHC haplotypes (promiscuous epitopes). This has been demonstrated in the recognition of mycobacterial antigens by murine, human, and bovine CD4+ T cells (33, 40, 41). In the present study, cattle of eight different breeds and cross-breeds from herds in different parts of Great Britain were used to define and subsequently validate the peptide epitopes, and ca. 78% of Great Britain field reactors tested responded to the peptide cocktail. It is therefore likely that we have defined truly promiscuous peptides within the antigens mapped in this study. However, we have not formally demonstrated the association of these peptides with multiple bovine MHC (BoLA) class II haplotypes, since this was beyond the applied objectives of this study. We also cannot exclude the existence of more such epitopes within the sequences of CFP-10 and ESAT-6. In addition, for obvious reasons we have tested only cattle from the Great Britain national herd, and we appreciate that further studies will be necessary to determine if those peptides are recognized by cattle from other national herds, particularly in Africa or Asia, where different breeds of cattle are more common.
Our strategy of using a large number of highly overlapping peptides prepared by pin synthesis to screen for regions of the sequence containing T-cell epitopes has been successful. A large number of peptides can be produced by this method at relatively low costs. However, the amount of each peptide produced is limited, as is the ability to perform quality assurance procedures. It is therefore important to confirm the results of such epitope scans by preparing peptide candidates employing conventional synthesis. Our chosen strategy was vindicated since we were able to confirm that T-cell epitopes were present within all of the regions identified by the pin-synthesized overlapping peptide set, with the exception of one peptide (residues 21 to 44), where we attempted to cover two adjacent sequence regions (residues 21 to 36 and 27 to 44) with a single peptide. This might have led to interference of MHC binding by the presence of secondary structures within this peptide linking at least two epitope cores and preventing MHC binding by steric hindrance (20, 37). Alternatively, the MHC haplotype(s) associated with residues 21 to 44 might not have been represented in the animals tested. The latter hypothesis is less likely, however, since two animals that had originally responded to peptides within the sequence regions 21 to 36 and 27 to 44 did not respond to the peptide containing residues 21 to 44.
The ESAT-6–CFP-10 peptide cocktail described in this study detected a similar proportion of infected cattle (77.9%) as that described for the respective recombinant proteins in the study by van Pinxteren and coworkers (49), who detected 75% of infected cattle using ESAT-6 and CFP-10 individually. This suggests that mixtures of synthetic peptides can have potencies in cattle equivalent to those of whole recombinant proteins. Supporting this notion, Arend and coworkers (3) have recently used M. tuberculosis-specific human T-cell lines of different HLA-DR types as well as ex vivo PBMC assays measuring IFN-γ production. They could demonstrate that mixtures of synthetic overlapping peptides spanning the complete amino acid sequence of each antigen have potencies equivalent to those of whole ESAT-6 and CFP-10 for sensitive and specific detection of human infection with M. tuberculosis. The ESAT-6–CFP-10 peptide cocktail also constitutes an improvement on the peptide pool composed of peptides from ESAT-6, MPB83, MPB64, and MPB70 that we have described previously (50): 88.3% of all SICCT-positive reactors that were identified as positive for BTB by comparison of the in vitro IFN-γ production with PPD-B and PPD-A were also identified using the ESAT-6–CFP-10 cocktail, which compares favorably to a 69.9% congruence level between peptides and tuberculin responses observed with the previously described peptide pool.
In conclusion, this study demonstrates that diagnostic cocktails based on synthetic peptides derived from antigens expressed in M. bovis but not in BCG can distinguish between vaccinated and infected cattle in blood-based assays. Such antigens could form the basis of next-generation diagnostic reagents whose sensitivity might reach or even surpass that of tuberculin PPD and which could be employed alongside vaccination.
ACKNOWLEDGMENTS
This work performed was funded by the Ministry of Agriculture, Fisheries and Food, Great Britain.
We express our appreciation to the staff of the Animal Services Unit at the Veterinary Laboratories Agency for their dedication to animal welfare. We acknowledge the valuable assistance of Veterinary Field Service staff of the Ministry of Agriculture, Fisheries and Food in the collection of blood samples from field reactor cattle for use in this study.
REFERENCES
- 1.Anonymous. The Government's response to the Krebs report on bovine tuberculosis in cattle and badgers. London, United Kingdom: Ministry of Agriculture, Fisheries and Food Publications; 1997. [Google Scholar]
- 2.Arend S M, Andersen P, van Meijgaarden K E, Skjot R L, Subronto Y W, van Dissel J T, Ottenhoff T H. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–1854. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 3.Arend S M, Geluk A, Van Meijgaarden K E, Van Dissel J T, Theisen M, Andersen P, Ottenhoff T H M. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. 2000;68:3314–3321. doi: 10.1128/iai.68.6.3314-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berggren S A. Field experiment with BCG vaccine in Malawi. Br Vet J. 1981;137:88–94. doi: 10.1016/s0007-1935(17)31792-x. [DOI] [PubMed] [Google Scholar]
- 5.Berthet F X, Rasmussen P B, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 6.Buddle B, Parlane N A, Keen D L, Aldwell F E, Pollock J M, Lightbody K, Andersen P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle using recombinant mycobacterial antigens. Clin Diagn Lab Immunol. 1999;6:1–5. doi: 10.1128/cdli.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buddle B M, de Lisle G W, Pfeffer A, Aldwell F E. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–1130. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 8.Buddle B M, Keen D, Thomson A, Jowett G, McCarthy A R, Heslop J, De Lisle G W, Stanford J L, Aldwell F E. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 9.Chambers A M, Vordermeier H M, Commander N, Tascon R E, Lowrie D B, Hewinson R G. Vaccination of mice and cattle with plasmid DNA encoding the Mycobacterium bovis antigen MBP83. Clin Infect Dis. 2000;30:S283–S287. doi: 10.1086/313875. [DOI] [PubMed] [Google Scholar]
- 10.Clarke G M. Statistics and experimental design. London, United Kingdom: Arnold; 1994. p. 88. [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Cosivi O, Grange J M, Daborn C J, Raviglione M C, Fujikura T, Cousins D, Robinson R A, Huchzermeyer H F, de Kantor I, Meslin F X. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosivi O, Meslin F X, Daborn C J, Grange J M. Epidemiology of Mycobacterium bovis infection in animals and humans, with particular reference to Africa. Rev Sci Technol. 1995;14:733–746. doi: 10.20506/rst.14.3.875. [DOI] [PubMed] [Google Scholar]
- 14.Daborn C J, Grange J M, Kazwala R R. The bovine tuberculosis cycle—an African perspective. Soc Appl Bacteriol Symp Ser. 1996;25:27S–32S. doi: 10.1111/j.1365-2672.1996.tb04595.x. [DOI] [PubMed] [Google Scholar]
- 15.Dankner W M, Waecker N J, Essey M A, Moser K, Thompson M, Davis C E. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Medicine (Baltimore) 1993;72:11–37. [PubMed] [Google Scholar]
- 16.Elhay J M, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–3456. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Economic Community. EEC directive 80/219, amending directive 64/432 annexe B. Official Journal. 1980;L047:25–32. [Google Scholar]
- 18.Fifis T, Corner L A, Rothel J S, Wood P R. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand J Immunol. 1994;39:267–274. doi: 10.1111/j.1365-3083.1994.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 19.Fifis T, Rothel J S, Wood P R. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet Microbiol. 1994;40:65–81. doi: 10.1016/0378-1135(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 20.Grewal I S, Moudgil K D, Sercarz E E. Hindrance of binding to class II major histocompatibility complex molecules by a single amino acid residue contiguous to a determinant leads to crypticity of the determinant as well as lack of response to the protein antigen. Proc Natl Acad Sci USA. 1995;92:1779–1783. doi: 10.1073/pnas.92.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haga S, Yamaguchi R, Nagai S, Matsuo K, Yamazaki A, Nakamura R M. Delayed-type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovis BCG. J Leukoc Biol. 1995;57:221–225. doi: 10.1002/jlb.57.2.221. [DOI] [PubMed] [Google Scholar]
- 22.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie R M, Watson J M. Mycobacterium bovis in England and Wales: past, present and future. Epidemiol Infect. 1992;109:23–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Harris D P, Vordermeier H M, Friscia G, Roman E, Surcel H M, Pasvol G, Moreno C, Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993;150:5041–5050. [PubMed] [Google Scholar]
- 25.Hart P D, Sutherland I, Thomas J. The immunity conferred by effective BCG and vole bacillus vaccines, in relation to individual variations in induced tuberculin sensitivity and to technical variations in the vaccines. Tubercle. 1967;48:201–210. [Google Scholar]
- 26.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W J. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 27.Hubrig T, Kruger W. Untersuchungen zur Tuberkuloseschutzimpfung bei Rindern. Monatshefte Veterinaermedizin. 1958;13:513–519. [Google Scholar]
- 28.Johnson P D R, Stuart R L, Grayson M L, Olden D, Clancy A, Ravn P, Andersen P, Britton W J, Rothel J S. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin Diagn Lab Immunol. 1999;6:934–937. doi: 10.1128/cdli.6.6.934-937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs J R. Bovine tuberculosis in cattle and badgers. London, United Kingdom: Ministry of Agriculture, Fisheries and Food Publications; 1997. [Google Scholar]
- 30.Lathigra R, Zhang Y, Hill M, Garcia M J, Jackett P S, Ivanyi J. Lack of production of the 19-kDa glycolipoprotein in certain strains of Mycobacterium tuberculosis. Res Microbiol. 1996;147:237–249. doi: 10.1016/0923-2508(96)81384-2. [DOI] [PubMed] [Google Scholar]
- 31.Lightbody K A, Girvin R M, Mackie D P, Neill S D, Pollock J M. T-cell recognition of mycobacterial proteins MPB70 and MPB64 in cattle immunized with antigen and infected with Mycobacterium bovis. Scand J Immunol. 1998;48:44–51. doi: 10.1046/j.1365-3083.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 32.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T-cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lightbody K A, Skuce R A, Neill S D, Pollock J M. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec. 1998;142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 34.Lowrie D B, Tascon R E, Bonato V L, Lima V M, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 35.Maeji N J, Bray A M, Geysen H M. Multi-rod peptide synthesis strategy for T cell determinant analysis. J Immunol Methods. 1990;134:23–31. doi: 10.1016/0022-1759(90)90108-8. [DOI] [PubMed] [Google Scholar]
- 36.Motulsky H. Intuitive biostatistics. New York, N.Y: Oxford University Press; 1995. [Google Scholar]
- 37.Moudgil K D, Grewal I S, Jensen P E, Sercarz E E. Unresponsiveness to a self-peptide of mouse lysozyme owing to hindrance of T cell receptor-major histocompatibility complex/peptide interaction caused by flanking epitopic residues. J Exp Med. 1996;183:535–546. doi: 10.1084/jem.183.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustafa A S, Amoudy H A, Wiker H G, Abal A T, Ravn P, Oftung F, Andersen P. Comparison of antigen-specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:535–543. doi: 10.1046/j.1365-3083.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 39.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 40.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 41.Pollock J M, Douglas A J, Mackie D P, Neill S D. Peptide mapping of bovine T-cell epitopes for the 38 kDa tuberculosis antigen. Scand J Immunol. 1995;41:85–93. doi: 10.1111/j.1365-3083.1995.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 42.Pollock J M, Girvin R M, Lightbody K A, Clements R A, Neill S D, Buddle B M, Andersen P. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet Rec. 2000;146:659–665. doi: 10.1136/vr.146.23.659. [DOI] [PubMed] [Google Scholar]
- 43.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 44.Rhodes S G, Gavier-Widen D, Buddle B M, Whelan A O, Singh M, Hewinson R G, Vordermeier H M. Antigen specificity in experimental bovine tuberculosis. Infect Immun. 2000;68:2573–2578. doi: 10.1128/iai.68.5.2573-2578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skjot R L, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun. 2000;68:214–220. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tekaia F, Gordon S V, Garnier T, Brosch R, Barrell B G, Cole S T. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 47.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H E. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 48.van Drunen Littel-van den Hurk S, Braun R P, Lewis P J, Karvonen B C, Baca-Estrada M E, Snider M, McCartney D, Watts T, Babiuk L A. Intradermal immunization with a bovine herpesvirus-1 DNA vaccine induces protective immunity in cattle. J Gen Virol. 1998;79:831–839. doi: 10.1099/0022-1317-79-4-831. [DOI] [PubMed] [Google Scholar]
- 49.van Pinxteren L A, Ravn P, Agger E M, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immunol. 2000;7:155–160. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vordermeier H M, Cockle P C, Whelan A, Rhodes S, Hewinson R G. Towards the development of diagnostic assays to discriminate between Mycobacterium bovis infection and BCG vaccination in cattle. Clin Infect Dis. 2000;30:S291–S298. doi: 10.1086/313877. [DOI] [PubMed] [Google Scholar]
- 51.Vordermeier H M, Cockle P C, Whelan A, Rhodes S, Palmer N, Bakker D, Hewinson R G. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–682. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vordermeier H M, Cockle P J, Whelan A O, Rhodes S, Chambers M A, Clifford D, Huygen K, Tascon R, Lowire D, Colston M J, Hewinson R G. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine. 2001;19:1246–1255. doi: 10.1016/s0264-410x(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 53.Vordermeier H M, Harris D P, Friscia G, Roman E, Surcel H M, Moreno C, Pasvol G, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 54.Vordermeier H M, Harris D P, Moreno C, Ivanyi J. Promiscuous T cell recognition of an H-2 IA-presented mycobacterial epitope. Eur J Immunol. 1994;24:2061–2067. doi: 10.1002/eji.1830240919. [DOI] [PubMed] [Google Scholar]
- 55.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson R J, Vordermeier H M, Wilkinson K A, Sjolund A, Moreno C, Pasvol G, Ivanyi J. Peptide specific response to M. tuberculosis: clinical spectrum, compartmentalization and effect of chemotherapy. J Infect Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 57.Wood P R, Rothel J S. In vitro immunodiagnostic assays for bovine tuberculosis. Vet Microbiol. 1994;40:125–135. doi: 10.1016/0378-1135(94)90051-5. [DOI] [PubMed] [Google Scholar]