Abstract
Background:
The present article aims to introduce the endolymphatic duct and sac decompression technique (DASD) and to give a spotlight on its benefits in Ménière’s disease (MD) treatment.
Methods:
Eighty-two patients with intractable MD which met the inclusion criteria were recruited and underwent DASD. This technique allows a meningeal decompression of the duct and the sac from the posterior cranial fossa to the labyrinthine block. The authors considered as main outcomes, the change of the dizziness handicap inventory (DHI) results, with the evaluations of the three sub-scales (Functional scale, Physical scale, and Emotional scale); ear fullness and tinnitus change on the perceptions of the patient; and hearing stage with four-Pure Tone Average (500 hz-1000 hz-2000 hz-4000 hz). The differences between the preoperative and the postoperative score were evaluated. A comparison with the literature was conducted.
Results:
After a 14-month follow-up, patients that underwent DASD reported a remarkable improvement of the symptoms in all three functional scales, confirmed by the total DHI. The difference between preoperative and postoperative scores is statistically significant. The data describe an ear fullness and tinnitus improvement. The multi-frequency tonal average before and after the surgery does not suggest a worsening of the value for any of 82 patients.
Conclusion:
The modification of sac surgery includes the endolymphatic duct in the decompression area allowing inner ear functional improvement, vertigo control, ear fullness improvement with minimal risk of facial nerve paralysis, and hearing loss. DASD is an improved old surgical technique.
Keywords: Endolymphatic decompression, Endolymphatic hydrops, Ménière’s disease, Vertigo

INTRODUCTION
Ménière’s Disease (MD) is an idiopathic pathology of the inner ear clinically characterized by spontaneous and recurrent episodes of dizziness, vertigo, fluctuating neurosensory hearing loss, tinnitus, and ear fullness. Years before the episodes of vertigo, the patient can experience tinnitus, ear fullness, and hearing loss of the affected ear. Hearing loss is associated with episodes of vertigo in 77% of patients.[3] It appears fluctuating and, in the first period of the disease, reversible after a crisis, however, with the progression of the pathology, hearing loss worsens and gradually becomes permanent. Tinnitus can be an initial symptom of MD, preceding the other symptoms by months. Over time it tends to become permanent and can increase or change in tone, heavily worsening quality of life. Nowadays, MD treatment is highly controversial. The authors propose a new technique for MD treatment named endolymphatic duct and sac decompression (DASD), the results of which are shown in this article, based on the idea that the endolymphatic duct (ED) could be responsible for the endolymphatic hydrops as much as the sac.
Surgical technique
DASD is performed in substitution of plain endolymphatic sac surgery (ELSS). The technique is not limited to sac decompression but extended to duct decompression. To get around the difficulty of recognizing the endolymphatic sac, it is mandatory to drill out all the bone over the dura, from the superior petrosal sinus to the superior limit of the jugular bulb. To decompress the ED with reasonable certainty, the dura has to be exposed as far as the arch of the posterior semicircular canal, where the ED passes. The bone paté-surgical bone dust mixed with fibrin glue-is then positioned superiorly and inferiorly to the area, where the ED resides [Figure 1]. Physiologically, the obstruction of the ED can hinder the success of any technique, it is therefore important to include this fundamental anatomical structure in the decompression zone.[14]
Figure 1:
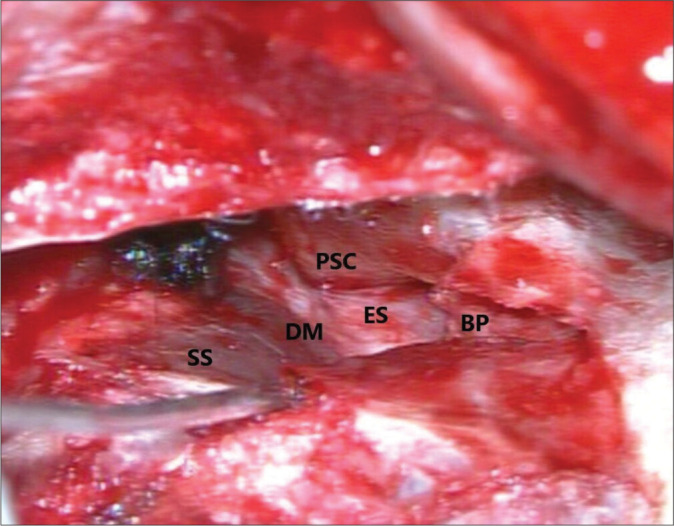
Decompression zone, BP: bone pate, PSC: posterior semicircular canal, D: dura mater, SS: sigmoid sinus, and ES: endolymphatic sac.
In consideration of the rounded-up intraosseous ES average length of 15 mm,[12] the duct average length of 1.64 mm,[13] and the MD ED average volume of 0.34,[17] the authors can demonstrate the height of bone paté necessary to decompress the ED.
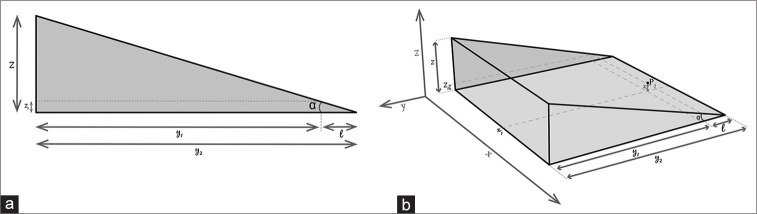
Considering the ED as a cylindrical geometric figure:
Considering [Figure 2a]
Figure 2:
(a and b) Demonstration of endolymphatic duct decompression.
y1 = 15 mm ES length
y2 = 15 mm + 1.64 mm = 16.64 = decompression zone length (length ES + length ED)
z1 = 0.48 = minimum height to be reached at the level of the duct (point P) considered as the diameter of the cylinder.
z=decompression zone height (bone-patè)
For l = y2-y1
The authors hypothesize that to decompress the ED (P1), it is necessary to have a height of about 4.8 mm of bone-pate to be placed between the posterior semi-circular canal and the upper extremity and dura mather (z). Using the currently available data in the literature regarding the ES,[14] its maximum intraosseous length has been considered 15 mm. It is important to report that an excessive bone-paté height constitutes a risk for CSF fistula.
Furthermore, by arranging bone-paté in two points, beyond ES cranial margin, and beyond ES caudal margin (x), it is possible to include these structures in the decompression area. The authors have identified a prismatic area that represents the approximate decompression zone [Figure 2b].
MATERIALS AND METHODS
The observational self-controlled case study was conducted retrospectively at the Otolaryngology Department of the Campus Bio-Medico University on patients that underwent DASD. All the patients who met the criteria for MD according to the Classification Committee of the Bárány [16] were subject beforehand to medical treatment (vestibular suppressors, diuretics, betahistine, and diet) without benefits for at least 6 months.
Exclusion criteria for DASD are patients older than 75 years (due to meningeal fragility), patients that cannot sustain general anesthesia, or suffering complete hearing loss of the affected ear. All patients underwent an ear Computed Tomography scan (CT scan) and brain magnetic resonance imaging to exclude inner ear pathologies and APC expansive formations.
Two patients showing signs of chronic otitis media will first undergo tympanoplasty and then DASD; the presence of a not well-pneumatized mastoid increases the difficulty of the intervention but does not represent a criterion of exclusion.
The authors administered the dizziness handicap inventory (DHI) questionnaire in an 82 patients consecutive cohort to evaluate postoperative and preoperative condition retrospectively. The DHI is a questionnaire validated in Italian[19] including three scales: functional, emotional, and physical; it is used to assess the impact of vertigo and instability on the quality of life. Ear fullness and tinnitus were marked as “absent, reduced, unchanged, or worsened.” Preoperative and postoperative audiometric examinations were evaluated with 4-PTA (Pure Tones Averege) (500Hz-1000Hz-2000hz-4000hz), the authors considered bone conduction average, the hearing threshold was considered stable with a ≤10-dB PTA improvement or worsening-based American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS = American Academy of Otolaryngology–Head and Neck Surgery) criteria for hearing level assessment. The age of the study population at the time of surgery ranged from 25 to 71, averaging 50.5 years, while the male/female ratio was 1.3:1. Of the 82 patients, 10.9% (9) presented the disease bilaterally, only one patient reported undiagnosed migraine, and one patient had a son with MD. The average time of the first vertiginous crisis was 8 (1–35) years earlier. Over the 6 months before the intervention, patients described the number of crises with wide variability 12 ± 10 (1–30).
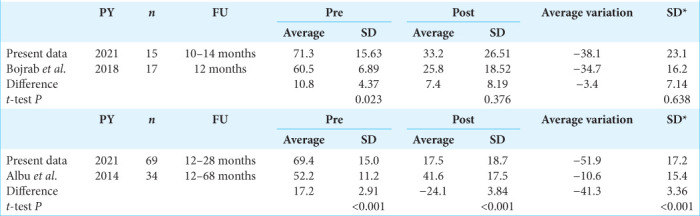
The average follow-up from the intervention date is 14.96 months. Statistical analysis was carried out with GraphPad software Inc. and the Shapiro–Wilk test was used to evaluate the normality of the sample. Discontinuous variables are listed with median and range (IQR). The difference between the preoperative and postoperative values was analyzed with the Wilcoxon signed–rank test. The authors performed a comparison with the literature and found two publications, in which DHI was used to evaluate ELSS,[1,2] the T-test was performed to compare the data of these studies with those of this article.
In the case of the study by Bojrab et al., the authors do not report data on the standard deviations, these data were extracted from “Figure 2” of the Web Plot Digitizer program. We included patients with similar follow-ups. Statistical significance was considered for P < 0.05.
RESULTS
The Wilcoxon signed–rank test showed that the scoring difference between the preoperative and the postoperative of each DHI question is statistically significant (P < 0.001) [Figure 3]. The average preoperative DHI score was 70 ± 15.19 against the postoperative one that turned out to be 18 ± 20 and the median score difference was 54 ± 18. The sub-scale comparison shows a preoperative average score of 24.9 against 6.52 in the postoperative for functional scale, 25.26 against 6.48 for the emotional scale, 19.8 against five for the physical scale. The postoperative situation shows fullness worsened in 4% of the case, unchanged 13% of the case, absent in 40% of the case, and reduced in 43% of the case. Postoperative tinnitus valuation worsened in 4% of the cases, unchanged in 36% of the cases, reduced in 38% of the cases, and absent in 22% of the cases.
Figure 3:
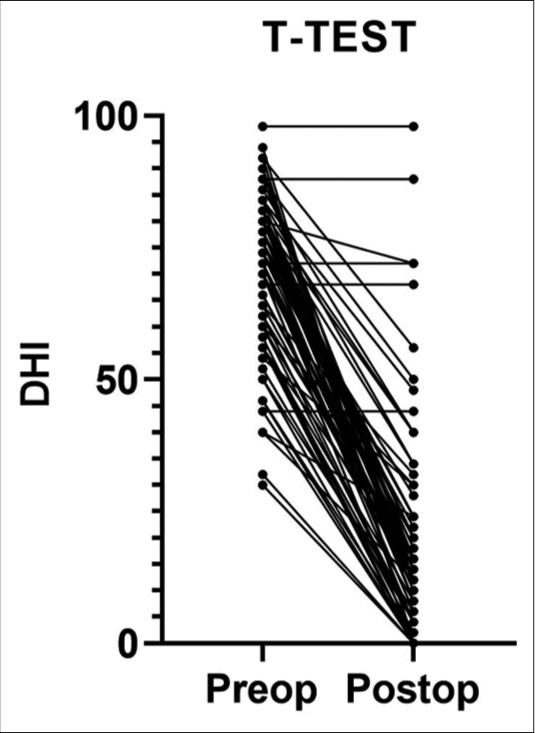
Comparison of total dizziness handicap inventory for each patient before and after surgery.
Considering the follow-up obtained, 89% (73 of 82) of the patients achieved control of vertigo, 8% (7 of 82) underwent further surgery (neurectomy, Intratympanic Gentamicin), the remaining 3% (2 of 82) did not achieve symptoms control but refused to undergo further interventions. Among the residual symptoms, the “blocked crises” are reported: the patient experiences prodromal symptoms (tinnitus and fullness that increase in intensity) with brief light-headedness, without leading to vertigo. The comparison of the multi-frequency average of hearing thresholds is described [Figure 4]. In each DASD treatment, no paralysis of the facial nerve and no hearing loss were observed. The literature comparative results are presented [Table 1]; the baseline condition in DHI terms of the patients in this study is worse than in the patients of the other two studies that are considered; hence, it is not possible to draw a comparison without significant bias.
Figure 4:
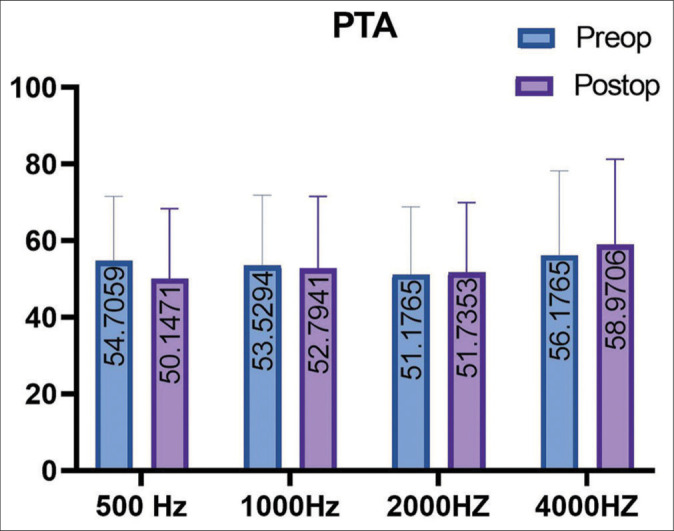
Four Pure Tone average (500 Hz-1000 Hz-2000 Hz-4000 Hz) before and after surgery.
Table 1:
Literature comparison in ELSS techniques using DHI questionnaire results in patients with similar follow-up.
DISCUSSION
The literature describes the different ELSS techniques: endolymphatic mastoid sac shunt and endolymphatic sac decompression without incision are described in some works with similar results among them;[6,27] the different ELSS techniques are described by a meta-analysis that reported vertigo control in 81.6% of cases in 36-month follow-up regarding sac decompression surgery, and 75.7% regarding mastoid shunt.[6] Furthermore, according to a review and a meta-analysis that did use the PubMed-NCBI database from 1970 to 2013, sac decompression and mastoid shunt are effective in controlling symptomatology with a 24-month follow-up in 75% of patients; the same studies reported better hearing performance without silastic application.[27]
Failure of ELSS is related to the intrinsic intraoperative difficulty to recognize the endolymphatic sac; landmarks for its recognition are described in dissections of temporal bones reports.[15]
The wide decompression of the sigmoid sinus is fundamental to allow vertigo control in 90% of cases.[7] Other studies describe an improvement of the symptoms in 95% of cases[10,25] or the significant improvement in the quality of life of the patient.[4] According to the authors, the improvement of the technique involves meningeal decompression of the ED, as well as the endolymphatic sac and the coverage of the decompressed area with bone wax and temporal fascia graft to avoid bone regrowth in the decompressed area.
Considering the young age of many of the patients, despite the good results in the literature on intratympanic gentamicin administration (ITG), the authors prefer not to expose patients even to the related 10% risk of hearing loss. Studies report different percentages of symptoms control for ITG: 75[24]–80%–83.1%[21] with a 24%,[24] 60.5%.[21] In a 2018 review, short-time intervals of ITG= Intratympanic Gentamicin showed vertigo control in 90% of patients on average, burdened by an average rate of hearing loss of 36.5%. In the same study, considering a wider time interval for this procedure, the control of the vertiginous symptomatology is reported in 85% of cases on average with a hearing loss in 12% of cases.[29] Many patients refuse to undergo ITG fearing auditory consequences.
Todays’, debate is still based on the placebo effect related to any surgical procedure on the ear of the MD patient.[28] The Cochrane review describes insufficient statistical evidence regarding the success of ELSS.[12,22] However, carrying out a double-blind study with randomized recruitment of the two groups arises ethical objections. Moreover, no one of the MD surgical treatment options was ever evaluated in a randomized controlled study. Even in the international consensus on the treatment of MD, ELSS is considered a third-line treatment before ITG (fourth-line treatment).[18,26] Despite guidelines, a decreasing number of ELSS and an increasing number of ITG are observed around the world, especially in the USA.[18]
This trend is possibly due to inherent difficulties with ELSS. Furthermore, ITG is supposedly performed more often for its easiness, replicability, and chain of work optimization. A consequent bias loop is established where less ELSS are performed, less data are provided, and less research is published.
For the authors, the goal and innovation of this technique are represented by the ED decompression. The duct and sac should be seen as a unitary system, but their organic and structural link is often underestimated. This hypothesis is based on the histopathological evidence of Linthicum et al.[13] that reported an osseous obstruction of the ED during temporal bones dissections of patients with MD at the House Ear Institute. Additional extra-structural research sustains a central role of the duct in endolymph homeostasis.[5,23] The ED communicates with the endolymphatic sac located in a dura duplicate on the posterior slope. Failures of endolymphatic sac shunt depend on the difficulty in finding this structure. Several animal studies show that ED obstruction can cause endolymphatic hydrops.[11] Inflammatory phenomena can cause periductal fibrosis and interstitial cellularity increase that can slow down the lymph capillary flow to the complete obstruction, with an altered endolymphatic homeostasis.[8,9] Moreover, a recent study about the ED conducted on micro-CT scan hypothesizes an endolymph inflow and outflow model. In this model, the sinusoidal and lymphovenous plexus of the ED are the main system for endolymph reabsorption. This network is responsible for endolymph drainage to the vestibular aqueduct and cranial sinus; its obstruction can cause endolymph hydrops.[20]
In this case series, treatment failure in 11% of cases are not associated with the frequency of preoperative vertigo nor with the duration of the disease; however, further data are needed.
The authors compared the results of this study with the literature using the DHI scale as a common differentiator. The initial good results appear to worsen during follow-up.[1] The articles found in literature examine the ELSS, although the technique is not described in the text, because it is considered as an already codified intervention.[2] The authors hypothesize that the technique failure is due to the lack of knowledge of the anatomical area that it is not exposed during surgery. Increasing accuracy and mastery of the anatomical area could improve the technique. To refute the hypothesis of symptoms worsening along the time, more than 2 years of follow-up will be performed.
CONCLUSION
DASD allows MD patients to obtain vertigo control, as well as improvement of the inner ear function. In addition, DASD helps to reduce ear fullness, with no relevant risk of hearing worsening in the postoperative time, lower rate of intraoperative complication, and shorter postoperative hospital stay and no irreversible vestibular deficits as expected from other techniques. DASD reduces the destructive effects of the MD on the cochlea and the labyrinths.
Footnotes
How to cite this article: Salvinelli F, Bonifacio F, Greco F, Cavicchioni G, Frari V, Pierri M, et al. Endolymphatic duct and sac decompression: A new technique for Ménière’s disease treatment. Surg Neurol Int 2022;13:418.
Contributor Information
Fabrizio Salvinelli, Email: f.salvinelli@policlinicocampus.it.
Francesca Bonifacio, Email: f.bonifacio@policlinicocampus.it.
Fabio Greco, Email: f.greco@policlinicocampus.it.
Giulio Cavicchioni, Email: g.cavicchioni@unicampus.it.
Valeria Frari, Email: v.frari@policlinicocampus.it.
Michelangelo Pierri, Email: michelangelo.pierri@unicampus.it.
Maurizio Trivelli, Email: m.trivelli@policlinicocampus.it.
Maurizio Iacoangeli, Email: neurotra@tiscali.it.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Albu S, Babighian G, Amadori M, Trabalzini F. Endolymphatic sac surgery versus tenotomy of the stapedius and tensor tympani muscles in the management of patients with unilateral definite Meniere’s disease. Eur Arch Otorhinolaryngol. 2015;272:3645–50. doi: 10.1007/s00405-014-3428-1. [DOI] [PubMed] [Google Scholar]
- 2.Bojrab DI 2nd, LaRouere MJ, Bojrab DI, Babu SC, Sargent EW, Chan EY, et al. Endolymphatic sac decompression with intra-sac dexamethasone injection in Menière’s disease. Otol Neurotol. 2018;9:616–21. doi: 10.1097/MAO.0000000000001810. [DOI] [PubMed] [Google Scholar]
- 3.Canale A, Caranzano F, Lanotte M, Ducati A, Calamo F, Albera A, et al. Comparison of VEMPs, VHIT and caloric test outcomes after vestibular neurectomy in Menière’s disease. Auris Nasus Larynx. 2018;45:1159–65. doi: 10.1016/j.anl.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Convert C, Franco-Vidal V, Bebear JP, Darrouzet V. Outcome-based assessment of endolymphatic sac decompression for Ménière’s disease using the Ménière’s disease outcome questionnaire: A review of 90 patients. Otol Neurotol. 2006;27:687–96. doi: 10.1097/01.mao.0000227661.52760.f1. [DOI] [PubMed] [Google Scholar]
- 5.Friberg U, Rask-Andersen H, Bagger-Sjöbäck D. Human endolymphatic duct. An ultrastructural study. Arch Otolaryngol. 1984;110:421–8. doi: 10.1001/archotol.1984.00800330003001. [DOI] [PubMed] [Google Scholar]
- 6.García MD, Segura CD, Lesser JC, Pianese CP. Endolymphatic sac surgery for Ménière’s disease-current opinion and literature review. Int Arch Otorhinolaryngol. 2017;21:179–83. doi: 10.1055/s-0037-1599276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang TS. Endolymphatic sac surgery for Meniere’s disease: Experience with over 3000 cases. Otolaryngol Clin North Am. 2002;35:591–606. doi: 10.1016/s0030-6665(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 8.Hultgård-Ekwall AK, Couloigner V, Rubin K, Rask-Andersen H. Network organization of interstitial connective tissue cells in the human endolymphatic duct. J Histochem Cytochem. 2003;51:1491–500. doi: 10.1177/002215540305101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hultgård-Ekwall AK, Mayerl C, Rubin K, Wick G, Rask-Andersen H. An interstitial network of podoplanin-expressing cells in the human endolymphatic duct. J Assoc Res Otolaryngol. 2006;7:38–47. doi: 10.1007/s10162-005-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaylie DM, Jackson CG, Gardner EK. Surgical management of Meniere’s disease in the era of gentamicin. Otolaryngol Head Neck Surg. 2005;132:443–50. doi: 10.1016/j.otohns.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1967;76:664–87. doi: 10.1177/000348946707600311. [DOI] [PubMed] [Google Scholar]
- 12.Lim MY, Zhang M, Yuen HW, Leong JL. Current evidence for endolymphatic sac surgery in the treatment of Meniere’s disease: A systematic review. Singapore Med J. 2015;56:593–8. doi: 10.11622/smedj.2015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linthicum FH, Doherty J, Webster P, Makarem A. The periductal channels of the endolymphatic duct, hydrodynamic implications. Otolaryngol Head Neck Surg. 2014;150:441. doi: 10.1177/0194599813516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo WW, Daniels DL, Chakeres DW, Linthicum FH, Ulmer JL, Mark LP, et al. The endolymphatic duct and sac. AJNR Am J Neuroradiol. 1997;18:881. [PMC free article] [PubMed] [Google Scholar]
- 15.Locke RR, Shaw-Dunn J, O’Reilly BF. Endolymphatic sac surgical anatomy and transmastoid decompression of the sac for the management of Ménière’s disease. J Laryngol Otol. 2014;128:488–93. doi: 10.1017/S0022215114001017. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 17.Monsanto RC, Pauna HF, Kwon G, Schachern PA, Tsuprun V, Paparella MM, et al. A three-dimensional analysis of the endolymph drainage system in Ménière disease. Laryngoscope. 2017;127:E170–5. doi: 10.1002/lary.26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevoux J, Barbara M, Dornhoffer J, Gibson W, Kitahara T, Darrouzet V. International consensus (ICON) on treatment of Ménière’s disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135:S29–32. doi: 10.1016/j.anorl.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Nola G, Mostardini C, Salvi V, Ercolani AP, Ralli G. Validity of Italian adaptation of the dizziness handicap inventory (DHI) and evaluation of the quality of life in patients with acute dizziness. Acta Otorhinolaryngol Ital. 2010;30:190–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Nordström CK, Li H, Ladak HM, Agrawal S, Rask-Andersen H. A micro-CT and synchrotron imaging study of the human endolymphatic duct with special reference to endolymph outflow and Meniere’s disease. Sci Rep. 2020;10:8295. doi: 10.1038/s41598-020-65110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez N, Martín E, García-Tapia R. Intratympanic gentamicin for intractable Meniere’s disease. Laryngoscope. 2003;113:456–64. doi: 10.1097/00005537-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Pullens B, Verschuur HP, Van Benthem PP. Surgery for Ménière’s disease. Cochrane Database Syst Rev. 2013;2013:CD005395. doi: 10.1002/14651858.CD005395.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rask-andersen H, Friberg U, Bagger-Sjöbäck D. The ultrastructure of the human endolymphatic duct. Acta Otolaryngol Suppl. 1984;406:61–6. doi: 10.3109/00016488309123005. [DOI] [PubMed] [Google Scholar]
- 24.Rauch SD, Oas JG. Intratympanic gentamicin for treatment of intractable Meniere’s disease: A preliminary report. Laryngoscope. 1997;107:49–55. doi: 10.1097/00005537-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Sajjadi H, Paparella MM, Williams T. Endolymphatic sac enhancement surgery in elderly patients with Meniere’s disease. Ear Nose Throat J. 1998;77:975–82. [PubMed] [Google Scholar]
- 26.Sennaroglu L, Sennaroglu G, Gursel B, Dini FM. Intratympanic dexamethasone, intratympanic gentamicin, and endolymphatic sac surgery for intractable vertigo in Meniere’s disease. Otolaryngol Head Neck Surg. 2001;125:537–43. doi: 10.1067/mhn.2001.119485. [DOI] [PubMed] [Google Scholar]
- 27.Sood AJ, Lambert PR, Nguyen SA, Meyer TA. Endolymphatic sac surgery for Ménière’s disease: A systematic review and meta-analysis. Otol Neurotol. 2014;35:1033–45. doi: 10.1097/MAO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen J, Bonding P, Becker B, Stage J, Tos M. The non-specific effect of endolymphatic sac surgery in treatment of Meniere’s disease: A prospective, randomized controlled study comparing “classic” endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane. Acta Otolaryngol. 1998;118:769–73. doi: 10.1080/00016489850182413. [DOI] [PubMed] [Google Scholar]
- 29.Yetişer S. Intratympanic Gentamicin for Intractable Ménière’s Disease-a review and analysis of audiovestibular impact. Int Arch Otorhinolaryngol. 2018;22:190–4. doi: 10.1055/s-0037-1604064. [DOI] [PMC free article] [PubMed] [Google Scholar]