Abstract
Mosquito control is becoming more difficult as a result of the rise in resistance to toxic chemical insecticides. The insecticides of bio-fabrication sources may serve as a convenient alternative to environmentally acceptable methods in the future. The larvicidal and pupicidal activities of bio-fabricated zinc oxide nanoparticles (ZnO NPs) on the different instar larvae and pupae of Anopheles subpictus Grassi (Malaria vector) and Culex quinquefasciatus Say (lymphatic filariasis) were investigated in this study. The results recorded from XRD, FTIR, SEM-EDX, and TEM analyses confirmed the bio-fabrication of ZnO NPs. Such nanoparticles were nearly spherical and agglomerated with a size of 34.21 nm. GC-MS analysis of methanol extract revealed the compound, stigmasterol (C29H48O) as major one. Mosquito larvae and pupae of targeted mosquito were tested against varied concentrations of the bio-fabricated ZnO NPs and methanol extract of Vitex negundo for 24 h. The maximum activity was recorded from ZnO NPs against the larvae and pupae of A. subpictus LC50 which were 1.70 (I), 1.66 (II), 1.93 (III), 2.48 (IV), and 3.63 mg/L (pupa) and C. quinquefasciatus LC50 were 1.95 (I), 2.63 (II), 2.90 (III), 4.32 (IV), and 4.61 mg/L (pupa) respectively. ZnO NPs exhibited strong DPPH radical and FRAP scavengers compared to the aqueous extract of V. negundo. Also, V. negundo leaf methanol extract (VNLME) and ZnO NPs were evaluated for their cytotoxicity on HeLa cells, which exhibited the IC50 values of 72.35 and 43.70μg/mL, respectively. The methylene blue (MB) dye, which is harmful to both aquatic and terrestrial life, was degraded using the biosynthesized ZnO nanoparticles. At 664 nm, 81.2% of the MB dye had degraded after 120 min of exposure to sunlight. Overall, our results revealed that ZnO NPs are the perfect biological agent and economical for the control of malaria, filariasis vectors, antioxidant, HeLa cells, and MB blue dye degradation under sunlight irradiation.
Graphical abstract

Keywords: Vitex negundo, XRD, Larvicidal, Pupicidal, DPPH, HeLa cells, Solar irradiation
Introduction
Mosquito-borne illnesses (MBIs) are spreading and distributing over the world, mainly after the COVID-19 pandemic period [1]. Malaria, dengue, lymphatic filariasis, yellow fever, chikungunya, Zika virus, dengue fever, and Japanese encephalitis are among the dreadful mosquito-borne diseases (MBD) (Diptera: Culicidae) that threaten millions of people worldwide [2–4]. The spread of MBD depends on the intricate interactions between the environment and susceptibility, exposure, and adaptive capability of the people. There is evidence that climatic conditions, such as temperature and precipitation, impact the spatial and temporal distribution of vectors, hosts, and infections [5]. Dengue and malaria, the two most frequent MDBs, are quickly increasing their geographic ranges, arriving in previously unaffected regions and re-emerging in places where they had been eradicated for many years. There are millions of deaths caused by mosquito bites every year, especially in the tropics [6].
Malaria’s primary vector, Anopheles subpictus Grassi, is expanding its range across India. As far as global health is concerned, malaria is still a top priority [7]. India contributes significantly to the worldwide malaria burden, with 1.5–2 million confirmed malaria cases and yearly fatality estimates between 15,000 and 95,000 [7–9]. The World Health Organization (WHO) sought new vector control approaches in 2021 in an effort to enhance the effectiveness and sustainability of existing control initiatives [7]. Anopheles mosquitoes transfected with a nano-biotechnology tool are one novel strategy for bio-controlling malaria. A mosquito known as Culex quinquefasciatus found commonly in India’s metropolitan and outlying regions. More than 1.4 billion individuals in 73 countries are afflicted with lymphatic filariasis, a neglected tropical illness. Approximately 25 million males and more than 15 million individuals worldwide suffer from genital illness and lymphedema, respectively [10]. Despite its crippling consequences, lymphatic filariasis is accorded a low priority for control [11].
Because mosquito larvae are less mobile in the breeding habitat, it is easier to devise control measures during this stage [12]. Mosquito control methods that are more environmentally friendly are needed in order to overcome these issues [13–15]. Currently, synthetic pesticides are commonly used because of their rapid response against parasites. But the major problem of those synthetic compounds may cause toxic effects to non-targeted organisms and also pollute the environment. In this study, we try to overcome the problem that bio-synthesized NPs are used as pesticide agent as an alternative of chemical compounds because the biosynthesis of nanoparticles is a reasonable, eco-friendly, and non-hazardous process.
Cancer kills around 8.8 million people per year across the globe, accounting for one in six fatalities and surpassing the number of deaths caused by HIV/AIDS and malaria combined. In low- and middle-income countries (LMICs), where over 75% of cancer fatalities occur and the number of cancer patients is continually increasing, disease treatment remains tough and onerous. By the year 2035, the incidence of cancer is expected to increase by a factor of two [16]. According to the American Cancer Society, breast cancer (BC) and cervical cancer are the fourth most prevalent malignancies in women. As a consequence of an estimated 570,000 new cases and 311,000 fatalities, cervical cancer took the lives of 311,000 women globally in 2018. BC, the biggest cause of cancer-related deaths worldwide, was diagnosed in 2.6 million women, resulting in around 685,000 deaths. BC is the leading cause of cancer-related mortality, accounting for 30% of all cancer-related deaths [2, 17–20].
In the recent two decades, nanotechnology has emerged as a potential subject for multidisciplinary study due to its extensive variety of applications. Thousands of tons of metallic nanoparticles (MNPs) are now used in nano-enabled currencies, personal care items, pharmaceuticals, food, and agricultural goods [21, 22]. Chemical sensors, electronic devices, sunscreen UV filters, textile UV filters, and more have all used zinc oxide nanoparticles (ZnO NPs), a common ingredient in a wide variety of commercial products [23, 24]. Plant growth and vitality, antibacterial potential, photocatalytic activity, and mosquito larvicidal activity have all benefited from zinc nanoparticle inclusion in nanofertilizer in recent years [25–28]. Insecticides are not used in green synthesis methods, so they are suitable for pharmaceutical and other biomedical applications [29, 30]. Nirgundi is the Hindi name for the Verbenaceae family’s Vitex negundo Linn. The leaves of V. negundo are between 4 and 10 cm long and include five lanceolate leaflets with a hairy underside and pointy at both ends [31]. There is Ayurvedic evidence that the plant leaves of V. negundo, taken orally, can combat implantation, bacteria, diabetes, inflammation, and asthma [32]. Flavonoids, steroids, volatile oils, terpenes, and lignans are just a few of the many compounds discovered through phytochemical research on V. negundo [33, 34]. The synthesized ZnO NPs and V. negundo leaf methanol extract (VNLME) exhibited larvicidal and pupicidal activity against Anopheles subpictus and C. quinquefasciatus, respectively. Also, HeLa cells were used to assess cytotoxicity of ZnO NPs and methanol extract. By using XRD, FTIR, SEM-EDX, and TEM, ZnO NPs were characterized. The major compounds were discovered through GC-MS analysis of VNLME. In the development of new control tools for larvicidal and pupicidal (A. subpictus and C. quinquefasciatus) activity, antioxidant HeLa cell and dye degradation of methylene blue under solar sun light irradiation, the V. negundo-biofabricated ZnO NPs could be useful in stimulating research.
Materials and methods
Materials
The leaves of Vitex negundo Linn. were collected from the village of Thandrampet under Thiruvannamalai district in Tamil Nadu state, India (12° 9′ 15″ N 78° 56′ 48″ E), and Dr. S. Isabella Rosaline, Department of Botany, Auxilium College, Vellore, India, has done the taxonomic identification. The specimens were numbered and stored for future reference in the research laboratory. Sigma-Aldrich, India, supplied 99.9% pure zinc nitrate (Zn (NO3)2) for this study. The MPM Scientific Co., Vellore, India, provided methanol.
Preparation of plant crude extract
Leaf powder (100 g) from V. negundo was ground in an electric grinder (28 °C) and extracted with methanol (500 mL, Qualigens) for 3 h in the Soxhlet apparatus (boiling point range of 85 °C) for 6–10 days in the shade. Rotavapor was then used to completely evaporate the solvent to dryness at 60 °C. Methanol extract residues were identified and stored at 10 °C for further analysis.
V. negundo-synthesized ZnO NPs
Fresh V. negundo leaves were cleaned with tap water for 5 min before being rinsed with distilled water to eliminate dust. The plant leaf aqueous solution was prepared by boiling 12 g of washed and finely chopped leaves in a 300-mL Erlenmeyer flask with 100 mL of sterile distilled water for 15 min at 70°C. For 24 h, 20 mL of V. negundo aqueous extract was subjected to 80 mL of Zn (NO3)2. 5H2O (5 mM) after decanting the solution. V. negundo’s leaf extract (VNLE) and zinc nitrate Zn (NO3)2 did not change in color. Upon reaction with zinc nitrate, the color of the extract of V. negundo changed to yellow, and the extract was filtered through Whatman filter paper no. 1 [35].
Characterization of ZnO NPs
X-ray diffraction (XRD) spectroscopy confirmed the presence of bio-fabricated ZnO NPs in the KBr pellets and ZnO NP crystal structure. The Bruker AXS D8 Advance XRD instrument was used to conduct the XRD analyses. According to FTIR measurements, the ZnO nanoparticles’ functional groups have been confirmed qualitatively. Thermo Nicolet Avatar 370 FTIR spectrophotometer was used for these measurements. ZnO NP solution was sputter coated on a copper stub to study the morphology with a scanning electron microscope (JEOL Model JSM-6390LV) equipped with an energy-dispersive spectrometer (EDX; JEOL Model JED-2300). Transmission electron microscopy measurements (TEM; JEOL/JEM 2100) used a 200-kV acceleration voltage, with a resolution of 0.23 nm and lattice spacing of 0.14 nanometers.
Parasites rearing
At the Vellore Zonal Entomological Research Centre (12°55′48′′ N, 79°7′48′′ E), mosquito larvae from Gandhinagar Vellore (12.953°N, 79.141°E) stagnant and rice field water areas were collected and identified as A. subpictus and C. quinquefasciatus. The mosquitoes were kept in 12-h light/dark cycles at 28 ± 2 °C and 40 to 63% relative humidity throughout the study. Larvae were fed dog biscuits and brewer’s yeast in a 3:1 ratio during the experiments. A 10% sucrose solution was fed to adult male mosquitoes after 6 days of development. According to Kamaraj et al. [36], female mosquitos use the white albino rat’s blood to produce eggs. In accordance with Dinesh et al. [37], fresh larvae and pupae were cultured prior to being used in the experiments.
Larvicidal and pupicidal experiments against parasites
In total, 25 C. quinquefasciatus and A. subpictus larvae and pupae were put in a beaker with 250 mL of distilled water and different amounts of V. negundo leaf methanol extract (20–100 mg/L) and bio-fabricated ZnO NPs (2–10 mg/L) for 24 h. The beaker contained the various concentrations of V. negundo. Each concentration was tested with larval food (0.5 mg), and the method was evaluated in accordance with [38]. Larvae and pupae were exposed to each concentration three times each. Mortality rates were determined as follows:
Gas chromatography-mass spectrometry analysis
To conduct GC-MS analysis on the V. negundo leaf methanol extract (VNLME) using the Varian 1200 L Single Quadrupole mass spectrometer, we followed the steps mentioned below: specifications of the columns: WCOT fused silica is used for the columns. VF-5MS in stationary phase and the column is 30 m long. At a temperature of 325 °C, helium has a mass range of 10–800 U, making it an ideal carrier gas. The components were identified by using the National Institute of Standards and Technology Library.
Antioxidant assay
DPPH radical scavenging assay
According to the procedure of the antioxidant assays, the ability of ZnO NPs and the extract of V. negundo leaf reduce DPPH activity [39] with minor modifications. The standard ascorbic acid and extract (100–500μg/mL) were reacted with DPPH, allowed to incubate for 15 min in minimum light. After incubation, samples’ optical density was measured at 517 nm. The DPPH inhibition was calculated using the samples’ average optical absorbance value, and the reduced percent was calculated as follows.
where A0 is the blank absorbance, and A1 is the sample absorbance. All the experiments were evaluated in triplicates.
FRAP assay
Ferric ion reducing antioxidant power (FRAP) activity was determined according to the procedure of Bhagat et al. [40]. Briefly, acetate buffer (300 mM, pH 3.6), TPTZ (2,4,6-tripyridyl-s-triazine) 10 mM in 40 mM HCl, FeCl3·6H2O (20 mM). The three solutions were mixed in a 10:1:1 ratio to make the freshly prepared effective FRAP reagents. Biosynthesized ZnO NPs (100–500 μg/mL) and standard butylated hydroxytoluene (BHT) were dissolved with 3 mL of having to work FRAP reagent, and the optical density was recorded at 593 nm after stirring. The calibration curve with known Fe2+ dose was established utilizing methanol solutions of FeSO4·7H2O varied from 100 to 2000 μM. The amount of antioxidant with a Ferric-TPTZ reducing ability equal to that of 1 mM FeSO4·7H2O was defined as the parameter equivalent concentration.
Cytotoxic study
From the National Center for Cell Sciences, human cervical (HeLa) cancer cells were bought (NCCS, Pune, India). Using HeLa cells, a functional assay [41] was used to test how cytotoxic VNLME and bio-made ZnO NPs were to host cells. The cell toxicity was figured out by comparing the number (no.) of living cells at different concentrations of the test sample to the no. of living cells in the control group, which were not treated. By estimating the concentration needed to slow down the growth of cells in a culture by 50% compared to untreated cells, the IC50 was determined.
Photocatalytic activity of methylene blue (MB) using V. negundo ZnO NPs
The photocatalytic degradation performance of the green treated V. negundo ZnO NPs was assessed by removal of methylene blue (MB) dye under sunlight irradiation. In a typical procedure, different weight percentages (2, 4, 6, 8, and 10 mg/L) of the prepared catalysts were added to 100 mL MB (30 ppm) aqueous solution. After that, the mixed solution was kept under sunlight irradiation with continuous stirring. Subsequently, 5 mL of the mixed suspension was collected at regular interval time of every 20 min and centrifuged at 6000 rpm to separate the catalyst and investigated by a UV-Vis spectrometer. Finally, degradation efficiency of MB dye was calculated from the decrease in the intensity of the associated characteristic band absorption at 664 nm [42].
Data analysis
All studies used version 20.0 of the SPSS software package (SPSS 2007). Before the analysis, the formula by Abbott [43] was used to change the death rate in the treatments to match that of the controls. Probit analysis was used to determine LC50 (the lethal concentration (LC) that kills 50% of the organisms exposed) and LC90 (the LC that kills 90% of the organisms exposed) from larvicidal and pupicidal experiments [44]. At a p < 0.05 level, the results are statistically significant. All other graphs were drawn by Origin v 8.5 software.
Results and discussion
Characterization of V. negundo-synthesized ZnO NPs
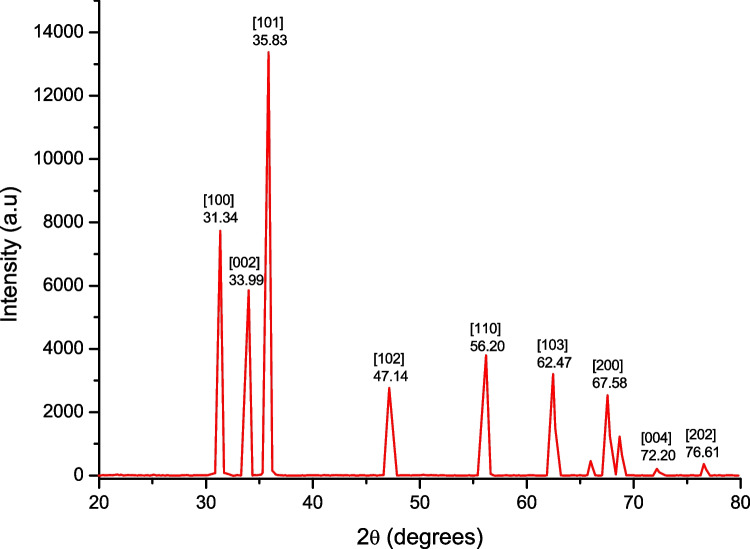
Bio-fabricated ZnO nanoparticles synthesized using LE from V. negundo were analyzed by XRD. The peaks at 2θ values of 31.34°, 33.99°, 35.83°, 47.14°, 56.20°, 62.47°, 67.58°, 72.20°, and 76.61° can be indexed to the (100), (002), (101), (102), (110), (103), (200), (004), and (202), respectively, confirming the presence of ZnO NPs (Fig. 1). According to previous research, crystalline planes are also well indexed. ZnO nanostructures were biosynthesized from P. trifoliata fruit extract XRD patterns, which showed diffraction peaks of the 2 values at 31, 34 ° (100), (002), (101), (102), (110), (103), and (202) planes for ZnO NPs, respectively. The synthesis of ZnO NPs from Aloe barbadensis miller LE was reported by Sangeetha and colleagues (2011). The XRD peaks of the aloe extract correspond to ZnO’s wurtzite structure planes (100), (002), (110), (102), (110), (103), (200), (112), (201), (004), and (202) (in order of increasing thickness). According to the Scherer formula, the average particle size of ZnO nanoparticles was around 33.4 nm.
Fig. 1.
XRD pattern of ZnO synthesized using Vitex negundo leaf broth
To identify functional groups in V. negundo that might be responsible for bio-fabrication and stabilization of ZnO nanoparticles, FTIR spectroscopy was used (Fig. 2a) and the bands at 3387.79, 1601.11, 1395.86, 1273.11, 1046.17, and 424.52 cm−1. Figure 2b represents the leaf aqueous extract of V. negundo with similar bands present at 3418.65, 1632.56, 1255.83, and 1106.78 cm−1. Peaks at 3387 and 1395 cm−1 imply intramolecular hydrogen bond OH stretching and alkane C–C stretching, respectively [45–47]. At 1601 cm−1, bending vibrations of the amide I of proteins were seen in a medium-charge, cis-tri-substituted vinyl band with a medium charge. A monosubstituted alkyne signal at 1273 cm−1, a phenolic group peak at 1046 cm−1, and a peak in the region of 424 cm−1 are assigned to Zn-O [48, 49].
Fig. 2.
a FTIR spectra of the synthesized ZnO NPs and b Leaf aqueous extract of V. negundo.
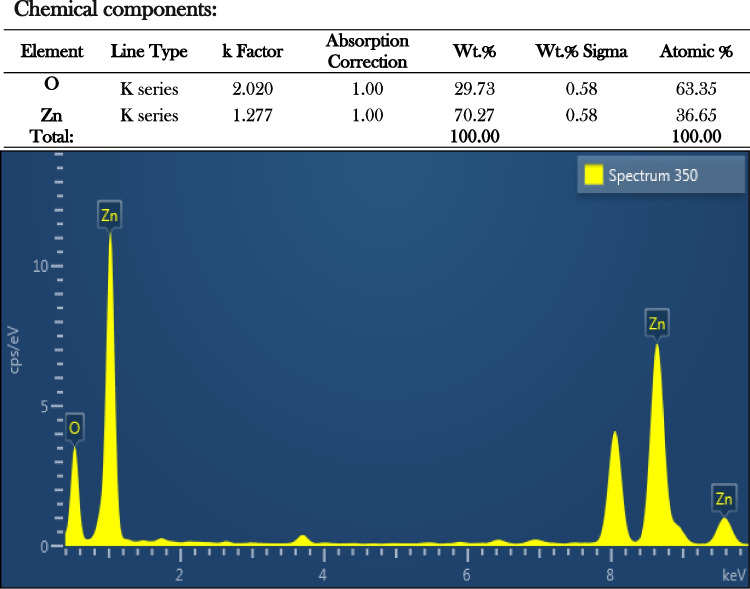
SEM micrographs of the bio-fabricated ZnO NPs showed an irregular shape. Figure 3a and b are close-ups of a few closely spherical particles that have clumped together. They were blown up 10,000 and 15,000 times. Similar outcome of the ZnO NPs shape was reported earlier by Vanathi et al. [50] and Jayaseelan et al. [51]. There was a strong Zn signal in an EDX profile that indicated the purity of the chemical components present in the sample, as well as several other peaks (Fig. 4) [45, 52].
Fig. 3.

a, b SEM micrograph leaf of V. negundo synthesized ZnO with magnification at 10,000× and 15,000×
Fig. 4.
Energy-dispersive X-ray spectroscopy exhibiting the chemical components of the synthesized ZnO
ZnO NPs fabricated using V. negundo have been studied by TEM to determine their size, shape, and morphology. Figure 5a and b show the TEM and SAED patterns that were generated from morphological characteristic studies. ZnO NPs have a spherical shape and agglomeration, and their size ranges from 28.48 to 42.14 nm, according to the image. ZnO NPs had a 34.21-nm mean size. The morphology of ZnO NPs was studied by Nagajyothi et al. [53] using TEM; the particles were in the size range between 8.48 and 36.2 nm with spherical morphology, where the average mean diameter is of 21.12 nm. Most ZnO NPs were found to be quasi-spherical, as evidenced by the SAED pattern obtained via TEM [54].
Fig. 5.

a, b Transmission electron microscopic image showing synthesized ZnO NPs from V. negundo. a 500 nm and b SAED pattern
Mosquitocidal toxicity of VNLME
V. negundo leaf methanol extract (VNLME) was found to be toxic to A. subpictus and C. quinquefasciatus under laboratory conditions (Table 1). The larvae and pupae of A. subpictus with susceptible LC50 values of 20.55 (I instar), 23.65 (II), 29.47 (III), 36.89 (IV), and 45.76 mg/L (pupa) and LC90 of 69.22 (I instar), 87.87 (II), 93.79 (III), 100.25 (IV), and 104.22 mg/L (pupa) were recorded. LC50 values for C. quinquefasciatus were 19.13 (I), 22.45 (II), 28.26 (III), 34.48 (IV), and 44.23 mg/L (pupa), while LC90 values were 69.66 (I instar), 85.54 (II), 97.29 (III), 107.13 (IV), and 117.42 mg/L (pupa). In the past 2 decades, several herbal extracts have been considered as possible sources against C. quinquefasciatus and A. subpictus larvae and pupae. For instance, [13, 55–57]. In an earlier study, Solanum xanthocarpum was reported to be toxic to the larvae and pupae of C. quinquefasciatus at LC50 levels of 155.29, 198.32, 271.12, 377.44, and 448.41 ppm, respectively (Kumar et al., 2012). The larvicidal properties of alkaloids isolated from neem seeds were tested against A. stephensi, according to Arya [58]. The larvae and pupae of A. stephensi were found to be severely harmed by LEs of Ocimum basilicum and Senna occidentalis in mosquito-killing experiments [59].
Table 1.
Acute toxicity of V. negundo leaf methanol extract against larvae and pupae of the A. subpictus and C. quinquefasciatus on 24-h exposure
| Species | Larvae instars | LC50 (mg/L) | 95 % confidence limit | LC90 (mg/L) | 95 % confidence limit | r2 | χ2 (d.f. = 4) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| A. subpictus | I | 20.55 | 11.18 | 27.26 | 69.22 | 62.76 | 78.12 | 0.982 | 3.780 |
| II | 23.65 | 12.22 | 31.63 | 87.87 | 78.87 | 101.24 | 0.980 | 0.228 | |
| III | 29.47 | 19.48 | 36.74 | 93.79 | 84.33 | 107.85 | 0.980 | 0.380 | |
| IV | 36.89 | 28.55 | 43.36 | 100.25 | 90.38 | 114.83 | 0.977 | 0.609 | |
| Pupa | 45.76 | 39.29 | 51.31 | 104.22 | 94.74 | 117.81 | 0.955 | 3.130 | |
| C. quinquefasciatus | I | 19.13 | 9.07 | 26.25 | 69.66 | 63.07 | 78.70 | 0.913 | 1.160 |
| II | 22.45 | 10.90 | 30.49 | 85.84 | 76.89 | 98.27 | 0.917 | 2.199 | |
| III | 28.26 | 17.11 | 36.15 | 97.29 | 86.94 | 113.09 | 0.939 | 1.751 | |
| IV | 34.48 | 24.18 | 42.02 | 107.13 | 95.28 | 125.64 | 0.933 | 2.667 | |
| Pupa | 44.23 | 35.73 | 51.05 | 117.42 | 104.31 | 137.99 | 0.929 | 4.057 | |
No mortality was observed in the control, significant at P < 0.05 level. Mean value of three replicates
LC50 lethal concentration that kills 50% of the exposed organisms, LC90 lethal concentration that kills 90 % of the exposed organisms, r2 regression coefficient, χ2 chi-square value d.f. degrees of freedom,
Mosquitocidal toxicity of V. negundo-synthesized ZnO NPs
The V. negundo-synthesized ZnO NPs were found to be highly detrimental to C. quinquefasciatus and A. subpictus even at low dosages (Table 2). There was a significant sensitivity with LC50 values for A. subpictus larvae and pupae exhibited by ZnO NPs which were 1.70 (I instar), 1.66 (II), 1.93 (III), 2.48 (IV), and 3.63 mg/L (pupa) and LC90 of 6.13 (I instar), 7.64 (II), 8.63 (III), 9.45 (IV), and 9.78 mg/L (pupa). In a similar manner, for the C. quinquefasciatus, the LC50 values recorded were 1.95 (I), 2.63 (II), 2.90 (III), 4.32 (IV), and 4.61 mg/L (pupa), and LC90 were 7.25 (I instar), 7.73 (II), 8.30 (III), 10.51 (IV), and 11.46 mg/L (pupa), respectively. In recent years, a rising variety of bio-fabricated nanoparticles has been explored for improved larvicidal and pupicidal efficacy against mosquito vectors [35, 60–63]. Euphorbia hirta LE has been used to synthesize Ag NP that showed toxicity to A. stephensi’s larvae and pupae with the LC50 values of 10.14 ppm and 34.52 ppm, respectively [64]. According to Govindarajan et al. [65], Malva sylvestris LE and green-synthesized Ag NPs have been recently found to be effective against three targeted mosquitoes. Ag NPs outperformed the aqueous LE in terms of highest larvicidal activity against C. quinquefasciatus, A. aegypti, and A. stephensi with the LC50 values of 12.19, 11.23, and 10.33 mg/L, respectively. Murugan et al. [66] found that bio-synthesized AgNPs were very efficacious against I to IV instar larvae of A. stephensi and its pupae with the LC50 values of 2.111, 3.090, 4.629, 5.261, and 6.860 ppm, respectively.
Table 2.
Acute toxicity of biosynthesized ZnO NPs against larvae and pupae of A. subpictus and C. quinquefasciatus on 24-h exposure
| Species | Larvae instars | LC50 (mg/L) | 95 % confidence limit | LC90 (mg/L) | 95 % confidence limit | r2 | χ2 (d.f. = 4) | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| A. subpictus | I | 1.70 | 0.75 | 2.37 | 6.13 | 5.55 | 6.92 | 0.910 | 1.657 |
| II | 1.66 | 0.40 | 2.52 | 7.64 | 6.86 | 8.76 | 0.913 | 2.650 | |
| III | 1.93 | 2.55 | 3.61 | 8.63 | 6.91 | 13.92 | 0.850 | 5.424 | |
| IV | 2.48 | 1.27 | 3.32 | 9.45 | 8.43 | 11.00 | 0.876 | 4.795 | |
| Pupa | 3.63 | 0.70 | 5.07 | 9.78 | 7.86 | 15.13 | 0.886 | 6.880 | |
| C. quinquefasciatus | I | 1.95 | 0.90 | 2.68 | 7.25 | 6.56 | 8.23 | 0.978 | 4.466 |
| II | 2.63 | 1.79 | 3.25 | 7.73 | 7.03 | 8.69 | 0.974 | 1.103 | |
| III | 2.90 | 2.06 | 3.53 | 8.30 | 7.55 | 9.34 | 0.937 | 1.095 | |
| IV | 4.32 | 3.61 | 4.92 | 10.51 | 9.50 | 11.99 | 0.983 | 2.117 | |
| Pupa | 4.61 | 3.85 | 5.24 | 11.46 | 10.24 | 13.31 | 0.980 | 1.991 | |
No mortality was observed in the control, significant at P < 0.05 level. Mean value of three replicates
LC50 lethal concentration that kills 50 % of the exposed organisms, LC90 lethal concentration that kills 90% of the exposed organisms, r2 regression coefficient, χ2 chi-square value d.f. degrees of freedom
GC-MS analysis
The results of the GC-MS analysis are included in this section (Table 3; Fig. 7). The VNLME contained eight compounds. In the VNLME, stigmasterol (C29H48O) with RT 23.54 was identified as a major constituent. Currently, the GC-MS analysis indicated the key components included in the methanol extract namely N-nexadecanoic acid (C16H32O2), 1,5,9-decatriene, 2,3,5,8-tetramethyl-(C14H24), 1,3,3-trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-(C15H26O), 5,9,13-pentadecatrien-2-one, 6,10,14-trimethyl-(C18H30O), diallylphenylmethylsilane (C13H18Si), 1H-3A, 7-methanoazulen-6-Ol, octahydro-3,6,8,8-tetramethyl-, acetate (C17H28O2), and phytol (C20H40O)
Table 3.
Important compounds identified in the GC-MS analysis of V. negundo leaf methanol extract
Fig. 7.
GC-MS analysis of leaf methanol extract of V. negundo
Stigmasterol is the most common component in all samples. It is also found in Phyllanthus amarus, Mimosa pudica, and Ricinus communis. In a previous study, the anti-snake venom activity of an extracted and purified combination of β-sitosterol and stigmasterol in a 3:1 ratio from the root extract of P. indica was evaluated [64]. In an in vitro study, Mors et al. [67] observed that ethanol extracts of the Eclipta prostrata plant prevented rattlesnake venom from killing or myotomizing rats.
Antioxidant assay of V. negundo ZnO NPs
The column diagram represented in Fig. 6a indicates DPPH scavenging efficiency of aqueous extract and fabricated ZnO NPs mediated by V. negundo. The findings of the DPPH assay demonstrated that fabricated ZnO NPs effectively block free radicals. The percentage of manufactured ZnO NPs was (83%) compared to that of standard ascorbic acid (96%) and V. negundo aqueous extract (19%) at the various concentrations utilized in this investigation, and the activity increased with higher concentrations of ZnO NPs. DPPH radicals react with optimum reducing agents, pairing off electrons and producing stoichiometric color loss [ 68]. Likewise, Rajeshkumar et al. [69] studied that the ZnO NPs manufactured from Cassia fistula plant extracts exhibited antioxidant potential.
Fig. 6 .
a DPPH scavenging efficiency of aqueous extract and fabricated ZnO NPs mediated by V. negundo. b FRAP antioxidant activity of aqueous extract and fabricated ZnO NPs mediated by V. negundo
FRAP is the ability of antioxidant to reduce Fe3+ to Fe2+ in the presence of TPTZ, forming an intense blue (Fe2+-TPTZ complex). NPs reduced the ferric ions (Fe3+) by 165.2 ± 0.5 μmol Fe (II)/g. BHT was used as a standard reducing agent assessed extremely strong reducing activity (200.23 ± 2.5 μmol Fe (II)/g) (Fig. 6b). Similarly, Senthilkumar et al. [70] demonstrated that the gold nanoparticles derived from Gelidiella acerosa had the highest antioxidant activity in the DPPH and FRAP assays (Fig. 7).
Cytotoxic study
The cytotoxicity study was assessed on HeLa cells. The inhibitory concentration (IC50) of cell growth was recorded as 72.35 and 43.70 μg/mL for VNLME and the bio-fabricated ZnO NPs, respectively. Recently, Thomas et al. [71] have reported that the Solanum nigrum-mediated biosynthesis ZnO NPs shows good inhibitory activity by decreasing the viability of the HeLa cancerous cells in a dose- and time-dependent manner with an IC50 value of 31.6 μg/mL. Significant anticancer activity was observed by ZnO NPs from Camellia sinensis tea leaf extract against HeLa cancer cells and considerably reduced cell viability by 90.37%, 78.01%, 40.50%, and 17.11% after post-treatment with 25 to 100 μg/mL [72]. Hayat et al. [73] reported that Acacia arabica-mediated ZnO NPs exhibited the cell viability of HeLa cancer cells that was found to be 88.6% at the highest concentration tested at 100 μg/mL. Delonix regia LE-mediated ZnO NPs exhibited optimal cytotoxic effects on HeLa cells [74]. Previous research clearly shows that ZnO NPs have strong antioxidant properties, causing cell wall and membrane damage, as well as oxidative damage in cancerous cells. Additionally, the present MTT experiment demonstrates that synthesized ZnO NPs have no effect on the ingenuity and viability of HeLa cell lines. Therefore, ZnO NPs will play a significant role in cancer therapy, resulting in enhanced anticancer activity [75–77]. Finally, our reports suggest that bio-fabricated NPs exhibit the best cytotoxic effect on human cervical cancer cells.
There are considerable distinctions between measuring the deadly potential of nanoparticles and the impact of synthetic pesticides. The current bio-fabrication demonstrates the use of the ecologically friendly and bio-resourceful V. negundo as an active reducing and stabilizing agent in the synthesis of ZnO NPs. This bio-fabricated metal reduction would be beneficial for the development of a clean, safe, and ecologically acceptable “bio approach” to generating metal oxide nanoparticles, using creatures as high as plants.
Photocatalytic activity
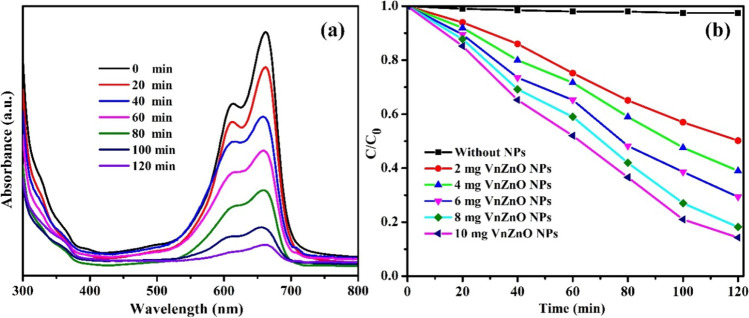
The photocatalytic degradation performances of the resultant catalysts were evaluated by assessing the degradation of methylene blue (MB) dye under sunlight irradiation at different time intervals. The degradation efficiency of green-treated V. negundo ZnO NPs was calculated by plotting C/C0 versus time t, and the results showed that the photocatalytic removal of MB could be neglected under sunlight irradiation without the prepared catalysts, which was the stability of MB under self-photolysis. Figure 8a shows the degradation progress of the typical UV-Vis absorption peak at 664 nm decreased progressively with the increasing sunlight irradiation time, proposing the degradation of MB by 10 mg/L of green-treated V. negundo ZnO NPs. The absorption peak almost vanishes after irradiation of 120 min, representing the complete photocatalytic degradation of MB chromophore owing to the disintegration of its aromatic ring. The percentage of the decolorization percentage was calculated by the following formula
Fig. 8.
a Photocatalytic degradation of MB dye using 10 mg/L of green-treated V. negundo ZnO NPs. b Photocatalytic degradation percentage of 2, 4, 6, 8, and 10 mg/L of green-treated V. negundo ZnO NPs
where A0 is the initial absorbance of MB corresponding to the initial concentration C0, and At is the absorbance with concentration Ct at time interval t.
Figure 8b exhibits the 10-mg green-treated V. negundo ZnO NP photocatalytic degradation spectra. The degradation results showed the enriched photocatalytic activity compared to that of 2, 4, 6, and 8 mg of green-treated V. negundo ZnO NPs. After photodegradation up to 120 min, the degradation efficacy of MB over 10 mg of biogenic V. negundo-mediated ZnO NPs exhibited ~ 87%, while the degradation rates of 2, 4, 6, and 8 mg/L of green-treated V. negundo ZnO NPs only reached 49.8, 61, 70.65, and 81.2%, respectively. The probable reason is that increasing the catalytic dosage may produce more reactive sites which produce more electrons and holes, thus increasing the photocatalytic efficiency.
Reusability of 10 mg of green-treated V. negundo ZnO nanoparticles
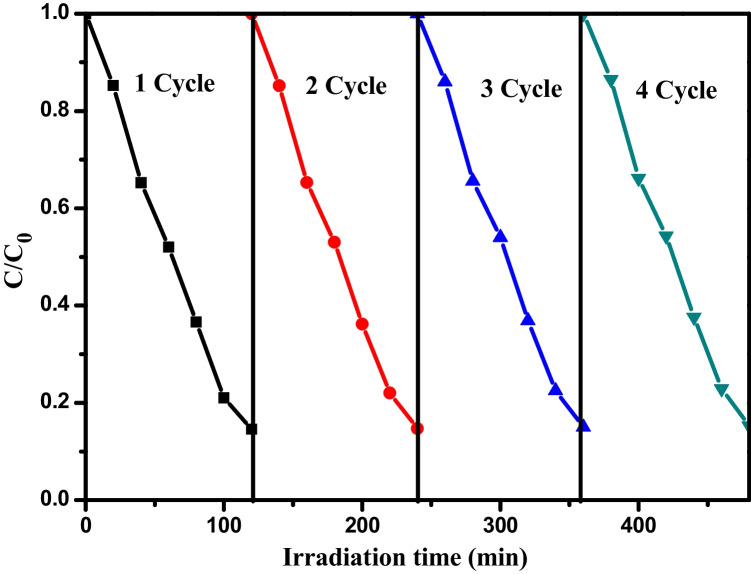
To review the photostability and reusability of the green-treated V. negundo ZnO NPs in the photocatalytic removal of MB dye under sunlight irradiation, consecutive tests were studied using an oven drying method at 100 °C between reaction cycles. After successive four cycles of photo decolorization, there is tiny change in the catalytic efficiency of the green-treated V. negundo ZnO NPs. The degradation efficiency for the four runs appeared to be slightly reduced from 87 to 85%, showing greater stability and reusability of green-treated V. negundo ZnO NPs for degradation of MB (Fig. 9).
Fig. 9.
The reusability experiments of 10 mg/L green-treated V. negundo ZnO NPs
A possible mechanism for photocatalytic degradation of MB Dye
The photocatalytic mechanism of green-treated V. negundo ZnO NPs is shown in Fig. 10. We have already studied the relationship between the time and the degradation process; now, we need to know how this degradation process happens. Figure 10 elucidates the probable mechanism for photocatalytic degradation of MB dye on green-treated V. negundo ZnO NPs catalyst. First, the dye is adsorbed on the surface of the catalyst, and then, it is exposed to sunlight irradiation to excite valence electrons. The electron-hole pairs are produced between the valence band and conduction band of green-treated V. negundo ZnO NPs. During the process, the photogenerated electrons (e−) produced in the conduction band of ZnO is hunted by O2 to form anion radical () which on protonation (H+) gives HOO∙. The positive holes and free electrons will react on the surface of the photocatalyst along with adsorbed water molecules, and as a result, the positive holes will react with water to produce ∙OH radicals, and the free electrons reduce the dissolved oxygen to superoxide anion radicals. These light-generated radicals degrade the dye molecules into simple molecules such as CO2 and H2O.
Fig. 10.
The possible photocatalytic mechanism of V. negundo-derived ZnO NPs
The mechanism of photodegradation of MB dye with ZnO NPs under sunlight may be explained using the following steps.
Agreeing to the above-mentioned equations, the photodegradation of pollutant dye is accomplished over the green-treated V. negundo-fabricated ZnO NP photocatalyst.
Conclusion
To sum up, the ZnO NP synthesis and extraction of metabolites from V. negundo leaves using the polar methanol solvent were achieved successfully. The lethal effects of synthesized ZnO NPs and leaf methanol extract of V. negundo were examined against the larvae and pupae of the malaria vector, A. subpictus, and the lymphatic filariasis vector, C. quinquefasciatus. As compared to the methanol extract, the ZnO NPs exhibited preponderant larvicidal and pupicidal activity. Methanol extract contains the compound, stigmasterol (C29H48O), as a major one. In addition, VNLME was composed of 7 different compounds. V. negundo-derived ZnO NPs exhibited the highest antioxidant capacity. Moreover, it was observed that the ZnO NPs exhibit a prominent photocatalytic activity by decolorizing the methylene blue dye in 3 h without the support of any other external catalyst. As an outcome, the fabrication of ZnO NPs using V. negundo leaf extract can be investigated for various agricultural uses and waste water management systems to mitigate such harmful pollutants.
The biosynthesized ZnO NPs were cytotoxic to human cervical cancer cells. Overall, our findings suggest that bio-fabricated ZnO NPs could be used to control blood-sucking parasites and cancer cells. The results presented here make it possible for more research to be done on how the nano-bio-fabricated product kills mosquitoes. Moreover, it was observed that the ZnO NPs exhibit a prominent photocatalytic activity by decolorizing the methylene blue dye in 3 h without the support of any other external catalyst. As an outcome, the fabrication of ZnO NPs using V. negundo leaf extract can be investigated for various agricultural uses and waste water management systems to mitigate such harmful pollutants.
Acknowledgements
We are grateful to the Sophisticated Test and Instrumentation Centre (STIC) at Cochin University of Science and Technology in Cochin, Kerala, India, for allowing us to conduct characterization studies such as XRD, FTIR, and SEM-EDX, TEM analyses. CK expresses gratitude to the SRM IST for providing the facility. PRG thanks the Auxilium College administration, the Principal, and the HOD of Zoology for their assistance and support in carrying out some work.
Author contribution
Chinnaperumal Kamaraj: study conception and design, provided funding, data collection, analysis, and interpretation of results, and draft manuscript preparation. Pachiyappan Rajiv Gandhi: study conception and design, data collection, analysis, interpretation of results, and draft manuscript preparation. Chinnasamy Ragavendran: analysis and interpretation of results. Vimal Sugumar: study conception and draft manuscript preparation. R. C. Satish Kumar: study conception and draft manuscript preparation. Rajendran Ranjith and A. Priyadharsan: performed and conducted the dye degradation experiments. Tijo Cherian: evaluation of antioxidant assay. All the authors reviewed the results and approved the final version of the manuscript.
Data availability
The data supporting this study’s findings are available from the corresponding authors on special request.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chinnaperumal Kamaraj, Email: kamarajc@srmist.edu.in.
Pachiyappan Rajiv Gandhi, Email: raju87msc@gmail.com.
Chinnasamy Ragavendran, Email: ragavan889@gmail.com.
Vimal Sugumar, Email: vimalbiotechs@gmail.com.
R. C. Satish Kumar, Email: dean.iiism@srmist.edu.in.
Rajendran Ranjith, Email: ranjith0591@gmai.com.
A. Priyadharsan, Email: dharsan69@gmail.com
Tijo Cherian, Email: tvarghese891@gmail.com.
References
- 1.Ong SQ, Pauzi MBM, Gan KH. Text mining in mosquito-borne disease: a systematic review. Acta Trop. 2022;231:106447. doi: 10.1016/j.actatropica.2022.106447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Vectore borne diseases. 2020. [Google Scholar]
- 3.Mehlhorn H, Al-Rasheid KA, Al-Quraishy S, Abdel-Ghaffar F. Research and increase of expertise in arachno-entomology are urgently needed. Parasitol Res. 2012;110:259–265. doi: 10.1007/s00436-011-2480-7. [DOI] [PubMed] [Google Scholar]
- 4.Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res. 2016;115(5):1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 5.Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, Caminade C, Lowe R. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lan Planet Heal. 2021;5(7):e404–e414. doi: 10.1016/S2542-5196(21)00132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morens MV, Fauci AS. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9(7):1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Malaria [Internet]. World Health Organ. 2021. [Google Scholar]
- 8.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77:69–78. doi: 10.4269/ajtmh.2007.77.69. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Hosmani R, Jadhav S, et al. Anopheles subpictus carry human malaria parasites in an urban area of Western India and may facilitate perennial malaria transmission. Malar J. 2016;15:124. doi: 10.1186/s12936-016-1177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO (2014) Neglected tropical diseases. PCT databank, Lymphatic filariasis
- 11.Ramaiah KD, Das PK, Michael E, Guyatt H. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16(6):251–253. doi: 10.1016/S0169-4758(00)01643-4. [DOI] [PubMed] [Google Scholar]
- 12.Howard AF, Zhou G, Omlin FX. Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health. 2007;7(1):199. doi: 10.1186/1471-2458-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benelli G. Research in mosquito control: current challenges for a brighter future. Parasitol Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- 14.Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M. Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res. 2015;114:391–397. doi: 10.1007/s00436-014-4286-x. [DOI] [PubMed] [Google Scholar]
- 15.Pavela R, Benelli G. Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors - a review. Exp Parasitol. 2016;167:103–108. doi: 10.1016/j.exppara.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Prager GW, Braga S, Bystricky B, Qvortrup C, Criscitiello C, Esin E, Sonke GS, Martínez G, Frenel JS, Karamouzis M, Strijbos M. Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open. 2018;3(2):e000285. doi: 10.1136/esmoopen-2017-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh HS. Can visual inspection with acetic acid be used as an alternative to Pap smear in screening cervical cancer? Middle East Fertil Soc J. 2014;19(3):187–191. doi: 10.1016/j.mefs.2013.10.003. [DOI] [Google Scholar]
- 18.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 19.Mobarez AM, Soleimani N, Esmaeili SA, Farhangi B. Nanoparticle-based immunotherapy of breast cancer using recombinant Helicobacter pylori proteins. Euro J Pharma Biopharmaceut. 2020;155:69–76. doi: 10.1016/j.ejpb.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Anwer MK, Fatima F, Ahmed MM, Aldawsari MF, Alali AS, Kalam MA, Alshamsan A, Alkholief M, Malik A, Az A, Al-shdefat R (2022) Abemaciclib-loaded ethylcellulose based nanosponges for sustained cytotoxicity against MCF-7 and MDA-MB-231 human breast cancer cells lines. Sau Pharma J. 10.1016/j.jsps.2022.03.019 [DOI] [PMC free article] [PubMed]
- 21.Chan WC. Bionanotechnology progress and advances. Biol Blood Mar Trans. 2006;12:87–91. doi: 10.1016/j.bbmt.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Kamaraj C, Karthi S, Reegan AD, Balasubramani G, Ramkumar G, Kalaivani K, Zahir AA, Deepak P, Senthil-Nathan S, Rahman MM, Islam ARMT (2022) Green synthesis of gold nanoparticles using Gracilaria crassa leaf extract and their ecotoxicological potential: issues to be considered. Environ Res 113711 [DOI] [PubMed]
- 23.Serpone N, Dondi D, Albini A. Inorganic and organic UV filters: their role and efficacy in sunscreens and suncare products. Inorg Chim Acta. 2007;360:794–802. doi: 10.1016/j.ica.2005.12.057. [DOI] [Google Scholar]
- 24.Becheri A, Durr M, Lo Nostro P, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res. 2008;10:679–689. doi: 10.1007/s11051-007-9318-3. [DOI] [Google Scholar]
- 25.Vinotha V, Yazhiniprabha M, Raj DS, Mahboob S, Al-Ghanim KA, Al-Misned F, Govindarajan M, Vaseeharan B. Biogenic synthesis of aromatic cardamom-wrapped zinc oxide nanoparticles and their potential antibacterial and mosquito larvicidal activity: an effective eco-friendly approach. J Envi Chem Engi. 2020;8(6):104466. doi: 10.1016/j.jece.2020.104466. [DOI] [Google Scholar]
- 26.Bhatt K, Jain VK, Khan F. Antibacterial study of Eucalyptus grandis fabricated zinc oxide and magnesium doped zinc oxide nanoparticles and its characterization. J Indian Chemi Soci. 2022;99(5):100441. doi: 10.1016/j.jics.2022.100441. [DOI] [Google Scholar]
- 27.Alsuwayyid AA, Alslimah AS, Perveen K, Bukhari NA, Al-Humaid LA. Effect of zinc oxide nanoparticles on Triticum aestivum L. and bioaccumulation assessment using ICP-MS and SEM analysis. J King Sau Univ Sci. 2022;34(4):101944. doi: 10.1016/j.jksus.2022.101944. [DOI] [Google Scholar]
- 28.Maheo AR, Vithiya BSM, Prasad TAA. Biosynthesis of chitosan and Eupatorium adenophorum mediated zinc oxide nanoparticles and their biological and photocatalytic activities. Materials Today: Proceedings. 2022;65(1):298–312. [Google Scholar]
- 29.Willner B, Basnar B. Nanoparticle-enzyme hybrid system for nanobiotechnology. J FEBS. 2007;274:302–309. doi: 10.1111/j.1742-4658.2006.05602.x. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi PR, Jayaseelan C, Vimalkumar E, Mary RR. Larvicidal and pediculicidal activity of synthesized TiO2 nanoparticles using Vitex negundo leaf extract against blood feeding parasites. J Asia Pac Entomol. 2016;19(4):1089–1094. doi: 10.1016/j.aspen.2016.10.001. [DOI] [Google Scholar]
- 31.Meena AK, Niranjan US, Rao MM, Padhi MM, Babu R. A review of the important chemical constituents and medicinal uses of Vitex genus. Asian J Tradit Med. 2011;6(2):54–60. [Google Scholar]
- 32.Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86(1):75–80. doi: 10.1016/S0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 33.Chandramu C, Manohar RD, Krupadanam DGL, Dashavantha RV. Isolation characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res. 2003;17(2):129–134. doi: 10.1002/ptr.1088. [DOI] [PubMed] [Google Scholar]
- 34.Maurya R, Shukla PK, Ashok K. New antifungal flavonoid glycoside from Vitex negundo. Bioorg Med Chem Lett. 2007;17(1):239–242. doi: 10.1016/j.bmcl.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi PR, Jayaseelan C, Mary RR, Mathivanan D, Suseem SR. Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordica charantia leaf extract against blood feeding parasites. Exp Parasitol. 2017;181:47–56. doi: 10.1016/j.exppara.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Kamaraj C, Rahuman AA, Bagavan A. Antifeedant and larvicidal effects of plant extracts against Spodoptera litura (F.), Aedes aegypti L. and Culex quinquefasciatus Say. Parasitol Res. 2008;103:325–331. doi: 10.1007/s00436-008-0974-8. [DOI] [PubMed] [Google Scholar]
- 37.Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U. Mosquitocidal and antibacterial activity of green synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res. 2015;114:1519–1529. doi: 10.1007/s00436-015-4336-z. [DOI] [PubMed] [Google Scholar]
- 38.WHO . Guidelines for laboratory and field-testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005. 2005. pp. 241–244. [Google Scholar]
- 39.Anjum SM, Riazunnisa K. Fine ultra-small ruthenium oxide nanoparticle synthesis by using Catharanthus roseus and Moringa oleifera leaf extracts and their efficacy towards in vitro assays, antimicrobial activity and catalytic: adsorption kinetic studies using methylene blue dye. J Clus Sci. 2022;33(3):1103–1117. doi: 10.1007/s10876-021-02037-0. [DOI] [Google Scholar]
- 40.Bhagat M, Anand R, Datt R, Gupta V, Arya S. Green synthesis of silver nanoparticles using aqueous extract of Rosa brunonii Lindl and their morphological, biological and photocatalytic characterizations. J Inorg Organ Poly Mate. 2019;29(3):1039–1047. doi: 10.1007/s10904-018-0994-5. [DOI] [Google Scholar]
- 41.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 42.Ragupathy S, Manikandan V, Devanesan S, Ahmed M, Ramamoorthy M, Priyadharsan A. Enhanced sun light driven photocatalytic activity of Co doped SnO2 loaded corn cob activated carbon for methylene blue dye degradation. Chemosph. 2022;295:133848. doi: 10.1016/j.chemosphere.2022.133848. [DOI] [PubMed] [Google Scholar]
- 43.Abbott WS. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 44.Finney DJ. Cambridge University Press, Cambridge. ISBN 052108041X. OCLC 174198382. 3 1971. Probit analysis. [Google Scholar]
- 45.Sangeetha G, Rajeshwari S, Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: structure and optical properties. Mater Res Bull. 2011;46(12):2560–2566. doi: 10.1016/j.materresbull.2011.07.046. [DOI] [Google Scholar]
- 46.Tas AC, Majewski PJ, Aldinger F. Chemical preparation of pure and strontium-and/or magnesium-doped Lanthanum gallate Powders. J Am Ceram Soc. 2000;83(12):2954–2960. doi: 10.1111/j.1151-2916.2000.tb01666.x. [DOI] [Google Scholar]
- 47.Rajiv P, Rajeshwari S, Venckatesh R. Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta A Mol Biomol Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 48.Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B. 2014;140:194–204. doi: 10.1016/j.jphotobiol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Vanathi P, Rajiv P, Narendhran S, Rajeshwari S, Rahman PK, Venckatesh R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: a green chemistry approach. Mater. Lett. 2014;134:13–15. doi: 10.1016/j.matlet.2014.07.029. [DOI] [Google Scholar]
- 50.Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthua S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Bhaskara Rao KV. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta Part A. 2012;90:78–84. doi: 10.1016/j.saa.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Salam HA, Sivaraj R, Venckatesh R. Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater Lett. 2014;131:16–18. doi: 10.1016/j.matlet.2014.05.033. [DOI] [Google Scholar]
- 52.Nagajyothi PC, An TM, Sreekanth TVM, Lee JI, Lee DJ, Lee KD. Green route biosynthesis: characterization and catalytic activity of ZnO nanoparticles. Mater Lett. 2013;108:160–163. doi: 10.1016/j.matlet.2013.06.095. [DOI] [Google Scholar]
- 53.Ramesh M, Anbuvannan M, Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136:864–870. doi: 10.1016/j.saa.2014.09.105. [DOI] [PubMed] [Google Scholar]
- 54.Kamaraj C, Rahuman AA, Bagavan A. Screening for antifeedant and larvicidal activity of plant extracts against Helicoverpa armigera (Hubner), Sylepta derogata (F.) and Anopheles stephensi (Liston) Parasitol Res. 2008;103:1361–1368. doi: 10.1007/s00436-008-1142-x. [DOI] [PubMed] [Google Scholar]
- 55.Vincent S, Kovendan K, Chandramohan B, Kamalakannan S, Kumar PM, Vasugi C, Praseeja C, Subramaniam J, Govindarajan M, Murugan K, Benelli G (2016) Swift fabrication of silver nanoparticles using Bougainvillea glabra: potential against the Japanese encephalitis vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). J Clust Sci:1–22
- 56.Mathivanan D, Gandhi PR, Mary RR, Suseem SR (2017) Larvicidal and acaricidal efficacy of different solvent extracts of Andrographis echioides against blood-sucking parasites. Physiol Mol Plant Pathol:1–10
- 57.Arya N. Evolution of mosquito larvicidal activity of neem seed kernel alkaloid against Anopheles stephensi. Inte J Life Scis Biotech Pharma Res. 2014;3(4):129. [Google Scholar]
- 58.Murugan K, Aarthi N, Kovendan K, Panneerselvam C, Chandramohan B, Kumar PM, Amerasan D, Paulpandi M, Chandirasekar R, Dinesh D, Suresh U. Mosquitocidal and antiplasmodial activity of Senna occidentalis (Cassiae) and Ocimum basilicum (Lamiaceae) from Maruthamalai hills against Anopheles stephensi and Plasmodium falciparum. Parasitol Res. 2015;114(10):3657–3664. doi: 10.1007/s00436-015-4593-x. [DOI] [PubMed] [Google Scholar]
- 59.Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vect bor zoon dis. 2012;12(3):262–268. doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- 60.Roni M, Murugan K, Panneerselvam C, Subramaniam J, Hwang JZ. Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae) Parasitol Res. 2013;112:981–990. doi: 10.1007/s00436-012-3220-3. [DOI] [PubMed] [Google Scholar]
- 61.Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Mahesh Kumar P, Subramaniam J, Dinesh D, Chandramohan B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasitol Res. 2015;114:1551–1562. doi: 10.1007/s00436-015-4339-9. [DOI] [PubMed] [Google Scholar]
- 62.Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 2016;115:23–34. doi: 10.1007/s00436-015-4800-9. [DOI] [PubMed] [Google Scholar]
- 63.Priyadarshini A, Murugan K, Panneerselvam C, Ponarulselvam S, JiangShiou H, Nicoletti M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae) Parasitol Res. 2012;111:997–1006. doi: 10.1007/s00436-012-2924-8. [DOI] [PubMed] [Google Scholar]
- 64.Govindarajan M, Hoti SL, Rajeswary M, Benelli G. One-step synthesis of polydispersed silver nanocrystals using Malva sylvestris. Parasitol Res. 2016;115(7):2685–2695. doi: 10.1007/s00436-016-5038-x. [DOI] [PubMed] [Google Scholar]
- 65.Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, Chandramohan B, Subramaniam J, Madhiyazhagan P, Dinesh D, Rajagansh R. Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol Res. 2015;114(11):4087–4097. doi: 10.1007/s00436-015-4638-1. [DOI] [PubMed] [Google Scholar]
- 66.Gomes A, Saha A, Chatterjee I, Chakravarty AK. Viper and cobra venom neutralization by β-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae). Phytomed. 2007;14(9):637–643. doi: 10.1016/j.phymed.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 67.Mors WB, Nascimento MCD, Parente JP, Da Silva MH, Melo PA, Suarezkurtz G. Neutralization of lethal and myotoxic activities of south American rattle snake venom by extracts of Eclipta prostrate (Asteraceae) Toxicon. 1989;27(9):1003–1009. doi: 10.1016/0041-0101(89)90151-7. [DOI] [PubMed] [Google Scholar]
- 68.Kokila T, Ramesh PS, Geetha D. Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: a novel biological approach. Applied Nanosci. 2015;5(8):911–920. doi: 10.1007/s13204-015-0401-2. [DOI] [Google Scholar]
- 69.Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM. Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzy Micro Technol. 2018;117:91–95. doi: 10.1016/j.enzmictec.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Senthilkumar P, Surendran L, Sudhagar B, Ranjith Santhosh Kumar DS. Facile green synthesis of gold nanoparticles from marine algae Gelidiella acerosa and evaluation of its biological potential. SN. Applied Sci. 2019;1(4):1–12. doi: 10.1007/s42452-019-0284-z. [DOI] [Google Scholar]
- 71.Thomas S, Gunasangkaran G, Arumugam VA, Muthukrishnan S. Synthesis and characterization of zinc oxide nanoparticles of solanum nigrum and its anticancer activity via the induction of apoptosis in cervical cancer. Biolog Trace Ele Res. 2022;200(6):2684–2697. doi: 10.1007/s12011-021-02898-6. [DOI] [PubMed] [Google Scholar]
- 72.Al-Ghamdi SA, Alkathiri TA, Alfarraj AE, Alatawi OM, Alkathiri AS, Panneerselvam C, Vanaraj S, Darwish AA, Hamdalla TA, Pasha A, Khasim S. Green synthesis and characterization of zinc oxide nanoparticles using Camellia sinensis tea leaf extract and their antioxidant, anti-bactericidal and anticancer efficacy. Res Chem Intermediates. 2022;3:1–5. [Google Scholar]
- 73.Hayat S, Ashraf A, Zubair M, Aslam B, Siddique MH, Khurshid M, Saqalein M, Khan AM, Almatroudi A, Naeem Z, Muzammil S. Biofabrication of ZnO nanoparticles using Acacia arabica leaf extract and their antibiofilm and antioxidant potential against foodborne pathogens. PLoS One. 2022;17(1):e0259190. doi: 10.1371/journal.pone.0259190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Begum JS, Manjunath K, Pratibha S, Dhananjaya N, Sahu P, Kashaw S. Bioreduction synthesis of zinc oxide nanoparticles using Delonix regia leaf extract (Gul Mohar) and its agromedicinal applications. J Sci Advan Mat Devi. 2020;5(4):468–475. [Google Scholar]
- 75.Sahu P, Sushil KK, Samaresh S, Arun KI. Stimuli-responsive bio-hybrid nanogels: an emerging platform in medicinal arena. J Controlled Rele. 2017;107(1):143–157. [Google Scholar]
- 76.Malaikozhundan B, Vaseeharan B, Vijayakumar S, Pandiselvi K, Kalanjiam MAR, Murugan K, Benelli G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathog. 2017;104:268–277. doi: 10.1016/j.micpath.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 77.Kamaraj C, Gandhi PR, Satish Kumar RC, Balasubramani G, Malafaia G (2022) Biosynthesis and extrinsic toxicity of copper oxide nanoparticles against cattle parasites: an eco-friendly approach. Environmental Res 114009. 10.1016/j.envres.2022.114009 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding authors on special request.