Abstract
Background:
Past research has found high rates of hyperventilation in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), but hyperventilation can be influenced by psychological factors. Clinical respiratory rates have been less frequently assessed.
Aim:
This study aimed to identify the predictors of rapid respiratory rates in patients referred to an outpatient clinic specializing in ME/CFS.
Methods:
Adults (n = 216) referred to an outpatient clinic specializing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) participated in a two-day cardiopulmonary exercise test. As part of that evaluation, subjects had resting respiratory rates measured on two consecutive days. The current study used questionnaires to assess the relationship between tachypnea (rapid respiratory rates) and a variety of domains including post-exertional malaise (PEM), a common complaint in patients with ME/CFS, and psychiatric/somatic symptoms, using hierarchical logistic regression analysis.
Results:
PEM was a significant predictor of tachypnea, while psychological/somatic assessments and sedentary behaviors were not significantly predictive of tachypnea.
Conclusions:
These findings suggest that respiratory rate may be useful as an objective clinical metric of PEM, and potentially ME/CFS.
Keywords: Post-exertional malaise, tachypnea, somatization, respiratory rate
Introduction
Severe and debilitating fatigue is characteristic of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) [1], which also involves disabling musculoskeletal and cognitive symptoms, including post-exertional malaise (PEM). PEM is defined as an exacerbation of some, or all, of these musculoskeletal or cognitive symptoms after physical or cognitive exertion, leading to a reduction in functional ability, which often lasts more than 24 h [1,2]. PEM occurs in patients with ME/CFS at higher rates than those with multiple sclerosis or post-polio syndrome [3–5]. PEM has been studied through several self-report evaluations, actigraph activity data, and exercise challenge [3,6–10]. PEM has also been assessed using routine exercise testing in patients with ME/CFS [11–13]. One such study, using cardiopulmonary exercise testing, showed that patients with PEM have a depressed total oxygen volume uptake at maximum exertion [11,12]. Three studies have also examined hyperventilation in patients with ME/CFS, with two showing an increased incidence compared with controls [14–16]. Hyperventilation is marked by both an excessive breathing rate and depth of ventilation leading to the loss of carbon dioxide, whereas tachypnea is only abnormal rapid breathing [17–19].
Tachypnea is commonly caused by asthma, chronic obstructive pulmonary disease, or lung infections. Hyperventilation, on the other hand, is more commonly associated with anxiety disorders, acclimatization, and other somatic issues [17–20]. Therefore, previous studies examining the relationship between fatigue and hyperventilation may be confounded by anxiety, distress, or other somatic illnesses. Previous studies of tachypnea have also been hampered by inaccuracy, as objective measurements of respiratory rates were not used [21,22], and respiratory rates were often subjectively assessed, which leads to high false negative rates of sustained tachypnea, even in patients with severe respiratory illnesses [22]. Therefore, we objectively measured sustained, elevated respiratory rates, PEM and somatic complaints in patients referred to a clinic for ME/CFS to better understand their inter-relationships.
Methods
Subjects
Subjects were selected from a group of 373 individuals with a physician report of ME/CFS referred to an outpatient clinic in the Netherlands (the CFS Medical Center in Amsterdam) specializing in ME/CFS for two days of exercise testing. Ten were excluded because they were under the age of 18 or over the age of 65. Patients (n = 16) with a BMI greater than 35 (morbidly obese) were also excluded. Of the recruited sample who completed the first day of testing, 65% returned the next day. One participant reported being diagnosed with type 2 diabetes. However, diabetes is not a listed exclusionary comorbidity of ME/CFS [1,2], therefore this participant was included. Additionally, participants included in the study reported no exclusionary comorbidities explaining their fatigue. Participants who did not complete the second day of testing were included in the analysis, but their respiratory rate on day 2 was classified as missing. Self-assessment questionnaires (listed below) were administered on the first day. Only subjects who completed the self-assessment questionnaires were included in the analysis (n = 216). IRB approval was obtained from all relevant institutions. All subjects signed informed consent.
Measures
Respiratory rate and tachypnea
Respiratory rates were continuously measured over a period of 3 min using the METALYZER 3B [23], a reliable measure of respiration [24]. Resting respiratory rates were highly correlated between both days of testing (r = .80). Tachypnea was defined as a resting respiratory rate of greater than 20 breaths per minute in our non-morbidly obese resting adults.
DePaul Symptom Questionnaire
The DePaul Symptom Questionnaire is a 54-item self-report measure that includes a subsection of PEM (see below), and also includes items assessing sociodemographic, medical, occupational, engagement in exercise and social history [10]. For each symptom, patients were asked to rate their symptom frequency and severity on a scale from 0 to 4. For frequency: 0 = ‘none of the time,’ 1 = ‘a little of the time,’ 2 = ‘about half the time,’ 3 = ‘most of the time,’ 4 = ‘all of the time.’ For severity: 0 = ‘symptom not present,’ 1 = ‘mild,’ 2 = ‘moderate,’ 3 = ‘severe,’ 4 = ‘very severe.’ DePaul Symptom Questionnaire composite scores were calculated by multiplying both the frequency and severity scores by 25 to create 100-point scales. The DSQ is available at REDCap’s shared library: https://redcap.is.depaul.edu/surveys/?s=tRxytSPVVw, and has good internal reliability (internal consistency α = 0.80) and test–retest reliability [25].
Post-exertional malaise.
Post-exertional malaise is measured in a dedicated portion of the DePaul Symptom Questionnaire by asking about the following symptoms: ‘Dead, Heavy feeling after starting to exercise’, ‘Next day soreness or fatigue after non-strenuous, everyday activities’, ‘Mentally tired after the slightest effort’, ‘Minimum exercise makes you physically tired’, and ‘Physically drained or sick after mild activity’ [25]. The frequency and severity of each of the 5 items were averaged individually, and then collectively averaged and multiplied by 25 to create a 100-point scale.
Four-dimensional symptom Questionnaire
The four-dimensional symptom questionnaire (4DSQ) [26] is a 50-item self-assessment comprised of four subscales: Somatization, Distress, Depression, and Anxiety. The assessment has shown good test–retest reliability and construct validity [26].
4DSQ somatization
The somatization subscale of the 4DSQ is a 16-item scale that measures the tendency of individuals to experience medically unexplained somatic symptoms, to attribute them to physical illness, and to seek medical help for them. Each item is a Yes/No statement (i.e. ‘Have you experienced shortness of breath?’). The scale ranges from 0 to 2, and is additive with each ‘Yes’ response adding 0.1875 to the total score.
4DSQ distress
The distress subscale of the 4DSQ is a 16-item scale that measures individual’s worry, irritability, tension, listlessness, poor concentration, sleeping problems and demoralization. Each item is a Yes/No statement (i.e. ‘During the past week have you felt you can’t cope anymore?’). The scale ranges from 0 to 2, and is additive with each ‘Yes’ response adding 0.33 to the total score.
4DSQ depression
The depression subscale of the 4DSQ is a 6-item scale that measures the tendency of individuals to experience depressive thoughts and anhedonia. Each item is a Yes/No statement (i.e. ‘Have you felt that everything feels meaningless?’). The scale ranges from 0 to 2, and is additive with each ‘Yes’ response adding 0.1875 to the total score.
4DSQ anxiety
The anxiety subscale of the 4DSQ is a 12-item scale that measures the tendency of individuals to experience irrational fears, anticipation anxiety and avoidance behavior. Each item is a Yes/No statement (i.e. ‘Have you felt that everything feels meaningless?’). The scale ranges from 0 to 2, and is additive with each ‘Yes’ response adding 0.1667 to the total score.
Procedure
Data were collected from patients who attended the CFS Medical Centre in Amsterdam. Sociodemographic, 4DSQ, and DePaul Symptom Questionnaire assessments were collected on the first day prior to respiratory testing. Resting respiratory rates were then measured using the Metalyzer 3B [23] over a period of 3 min. Resting respiratory rate was averaged over the three minutes to provide a resting respiratory rate per each day of testing. Day 1 resting respiratory rate was calculated as the 3-minute average on the first testing day. Subjects were invited back the next day for a second of testing following the same procedures as the first day, excluding the self-assessment questionnaires.
Statistical analysis
Self-assessment questionnaires were collected once, and resting respiratory rates collected twice, on two consecutive days. To account for within-subject variance in respiratory rates across both time points, we used a hierarchical logistic regression to assess the probability of tachypnea as a function of PEM, somatization, depression, distress and anxiety, using the package ‘lme4’ [27] for R statistical programming. Because we gathered respiratory rate data on two different days, we analyzed the data using two-level hierarchical logistic regression. The first level was organized at the individual case level, second level was organized by the testing day (day 1 vs day 2). As the number of observations was greater than the number of individuals due to multiple testing, we clustered the standard errors at the individual case level. We resampled the data using hierarchical cluster bootstrap analysis to obtain model estimates [28; see Appendix A]. Significance testing was examined at p < .05. Finally, we examined variance inflation factors (VIF) to test whether the predictors are predictive of one another (i.e. for multicollinearity).
Results
Subject statistics
By design, subjects’ age ranged from 18 to 65 (Mean = 36.13, Standard Deviation = 11.22). Women constituted 79.5% (n = 171) of the patients while men constituted 20.5% (n = 44), consistent with most studies of ME/CFS in which the sample is majority women [29–31]. One subject chose not to report their gender. Participants with a BMI >29 comprised 12% of the sample. A subset of participants with a BMI approaching 35 (>34) comprised 1.4% of the sample. Additionally, 36% of participants reported engaging in regular exercise. Importantly, there was no relationship between BMI category and engagement in exercise [χ2 (1, N = 216) = 2.19, p > .05]. Subjects’ resting respiratory rate on the first day ranged from 10.1 breaths per minute to 27.9 breaths per minute (Mean = 18.32, Standard Deviation = 3.76). On the second day, resting respiratory rates ranged from 9.10 breaths per minute to 30.3 breaths per minute (Mean = 36.13, Standard Deviation = 11.22). On the first day, 32.9% of the sample (n = 72) met the criteria for tachypnea. On the second day 30.1% (n = 65) met the criteria for tachypnea. A McNemar test was conducted to evaluate consistency of tachypnea over both days. Of the participants who tested on multiple days, 88.1% of those who were non-tachypneic on the first day were consistently non-tachypneic on the second day. For those who were tachypneic on the first day, 73.1% were tachypneic on the second day. These differences were found to be non-significant (p > .05), indicating that subjects’ tachypnea categorization was consistent over the two days of testing.
Regression analysis
A hierarchical logistic regression was conducted to evaluate the probability of having tachypnea. The model of tachypnea included the following predictors: PEM, engagement in exercise (‘Yes’/ ’No’), BMI (≤29/>29), somatization, anxiety, depression, and distress. Bootstrapped estimates and confidence intervals of each predictor are shown in Table 1.
Table 1.
Bootstrapped estimates predicting tachypnea.
| 95% CI for odds ratio | ||||
|---|---|---|---|---|
| Estimate (SE) | Bootstrap mean | Lower | Upper | |
| Intercept level 1 (cases) | −1.94 (0.50)** | −1.99 | −3.02 | −1.08 |
| Intercept level 2 (days) | 0.00 (0.00) | 0.03 | 0.00 | 0.20 |
| PEM | .02 (0.01)** | 0.02 | 0.01 | 0.04 |
| Exercise | 0.04 (0.25) | −0.33 | −1.04 | 0.39 |
| BMI | 0.02 (0.34) | 0.17 | −0.58 | 0.91 |
| Depression | −0.27(0.27) | −0.31 | −0.96 | 0.29 |
| Anxiety | 0.43 (0.41) | 0.02 | −0.57 | 0.58 |
| Somatization | −0.31 (0.32) | 0.01 | −0.66 | 0.66 |
| Distress | 0.17 (0.36) | 0.45 | −0.46 | 1.45 |
Notes: LL = −258.2, AIC = 530.5, BIC = 558.7, Number of obs: 418. LL (Log Likelihood), AIC (Akaike Information Criteria), and BIC (Bayesian Information Criteria) are relative fit-statistic measures. The intercepts represent the log odds of tachypnea.
p < .05
p < .01.
A Hosmer and Lemeshow test indicated strong goodness-of-fit [χ2 = 8.80, p > .05]. Differences in likelihood-ratio tests also indicated that the addition of PEM improved the model significantly over and intercepts-only model (χ2 = 7.35, p<.01). PEM was the only significant predictor of tachypnea with a positive odds ratio, as the four somatic predictors, exercise, and BMI had upper and lower confidence intervals which crossed 0, indicating non-predictiveness. PEM was thus found to be a good predictor of tachypnea; every unit increase in PEM score corresponded to a 2.1% increase in the probability of the subject having tachypnea. Examining specific PEM scores of the logit function shows that a PEM score of 25 is predicted to have a 20% probability of tachypnea, a score of 50 is predicted to have a 28% probability of tachypnea, a score of 75 is predicted to have 39% probability to have tachypnea, and a maximum score of 100 is predicted to have a 50% probability of tachypnea. Somatization, distress, depression, and anxiety were not significant predictors of tachypnea. See Figure 1.
Figure 1.
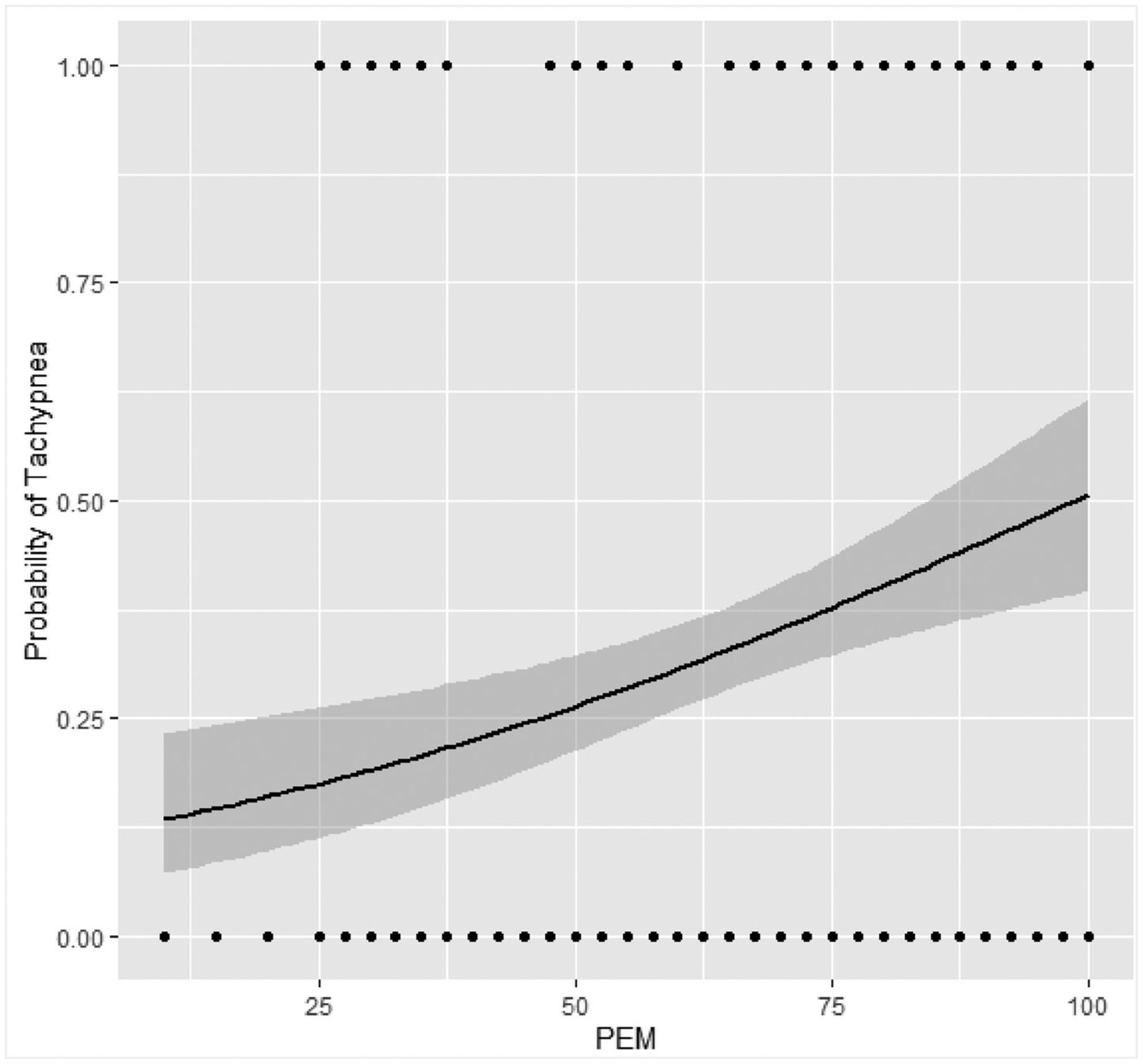
Tachypnea probability estimates. The Y axis represents the probability of a study subject being tachypneic and the X axis represents the PEM score as calculated (see Methods). Each dot represents one or more subjects’ PEM score.
Multicollinearity was assessed using variance inflation factor quotients (VIFs) (Table 2). VIFs >10 or <0.2 indicate significant confounds [32]. As all VIFs were between 1 and 3, we concluded that there were no significant confounders among the predictors.
Table 2.
Variance inflation factors.
| Predictor | VIF |
|---|---|
| PEM | 1.13 |
| Exercise | 1.14 |
| BMI | 1.05 |
| Distress | 2.55 |
| Depression | 2.09 |
| Anxiety | 2.18 |
| Somatization | 1.44 |
Discussion
Patients with ME/CFS often complain of shortness of breath even with the slightest physical or mental effort [33], but this is often interpreted as a symptom of anxiety or mental distress [34]. Previous studies examining respiratory rates in patients with ME/CFS used hyperventilation as a measure of respiratory distress [12–16]; however, hyperventilation can be influenced by somatization [18,19].
A multilevel logistic regression framework was implemented in the current study. We objectively measured tachypnea alone as a sign of respiratory distress, and showed that PEM was predictive of tachypnea in patients referred to a specialist for ME/CFS, while engagement in exercise, obese (BMI >29), somatization, distress, anxiety, and depression were not. These data argue that PEM is a physical symptom of fatigue and likely ME/CFS as well, and may be related to underlying physiologic, not psychologic dysfunction.
PEM and tachypnea probability appeared to have a moderate linear relationship as seen in Figure 1. The lack of sigmoidal relationship typically seen in logistic regression can be explained as there were no extreme probabilities of tachypnea associated with PEM; even with a maximum PEM score, the probability of tachypnea was approximately 50%. While a probability of 50% might be considered no better than chance for behavioral studies, because tachypnea is a low probability event [17,21,22], it is likely indicative of an underlying physiological abnormality in our subjects due the large change in probability.
The current study has a few limitations. Although we incorporated measures of sedentary lifestyle including obesity (BMI >29) and engagement in exercise into the model, without a measure of daily activity behaviors it may still be possible for sedentary lifestyles to be a confounding variable. Yet, the inclusion of such features provides evidence that PEM is a distinct symptom not associated with obesity or lack of exercise. Additional research accounting for logging regular activity will be needed to further separate PEM from deconditioning [35]. A second limitation was lack of confirmation of the reported physician diagnosis of ME/CFS. Third, though assessing respiratory rates during both testing days was beneficial for examining within-subject changes over the course of two days, it would have also been useful to examine the self-assessment questionnaires over the course of two days, to evaluate within-subject variance of responses.
The current study focused on the association between tachypnea and PEM to highlight an easily measured metric which can be utilized to assess PEM, its severity and overall debilitation in at least some patients with ME/CFS. PEM, and its exacerbation of symptoms, should therefore not be dismissed as being due to psychological factors, as reports of clinicians’ experiences have shown [36]. Careful measurements of the respiratory rate at rest or after minimal activity thus may provide objective evidence of disability. Future research should more closely examine the physiologic relationship between PEM and respiratory dysfunction in patients with ME/CFS.
Funding
This clinical research study was supported by the National Institutes of Health [grant number 1R01AI105781-01A1].
Biographies
Joseph Cotler is a project director of ME/CFS research at the Center for Community Research at DePaul university. He has been conducting research on ME/CFS since 2018.
Dr Ben Z. Katz is a Professor of Pediatrics at the Northwestern University Feinberg School of Medicine and an Attending Physician in the Division of Infectious Diseases at the Ann & Robert H Lurie Children’s Hospital of Chicago. He has been studying chronic fatigue syndrome with the group from DePaul since 2002.
Corine Reurts-Post is a doctor’s research assistant at the CVS/ME Medisch Centrum in Amsterdam, specializing in the treatment of patients with chronic fatigue.
Ruud Vermeulen heads the Research department and the research and treatment of chronic fatigue and exercise physiology at the CVS/ME Medisch Centrum.
Leonard A. Jason has been the principle investigator of ME/CFS research at DePaul university and the Center for Community Research for 25 years.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Carruthers BM, Sande MI, Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus criteria. J Intern Med. 2011;270:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].IOM. (2015). Beyond myalgic encephalomyelitis/chronic fatigue syndrome. [Google Scholar]
- [3].Cotler J, Holtzman C, Dudun C, et al. A brief Questionnaire to assess post-exertional malaise. Diagnostics. 2018;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013;11, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McManimen SL, Jason LA. Differences in ME and CFS symptomatology in patients with normal and abnormal exercise test results. Int J Neurol Neurother. 2017;4:066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jason LA, Boulton A, Porter NS, et al. Classification of myalgic encephalomyelitis/chronic fatigue syndrome by types of fatigue. Behav Med. 2010;36:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brown AA. Investigating post-exertional malaise as a core symptom of myalgic encephalomyelitis and chronic fatigue syndrome: a meta-analytic approach (2017). College of Science and Health Theses and Dissertations, 234. Available from: https://via.library.depaul.edu/csh_etd/234. [Google Scholar]
- [8].Hall DL, Lattie EG, Antoni MH, et al. Stress management skills, cortisol awakening response, and post-exertional malaise in chronic fatigue syndrome. Psychoneuroendocrinology. 2014;49:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stevens S, Snell C, Stevens J, et al. Cardiopulmonary exercise test methodology for assessing exertion intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jason LA, et al. The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. Am J Biochem Biotechnol. 2010;6(2):120–135. DOI: 10.3844/ajbbsp.2010.120.135. [DOI] [Google Scholar]
- [11].VanNess JM, Snell CR, Stevens SR. Diminished cardiopulmonary capacity during post-exertional malaise. J Chronic Fatigue Syndrome. 2007;14:77–85. [Google Scholar]
- [12].Vermeulen RC, Kurk RM, Visser FC, et al. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vermeulen RC, Eck IW. Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J Transl Med. 2014;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bazelmans E, Bleijenberg G, Vercoulen J, et al. The chronic fatigue syndrome and hyperventilation. J Psychosom Res. 1997;43:371–377. [DOI] [PubMed] [Google Scholar]
- [15].Riley MS, Obrien CJ, Mccluskey DR, et al. Aerobic work capacity in patients with chronic fatigue syndrome. Br Med J. 1990;301(6758):953–956. DOI: 10.1136/bmj.301.6758.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saisch SG, Deale A, Gardner WN, et al. Hyperventilation and chronic fatigue syndrome. Q J Med. 1994;87(1):63–67. [PubMed] [Google Scholar]
- [17].Des Jardins T, Burton GG. Clinical manifestations and assessment of respiratory disease. St. Louis (MO): Elsevier; 2020. [Google Scholar]
- [18].Hutchison R, Glynn M, Drake WM. Hutchisons clinical methods: an integrated approach to clinical practice. Edinburgh: Saunders/Elsevier; 2012. [Google Scholar]
- [19].Hamilos DL, Nutter D, Gershtenson J, et al. Core body temperature is normal in chronic fatigue syndrome. Biol Psychiatry. 1998;43:293–302. [DOI] [PubMed] [Google Scholar]
- [20].Ringsberg KC, Åkerlind I. Presence of hyperventilation in patients with asthma-like symptoms but negative asthma test responses: Provocation with voluntary hyperventilation and mental stress. J Allergy Clin Immunol. 1999;103(4):601–608. DOI: 10.1016/s0091-6749(99)70231-9. [DOI] [PubMed] [Google Scholar]
- [21].Badawy J, Nguyen OK, Clark C, et al. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26:832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bianchi W, Dugas AF, Hsieh Y, et al. Revitalizing a vital sign: improving detection of tachypnea at primary triage. Ann Emerg Med. 2013;61:37–43. [DOI] [PubMed] [Google Scholar]
- [23].METALYZER® 3B. (2020). Available from: https://cortex-medical.com/EN/METALYZER-3B-en.htm.
- [24].Meyer T, Georg T, Becker C, et al. Reliability of gas exchange measurements from two different spiroergometry systems. Int J Sports Med. 2001;22:593–597. [DOI] [PubMed] [Google Scholar]
- [25].Jason LA, Sunnquist M. The development of the DePaul symptom Questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr. 2018;6, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Terluin B, Marwijk HWV, Adèr HJ, et al. The four-dimensional symptom Questionnaire (4DSQ): a validation study of a multidimensional self-report questionnaire to assess distress, depression, anxiety and somatization. BMC Psychiatry. 2006;6:1. DOI: 10.1186/1471-244x-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bates D, Maechler M, Bolker B, et al. (2020, April 7). PDF. Available from: https://cran.r-project.org/web/packages/lme4/lme4.pdf.
- [28].Department of Biostatistics, Applied Nonparametric Bootstrap with Hierarchical and Correlated Data. (2015). Available from: http://biostat.mc.vanderbilt.edu/wiki/Main/HowToBootstrapCorrelatedData. [Google Scholar]
- [29].Bakken IJ, Tveito K, Gunnes N, et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: A population based registry study from Norway 2008–2012. BMC Med. 2014;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jordan KM, Ayers PM, Jahn SC, et al. Prevalence of fatigue and chronic fatigue syndrome-like illness in children and adolescents. J Chronic Fatigue Syndrome. 2000;6(1):3–21. DOI: 10.1300/J092v06n01_02. [DOI] [Google Scholar]
- [31].Lloyd AR, Hickie I, Boughton CR, et al. Prevalence of chronic fatigue syndrome in an Australian population. Med J Aust. 1990;153:522–528. [DOI] [PubMed] [Google Scholar]
- [32].Tabachnick BG, Fidell LS. Using multivariate statistics (7th ed.). New York, NY: Pearson; 2019. [Google Scholar]
- [33].Jason LA, Holtzman CS, Sunnquist M, et al. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J Health Psychol. Published online: October 24, 2018. 10.1177/1359105318805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mccue P, Buchanan T, Martin CR. Screening for psychological distress using internet administration of the hospital anxiety and depression scale (HADS) in individuals with chronic fatigue syndrome. Br J Clin Psychol. 2006;45(4):483–498. DOI: 10.1348/014466505(82379. [DOI] [PubMed] [Google Scholar]
- [35].Clark LV, White PD. The role of deconditioning and therapeutic exercise in chronic fatigue syndrome (CFS). J Mental Health. 2005;14(3):237–252. DOI: 10.1080/09638230500136308. [DOI] [Google Scholar]
- [36].Stenhoff AL, Sadreddini S, Peters S, et al. Understanding medical students’ views of chronic fatigue syndrome: a qualitative study. J Health Psychol. 2013;20(2):198–209. DOI: 10.1177/1359105313501534. [DOI] [PubMed] [Google Scholar]


