Abstract
Background:
The awake craniotomy (AC) procedure allows for safe and maximal resection of brain tumors from highly eloquent regions. However, geriatric patients are often viewed as poor candidates for AC due to age and medical comorbidities. Frailty assessments gauge physiological reserve for surgery and are valuable tools for preoperative decision-making. Here, we present a novel case illustrating how frailty scoring enabled an elderly but otherwise healthy female to undergo successful AC for tumor resection.
Case Description:
A 92-year-old right-handed female with history of hypertension and basal cell skin cancer presented with a 1-month history of progressive aphasia and was found to have a ring-enhancing left frontoparietal mass abutting the rolandic cortex concerning for malignant neoplasm. Frailty scoring with the recalibrated risk analysis index (RAI-C) tool revealed a score of 30 (of 81) indicating low surgical risk. The patient and family were counseled appropriately that, despite advanced chronological age, a low frailty score predicts favorable surgical outcomes. The patient underwent left-sided AC for resection of tumor and experienced immediate improvement of speech intraoperatively. After surgery, the patient was neurologically intact and had an unremarkable postoperative course with significant improvements from preoperatively baseline at follow-up.
Conclusion:
To the best of our knowledge, this case represents the oldest patient to undergo successful AC for brain tumor resection. Nonfrail patients over 90 years of age with the proper indications may tolerate cranial surgery. Frailty scoring is a powerful tool for preoperative risk assessment in the geriatric neurosurgery population.
Keywords: Awake craniotomy, Frailty, Malignant tumor, Risk analysis index, Tumor resection

INTRODUCTION
Techniques utilized by neurosurgeons to maximize resection of brain tumors, while minimizing morbidity has improved significantly over time.[7,17] The awake craniotomy (AC) procedure is the gold standard for safe and maximal resection of intracranial lesions in supratentorial eloquent regions, with multiple studies demonstrating superior outcomes.[7,16,23] Geriatric patients are often denied AC and/or other aggressive brain tumor treatments due to concerns for poor outcomes related to chronological age.[4,13,22,27] However, with modern techniques and proper patient selection, there is evidence that AC for gross total resection or maximal safe resection is well-tolerated in elderly patients.[13] Frailty, a measure of physiological reserve, is an emerging tool in the preoperative risk assessment of patients with brain tumors and numerous other conditions.[3,8,19,24,28] There is rapidly accumulating evidence that frailty assessments are superior to chronological age alone for risk stratification in the preoperative setting.[9,10,29] The growing consensus that frailty should inform surgical decision-making prompted the design of novel scoring systems, such as the Risk Analysis Index (RAI).[14] The RAI was developed to measure preoperative frailty in surgical patients and has been successfully validated and shown to outperform prior indices for postoperative outcomes.[2,21] However, real-world examples integrating frailty tools into clinical practice in the geriatric brain tumor population are sparse. Here, we present a novel case example illustrating how frailty scoring enabled a 92-year-old healthy female to undergo and successfully recover from AC for resection of intracranial tumor.
CASE REPORT
History and examination
A 92-year-old right-handed female with a history of hypertension and basal cell skin cancer presented with a 1-month history of progressively worsening speech difficulties. The patient was brought to clinic by her daughter who first noticed the mild changes in sentence syntax. The patient is a retired schoolteacher who at baseline takes no medication, exercises daily, and lives independently. The progressive speech symptoms prompted medical workup including magnetic resonance imaging (MRI) of the brain and subsequent referral to our institution for neurosurgical evaluation. Symptoms at time of consultation included speech difficulties and intermittent right-hand numbness. Neurological examination was positive for expressive aphasia and otherwise unremarkable.
Preoperating neuroimaging
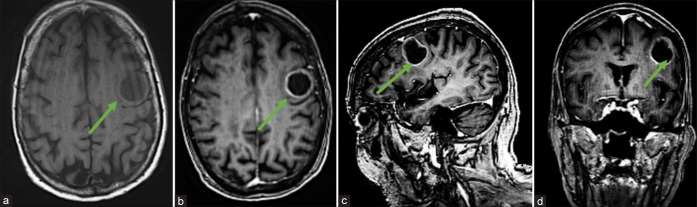
MRI brain with/without contrast revealed a T1 hypointense, rim-enhancing, and intra-axial mass centered in the left precentral gyrus concerning for a malignant neoplasm involving the eloquent rolandic cortex [Figure 1].
Figure 1:
Preoperative neuroimaging. (a) Axial magnetic resonance imaging (MRI)-brain T1 image showing hypointense left frontoparietal mass centered in the left precentral gyrus with Gadolinium-enhanced T1 MRI axial (b), sagittal (c), and coronal (d) images demonstrating rim-enhancement concerning for malignant intracranial neoplasm. Green arrows represent tumor.
Preoperative assessment and patient counseling
The clinical symptoms and imaging findings were strongly suggestive of intermediate to high grade tumor involving the eloquent motor cortex responsible for speech expression. AC would be offered as a primary treatment option given acceptable surgical risk. The risks associated with surgery in the patient’s age group were considered. However, frailty assessment as measured by the recalibrated RAI-C scoring system deemed her a low-risk surgical candidate.[2,14] The RAI-C is a 14-question questionnaire assessing 11 variables, including age, sex, several medical comorbidities, and ability to complete activities of daily living (ADL’s).[2] Answers for each question are assigned a score, with a minimum total score of 0 and maximum score of 81. Higher scores are associated with more severe frailty and associated morbidity/mortality. Given her excellent baseline functional status, low frailty score (RAI-C of 30), location of tumor, and risk of tumor-related progression, the patient was offered AC for resection of tumor. The patient and family elected to proceed with the operation after extensive discussion of risks/benefits/alternatives.
Operative details
A left-sided frontoparietal temporal AC was performed for resection of tumor with the use of the operative microscope and Medtronic Stealth neuronavigation. Awake speech mapping with intraoperative electrophysiology mapping was also performed with assistance from neuropsychology colleagues. Gross-total resection with achieved without any speech arrest. Speech improved intraoperatively on removal of the cystic portion of tumor.
Histopathological findings
Samples taken during tumor resection were sent to pathology, where microscopic examination of the tissue sections demonstrated a poorly differentiated population of cells with multiforme cytologic features and abundant atypical mitotic figures with vascular proliferation and necrosis, providing a histopathological diagnosis of the WHO Grade IV glioblastoma. Figure 2 depicts the photomicrographs of hematoxylin and eosin stained tissue sections.
Figure 2:
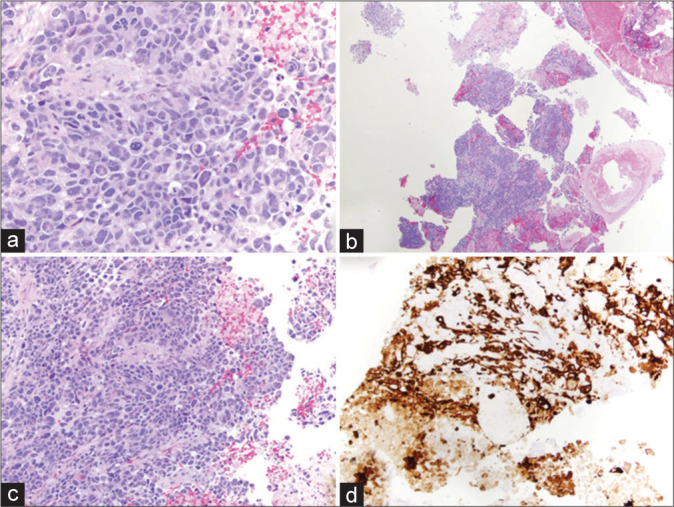
Histopathology of grade IV glioblastoma multiforme tumor. (a-c) Hematoxylin and eosin staining showing a poorly differentiated population of cells with multiforme cytologic features and some vaguely astrocytic features. There are abundant, often atypical mitotic figures, vascular proliferation, and necrosis. (d) Positive for GFAP immunoreactivity.
Postoperative imaging and course
Postoperatively, the patient was admitted to the neurosurgical ICU for routine monitoring. The patient was neurologically intact with exception of mild expressive aphasia that improved compared to preoperative assessment. The postoperative MRI with and without contrast was negative for acute complications and showed no residual mass [Figure 3]. The patient had an uncomplicated postoperative course resulting in discharge to acute rehab facility on postoperative day 3. At follow-up, the patient was doing well with continued improvement in aphasia.
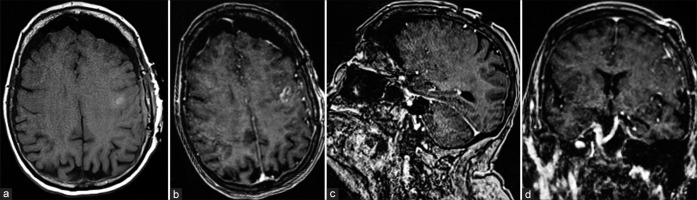
Figure 3:
Postoperative neuroimaging. MRI brain images, pre- (a) and post-contrast (b-d), showing no acute complications and no residual mass.
DISCUSSION
With current life expectancy trends, octogenarians are expected to triple globally by 2050.[12,20] The incidence of neurosurgical pathology, including brain tumors, increases with age, leading to a rapidly growing census of geriatric patients presenting for surgical consultation.[25] Correspondingly, there is a critical need for the development, validation, and adoption of easy-to-use preoperative risk assessment tools to select candidates for surgery.[10,25] The present case illustrates how the RAI-C was implemented during preoperative evaluation to select a 92-year-old patient for cranial surgery. To the best of our knowledge, this was the oldest reported patient to undergo successful AC for resection of malignant tumor with no postoperative complications. The eloquent region tumor was safely removed resulting in significant improvement of aphasia symptoms.
Based on traditional preoperative evaluations, our patient may have been refused elective AC due to advanced chronological age alone. Similar to chronological age, older comorbidity-based indices[6,11] used to quantify preoperative risk fail to directly measure frailty and a patient’s likelihood of successfully recovering from surgery (physiological reserve).[31] The present case experience emphasizes the importance of using high quality, validated frailty tools in the preoperative risk assessment process. Frailty and outcomes following craniotomy for brain tumor have been analyzed extensively. Multiple studies (single-center and administrative database) demonstrated that increasing frailty, as measured by a variety of assessments, is associated with increased risk of postoperative complications, adverse discharge disposition, readmission, and mortality in patients undergoing resection of brain tumors.[3,5,8,15,18,24] Dicpinigaitis et al. found that frailty status (measured by mFI-11), compared to chronological age, was a robust predictor of postoperative in a population of 13,650 patients who underwent metastatic brain tumor resection.[10] Despite the popularity of frailty in the literature, implementation in the clinical setting lags behind and underscores the importance of selecting a frailty tool that seamlessly integrates into clinical practice and predicts postoperative outcomes with exceptional accuracy.[14]
The RAI is a powerful frailty tool developed and validated to improve the selection of patients for surgery.[14] A recent quality improvement initiative at a large quaternary center demonstrated that the RAI frailty screening tool can be effectively implemented in healthcare systems including screening within surgical clinics.[30] The RAI is superior to other commonly utilized frailty tools, such as the modified frailty index-5 (mFI-5), for a number of reasons. The RAI accounts for functional dependence (ADL’s) and incorporates patient age, which is both not directly measured by the mFI-5. This was particularly important in our patient’s case given the exceptional degree of functional independence for a 92 years old. The RAI was validated for bedside use as a clinical survey (RAI-C)[26] and for administrative data (RAI-A), which is invaluable for future research efforts and quality improvement interventions.[2] However, as a newer frailty tool, the patient population specific RAI analyses are sparse. The RAI score was recently adopted to predict outcomes following spine surgery with superior discriminative ability for postoperative morbidity/mortality.[1] However, neurosurgery patient populations are heterogeneous and thus warrant pathology-specific analyses (e.g., malignant glioma, meningioma, and cranial trauma) for validation and, if indicated, recalibration of RAI.
Outcomes data in elderly patients undergoing AC are scarce. A single study by Grossman et al., in 2013, reported outcomes after AC for tumor resection in a series of 334 young (45.4 ± 13.2 years, mean ± SD) and 90 elderly (71.7 ± 5.1 years) patients.[13] They found that AC was safe and effective in elderly patients with no difference in rate of short-term complications or mortality compared to younger patients. However, “elderly” was defined as age ≥ 65 and the study did not specifically describe or discuss outcomes for octogenarians or nonagenarians. Schär et al., in 2020, conducted a prospective cohort study of 1452 consecutive elective craniotomies to assess the safety of the procedure in elderly patients (<65 years vs. ≥65–<75 years vs. ≥75 years).[25] Mortality rates were low in all age groups, but increasing age was associated with discharge to other hospitals and postacute care facilities.[25] Future studies are indicated to further analyze the safety and efficacy of AC in a larger cohort of patients.
CONCLUSION
The present case represents the first successful AC for tumor resection in a 92 years old. The case illustrates how robust frailty scoring tools can be integrated into the clinical workflow to select elderly but otherwise healthy patients for surgery. Nonfrail patients over 90 years of age with the proper indications may tolerate cranial surgery. Frailty scoring is a powerful tool for preoperative risk assessment in the geriatric population.
Footnotes
How to cite this article: Cole KL, Varela S, Rumalla K, Kazim SF, Rebbe RW, Carvajal M, et al. Advanced frailty assessment tool predicts successful awake craniotomy in a 92-year-old patient: A case report. Surg Neurol Int 2022;13:404.
Contributor Information
Kyril L. Cole, Email: kyril.cole@hsc.utah.edu.
Samantha Varela, Email: savarela@salud.unm.edu.
Kavelin Rumalla, Email: kavelinrumalla@gmail.com.
Syed Faraz Kazim, Email: skazim@salud.unm.edu.
Ryan W. Rebbe, Email: rrebbe@salud.UNM.edu.
Michael Carvajal, Email: mcarvajal@salud.unm.edu.
Karen S. SantaCruz, Email: ksantacruz@salud.unm.edu.
Rohini McKee, Email: rmckee@salud.unm.edu.
Cheryl Willman, Email: cwillman@salud.unm.edu.
Meic H. Schmidt, Email: mhschmidt@salud.unm.edu.
Christian A. Bowers, Email: cabowers@salud.unm.edu.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agarwal N, Goldschmidt E, Taylor T, Roy S, Dunn SC, Bilderback A, et al. Impact of frailty on outcomes following spine surgery: A prospective cohort analysis of 668 patients. Neurosurgery. 2021;88:552–7. doi: 10.1093/neuros/nyaa468. [DOI] [PubMed] [Google Scholar]
- 2.Arya S, Varley P, Youk A, Borrebach JD, Perez S, Massarweh NN, et al. Recalibration and external validation of the risk analysis index: A surgical frailty assessment tool. Ann Surg. 2020;272:996–1005. doi: 10.1097/SLA.0000000000003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonney PA, Chartrain AG, Briggs RG, Jarvis CA, Ding L, Mack WJ, et al. Frailty is associated with in-hospital morbidity and nonroutine disposition in brain tumor patients undergoing craniotomy. World Neurosurg. 2021;146:e1045–53. doi: 10.1016/j.wneu.2020.11.083. [DOI] [PubMed] [Google Scholar]
- 4.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–9. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloney M, D’Amico R, Lebovic J, Nazarian M, Zacharia BE, Sisti MB, et al. Frailty in geriatric glioblastoma patients: A predictor of operative morbidity and outcome. World Neurosurg. 2016;89:362–7. doi: 10.1016/j.wneu.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 6.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: The Charlson comorbidity index. Methods Inf Med. 1993;32:382–7. [PubMed] [Google Scholar]
- 7.De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010;66:1074–84. doi: 10.1227/01.NEU.0000369514.74284.78. discussion 1084. [DOI] [PubMed] [Google Scholar]
- 8.Dicpinigaitis AJ, Hanft S, Cooper JB, Gandhi CD, Kazim SF, Schmidt MH, et al. Comparative associations of baseline frailty status and age with postoperative mortality and duration of hospital stay following metastatic brain tumor resection. Clin Exp Metastasis. 2022;39:303–10. doi: 10.1007/s10585-021-10138-3. [DOI] [PubMed] [Google Scholar]
- 9.Dicpinigaitis AJ, Kalakoti P, Schmidt M, Gurgel R, Cole C, Carlson A, et al. Associations of baseline frailty status and age with outcomes in patients undergoing vestibular schwannoma resection. JAMA Otolaryngol Head Neck Surg. 2021;147:608–14. doi: 10.1001/jamaoto.2021.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicpinigaitis AJ, Kazim SF, Schmidt MH, Couldwell WT, Theriault BC, Gandhi CD, et al. Association of baseline frailty status and age with postoperative morbidity and mortality following intracranial meningioma resection. J Neurooncol. 2021;155:45–52. doi: 10.1007/s11060-021-03841-4. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–7. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman R, Nossek E, Sitt R, Hayat D, Shahar T, Barzilai O, et al. Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann Surg Oncol. 2013;20:1722–8. doi: 10.1245/s10434-012-2748-x. [DOI] [PubMed] [Google Scholar]
- 14.Hall DE, Arya S, Schmid KK, Blaser C, Carlson MA, Bailey TL, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152:175–82. doi: 10.1001/jamasurg.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harland TA, Wang M, Gunaydin D, Fringuello A, Freeman J, Hosokawa PW, et al. Frailty as a predictor of neurosurgical outcomes in brain tumor patients. World Neurosurg. 2020;133:e813–8. doi: 10.1016/j.wneu.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low-and high-grade glioma. J Neurooncol. 2016;130:269–82. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 17.Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L, et al. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J Neurosurg. 2015;123:325–39. doi: 10.3171/2014.10.JNS141520. [DOI] [PubMed] [Google Scholar]
- 18.Huq S, Khalafallah AM, Jimenez AE, Gami A, Lam S, RuizCardozo MA, et al. Predicting postoperative outcomes in brain tumor patients with a 5-factor modified frailty index. Neurosurgery. 2021;88:147–54. doi: 10.1093/neuros/nyaa335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huq S, Khalafallah AM, Jimenez AE, Gami A, Lam S, RuizCardozo MA, et al. Predicting postoperative outcomes in brain tumor patients with a 5-factor modified frailty index. Neurosurgery. 2020;88:147–54. doi: 10.1093/neuros/nyaa335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–9. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 21.McIsaac DI, Aucoin SD, van Walraven C. A Bayesian comparison of frailty instruments in noncardiac surgery: A cohort study. Anesth Analg. 2021;133:366–73. doi: 10.1213/ANE.0000000000005290. [DOI] [PubMed] [Google Scholar]
- 22.Patil CG, Veeravagu A, Lad SP, Boakye M. Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry. 2010;81:502–5. doi: 10.1136/jnnp.2009.185074. [DOI] [PubMed] [Google Scholar]
- 23.Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011;68:1192–8. doi: 10.1227/NEU.0b013e31820c02a3. discussion 1198-9. [DOI] [PubMed] [Google Scholar]
- 24.Sastry RA, Pertsch NJ, Tang O, Shao B, Toms SA, Weil RJ. Frailty and outcomes after craniotomy for brain tumor. J Clin Neurosci. 2020;81:95–100. doi: 10.1016/j.jocn.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Schär RT, Tashi S, Branca M, Söll N, Cipriani D, Schwarz C, et al. How safe are elective craniotomies in elderly patients in neurosurgery today? A prospective cohort study of 1452, consecutive cases. J Neurosurg. 2020;134:1113–21. doi: 10.3171/2020.2.JNS193460. [DOI] [PubMed] [Google Scholar]
- 26.Shah R, Borrebach JD, Hodges JC, Varley PR, Wisniewski MK, Shinall MC, Jr, et al. Validation of the risk analysis index for evaluating frailty in ambulatory patients. J Am Geriatr Soc. 2020;68:1818–24. doi: 10.1111/jgs.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana S, Omote M, Yamakage M. Successful awake craniotomy in an aged patient with a severe hearing impairment using a bone conduction voice amplifier: A case report. JA Clin Rep. 2019;5:37. doi: 10.1186/s40981-019-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theriault BC, Pazniokas J, Adkoli AS, Cho EK, Rao N, Schmidt M, et al. Frailty predicts worse outcomes after intracranial meningioma surgery irrespective of existing prognostic factors. Neurosurgical Focus. 2020;49:E16. doi: 10.3171/2020.7.FOCUS20324. [DOI] [PubMed] [Google Scholar]
- 29.Thommen R, Kazim SF, Cole KL, Olson GT, Shama L, Lovato CM, et al. Worse pituitary adenoma surgical outcomes predicted by increasing frailty, not age. World Neurosurg. 2022;161:e347–54. doi: 10.1016/j.wneu.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Varley PR, Borrebach JD, Arya S, Massarweh NN, Bilderback AL, Wisniewski MK, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274:e1230–7. doi: 10.1097/SLA.0000000000003808. [DOI] [PubMed] [Google Scholar]
- 31.Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE, et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019;67:1559–64. doi: 10.1111/jgs.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]