Abstract
Acute cholecystitis is an infectious disease of the gallbladder caused mainly by Escherichia coli, Klebsiella, and Enterococcus species. Streptococcus gallolyticus subsp. pasteurianus, previously known as Streptococcus bovis biotype II/2, rarely causes endocarditis, meningitis, and septicemia, mainly in children. Biliary tract infections by Streptococcus gallolyticus subsp. pasteurianus are extremely rare. There have been no reports of cases in Japan. Here, we describe the first case in Japan of acute calculous cholecystitis caused by Streptococcus gallolyticus subsp. pasteurianus infection. A 63-year-old man was admitted to our hospital with epigastric pain and vomiting. He had moderate tenderness and a full sensation in the epigastrium. Abdominal imaging revealed multiple stones in the gallbladder. After admission, he had a high fever that did not improve with antibiotics. Percutaneous transhepatic gallbladder drainage was performed. The patient underwent open cholecystectomy. During surgery, several small stones in the gallbladder and an abscess were observed at the gallbladder base. Streptococcus gallolyticus subsp. pasteurianus was detected by bacterial culture of the bile juice. The gallstones were bilirubin calcium stones. The endoscopic study showed three adenomas in the colon, but the histopathological examination demonstrated no malignant cells. Although infection by this bacterium may not be rare, this is the first reported case in Japan of acute calculous cholecystitis caused by Streptococcus gallolyticus subsp. pasteurianus infection.
Keywords: acute calculous cholecystitis, Streptococcus gallolyticus subspecies pasteurianus, Streptococcus bovis, bilirubin calcium stone, β-glucuronidase
1. Introduction
Streptococcus gallolyticus subsp. pasteurianus (SGSP) belongs to the group D Streptococcus, formerly known as Streptococcus bovis type II/2 [1]. Based on biochemical differences, S. bovis was originally classified into type I (mannitol-fermentation-positive), type II/1 (mannitol-fermentation-negative and β-glucuronidase-negative), and type II/2 (mannitol-fermentation-negative and β-glucuronidase-positive) [1]. In 2003, Schlegel et al. proposed a new taxonomy of S. bovis-group bacteria based on genetic differences [2]. Although this new taxonomy for the bacterium was accepted and is currently used, many clinicians remain unfamiliar with it and therefore still call the bacterium S. bovis. Organisms from the S. bovis group have been associated with endocarditis, meningitis, sepsis, and colorectal cancers [3,4,5,6]. However, reports of biliary tract infection caused by this group of bacteria are very rare worldwide [7]. Reports of biliary tract infection by SGSP are even rarer. Although there may be many cases, herein, we present the first reported case of acute calculous cholecystitis caused by SGSP in Japan and review the literature.
2. Case Presentation
A 63-year-old male with a history of herpes zoster at the age of 24 and acute gastroenteritis after the ingestion of raw egg at the age of 61 was admitted to our hospital for upper abdominal pain and vomiting. The day before admission, two hours after eating a pork cutlet for dinner, he felt a burning pain in the epigastrium. Less than 24 h after the start of his symptoms, the patient consulted our institution. He continued having epigastric pain and started vomiting after admission. Physical examination revealed marked tenderness in the epigastrium and right upper quadrant but no rebound tenderness. His vital signs were within the normal range except for a body temperature of 37.3 °C. The blood analysis showed a slight elevation of leukocytes, neutrophils, C-reactive protein, triglycerides, low-density lipoprotein cholesterol, and liver enzymes (Table 1). No anemia or jaundice was observed.
Table 1.
Blood analysis results.
| Peripheral Blood | Normal Range | Biochemistry | Normal Range | ||
|---|---|---|---|---|---|
| White blood cells | 9800/μL | 3900–9800 | Total protein | 7.7 g/dL | 6.5–8.5 |
| Neutrophils | 81.9% | 27.0–70.0 | Albumin | 4.7 g/dL | 4.1–5.3 |
| Eosinophils | 0.5% | 0.0–10.0 | Total bilirubin | 0.80 mg/dL | 0.2–1.3 |
| Basophils | 0.3% | 0.0–3.0 | AST | 37 IU/L | 10–35 |
| Lymphocytes | 15.0% | 19.0–59.0 | ALT | 54 IU/L | 10–35 |
| Monocytes | 1.8% | 0.0–12.0 | Lactate dehydrogenase | 174 IU/L | 110–225 |
| Red blood cells | 488 × 104/μL | 427–570 | Alkaline phosphatase | 222 IU/L | 110–340 |
| Hemoglobin | 15.6 g/dL | 13.5–17.6 | γ-GTP | 30 IU/L | 8–60 |
| Hematocrit | 44.5% | 39.8–51.8 | Cholinesterase | 424 IU/L | 214–466 |
| MCV | 91.3 fl | 82.7–101.6 | Amylase | 153 IU/L | 38–137 |
| MCH | 31.9 pg | 28.0–34.6 | Total cholesterol | 233 mg/dL | 150–219 |
| MCHC | 35.0% | 31.6–36.6 | HDL-C | 69 mg/dL | 40–96 |
| Platelets | 19.5 × 104/μL | 13.1–36.2 | LDL-C | 143 mg/dL | 70–139 |
| Coagulation> | Triglycerides | 177 mg/dL | 50–150 | ||
| APTT | 24.3 s | 27.0–38.5 | CRP | 0.139 mg/dL | 0.000–0.299 |
| PT | 107% | 70–140 | Blood urea nitrogen | 9.8 mg/dL | 9.0–22.0 |
| PT-INR | 0.95 | 0.85–1.15 | Creatinine | 0.64 mg/L | 0.50–1.10 |
| FDP | 3.2 μg/mL | 0–5.0 | |||
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; APTT, activated partial thromboplastin time; PT, prothrombin time; PT-INR, prothrombin time–international normalized ratio; FDP, fibrin degradation products; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γGTP; γ-glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
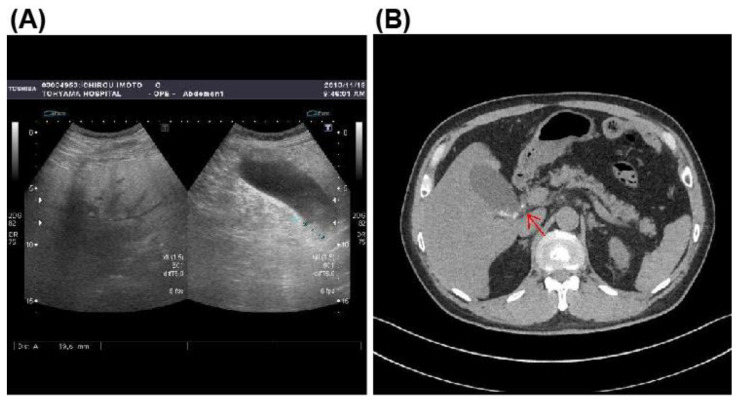
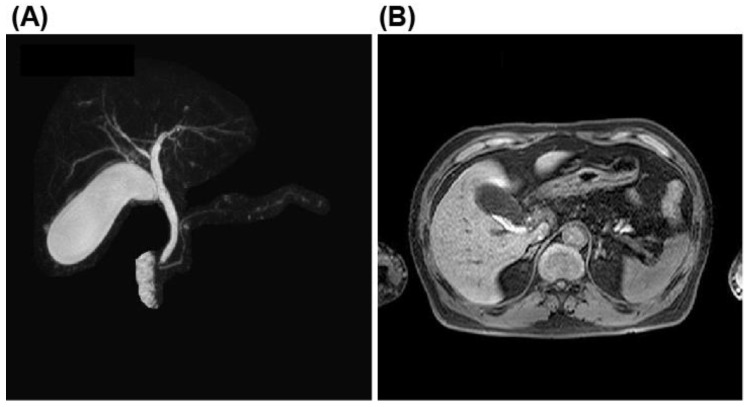
Abdominal ultrasonography and computed tomography (CT) findings showed a moderately fatty liver and multiple stones in an enlarged gallbladder with suspicion of stone impaction in the gallbladder neck (Figure 1A,B). However, on admission, the abdominal ultrasonography showed no typical findings of acute calculous cholecystitis, including gall bladder wall thickening, peri-cholecystic fluid, or sonographic-positive Murphy’s sign. This scarcity in typical findings was probably due to the early disease stage. Magnetic resonance cholangiopancreatography (MRCP) showed numerous stones in the gallbladder but no dilation of the common bile duct or stones in the common bile duct (Figure 2A,B). The diagnosis was acute calculous cholecystitis.
Figure 1.
Abdominal ultrasonography and computed tomography findings. (A), Abdominal ultrasonography revealed moderately fatty liver and numerous small stones and sludge in the gallbladder. (B), Abdominal CT revealed small stones in the enlarged gallbladder with suspicion of stone impaction (arrow) in the gallbladder neck.
Figure 2.
Magnetic resonance cholangiopancreatography findings. (A) No dilation and no stones in the common biliary duct. (B), Small stones and sludge in the distended gallbladder.
After admission, the patient received antibiotic therapy, including 1 g of ceftriaxone sodium hydrate (CTRX) twice daily. Percutaneous transhepatic gallbladder drainage (PTGBD) was performed on the same night. Sixty milliliters of turbid concentrated bile was aspirated from the PTGBD tube. The PTGBD is recommended by the Tokyo Guidelines 2018 in cases with complications or comorbidities [8]. Transhepatic needle puncture may injure hepatic blood vessels. Therefore, instead of PTGBD, percutaneous cholecystostomy is widely used for gallbladder drainage in European countries [9]. Cholecystography showed numerous small stones in the gallbladder. On the third day of hospitalization, levofloxacin hydrate 500 mg/day was added, and the fever resolved on the fourth day of hospitalization. Bile culture from the PTGBD tube revealed Gram-positive bacteria. SGSP was identified using a Vitec 2/Gram-positive (GP) identification card (Sysmex Biomérieux). The drug sensitivity test showed that the minimum inhibitory concentration of CTRX was 0.25 μg/mL. On the evening of the fifth day of hospitalization, antibiotics were switched from CTRX to 1 g of hydrochloride hydrate twice daily, which had a minimum inhibitory concentration of <0.06 μg/mL. Following these treatments, the patient’s subjective symptoms and objective findings improved.
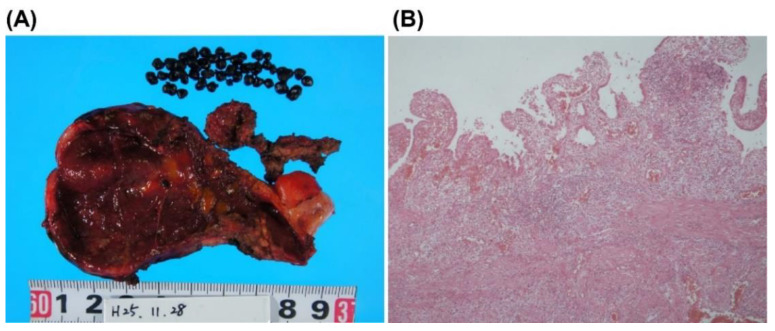
On the 11th day of hospitalization, the patient underwent an open cholecystectomy through a right subcostal incision. More than 30 dark brown stones 2–3 mm in diameter were found in the gallbladder, and the gallbladder base had partially melted due to necrotizing cholecystitis, forming an abscess, which was coated with a large mesh (Figure 3A). In addition, the necrotic mucosa of the gallbladder at the liver bed, which was highly inflamed, was removed as fragments. After intraoperative contrast studies confirmed the absence of residual stones, a Penrose drain was placed, and the abdomen was closed.
Figure 3.
Macroscopic and microscopic findings of the gallbladder. (A), Macroscopic finding of the resected gallbladder. More than 30 black stones of 2–3 mm in diameter were found in the gallbladder. The base of the gallbladder was partially melted due to necrotizing cholecystitis and abscess. (B), Microscopic findings revealed erosion, fibrosis, and moderate inflammatory cell infiltration with neutrophils, but no malignant cells.
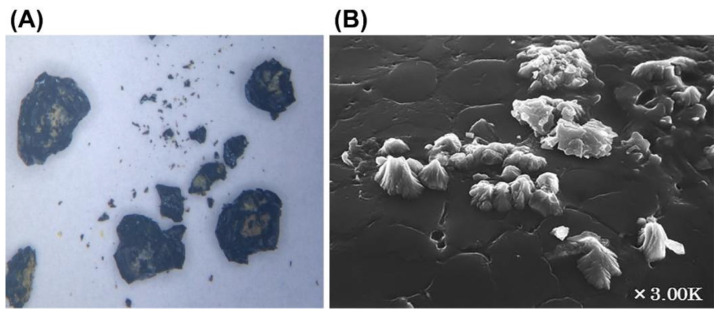
The histological specimens from the cholecystectomy showed erosions, fibrosis, and a moderate inflammatory cell infiltration with neutrophils, but no malignant findings (Figure 3B). Infrared spectroscopy revealed that the composition of the stones was >98% bilirubin calcium. Scanning electron microscopic analysis showed the presence of bacterial colonies on the surface of the calculus (Figure 4A). Infections caused by bacteria of the S. bovis group are commonly associated with colorectal cancer [6]. A colonoscopy showed the presence of three adenomas in the sigmoid colon but without malignant findings.
Figure 4.
Macroscopic and scanning electron microscopic findings. (A), Macroscopic findings of gallstones. Black stones ranging from the size of a grain of sand to 2 to 3 mm. Brownish areas on the irregular surface of the stones. (B), Scanning electron microscopic findings (×3.00 K). Bacterial colonies were observed on the surface of the stone.
After removing the drain on the 12th postoperative day, the patient was discharged on the 17th postoperative day.
3. Discussion
In this report, we described a case of acute calculous cholecystitis caused by an SGSP (formerly S.bovis II/2) infection in a 63-year-old male. Microorganisms of the S. bovis group are commensal intestinal bacteria that can infect humans and animals, including birds [10]. This group of bacteria colonizes the intestines in 10% of healthy individuals [11]. The rate of bacteremia by this group of microorganisms is significantly correlated with the cattle density, suggesting a possible transmission of S. bovis from cows to people [12]. In the current case, the patient used to drink milk and eat raw eggs during childhood, although he was not engaged in raising or fattening cattle. Furthermore, S. bovis group members are increasingly isolated as predominant species from fermented products in Europe, Asia, and Africa [13]. Therefore, dietary habits may be involved in infection acquisition, although the route of infection is unknown.
Microorganisms that degrade gallic acid have been isolated from koalas’ intestines and named S. gallolyticus [1]. This bacterium was classified into three subspecies: S. gallolyticus subs. gallolyticus, S. gallolyticus subs. macedonicus, and S. gallolyticus subs. pasteurianus (SGSP) [1]. The latter subspecies correspond to biotype II/2 (SGSP), which was the infectious agent in the present case. Streptococci of group D, including SGSP, can grow in bile esculin agar medium containing bile acids [14]. Therefore, bacteria from the S. bovis group, including SGSP, can live in bile. Lee et al. evaluated 37 cases of S. bovis bacteremia and found that 14 (38%) had biliary tract disease, indicating that biliary tract infection was the main source of bacteremia by S. bovis [15]. Ruoff et al. reported that among 38 cases of bacteremia by S. bovis, 9 cases (23.7%) of biotype II (II/1; 7 cases, II/2; 2 cases) had a hepatobiliary source of infection [16]. Similarly, Corredoira et al. reviewed several cases of bacteremia caused by S. bovis and S. salivarius and found that S. bovis-biotype-II-associated bacteremia had a hepatobiliary origin in 50% of the patients [17]. In 2013, Corredoira et al. evaluated bacteria of the S. bovis group in 30 cases of cholangitis and 21 cases of cholecystitis using the VITEK 2 g-positive (GP) identification card [7]. They found 29 (57%) cases of S. infantarius (biotype II/1), 20 (39%) cases of SGSP (biotype II/2), and 2 (4%) cases of S. gallolyticus subsp. gallolyticus (biotype I). They concluded that biotype II bacteria of the S. bovis group are predominant in biliary tract infection.
Acute cholecystitis is a complication of cholelithiasis in more than 90% of patients [18]. Gallstones are classified according to their major components in cholesterol, pigment (calcium bilirubin stones and black stones), and rare gallstones [18]. Brown-pigment stones containing bilirubin calcium are formed in bile infected by enteric bacteria [19]. Bae et al. reported that a bile culture was positive for the following bacteria: Escherichia coli (25.0%), Enterococcus spp. (13.4%). Klebsiella spp. (11.1%), Pseudomonas spp. (11.1%), and coagulase-negative Staphylococcus (9.7%) [20]. Most of these bacteria have β-glucuronidase, which hydrolyzes bilirubin glucuronides and is ultimately responsible for forming calcium bilirubin gallstones [21,22]. In the current case, the gallstones contained more than 98% bilirubin calcium, and the culture was positive for SGSP, which releases β-glucuronidase that promotes the formation of gallstones.
The S. bovis group of bacteria has long been associated with colorectal cancer, although not all genospecies are closely related to this malignant complication. The meta-analysis reported by Boleij et al. reported four subspecies of S. gallolyticus and demonstrated a strong association of S. bovis biotype I (S. gallolyticus sub. gallolyticus) with the development of colorectal adenoma/cancer [3]. The association of SGSP with colon cancer was not so high [3]. We performed a colonoscopy because they are related bacteria. The endoscopic study disclosed the presence of adenoma in the sigmoid colon. However, future studies in a large population should be performed before drawing any conclusion on the association between SGSP and colorectal tumors.
4. Conclusions
Although infection by this bacterium may not be rare, this is the first reported case of acute calculous cholecystitis caused by SGSP infection in Japan. In addition, careful clinical follow-up is recommended due to a potential association between SGSP and the development of colorectal cancer.
Author Contributions
Conceptualization, methodology, and formal analysis, S.O., T.S., T.K. and Y.I. (Yasuhiro Inoue); validation and resources, R.N., N.S., T.H., M.N. and K.T.; writing—original draft preparation, I.I., T.Y. and Y.I. (Yoshiyuki Ito); Surgical treatment of the patient, Y.I. (Yoshiyuki Ito); writing—review and editing, E.C.G., A.H., T.Y. and I.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. In addition, other material and information on this case report are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coykendall A.L. Classification and identification of the viridans streptococci. Clin. Microbiol. Rev. 1989;2:315–328. doi: 10.1128/CMR.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlegel L., Grimont F., Ageron E., Grimont P.A.D., Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: Description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Pt 3Int. J. Syst. Evol. Microbiol. 2003;53:631–645. doi: 10.1099/ijs.0.02361-0. [DOI] [PubMed] [Google Scholar]
- 3.Boleij A., van Gelder M.M., Swinkels D.W., Tjalsma H. Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: Systematic review and meta-analysis. Clin. Infect. Dis. 2011;53:870–878. doi: 10.1093/cid/cir609. [DOI] [PubMed] [Google Scholar]
- 4.Gavin P.J., Thomson R.B., Jr., Horng S.J., Yogev R. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): Report of a case and review. J. Clin. Microbiol. 2003;41:3433–3435. doi: 10.1128/JCM.41.7.3433-3435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A., Madani R., Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis. 2010;12:164–171. doi: 10.1111/j.1463-1318.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- 6.Klatte J.M., Clarridge J.E., 3rd, Bratcher D., Selvarangan R. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants. J. Clin. Microbiol. 2012;50:57–60. doi: 10.1128/JCM.05635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corredoira J., Alonso M.P., Garcia-Garrote F., Garcia-Pais M.J., Coira A., Rabunal R., Gonzalez-Ramirez A., Pita J., Matesanz M., Velasco D., et al. Streptococcus bovis group and biliary tract infections: An analysis of 51 cases. Clin. Microbiol. Infect. 2014;20:405–409. doi: 10.1111/1469-0691.12333. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K., Suzuki K., Takada T., Strasberg S.M., Asbun H.J., Endo I., Iwashita Y., Hibi T., Pitt H.A., Umezawa A., et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J. Hepatobiliary Pancreat. Sci. 2018;25:55–72. doi: 10.1002/jhbp.516. [DOI] [PubMed] [Google Scholar]
- 9.Marziali I., Cicconi S., Marilungo F., Benedetti M., Ciano P., Pagano P., D’Emidio F., Guercioni G., Catarci M. Role of percutaneous cholecystostomy in all-comers with acute cholecystitis according to current guidelines in a general surgical unit. Updates Surg. 2021;73:473–480. doi: 10.1007/s13304-020-00897-1. [DOI] [PubMed] [Google Scholar]
- 10.Pompilio A., Di Bonaventura G., Gherardi G. An Overview on Streptococcus bovis/Streptococcus equinus Complex Isolates: Identification to the Species/Subspecies Level and Antibiotic Resistance. Int. J. Mol. Sci. 2019;20:480. doi: 10.3390/ijms20030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R.S., Recco R.A., Catalano M.T., Edberg S.C., Casey J.I., Steigbigel N.H. Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 12.Corredoira J., Miguez E., Mateo L.M., Fernandez-Rodriguez R., Garcia-Rodriguez J.F., Perez-Gonzalez A., Sanjurjo A., Pulian M.V., Rabuñal R., GESBOGA Correlation between Streptococcus bovis bacteremia and density of cows in Galicia, northwest of Spain. Infection. 2019;47:399–407. doi: 10.1007/s15010-018-1254-x. [DOI] [PubMed] [Google Scholar]
- 13.Jans C., Kaindi D.W., Bock D., Njage P.M., Kouame-Sina S.M., Bonfoh B., Lacroix C., Meile L. Prevalence and comparison of Streptococcus infantarius subsp. infantarius and Streptococcus gallolyticus subsp. macedonicus in raw and fermented dairy products from East and West Africa. Int. J. Food Microbiol. 2013;167:186–195. doi: 10.1016/j.ijfoodmicro.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facklam R.R., Moody M.D. Presumptive identification of group D streptococci: The bile-esculin test. Appl. Microbiol. 1970;20:245–250. doi: 10.1128/am.20.2.245-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee R.A., Woo P.C.Y., To A.P.C., Lau S.K.P., Wong S.S.Y., Yuen K.Y. Geographical difference of disease association in Streptococcus bovis bacteraemia. Pt 10J. Med. Microbiol. 2003;52:903–908. doi: 10.1099/jmm.0.05199-0. [DOI] [PubMed] [Google Scholar]
- 16.Ruoff K.L., Miller S.I., Garner C.V., Ferraro M.J., Calderwood S.B. Bacteremia with Streptococcus bovis and Streptococcus salivarius: Clinical correlates of more accurate identification of isolates. J. Clin. Microbiol. 1989;27:305–308. doi: 10.1128/jcm.27.2.305-308.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corredoira J.C., Alonso M.P., Garcia J.F., Casariego E., Coira A., Rodriguez A., Pita J., Louzao C., Pombo B., Lopez M.J., et al. Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: A prospective 16-year study. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:250–255. doi: 10.1007/s10096-005-1314-x. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y., Takada T., Kawarada Y., Nimura Y., Hirata K., Sekimoto M., Yoshida M., Mayumi T., Wada K., Miura F., et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J. Hepatobiliary Pancreat. Surg. 2007;14:15–26. doi: 10.1007/s00534-006-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman H.S., Magnuson T.H., Lillemoe K.D., Frasca P., Pitt H.A. The role of bacteria in gallbladder and common duct stone formation. Ann. Surg. 1989;209:584–591; discussion 591–592. doi: 10.1097/00000658-198905000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae W.K., Moon Y.S., Kim J.H., Lee S.H., Kim N.H., Kim K.A., Lee J.S., Um T.H., Cho C.R. Microbiologic study of the bile culture and antimicrobial susceptibility in patients with biliary tract infection. Korean J. Gastroenterol. 2008;51:248–254. [PubMed] [Google Scholar]
- 21.Stewart L., Oesterle A.L., Erdan I., Griffiss J.M., Way L.W. Pathogenesis of pigment gallstones in Western societies: The central role of bacteria. J. Gastrointest. Surg. 2002;6:891–903; discussion 903–904. doi: 10.1016/S1091-255X(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 22.Vitek L., Carey M.C. New pathophysiological concepts underlying pathogenesis of pigment gallstones. Clin. Res. Hepatol. Gastroenterol. 2012;36:122–129. doi: 10.1016/j.clinre.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. In addition, other material and information on this case report are available from the corresponding author on reasonable request.