Abstract
In mice administered Lactobacillus casei strain Shirota (LcS) intranasally, potent induction of interleukin 12, gamma interferon, and tumor necrosis factor alpha, which play a very important role in excluding influenza virus (IFV), was evident in mediastinal lymph node cells. In this model of upper respiratory IFV infection, the titers of virus in the nasal wash of mice inoculated with 200 μg of LcS for three consecutive days (LcS 200 group) before infection were significantly (P < 0.01) lower than those of mice not inoculated with LcS (control group) (100.9 ± 0.6 versus 102.1 ± 1.0). The IFV titer was decreased to about 1/10 of the control level. Using this infection model with modifications, we investigated whether the survival rate of mice was increased by intranasal administration of LcS. The survival rate of the mice in the LcS 200 group was significantly (P < 0.05) greater than that of the mice in the control group (69% versus 15%). It seems that the decrease in the titer of virus in the upper respiratory tract to 1/10 of the control level was important in preventing death. These findings suggest that intranasal administration of LcS enhances cellular immunity in the respiratory tract and protects against influenza virus infection.
Influenza virus (IFV) infections continue to be a significant public health problem for which improved therapies and preventive treatments are urgently needed. Some of the therapeutic approaches used thus far have included antiviral compounds (9, 23), vaccines (10), and biological response modifiers (2, 8, 21). In recent years, there has been an increased tendency in modern medicine to apply preventive treatments involving the use of probiotics, such as Lactobacillus or Bifidobacterium.
A probiotic is defined by Havenaar (7) as a monoculture or mixed culture of living microorganisms that is applied to animals or man, beneficially affecting the host by improving the properties of the indigenous gastrointestinal microflora. It is restricted to products that contain living microorganisms, improve the health and well-being of man or animals, and can have an effect on all host mucosal surfaces, including the mouth, gastrointestinal tract (e.g., applied in food), upper respiratory tract (e.g., applied as an aerosol), or urogenital tract (local application). However, recently the definition of a probiotic has become even broader, and it has been concluded that dead bacteria and metabolic products with immunostimulatory activity or activity in prevention of infection by pathogens should be included in this category. Lactic acid bacteria and their products have been reported to have beneficial effects on host homeostasis and are effective in activating the immune system (4, 5).
Lactobacillus casei strain Shirota (LcS), a member of the lactic acid bacteria, was originally isolated from the human intestine and has been used commercially for a long time to produce fermented milk. Various aspects of the effects of LcS have been studied intensively. LcS exhibits remarkable activity against syngeneic and allogeneic tumors (12, 16) and against various pathogens, such as Pseudomonas aeruginosa and Listeria monocytogenes (18, 20). However, whether LcS exhibits activity against IFV has not been reported.
The purpose of the present study was to investigate the effects of intranasal administration of LcS on activation of the immune system in the respiratory tract and on IFV infection of the upper respiratory tract. Special attention was focused on the possibility of inhibiting IFV infection through intranasal administration of LcS.
MATERIALS AND METHODS
Mice.
BALB/c female mice, 10 and 11 weeks old, were obtained from Japan SLC, Inc. (Hamamatsu-shi, Japan), and used for the experiments.
LcS.
LcS was originally isolated from human feces at Yakult Central Institute for Microbiological Research (Tokyo, Japan). LcS cells were cultured for 24 h at 37°C in MRS medium (Difco Laboratories, Detroit, Mich.), collected by centrifugation, and washed several times with sterile distilled water. The LcS cells were killed by heating at 100°C for 30 min and lyophilized. The lyophilized preparation was suspended in saline and autoclaved (121°C, 20 min) before intranasal administration to mice. Mice received intranasal administration three times, i.e., 20 μl of LcS solution at a concentration of 0, 1, or 10 mg/ml (0, 20, or 200 μg per mouse) once daily for three consecutive days.
Virus.
Influenza A/PR/8/34 (PR8, H1N1) virus was grown in the allantoic sacs of 11-day-old chicken embryos at 34°C for 2 days by the method of Yasui et al. (27). The allantoic fluid was removed and stored at −80°C. The titer of virus in the allantoic fluid was expressed as the 50% egg-infecting dose (EID50) (26). Serial 10-fold dilutions of the allantoic fluid were injected into embryonated eggs, and the presence of virus in the allantoic fluid of each egg was determined on the basis of the hemagglutinating capacity 2 days after injection. The titer of virus was 109.2 EID50/ml.
PR8 was purified from the allantoic fluid from 11-day-old embryonated eggs. The details of the procedure for purification of PR8 have been described previously (27).
Mediastinal lymph node cell culture.
On the next day after completion of intranasal administration of the LcS solution at a concentration of 0 (control group), 1 (LcS 20 group), or 10 (LcS 200 group) mg/ml for three consecutive days, the mice were anesthetized with diethylether and killed by exsanguination. Mediastinal lymph nodes (MLNs) were aseptically removed, and single-cell suspensions were prepared after depletion of erythrocytes. In experiment 1, 2.5 × 105 cells were cultured in the absence or presence of concanavalin A (ConA) (Sigma) at 2 μg/ml in 0.1 ml of RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a 96-half-area-well culture plate (Corning, Corning, N.Y.). In experiment 2, 2.5 × 105 cells were cultured in the absence or presence of purified PR8 at doses ranging from 0.2 to 20 μg of protein/ml in 0.1 ml of RPMI containing 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a 96-half-area-well culture plate. Supernatants were collected on day 3 for determination of cytokine concentrations and stored at −80°C for further analysis.
ELISA for determination of cytokine concentrations.
Gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 4 (IL-4), and IL-12 concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA). IFN-γ, TNF-α, and IL-4 concentrations were measured using DuoSet mouse IFN-γ, TNF-α, and IL-4 ELISA kits (Genzyme), respectively, according to the recommended protocol. For measurement of IL-12 concentrations, rat anti-mouse IL-12 monoclonal antibody (C 15.6; Pharmingen) was used as the capture antibody and biotinylated rat anti-mouse IL-12 monoclonal antibody (clone C17.8; Pharmingen) was used as the detection antibody. Standard recombinant mouse IL-12 was purchased from Pharmingen.
Infection.
On the next day after completion of intranasal administration of LcS solution at a concentration of 0, 1 or 10 mg/ml for three consecutive days, mice were anesthetized by intraperitoneal injection of sodium amobarbital (0.25 μg per mouse) (27), and then the mice were infected by dropping 1 μl of fluid containing 103.5 EID50 of PR8 into each nostril (2 μl per mouse). Three days after inoculation, the titers of virus in the nasal wash were measured by the method of Tamura et al. (24). Thereafter, the mice were anesthetized with diethylether and killed by exsanguination. The head of each mouse was removed, and the lower jaw was cut off. A needle attached to a syringe was inserted into the posterior opening of the nasopharynx, and a total of 1 ml of phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin was injected. This procedure was repeated three times, and the outflow was collected each time as nasal wash. The nasal wash was centrifuged at 13,000 × g to remove cellular debris and used for virus titration. The titer of virus in each experimental group is expressed as the mean ± the standard deviation (SD) of the viral titer of each wash specimen from all mice in each group.
To examine the survival of mice inoculated with PR8 solution, PBS was administered intranasally 3 days after inoculation of the mice with PR8. After nasal inoculation with PR8, the mice were monitored for 14 days to assess the survival rate.
Statistical analyses.
Cytokine concentrations were compared by means of Student's t test and Dunnett's test. The differences in titers of virus in the nasal wash were examined by means of Dunnett's test. The differences in survival rates were examined by means of Fisher's exact test. Probability values of less than 5% were considered significant.
RESULTS
Effect of intranasal administration of LcS on production of various cytokines by MLN cells.
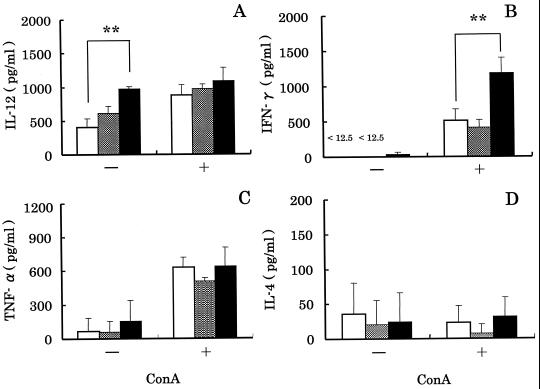
In experiment 1, we investigated the effect of intranasal administration of LcS on production of various cytokines by MLN cells (Fig. 1). When the cells were cultured in the absence of ConA, no significant differences in the concentrations of IFN-γ, TNF-α, or IL-4 (Fig. 1B, C, and D) were observed, but the concentrations of IL-12 were significantly (P < 0.01) different (Fig. 1A). The IL-12 concentrations in the case of the LcS 200 group and the control group were 970 ± 35 and 400 ± 130 pg/ml, respectively. The mean IL-12 concentration in the case of the LcS 200 group was about 2.5 times higher than that for the control group.
FIG. 1.
Effect of intranasal administration of LcS to mice on production of IL-12, IFN-γ, TNF-α and IL-4 by MLN cells. MLN cells from mice administered LcS 0 (control) (□), LcS 20 ( ), or LcS 200 (■) intranasally were cultured in the absence (−) or presence (+) of ConA. Supernatants were collected 72 h after the initiation of culture, and the concentrations of various cytokines were determined. Each bar represents the mean ± SD of triplicate samples. Double asterisk, statistically significant difference from the control by Dunnett's test at P < 0.01.
), or LcS 200 (■) intranasally were cultured in the absence (−) or presence (+) of ConA. Supernatants were collected 72 h after the initiation of culture, and the concentrations of various cytokines were determined. Each bar represents the mean ± SD of triplicate samples. Double asterisk, statistically significant difference from the control by Dunnett's test at P < 0.01.
In the next study, we measured production of various cytokines by MLN cells stimulated with ConA, which was T-cell mitogen, to prove the activation of T cells by intranasal administration of LcS (Fig. 1). The concentrations of IL-12, TNF-α, and IL-4 were not significantly different among the three groups (Fig. 1A, C, and D). However, the IFN-γ concentration in the case of the LcS 200 group was significantly (P < 0.01) different from that for the control group (Fig. 1B). The IFN-γ concentrations in the case of the LcS 200 and control groups were 1,180 ± 220 and 510 ± 160 pg/ml, respectively. The concentrations of IL-12, IFN-γ, TNF-α, and IL-4 in the case of the LcS 20 group were not different from those for the control group.
Effect of intranasal administration of LcS on production of various cytokines by MLN cells cultured in the presence of PR8.
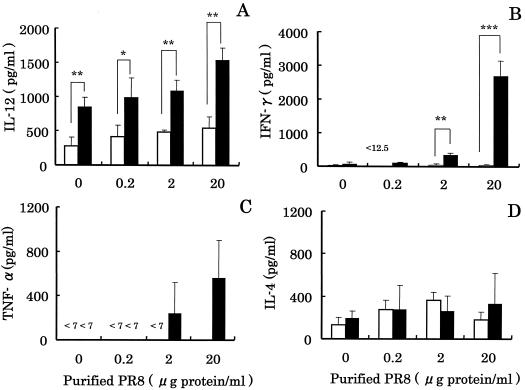
In experiment 2, we investigated the effect of intranasal administration of LcS on production of various cytokines by MLN cells stimulated with purified PR8 (Fig. 2). The most effective dose of purified PR8 was 20 μg of protein/ml. The IL-12 concentration in the case of the LcS 200 group was about 3 times higher than that for the control group, and the difference was statistically significant (P < 0.01). The IFN-γ concentrations for the control and LcS 200 groups were 34.0 ± 20 and 2,670 ± 450 pg/ml, respectively (Fig. 2B), and the difference was statistically significant (P < 0.001). The TNF-α concentration in the case of the LcS 200 group was more than 80 times higher than that for the control group (Fig. 2C). There was no difference in IL-4 production between the control and LcS 200 groups (Fig. 2D).
FIG. 2.
Effect of intranasal administration of LcS to mice on production of IL-12, IFN-γ, TNF-α, and IL-4 by MLN cells stimulated with purified PR8. MLN cells from mice administered LcS 0 (control) (□) or LcS 200 (■) intranasally were cultured in the absence (0 μg/ml) or presence of purified PR8 (0.2 to ∼20 μg of protein/ml). Collection of supernatants and determination of various cytokines were performed in the same manner as described in the legend to Fig. 1. Each bar represents the mean ± SD of triplicate samples. Single, double, and triple asterisk, statistically significant difference from the control by Student's t test at P < 0.05, P < 0.01, and P < 0.001, respectively.
Effect of intranasal administration of LcS on the titer of virus in the nasal wash of mice inoculated with PR8.
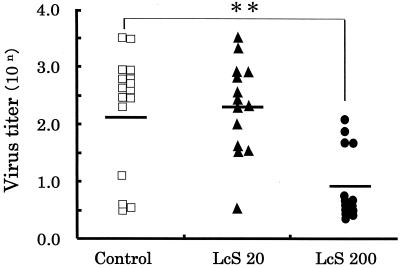
As shown in Fig. 3, the titers of virus for the control, LcS 20, and LcS 200 groups were 102.1 ± 1.0, 102.3 ± 0.8 and 100.9 ± 0.6, respectively. There was no difference in titers of virus between the LcS 20 and control groups, but the titer of virus in the case of the LcS 200 group was significantly (P < 0.01) lower than that for the control group.
FIG. 3.
Effect of intranasal administration of LcS on the titer of virus in nasal washes. Mice were administered 20 μl of LcS solution at the concentration of 0 (control) (□), 1 (LcS 20) (▴), or 10 (LcS 200) (●) mg/ml intranasally for three consecutive days. One day thereafter, the mice were intranasally infected with PR8, and 3 days later the titers of virus in the nasal washes from the infected mice were measured. Each bar represents the mean of values for 14 mice. Double asterisk, statistically significant difference from values for the control by Dunnett's test at P < 0.01.
Effect of intranasal administration of LcS on survival rate of mice inoculated with PR8.
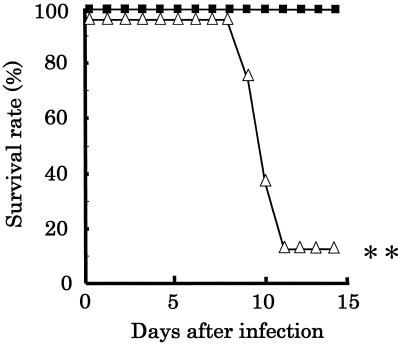
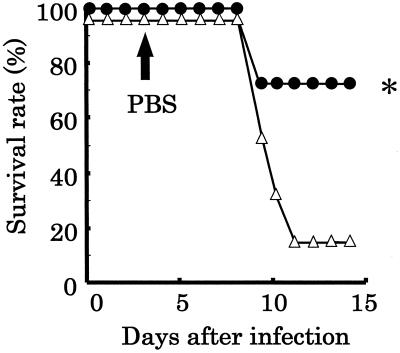
We investigated whether intranasal administration of PBS led to deaths of the PR8-infected mice. Mice were infected in the upper respiratory tract with 2 μl of inocula containing 103.5 EID50 of PR8. After 3 days, the mice were administered 20 μl of PBS intranasally to disseminate the increased virus from the nasal cavity to the lower respiratory tract (PBS-treated group). Infected mice not administered PBS served as controls. The survival rate of the mice in the control group was 100%. On the other hand, the survival rate of the mice in the PBS-treated group was 13% (Fig. 4). It was found that the survival rate of the mice in the PBS-treated group was significantly (P < 0.01) lower than that of the mice in the control group.
FIG. 4.
Survival of mice infected with PR8 in the upper respiratory tract. Mice were inoculated with 2 μl of fluid containing 103.5 EID50 of PR8, and PBS was administered intranasally at 3 days postinfection (n = 8) (▵). Infected mice not administered PBS intranasally served as controls (n = 6) (■). After nasal inoculation with PR8, the mice were observed for 14 days to assess the survival rates. Double asterisk, statistically significant difference from the control by Fisher's exact test at P < 0.01.
In the next study, we investigated whether intranasal administration of LcS affects the survival rate of mice infected with PR8, using the above-described PBS-treated model. The survival rates of the mice in the control and LcS 200 groups were 15 and 69%, respectively (Fig. 5), and the difference was statistically significant (P < 0.05). It was found that intranasal administration of LcS protected against death due to IFV infection.
FIG. 5.
Protection against mortality due to IFV infection in mice administered LcS intranasally. Mice administered LcS (●) (n = 13) intranasally and mice not administered LcS (▵) (n = 13) were inoculated with 2 μl of fluid containing 103.5 EID50 of PR8, and PBS was administered intranasally at 3 days postinfection. Assessment of the survival was performed in the same manner as described in the legend to Fig. 4. Asterisk, statistically significant difference from the control by Fisher's exact test at P < 0.05.
DISCUSSION
Recently it was reported that intranasal administration of Lactobacillus fermentum is effective in augmenting the respiratory immune system, especially through activation of macrophages (3). It was previously reported that LcS activates macrophages (18), NK cells (13), and cytotoxic T cells (14) and that activation of these cells contributes to antitumor and antibacterial activities. However, whether intranasal administration of LcS augments the respiratory immune system has not been reported. Therefore, we investigated this possibility and further examined whether antiviral activity was exhibited against IFV in mice.
It has been reported that MLN cells in the respiratory tract play an important role in preventing IFV infection (1, 6). Therefore, we investigated the production of various cytokines by MLN cells in mice administered LcS intranasally. It was found that the LcS administered intranasally strongly induced production of IL-12 in these cells. IL-12 is an important cytokine for cellular immunity. IL-12 potently stimulates cytotoxic T cells and NK cells and enhances the production of Th1 cytokines and the proliferation of Th1 cells. Shida et al. reported that LcS induces IL-12 production in splenocytes cultured in vitro (22). IL-12 is secreted by B cells, dendritic cells, and macrophages. Kato et al. reported that LcS activates X-ray-irradiated splenocytes, probably macrophages, and that these cells secreted IL-12 (15). It has been found in analysis of MLN cells from mice administered LcS intranasally that production not only of IL-12 but also of IFN-γ and TNF-α is induced upon addition of purified PR8 to the culture. These results obtained in vitro are very interesting, because they resemble the cytokine response observed in cases of IFV infection in vivo. IL-12, IFN-γ, and TNF-α are thought to play a very important role in preventing IFV infection. IFN-γ is produced by T cells, NK cells, and macrophages, and it has pleiotropic effects on various cells of the immune system. TNF-α is produced mainly by macrophages, and it has multiple effects of importance in terms of inflammation and immune functions. It has been reported that IL-12, IFN-γ, and TNF-α inhibit virus replication and are associated with protection against viral infection (11, 19, 25). In further studies, we are about to investigate whether these cytokines are produced in respiratory sites in mice administered LcS and infected with IFV and whether they inhibit IFV replication.
In elderly humans and in infants with low-level immune functions, IFV replicates in the upper respiratory tract and easily moves to the lower respiratory tract, and it frequently causes pneumonia. We tried to establish a murine model of IFV infection in which virus moves from the upper respiratory tract to the lower respiratory tract, and we used this model to study the effect of LcS. When mice were administered PBS intranasally 3 days after intranasal inoculation with IFV, the mortality rate for the mice was increased. Administration of PBS into the respiratory tract apparently enhanced viral spread to the lower respiratory tract and increased pulmonary damage, and as a result, it increased the mortality rate. Intranasal administration of LcS before inoculation of the mice with IFV resulted in a decrease in the mortality rate of the mice infected with IFV in the upper respiratory tract. A decrease in the titer of IFV in the upper respiratory tract is a very important phenomenon. In the mice administered LcS, the titer of IFV decreased to 1/10 of that in the control group, and this was a key point in preventing death of the host. It seems that intranasal administration of LcS augmented cellular immunity in the respiratory tract and decreased the titer of virus in the nasal wash, and as a result, the survival rate of the mice was increased.
Cangemi de Gutierrez et al. have reported that intranasal administration of L. fermentum activates macrophages in the respiratory tract of mice (3). No other reports on protection against respiratory infection by intranasal administration of probiotics have yet been published. In this paper, we demonstrate that intranasal administration of LcS activated cellular immunity in the respiratory tract and protected against IFV infection.
Since LcS is used to produce fermented milk and is safe, it may be useful to apply LcS as an aerosol or spray to prevent respiratory infections. In a preliminary experiment, polysaccharide-peptidoglycan, one component of LcS that was reported to possess antitumor activity (17), was found to be protective against IFV infection (unpublished data). It seems possible that the active constituents of LcS may include polysaccharide-peptidoglycan.
We have been studying two strains of lactic acid bacteria which modulated the different immune systems each in its own way (28). One strain was Bifidobacterium breve YIT 4064, which enhanced humoral immunity. We have previously reported that oral administration of this strain augmented production of antigen-specific immunoglobulin G in the serum and protected against IFV infection in mice (27). The other strain was LcS, which enhanced cellular immunity, inhibited the growth of tumors, and protected against bacterial infection. In this study, we examined whether intranasal administration of LcS activated cellular immunity in the respiratory immune system and protected against IFV infection. We are now studying whether oral administration of LcS activates the cellular immune system in the respiratory tract and whether this leads to effective protection against IFV infection.
ACKNOWLEDGMENTS
We thank S. Tamura, H. Asanuma, and T. Kurata (National Institute of Health, Tokyo, Japan) for help with the study and M. Watanuki for helpful discussions.
REFERENCES
- 1.Allan W, Tabi Z, Cleary A, Doherty P C. Cellular events in the lymph node and lung of mice with influenza. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 2.Bertagnolli M M, Lin B Y, Young D, Herrmann S H. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992;149:3778–3783. [PubMed] [Google Scholar]
- 3.Cangemi de Gutierrez R, Santos de Araoz V, Nader-Macias M E. Effect of intranasal administration of Lactobacillus fermentum on the respiratory tract of mice. Biol Pharm Bull. 2000;23:973–978. doi: 10.1248/bpb.23.973. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes C F, Shahani K M. Anticarcinogenic and immunological properties of dietary lactobacilli. J Food Prot. 1990;53:704–710. doi: 10.4315/0362-028X-53.8.704. [DOI] [PubMed] [Google Scholar]
- 5.Gilliland S E. Acidophilus milk products: a review of potential benefits to consumers. J Dairy Sci. 1989;72:2483–2494. doi: 10.3168/jds.S0022-0302(89)79389-9. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havenaar R, Brinck T, Huis I'nt Veld J H. Selection of strains for probiotic use. In: Fuller R, editor. Probiotics: the scientific basis. London, England: Chapman & Hall; 1992. pp. 209–224. [Google Scholar]
- 8.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 9.Herrmann J E, Bruns M, West K, Ennis F A. Efficacy of rimantadine hydrochloride in the treatment of influenza infection of mice. Antivir Res. 1989;11:127–135. doi: 10.1016/0166-3542(89)90024-7. [DOI] [PubMed] [Google Scholar]
- 10.Iinuma H, Nerome K, Yoshioka Y, Okinaga K. Characteristics of cytotoxic T lymphocytes directed to influenza-virus hemagglutinin elicited by immunization with muramyldipeptide-influenza liposome vaccine. Scand J Immunol. 1995;41:1–10. doi: 10.1111/j.1365-3083.1995.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 11.Karupiah G, Xie Q, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 12.Kato I, Kobayashi S, Yokokura T, Mutai M. Antitumor activity of Lactobacillus casei in mice. Gann. 1981;72:517–523. [PubMed] [Google Scholar]
- 13.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;27:209–217. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato I, Yokokura T, Mutai M. Correlation between increase in Ia-bearing macrophages and induction of T-cell-dependent anti-tumor activity by Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26:215–221. doi: 10.1007/BF00199932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato I, Tanaka K, Yokokura T. Lactic acids bacterium potently induces the production of interleukin-12 and interferon-γ by mouse splenocytes. Int J Immunopharmacol. 1999;21:121–131. doi: 10.1016/s0192-0561(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki T, Yokokura T, Mutai M. Antitumor effect of intrapleural administration of Lactobacillus casei in mice. Infect, Immun. 1988;48:209–214. doi: 10.1007/BF00199931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaki T, Nagaoka M, Nomoto K, Yokokura T. Antitumor effect of polysaccharide-peptideglycan complex (PS-PG) of Lactobacillus casei YIT 9018 on Meth A. Jpn Pharmacol Ther. 1990;18:51–57. [Google Scholar]
- 18.Miake S, Yokokura T, Yoshikai Y, Mutai M, Nomoto K. Protective effect of Lactobacillus casei on Pseudomonas aeruginosa infection in mice. Infect Immun. 1985;48:480–485. doi: 10.1128/iai.48.2.480-485.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteiro J M, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol. 1998;72:4825–4831. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomoto K, Miake S, Hashimoto S, Yokokura T, Mutai M, Yoshikai Y, Nomoto K. Augmentation of host resistance to Listeria monocytogenes infection by Lactobacillus casei. J Clin Lab Immunol. 1985;17:91–97. [PubMed] [Google Scholar]
- 21.Sarawar S R, Sangster M, Coffman R L, Doherty P C. Administration of anti-IFN-gamma antibody to beta 2-microglobulin-deficient mice delays influenza virus clearance but does not switch the response to a T helper cell 2 phenotype. J Immunol. 1994;153:1246–1253. [PubMed] [Google Scholar]
- 22.Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Amitani A, Sato T, Kumagai Y, Habu S, Kaminogawa S. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–287. doi: 10.1159/000069458. [DOI] [PubMed] [Google Scholar]
- 23.Sidwell R W, Huffman J H, Call E W, Alaghamandan H, Cook P D, Robins R K. Effect of selenazofurin on influenza A and B virus infections of mice. Antivir Res. 1986;6:343–353. doi: 10.1016/0166-3542(86)90016-1. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 25.VanCampen H. Influenza A virus replication is inhibited by tumor necrosis factor-α in vitro. Arch Virol. 1994;136:439–446. doi: 10.1007/BF01321073. [DOI] [PubMed] [Google Scholar]
- 26.Webster R G, Askonas B A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980;10:396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 27.Yasui H, Kiyoshima J, Hori T, Shida K. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT 4064. Clin Diagn Lab Immunol. 1999;6:186–192. doi: 10.1128/cdli.6.2.186-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasui H, Shida K, Matsuzaki T, Yokokura T. Immunomodulatory function of lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:383–389. [PubMed] [Google Scholar]