Abstract
Multidrug resistance (MDR) among pathogens and the associated infections represent an escalating global public health problem that translates into raised mortality and healthcare costs. MDR bacteria, with both intrinsic abilities to resist antibiotics treatments and capabilities to transmit genetic material coding for further resistance to other bacteria, dramatically decrease the number of available effective antibiotics, especially in nosocomial environments. Moreover, the capability of several bacterial species to form biofilms (BFs) is an added alarming mechanism through which resistance develops. BF, made of bacterial communities organized and incorporated into an extracellular polymeric matrix, self-produced by bacteria, provides protection from the antibiotics’ action, resulting in the antibiotic being ineffective. By adhering to living or abiotic surfaces present both in the environment and in the healthcare setting, BF causes the onset of difficult-to-eradicate infections, since it is difficult to prevent its formation and even more difficult to promote its disintegration. Inspired by natural antimicrobial peptides (NAMPs) acting as membrane disruptors, with a low tendency to develop resistance and demonstrated antibiofilm potentialities, cationic polymers and dendrimers, with similar or even higher potency than NAMPs and with low toxicity, have been developed, some of which have shown in vitro antibiofilm activity. Here, aiming to incite further development of new antibacterial agents capable of inhibiting BF formation and dispersing mature BF, we review all dendrimers developed to this end in the last fifteen years. The extension of the knowledge about these still little-explored materials could be a successful approach to find effective weapons for treating chronic infections and biomaterial-associated infections (BAIs) sustained by BF-producing MDR bacteria.
Keywords: multidrug resistance, bacterial BFs, fungi BFs, P. aeruginosa BFs, cationic antimicrobial agents, cationic dendrimers, antibiofilm agents
1. Introduction to Microbial Resistance
The incidence of microbial infections has expanded dramatically, mainly due to the increasing occurrence of resistance among diverse strains of bacteria. The resistance to drugs is described as the tolerance or insensitivity of a microbe to an antimicrobial drug despite earlier susceptibility to it [1,2]. Concerning drug resistance (DR), different definitions are used in the medical literature to characterize the different patterns of resistance found in healthcare-associated, antimicrobial-resistant bacteria. Particularly, bacteria can be multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) [3]. So, MDR bacteria are those that have acquired non-susceptibility to at least one antibiotic in three or more antimicrobial categories, XDR bacteria are defined as non-susceptible to at least one antibiotic in all but two or fewer antimicrobial categories, and PDR bacteria are those non-susceptible to all agents in all antimicrobial categories [3].
Resistant bacteria, fungi, viruses, and parasites are able to combat the attack of available drugs, which are no longer effective, thus resulting in the persistence and spreading of chronic infections. The development of MDR is a natural phenomenon; however, excessive use of antibiotics, among humans, animals, and plants, incessantly supports its expansion [4]. Additionally, the widespread increase in immunocompromised individuals, such as patients affected by HIV infection, diabetic patients, persons who have experienced organ transplantation, and severe burn patients, makes the human body an easy target for hospital-acquired infectious (HAIs), thus contributing to further spread of MDR pathogens. From studies on WHO reports concerning the rates of resistance in bacteria, such as Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, Nontyphoidal Salmonella, Shigella species, Neisseria gonorrhoeae, and Mycobacterium tuberculosis, several fungi, viruses, and parasites, against several different classes of antibiotics, it has emerged that they are responsible for severe infections such as urinary tract infections (UTIs), pneumonia, and bloodstream infections (BSIs) [5,6]. The most common MDR microbes, tolerated drugs, and related hospital-acquired infections are reported in Table 1.
Table 1.
Common drug-resistant microbes and diseases caused by them.
| Name of Bacterium | Drug(s) Resistant to | Typical Disease |
|---|---|---|
| E. coli | Cephalosporins, fluoroquinolones | UTI, BSI |
| K. pneumoniae | Cephalosporins, carbapenems | Pneumonia, BSI, UTI |
| S. aureus | Methicillin | Wound, BSI |
| S. pneumoniae | Penicillin | Pneumonia, meningitis, otitis |
| Nontyphoidal Salmonella | Fluoroquinolones | Foodborne diarrhea, BSI |
| Shigella spp. | Fluoroquinolones | Diarrhea * |
| N. gonorrhoeae | Cephalosporins | Gonorrhea |
| M. tuberculosis | Rifampicin, isoniazid, fluoroquinolone | Tuberculosis |
| Name of Fungi | ||
| Candida spp. | Fluconazole, echinocandins [7] | Candidiasis |
| Cryptococcus neoformans | Fluconazole [8] | Cryptococcosis |
| Aspergillus spp. | Azoles [9] | Aspergillosis |
| Scopulariopsis spp. Onychomycosis | Amphotericin B, flucytosine, azoles [10] | Onychomycosis |
| Name of Virus | ||
| Cytomegalovirus (CMV) | Ganciclovir, foscarnet [11] | AIDS and oncology patients |
| Herpes simplex virus (HSV) | Acyclovir, famciclovir, valacyclovir [12] | Herpes simplex |
| Human immunodeficiency virus (HIV) | Antiretroviral drugs [13] | AIDS |
| Influenza virus | Amantadine, rimantadine, neuraminidase inhibitors [14] | Influenza |
| Varicella zoster virus | Acyclovir, valacyclovir [12] | Chicken pox |
| Hepatitis B virus (HBV) | Lamivudine [15] | Hepatitis B |
| Name of Parasite | ||
| Plasmodia spp. | Chloroquine, artemisinin, atovaquone [16] | Malaria |
| Leishmania spp. | Pentavalent antimonials, miltefosine paromomycin, amphotericin B [17,18] |
Leishmaniasis |
| Schistosomes | Praziquantel, oxamniquine [19,20] | Schistosomiasis |
| Entamoeba | Metronidazole [21] | Amoebiasis |
| Trichomonas vaginalis | Nitroimidazoles [22] | Trichomoniasis |
| Toxoplasma gondii | Artemisinin, atovaquone, sulfadiazine [23,24,25] | Toxoplasmosis |
* Bacillary dysentery.
Antifungal drugs available for the treatment of chronic fungal infections are limited and resistance to drugs such as amphotericin B, ketoconazole, fluconazole, itraconazole, voriconazole, flucytosine, and echinocandins was found in isolates of Candida spp., Aspergillus spp., C. neoformans, Trichosporon beigelii, Scopulariopsis spp., and Pseudallescheria boydii [7,8,9,10]. Additionally, antiviral resistance has become a serious matter of concern in immunocompromised patients, including immunosuppressed transplant receivers and oncology patients infected by CMV, HSV, VZV, HIV, influenza A virus, hepatitis C (HCV), or HBV [11,12,13,14,15]. Parasitic MDR has been observed in isolates of Plasmodia, Leishmania, Entamoeba, T. vaginalis, Schistosomes [16,17,18,19,20,21,22,26,27], and T. gondii [23,24,25] against drugs such as chloroquine, pyrimethamine, artemisinin, pentavalent antimonials, miltefosine, paromomycin, and amphotericin B, in addition to atovaquone and sulfadiazine. A leading example of a parasite with a high tendency to develop MDR is P. falciparum, which is the cause of malaria. Other protozoan MDR parasites are Entamoeba spp., which causes amoebiasis, a major public health threat in many tropical and subtropical countries; and Schitosomes which is responsible for schistosomiasis, considered a global health concern similar to malaria and other chronic diseases [27].
2. Introduction to Biofilm (BF)
BFs are organized communities of microorganisms, including sessile cells, persistent cells, and dormant cells, which, during BF development, obtain physiological characteristics differentiating them from planktonic cells. Differently from the latter, which live suspended in a medium, sessile cells are cells attached to surfaces, forming highly coordinated microcolonies that lack motility [28]. They are incorporated into an extracellular polymeric matrix, self-produced by the bacteria, which adheres to living or abiotic surfaces present both in the environment and the healthcare environment [28]. The formation of BF by bacteria is a valid adaptation strategy that protects them from hostile environments, including host defenses, disinfectants, and antibiotics [28]. BF biomass consists of extracellular polymeric substances (EPSs), including a combination of enzymatic proteins; polysaccharides, such as cellulose, polyglucosamine (PGA), and exopolysaccharides; extracellular DNA (eDNA); and cationic and anionic glycoproteins and glycolipids, which allow real communication between the bacteria and stabilize the three-dimensional structure of the BF itself [29,30]. Table 2 summarizes the general composition of BFs.
Table 2.
General composition of BFs.
| BF Biomass | Organized communities of pathogens | Sessile cells | Cells attached to surfaces forming highly coordinated microcolonies, communicating by the QS system, and lacking motility |
| Persistent cells | Small subpopulation of microorganisms reversibly transformed into slowly growing cells | ||
| Dormant cells | |||
| Extracellular polymeric substances (EPSs) | Enzymatic proteins | Polypeptides | |
| Polysaccharides | Cellulose Polyglucosamine (PGA) Anexopolysaccharides |
||
| Extracellular DNA (eDNA) | |||
| Cationic and anionic glycoproteins | |||
| Cationic and anionic glycolipids |
Allow real communication between bacteria Stabilize the 3-D structure of BF |
||
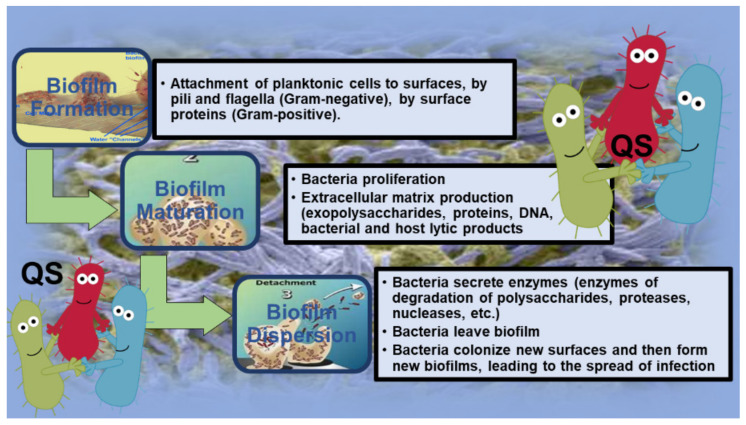
The nutrients within the matrix are used by bacteria, which in turn secrete enzymes capable of changing the composition of EPSs in response to changes in the nutrient availability, while water is retained through H bonds with hydrophilic polysaccharides [29,30]. In Gram-negative species, the formation of BF begins with the anchoring of planktonic cells to a surface by pili and bacterial flagella [31,32]. Differently, in Gram-positive species, anchoring occurs through surface proteins [33]. Following adhesion, the bacteria begin to proliferate and form microcolonies and produce the extracellular matrix, thus allowing the integrity of the BF, whose matrix, in addition to exopolysaccharides, proteins, and DNA, also includes both bacterial and host lytic products [34,35,36]. Following the maturation of BF, the dispersion phase occurs, in which the secretion of enzymes (enzymes of the degradation of polysaccharides, proteases, nucleases, etc.) responsible for the disintegration of the matrix is observed, allowing other bacteria to leave BF, colonize new surfaces, and then form new BFs, leading to the spread of infection [34,35,36]. As an example, the phases of bacterial BF development are shown in Figure 1.
Figure 1.
Phases of bacterial BF development.
The genesis of BF is related to quorum sensing (QS), a mechanism that allows intracellular communication and regulates gene expression in response to cell density [30]. QS occurs in both Gram-positive and Gram-negative bacteria, but the self-inductors that are produced, and/or the signal molecules that allow cell-to-cell communication are different. In general, QS allows bacteria to begin, synchronize, and create a synergy that allows the architecture of BF to be retained and the creation of an environment within it that is conducive to the survival of pathogens [37,38]. There are many abiotic or living surfaces, including tissues, industrial surfaces, medical devices, dental materials, and contact lenses, on which pathogens can form BF [37,38].
Note that BF succeeds in cancelling antibiotic activity because drugs are incapable of diffusing into its complex structure and reaching pathogens [39]. Additionally, several overcoming factors, including a high cell density, an increase in the number of resistant mutants, molecular exchanges, release of substances, an increase in the expression of efflux pumps, altered bacterial growth rates, different gene expression, and persistent and dormant cells, make it difficult for antibiotics to counteract BF production [29,40]. The formation of BFs occurs mainly in chronic infections, characterized by the persistence of etiological agents, while acute infections are generally sustained by planktonic bacteria [41,42,43,44]. BFs are involved in over 60% of chronic wound infections, which can be colonized by a single or several bacterial species [45]. In this scenario, the major bacteria involved are S. aureus and P. aeruginosa [46,47]. In this regard, chronic respiratory infections sustained by P. aeruginosa are one of the main causes of mortality in patients suffering from cystic fibrosis. Unfortunately, evidence attests that 80% of the patients affected by cystic fibrosis are likely to develop chronic respiratory infections [48]. Furthermore, P. aeruginosa, following the formation of BF on the surface of the endotracheal tubes, is responsible for the development of pneumonia in intubated patients [49]. Eradication of chronic infection is hindered by the growth mode of BF, the intrinsic resistance of P. aeruginosa to antibiotics, and a high presence of hypermutable strains [50,51]. Regarding S. aureus, it is mainly responsible for the formation of BF on medical devices and the onset of biomaterial-associated infections (BAIs). In BAIs, the development of the infection depends on the type of implant and the length of time the implant is in the patient. The adhesion of pathogens to permanent medical devices is favored by fibronectin and fibrinogen, which act as adhesion mediators for staphylococci [52]. Concerning fungi, Candida species, which are often found in the normal microbiota of humans, are commonly found on implanted biomaterials and medical instrumentation [53,54,55], where they can form BF structure, thus being one of the main causes of catheter-related infections [5] and posing an important health risk for hospitalized patients [55,56,57]. BFs of Candida species have significant resistance to antifungal agents and the ability to withstand harsh conditions, and BF cells are capable of evading the host’s immune response [58], thus representing a significant challenge for patients with immunodeficiency or medical-implanted devices [59]. Moreover, since Candida is a eukaryotic microorganism, similar to human cells, the successful design of new antifungal compounds is limited by its poor selectivity and tendency to present high cytotoxic levels [60]. Collectively, there is an urgent need to find alternative options to prevent and control, among other microorganisms, the formation of P. aeruginosa, S. aureus, and C. albicans BF and BF-related infections. In recent years, various antibacterial surfaces or antibacterial coatings for surfaces have been developed to attempt to counteract the adhesion and colonization of surfaces by pathogens producing BFs. In general, antibacterial surfaces can be divided into antifouling or bactericide, depending on the effects they are able to exert on the biological systems they encounter [61].
The antifouling activity of surfaces is achieved by the use of hydrophilic polymers or zwitterionic polymers while bactericidal activity usually leads to the inactivation of pathogens that adhere to the surface or are in suspension close to surfaces. In the latter case, depending on the pathogen-killing mechanism, there are two types of bactericidal surfaces: those that kill bacteria by contact, and those that kill bacteria by releasing bactericidal agents [61]. Unfortunately, currently, no antibacterial surface is capable of completely hampering the formation of BF, by either inhibiting its formation or causing its degradation. To address this need, researchers have developed novel methods for designing novel functional antibacterial surfaces with joint bactericidal and antifouling properties [62]. Interestingly, dendrimer compounds have appeared as interesting alternatives to conventional drugs in biomedicine and could be a new therapeutic approach to combat the infections caused by MDR pathogens, including those producing BFs [63]. Dendrimer systems have well-defined and monodispersed structures and are being widely studied for various biomedical applications, such as drug delivery systems, antivirals, and magnetic resonance imaging contrast agents, in addition to antibacterial and antitumor drugs [63,64,65,66,67,68,69]. Concerning cationic dendrimers, properties such as mono-dispersity, high water solubility, and multivalency allow them to act as powerful therapeutic agents, with significant potential for clinical applications as antibacterial agents [70,71,72]. Particularly, multivalency provides dendrimers with several functional groups on the surface capable of detrimentally interacting with bacterial membranes, causing disruption and killing of pathogens [70,71,72]. In this regard, cationic dendritic molecules have been studied against different planktonic cells [73,74,75,76] and BFs [62,77], showing a low tendency to induce antibiotic resistance in bacteria [78]. Furthermore, the synergistic combination of these structures with commercial drugs can improve the drug’s solubility and antibacterial activity [75,79,80] more than one therapeutic function and can allow a reduction in the administered doses, reducing the side effects of the drugs.
In this scenario, the main scope of this paper is to incite further development of new antibacterial agents capable of inhibiting BF formation and, hopefully, destroying mature BF. To this end, we first summarize the useful information concerning microbial resistance, BF, and specifically relating to BFs by P. aeruginosa, which is one of the most clinically relevant opportunistic bacteria producing BF on medical devices, responsible for untreatable infections. Secondly, we review all the dendrimers developed in the last fifteen years that have shown an ability to counteract this worrying form of bacterial resistance. We are confident that the extension of the knowledge about this still little-explored nanomaterial is a successful approach to find effective weapons for treating chronic infections and biomaterial-associated infections (BAIs) sustained by BFs produced by MDR bacteria.
2.1. BFs by P. aeruginosa
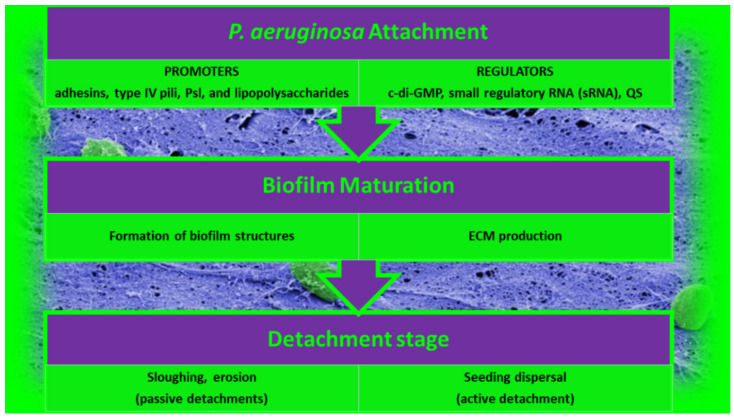
The formation of BF is an unceasing cycle, in which, as predicted in Section 2, organized communities of microorganisms are trapped in a matrix of EPSs that holds microbial cells together and attached to a surface [63]. BFs are symbolically called a “city of microbes” in which EPSs, representing 85% of the total BF biomass, constitute the “house of the BF cells”. BF cells handle 65–80% of all microbial infections. BFs have become a major issue in the medical sector since BFs are also a major cause of chronic infections due to their high resistance to antibiotics and the host immune response. Note that, in natural environments, BFs rarely exist as mono-species BF while interspecies interactions affect many genetic and phenotypic attributes in multispecies BFs. Consequently, it is crucial to focus mainly on the study of multispecies BFs to understand BFs better. Nevertheless, to date, BF research has mostly been limited to the study of mono-species BFs. In this regard, P. aeruginosa is a pathogenic opportunistic bacterium that has been widely studied for its high incidence in clinical settings and its ability to form strong BFs. P. aeruginosa is an opportunistic pathogen capable of adapting to various environments and developing resistance against multiple classes of antibiotics. The distinct main developmental stages of P. aeruginosa BF are shown in Figure 2.
Figure 2.
Main developmental stages of P. aeruginosa BF. PsL = extracellular polysaccharide expressed by non-mucoid P. aeruginosa strains; QS = quorum sensing; ECM = extracellular matrix.
2.1.1. Attachment of P. aeruginosa BFs
Although early studies suggested that simple chemical bonds such as Van der Waals forces contribute to the first attachment of bacteria of the BF-forming Pseudomonas genus, it has been shown that much more complex events and bacterial structures are involved in the early stage of BF development. Adhesins, type IV pili, and lipopolysaccharide (LPS) promote the attachment of bacteria to surfaces, and these bacterial structures are in turn specifically regulated by environmental signals [81,82]. Additionally, recent studies proved that the origins of BF formation occur simultaneously with an improvement in the levels of c-di-GMP, which is a second messenger [83,84,85,86,87] that activates the production of adhesins and various ECM products [83,87]. Moreover, BF formation is also regulated by sRNAs, which in turn control Psl, Pel, and alginate production, which are extracellular polysaccharides (EPSs) implicated in BF development and the motile-to-sessile switch of P. aeruginosa cells [88,89]. Particularly, Pel and Psl are produced by non-mucoid strains of P. aeruginosa, mainly playing an important role in surface attachment for most isolates, and there is significant strain-to-strain variability in the contribution of Pel and Psl to mature the BF structure [90].
2.1.2. Maturation of P. aeruginosa BFs
After adhering reversibly to surfaces or each other, bacterial cells undergo the switch from reversible to irreversible attachment. P. aeruginosa bacteria undergo a series of changes to adapt to the new mode of life, thus shifting from the status of planktonic cells (free-living cells) to that of sessile cells (not motile attached cells). They form a more structured architecture, termed microcolonies, in which bacteria are highly coordinated and start working together to produce ECMs and build structures and water channels. These microcolonies develop further into extensive three-dimensional mushroom-like structures, a hallmark of BF maturation. While BF matures, P. aeruginosa bacteria go through physiological modifications, thus becoming more resistant to environmental stresses and antibiotics. All this machinery is governed by a signaling system called quorum sensing (QS) [63].
2.1.3. Detachment of P. aeruginosa BFs
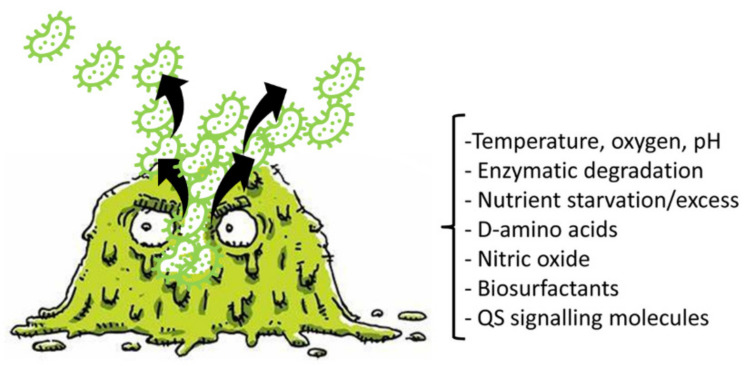
The costs associated with BF growth make it pivotal that bacteria have mechanisms to separate from BFs and return to planktonic life. This process is referred to as detachment or dispersion. Detachment is the final stage of BF development, essential to the creation of new BFs in new niches. Detachment can occur by several different mechanisms, such as sloughing, erosion, and seed dispersal [82,91,92]. While sloughing and erosion are passive detachments and are mediated by shear stress [82,91], seed dispersal consists in the active disengagement mechanism of P. aeruginosa BFs, during which single planktonic cells or microcolonies are released by the center of P. aeruginosa BFs, leaving an empty cavity (central hollowing) [91]. This mechanism includes the degradation of ECM and the autolysis of a BF subpopulation of cells, including dormant and persistent cells. Recently, it was reported that endonuclease EndA is needed for the dispersion of an existing BF via eDNA degradation. BF dispersion can also be promoted by environmental signals, such as variations in nutrients, the availability of oxygen, nitric oxide (NO), pH, and various chemicals. Collectively, signals that cause decreases in the levels of pyoverdine and intracellular c-di-GMP and increases in flagella production induce dispersal. Moreover, metal chelators and compounds such as cis-2-decenoic acid, anthranilate, or other surfactants can induce BF dispersal [93,94,95].
Figure 3 shows a BF in the dispersal phase and reports the main factors, cues, and signals that induce BF detachment.
Figure 3.
BF detachment.
Furthermore, in vitro and in vivo experiments have shown that the cells liberated by BFs are extremely cytotoxic to macrophages, more sensitive to iron diminution, and significantly more virulent to nematode hosts than planktonic bacteria. Additionally, it was reported that dispersed bacteria derived from BFs treated with glycoside hydrolase rapidly induced fatal septicemia in a mouse chronic wound infection model.
2.1.4. Important Characteristics of P. aeruginosa BF
Table 3 shows some important characteristics of BF produced by P. aeruginosa. Particularly, among the three identified types of EPSs in P. aeruginosa (Psl, Pel, and alginate) [96], Psl was named based on the locus of the polysaccharide synthesis, which was identified in 2004 [97,98]. In the late stage of BF maturation, Psl accumulates on the outside of structured BFs [99,100] but can also form a web of eDNA–Psl, thus supplying structural support to BF for its later dispersion. Interestingly, the eDNA–Psl interactions could increase the extent of P. aeruginosa in vivo by the use of neutrophil extracellular traps as a BF scaffold [101].
Table 3.
Important characteristics of P. aeruginosa BF.
| Functions | Components | Sub Components |
Molecules | Function |
|---|---|---|---|---|
| Matrix Adhesive material Protective barrier |
ECMs | EPS | PsL * | Initiation and maintenance of BF Supplies cell surface attachment Supplies intercellular interactions |
| PeL *, ** | Essential for forming pellicles at the air–liquid interface and solid surface-associated BFs [98,102] Platform for BF structure Supplies protection against aminoglycosides [100,103] Binding with eDNA of the BF [104,105]. Compensates for a lack of PsL in the BF periphery [104] |
|||
| Alginate *** | Factor used to distinguish mucoid or non-mucoid P. aeruginosa BFs Retains water and nutrients Supplies antibiotic resistance and immune evasion [105,106,107] |
|||
| eDNA | Formation of cation gradients Antibiotic resistance Nutrient source Early BF development [99,108,109,110] Major proinflammatory factor for P. aeruginosa BFs [111] |
|||
| Proteins | Flagella | Act as an adhesin to help initial bacterial attachment to the surface [112] |
||
| Type IV pili | Contribute to the formation of mushroom-like BF cap structures [113,114] | |||
| CdrA adhesin | Interacts with PsL and increases BF stability [115]. | |||
| Cup fimbriae | Important roles in cell-to-cell interaction during the first stage of BF formation [115] | |||
| Intercellular communication system enabling bacteria to sense their own population density. | QS system | NAHSL AI-2 |
las | Regulate several hundred genes in P. aeruginosa [116,117] Regulate the bacterial phenotype, spatial differentiation in BFs, motility, and BF formation [118] |
| Rhl | ||||
| PQS | ||||
| Integrated QS (IQS) | ||||
* Psl and Pel production occurs through the c-di-GMP signaling pathway owing to environmental signals; ** the Pel production mechanism is implicated in the association of LPS. The complete biochemical composition of Pel has not yet been discovered. Currently, Pel is known to be made of cationic amino sugars; *** mainly produced by strains of P. aeruginosa isolated from patients affected by cystic fibrosis (CF); NAHSL = N-acyl-homoserine lactones; AI-2 = autoinducer 2.
Among the most accredited hypothesis, it has been reported that e-DNA may be produced by active secretion, autolysis of bacteria, or release from small membrane vesicles [99,107]. Concerning the QS system, which is an intercellular communication system that allows bacteria to feel their own population density [63], it relies on small signaling molecules such as N-acyl-homoserine lactones (NAHSL) and the and autoinducer-2 (AI-2). Note that while the latter has also been found in Gram-positive bacteria, they use oligopeptides in place of lactones. Concerning the four types of QS systems in P. aeruginosa, while the las QS system is involved in the production of N-3-oxododecanoyl homoserine lactones (N-3-C12-HSL), the rhl QS system is implicated in the synthesis of N-butanoyl-L-homoserine lactone (C4-HSL). Additionally, the las and rhl QS systems control much gene expression, such as the production of elastase, protease, rhamnolipids, and other factors of virulence. Moreover, the PQS system controls the release of eDNA in BF formation and the production of membrane vesicles [119,120] and affects many other metabolic processes in P. aeruginosa, including iron chelation, redox homeostasis, the production of elastase and rhamnolipids, the formation of membrane vesicles, etc. [117,121]. IQS is a recently discovered QS system, which can integrate environmental stress cues into QS. These QS systems have hierarchical relationships among them, with the las system being in the highest position in the QS system while the rhl system is at the lowest level. Nevertheless, each QS system can also be initiated by environmental factors, including phosphate stress, starvation, low oxygen, low iron, and several host-derived factors [117,122,123,124,125].
2.1.5. P. aeruginosa BF-Associated Infections
Infections associated to BF produced by P. aeruginosa can be classified into two categories. The first category is represented by BF infections due to indwelling medical devices, such as central venous catheters, urinary catheters, prosthetic couplings, peritoneal dialysis catheters, pacemakers, contact lenses, and intrauterine devices. The second class consists of direct BF infections in host tissues, such as chronic pneumonia in patients with CF, chronic otitis media, endocarditis, chronic osteomyelitis, chronic prostatitis, palindromic urinary tract infections (UTIs), and gingivitis [99]. The major issue associated with the development of BF infections in diverse medical environments consists in their significant resistance against most antibiotics and other disinfectants. The specific characteristics of BFs hamper the diffusion of drugs, which become uncapable of reaching bacteria, thus nullifying the antibiotic activity [63]. To counteract bacteria capable of forming BFs, an antimicrobial agent must prevail over several additional obstacles, including an increased number of resistant mutants, high cell density, molecular exchanges, substance delivery, efflux pumps, persistent and dormant cells, altered bacteria growth rate, and different gene expression [63]. In this regard, Table 4 reports the mechanisms through which BFs hamper the activity of antibiotics, with examples of BF-producing microorganisms and the related inactivated antibiotics.
Table 4.
Bacteria BFs’ contribution in the inactivation of antibiotics and antibiotic treatment failure.
| Reasons for the Failure of Antibiotics | BF Function | Factors | Inactivated Antibiotics | Ref. |
|---|---|---|---|---|
| Hampered antibiotic penetration | Anti-spread barrier | EPS | Ampicillin Ciprofloxacin |
[126] |
| Presence of antibiotic-degrading enzymes | To provide β-lactamases (β-LS) |
↑ β-LS | Ampicillin | [127] |
| Imipenem Ceftazidime | ||||
| Increased BF resistance | To provide eDNA |
↑ eDNA ↓ Mg2+ |
Cationic Peptides Aminoglycosides |
[128,129,130] |
| Presence of persistent cells | To cause gradients in nutrients and oxygen concentration To promote differentiation in cell growth |
Endogenous stress TA 1-systems |
Rifampicin Aminoglycosides | [131] |
| Presence of dormant cells | ↓ Functions ↓ Energy ↓ Biosynthesis |
Fluoroquinolones | [132] | |
| ↑ Resistance to stress | To cause adaptive stress responses by heterogeneity | Changes in component/processes target of antibiotics | Ofloxacin Gentamicin Meropenem Colistin | [133] |
| Ofloxacin | [134] | |||
| ↑ Export of membrane proteins | To up-regulate the production of some efflux pumps | ↑ Efflux pumps QS |
Multidrugs | [135] |
| Azithromycin | [136] | |||
| Genetic diversity | To act as a reservoir of genetic diversity by promoting plasmids transfer | Horizontal gene transfer (HGT) eDNA QS |
Aminoglycosides | [137] |
1 TA = toxin/antitoxin; ↑ = improved, higher, increased; ↓ = reduced, decreased.
P. aeruginosa can use the mechanisms shown in Table 4 to infect and occupy various locations of the human body. P. aeruginosa is notorious for causing pneumonia in the lungs of CF patients, thus being the primary cause of death of CF patients [138]. P. aeruginosa BFs in the CF lung consist of small aggregates wrapped in EPSs. BFs induce inflammation of the infected lung by means of enrolling polymorphonuclear leukocytes. BF allows bacteria to survive inflammation and aggressive antibiotic treatments, thus causing persistent infection. The chronic inflammatory response against BF infections triggers tissue damage and leads to lung failure [139]. Otitis media is another infection sustained by P. aeruginosa BF. Particularly, it is the infection of the middle ear. Very common among children, it can cause serious inflammation that may lead to conductive hearing loss [140]. In this case, BF consists of small microcolonies of less than 100 bacteria. Additionally, P. aeruginosa can cause chronic bacterial prostatitis, which is an infection of the prostate gland, representing the major cause of relapsed UTIs in men, in which microcolonies of P. aeruginosa are associated with the ductal wall of the prostate duct and cause the disease [139,141]. One of the major complications of P. aeruginosa BF infection is represented by chronic wound infections. Chronic wounds are normally correlated with vascular abnormalities such as decubitus ulcers, ischemic injuries, diabetic foot ulcers, and venous leg ulcers [140,141]. Since the skin barrier is compromised, these chronic wounds generate suitable environments for bacteria attachment and colony formation. Microbial infections in chronic wounds are multispecies infections, consisting of both aerobic and anaerobic bacteria. Among the isolated bacteria from chronic wounds, P. aeruginosa and S. aureus are the most common ones [142,143]. P. aeruginosa exists in BFs in wounds, located in a deeper part of wounds than to S. aureus. Furthermore, chronic wounds with P. aeruginosa infection tend to be larger, more inflamed, and slower to recover [140,142]. This could be due to characteristics typical of P. aeruginosa BF, such as type IV pili and flagella-mediated motility, in addition to the production of virulence factors that protect the bacteria from host defense systems [139]. Another very important class of P. aeruginosa BF infections includes infections on medically implanted devices. In this regard, P. aeruginosa strains are often isolated from infected urinary catheters, intravascular catheters, artificial joints, and cochlear implants [139]. BFs have been isolated from almost all medical device-related infections and are very difficult to remove. These infections are at high risk of progression to systemic infections. Thus far, the only treatment of BF infections on medical devices is removal of the device.
3. Prevention and/or Eradication of BF by Dendrimers: A Possibility Still Little Explored
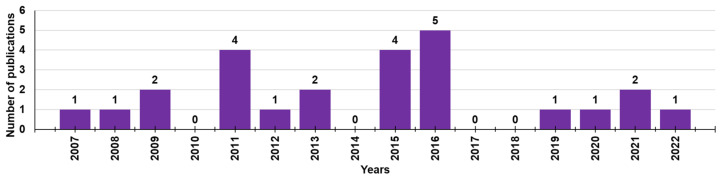
Although cationic dendrimers have been extensively studied as antibacterial agents with high potentiality, as shown by a search of Scopus using “cationic dendrimers” and “biofilm” as keywords, in the last 15 years (2007–2022), only 29 documents concerning this topic have been reported in the literature (Figure 4).
Figure 4.
Number of publications per year during the last fifteen years according to Scopus, concerning the development of dendrimers for contrasting BF.
This scenario shows that, although good premises exist, the possibility of using dendrimers to prevent the formation of BF or even to promote the breakdown of mature BF is, to date, still little explored. Unfortunately, the research in this field, instead of increasing, was shut down and after 2016, only five jobs were reported. This phenomenon highlights a diminished interest of a significant number of experts in developing new cationic dendrimers active against BF, further underlining the difficulty in being successful in such a challenge compared to being successful in developing new cationic materials, including dendrimers with bactericidal activity against bacteria with other types of resistances.
3.1. Antibacterial Cationic Macromolecules
Currently, there are few molecules under clinical development that are active against MDR pathogens and/or BF producers. Concerning the main agents in clinical development (Phase III) in 2020, only caspofungin, which is an antifungal drug, has been proven to inhibit the synthesis of polysaccharide components of the bacterial BF of S. aureus [144]. Concerning nanoparticles, some research groups are currently studying polymeric lipid nanoparticles, involving the conjugation of rhamnolipids (biosurfactants secreted by the pathogen P. aeruginosa) and polymer nanoparticles made of clarithromycin encapsulated in a polymeric core of chitosan. By the same principle, rhamnolipid-coated silver and iron oxide NPs have been developed, which have been shown to be effective in eradicating S. aureus and P. aeruginosa BFs [144]. Therefore, to counteract the phenomenon of drug resistance and meet the urgent need for new antibacterial agents that are also active in infections sustained by BF cells, the search for alternative therapeutic strategies capable of acting under mechanisms different from those of conventional drugs is a daily challenge of researchers in this field. In this regard, various natural cationic molecules called antimicrobial peptides (NAMPs) have shown significant capabilities to limit or inhibit bacterial growth and represent excellent candidates for replacing current antibiotics that are no longer effective. The amphiphilic structure and the net positive charge of NAMPs are the two most important requisites necessary for antibacterial effects and decide their mechanism of action [63,145,146,147]. It has, in fact, been shown that NAMPs’ action causes the death of the bacterial cell in a non-specific way from the outside, without having to enter the bacterial cell and interact with its metabolic processes, which is likely to genetically mute the conferring of resistance [63,145]. The action of NAMPs on bacteria is rapid, causes depolarization and destabilization of the membranes, and generates pores that gradually lead to an increase in their permeability, with consequent leakage of essential cations and other cytoplasmic materials and cell death. Since it is not connected to mutable bacterial processes, this mechanism of action generally allows the cationic peptides to induce a lower development of resistance in the target cell compared to traditional antibiotics [63,145]. Although the main target of NAMPs is bacterial membranes, it has been reported that some of these cationic antimicrobial peptides, following the increased permeability of bacterial envelopes, could also enter the cell and irreversibly damage molecules, such as DNA, RNA, and enzymes, thus leading to an improvement in their original therapeutic efficacy [63,146,147,148,149]. Unfortunately, the in vivo application of NAMPs is hampered by their early inactivation by peptidases, their high hemolytic toxicity, and high costs of production. Based on this, in the last decades, inspired by NAMPs, new cationic antibacterial macromolecules, such as polymers, copolymers, and dendrimers, have been developed, with proven interesting antibacterial effects. Such macromolecules, unlike small antibacterial compounds, in addition to possess multivalence, have other several advantages, including a limited residual toxicity, greater long-term activity, greater chemical stability, and lesser tendency to develop resistance [63,147,150]. Among polymers, cationic dendrimers (CDs) are nonpareil, nano-sized, hyper-branched cationic macromolecules with a tree-like architecture and a unique spherical shape [63,64,65,66,67,68,69,70,71,72]. Dendrimers, which have been extensively applied in biomedicine for years, have recently been found to act as potent antibacterial agents and also exploitable for coating surfaces [62,63,64,68,69,70,71,72].
Cationic Dendrimers
Dendrimers were first synthesized in the mid-1980s, but their use as antibacterial agents, mimicking the action of NAMPs for use as drugs, surface coating agents, or drug-delivery systems, has only recently been recognized [62,63,64,68,69,70,71,72]. In the last decade, dendrimers have mainly been synthesized for the treatment of infections caused by MDR pathogens and some of these have been shown to have antibiofilm action [151,152]. The most used and studied commercial dendrimers as antibacterial agents are poly(amidoamine) (PAMAM) and polypropylene imines (PPIs), which have shown wide activity in vitro; however, unless they are properly modified to improve their biodegradability, reduce their susceptibility to opsonization and toxicity against eukaryotic cells, and fast clearance, they are not clinically applicable [70,71,72]. Moreover, polymers such as linear and branched poly (ethylene imine)s (PEIs), in addition to requiring minor costs for production, although with less perfect architectures than PAMAMs and PPIs, are known for their ability to enter cells or permeabilize cell membranes. Accordingly, while a large number of studies have focused on the antibacterial activity of water-soluble PEI derivatives containing quaternized ammonium salt groups with long alkyl or aromatic groups, others reported the application of water-insoluble hydrophobic PEIs, including nanoparticles, as antibacterial coatings [153]. To improve and therefore obtain CDs with better characteristics, particular attention was paid to the synthesis of biodegradable polyester-based dendrimer scaffolds, peripherally modified with suitable amino acids, thus achieving shells that are highly cationic and conferring the obtained dendrimer NPs’ potent antibacterial properties [63,70,71,72,154,155]. In the worrying scenario where the weapons used to counteract MDR bacteria and above all infections sustained by BF-producing pathogens are dramatically decreased or non-existent, cationic dendrimer NPs could represent new valid promising tools for fighting MDR pathogens and also for the treatment of sustained BAIs of MDR BF-producing bacteria. The advantages associated with the use of cationic material with dendrimer structures mainly depend on their high multivalency deriving from their tree-like and generational structure, which allows a large abundance of active cationic moieties, thus greatly improving their antibacterial potency [63]. Aiming to inspire the realization of further synthetic strategies for developing new antibacterial dendrimers capable of inhibiting the first training and/or destroying mature BFs, we review the most recent investigations carried out to design and develop new antibiofilm dendrimer agents and the related results.
3.2. Recently Reported Case Studies
In the following part of the present paper, we report the dendrimer materials developed in the last fifteen years that were tested for their effects in preventing or limiting the development of fungi and bacterial BF, and causing its dispersal. In this regard, Table 5 shows the structures representative of the main class of dendrimers engineered.
Table 5.
Chemical structure representative of the cationic dendrimers developed in the last fifteen years that were active on pathogens’ BFs.
| Dendrimers Structure | Name | Activity |
|---|---|---|
|
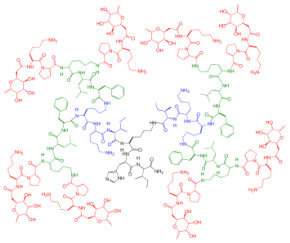
2G3 | IC50 = 0.025 µM P. aeruginosa (PA) LecB |
|
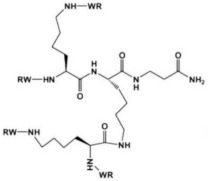
(RW)4D | Inactivate E. coli RP437 planktonic culture and BFs |
|
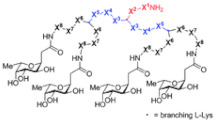
FD2 | LD50 LecB PA 0.14 µM P. aeruginosa BF formation (IC50 = 10 mM) |
| D-FD2 | LD50 LecB PA 0.66 µM |
|
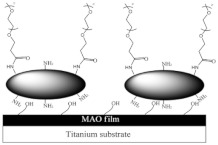
|
PEGylated PAMAM Film on MAO Substrate | BF by PA (strain PAO1) and S. aureus (SA). |
|
Nitric Oxide-Releasing amphiphilic PAMAM | Inhibited PA BFs |
|
14 | Inhibited C. albicans BF |
|
G4 PAMAMs decorated with C16-DABCO |
Inhibited E. coli and B. cereus BF |
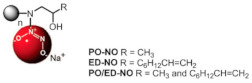
|
PEGylated AgNPs covered with cationic carbosilane dendrons | Inhibited E. coli and S. aureus BF |
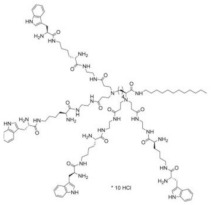
A research line started in the first decade of the 2000s concerned the synthesis and screening of libraries of dendrimer compounds with C-fucosyl and galactosyl residues with an affinity for fucose- and galactose-specific lectins. The scope was to detect potent fucose and/or galactose inhibitors of lectins LecA and LecB produced by P. aeruginosa, which are implicated in tissue binding and BF formation. In this regard, regarding the results obtained the previous year when a very large library of peptide dendrimers was screened to assess their affinity for LecB, the most potent LecB-ligands identified as dendrimers (FD2 (C-Fuc-LysProLeu)4(LysPheLysIle)2 LysHisIleNH2 (IC50 = 0.14 mM by ELLA) and PA8 (OFuc-LysAlaAsp)4(LysSerGlyAla)2 LysHisIleNH2 (IC50 = 0.11 mM by ELLA)) were tested on BF of P. aeruginosa by Johansson et al. in 2008 [156]. Particularly, FD2 led to complete inhibition of P. aeruginosa BF formation (IC50 = 10 mM) and induced complete dispersion of mature BFs in the wild-type strain and in numerous clinical P. aeruginosa isolates, thus indicating that LecB inhibition by high-affinity multivalent ligands such as FD2 could correspond to a curative approach against BF-associated chronic infection sustained by P. aeruginosa [156]. Subsequently, Kolomiets et al. (2009), building on promising results previously published, prepared 10 tetravalent and 3 octavalent water-soluble C-fucosyl peptide dendrimers by solid-phase peptide synthesis (SPPS). By determining the relative affinities of these ligands to LecB using an enzyme-linked lectin assay (ELLA), strong binding of up to a 440-fold enhancement in potency over fucose was observed for the octavalent cationic dendrimer 2G3, mainly due to the multivalency of the dendrimer. Collectively, due to the potent binding and inhibition action on LecB by 2G3 and the versatility and reliability of SPPS to produce tunable multivalent fucosylated peptide dendrimer ligands, the dendrimers reported by Kolomiets et al. seemed excellent candidates for the development of polyvalent inhibitors of P. aeruginosa adhesion and BF for use in therapy to prevent BF-associated infections [157]. Antibacterial dendrimer peptides, acting in this case as membrane disruptors due to their positive charge ((RW) 4D), were prepared by Hou et al. (2009), which were effective in preventing the formation of E. coli BFs, hindering their development and destroying mature forms [158]. In 2011, Johansson et al. carried out research on a potent LecB inhibitor active in the prevention and dispersal of BF by P. aeruginosa, proposing a strategy to limit the early in vivo deactivation of FD2 by proteases. In this regard, he synthetized the C-fucosyl peptide dendrimer D-FD2 (CFuc-lys-pro-leu)4(Lys-phe-lys-leu)2Lys-his-leu-NH2 using D-amino acids and obtained the stereoisomer of FD2 [159]. Although D-FD2 showed a lower affinity for the fucose-specific lectin LecB, it was active as a BF inhibitor and, in addition, showed high stability towards proteolysis with pure proteases and in human serum, thus being conceivable for future clinical applications. In summary, the peptide dendrimer D-FD2 was prepared by substituting D-amino acids for L-amino acids in the branches of the dendrimer FD2. Collectively, the exchanged geometry altered the binding affinity to LecB while retaining the P. aeruginosa BF inhibitory properties and supplying complete resistance to proteolysis [159]. Establishing that BF formation by P. aeruginosa is mediated in part by the galactose-specific lectin LecA (PA-IL) and the fucose-specific lectin LecB (PA-IIL), and understanding that the glycoconjugate–lectin interaction is a key feature in developing potent BF inhibitors, Kadam et al., deepening their research in the field, in 2011, reported the first case of P. aeruginosa BF inhibition by the β-phenylgalactosyl peptide dendrimer GalAG2 multivalent ligand, this time targeting the galactose-specific lectin LecA [160]. Since it was observed that hydrophobic groups in the sugar anomeric point improved the affinity of galactosides to LecA, the acetyl-protected 4-carboxyphenyl β-galactoside (GalA) was connected to the peptide dendrimer [160]. To investigate the effect of the sugar-dendrimer linker on the binding affinity, carboxypropyl β-thiogalactoside (GalB) was also introduced as the last building block in solid-phase peptide synthesis to give the dendrimers GalAG1-G2 and GalBG1-G2, and the linear peptides GalAG0 and GalBG0 [160]. Based on the reported results, the strongest binding was detected with the second-generation glycopeptide dendrimer GalAG2. Interestingly, both the GalAG2 and GalBG2 dendrimers exhibited potent BF inhibition while the G1 equivalents were much less active and the G0 analogs were ineffective [160]. In the same year (2011), Chen et al. assessed the activity of a dendrimer peptide with various numbers of Trp/Arg repeated residues ((RW)4D), which was previously found to have antibacterial effects by Hou et al. [158], against planktonic persister cells and BF-associated persister cells of E. coli HM22 [161]. The results were then compared with those obtained using three linear synthetic AMPs. Overall, (RW)4D exhibited potent activities in eradicating BF cells and associated antibiotic tolerance in a dose-dependent manner [161]. Particularly, after treatment for 1 h with 20, 40, and 80 μM (RW)4D, the total number of viable BF cells was reduced by 77.1%, 98.8%, and 99.3%, respectively, compared to the untreated control (p < 0.0001). The tolerance to ampicillin was also reduced by 57.2%, 96.9%, and 99.1%, respectively (p = 0.0012). Furthermore, no viable cell was found after treatment of the BFs with 40 or 80 μM (RW)4D followed by 5 μg/mL ofloxacin, suggesting that all persister cells were eliminated [161]. On these early results, (RW)4D may be a pioneer compound for the development of new therapeutics to treat BF-associated infections that is also able to improve the action of antibiotics, including ampicillin and ofloxacin. Wang et al. (2011) presented a report on the antibacterial activity and cytotoxicity of three types of titanium-based substrates with and without calcium phosphate coatings on which poly(amidoamine) (PAMAM) dendrimers were immobilized (named Ti-S(CaPO4)060-PEGPAMAMs and Ti-S-060-PEGPAMAMs in the table) [162]. The utilized amino-terminated PAMAM dendrimers were modified with various percentages (0–60%) of PEG to obtain strong absorption on the titanium-based substrates and the formation of dendrimer films. The obtained films effectively inhibited colonization by P. aeruginosa (strain PAO1) and, although to a lesser extent, S. aureus. The antibacterial activity of the films was maintained even after storage of the samples in PBS for up to 30 days [162]. In addition, the dendrimer-based films showed low cytotoxicity to human bone mesenchymal stem cells (hMSCs) and did not alter the osteoblast gene expression promoted by the calcium phosphate coating [162]. The next year (2012), Scorciapino et al., using an alternation of hydrophilic and lipophilic amino acids, synthetized a lipodimeric dendrimer (SB056), which demonstrated an antibacterial effect by acting as a membrane disruptor [163]. The antibacterial activity of SB056 was similar to that of colistin e polimixin B against isolated MDR Gram-negative species while SB056 showed poor activity against strains of Gram-positive species. Interestingly, SB056 showed good antibiofilm effects against BFs produced by S. epidermidis and P. aeruginosa [163], thus being a future therapeutic weapon against BF-associated infections. The group of Lu et al., in 2013, recovered PAMAM-based dendrimers capable of releasing NO to develop new antibacterial agents, which are hopefully also effective against BFs [164]. Particularly, a series of amphiphilic PAMAM dendrimers were synthesized by a ring-opening reaction between the primary amine groups on the dendrimers and propylene oxide (PO), 1,2-epoxy-9-decene (ED), or a ratio of the two [164]. The obtained PAMAM-based dendrimers with different functionalities were then reacted with NO at 10 atm to produce N-diazeniumdiolate-modified scaffolds with a total loading of NO of ~1 μmol/mg [164]. In sight of a future clinical application of such materials, structure–activity relationship (SAR) studies were carried out to evaluate the bactericidal efficacy of the prepared NO-releasing delivery systems against established BFs by P. aeruginosa as a function of the dendrimer exterior’s hydrophobicity (i.e., ratio of PO/ED), size (i.e., generation), and NO release [164]. It was revealed that both the size and exterior functionalization of the dendrimer were pivotal for dendrimer–bacteria interaction, the NO delivery efficiency, bacteria membrane disruption, migration within BF, and toxicity to mammalian cells [164]. In this regard, the best PO/ED ratios for BF eradication with minimal toxicity against L929 mouse fibroblast cells were 7:3 and 5:5 [164]. The inhibition of BF formation by blocking the action of LecA and LecB lectins from P. aeruginosa was also investigated by Reymond et al. [165]. So, the four glycopeptide dendrimers that were synthesized showed high affinity for the lectins and were efficient in both blocking P. aeruginosa BF formation and inducing its dispersal in vitro [165]. As mimics of natural cationic amphiphilic peptides (NAMPs) with antifungal activity, eight peptide dendrimers were designed and evaluated for their anti-Candida spp. effects against both the wild-type strains and mutants by Zielinska et al. in 2015 [166]. Among the synthetized macromolecules, dendrimer 14, containing four tryptophan (Trp) residues and a dodecyl tail, and dendrimer 9, decorated with four N-methylated Trp, was shown to inhibit 100 and 99.7% of Candida growth at 16 µg/mL, respectively [166]. On these promising outcomes, 9 and 14 were designated for their evaluation against C. albicans mutants with deactivated biosynthesis of aspartic proteases, which are responsible for host tissue colonization and morphogenesis during BF formation (sessile model). According to the reported results, 14 affected C. albicans BF viability and the hyphal and cell wall morphology by membranolytic mechanisms [166]. By affecting the cellular apoptotic pathway and damaging the cell wall formation in mature BF, 14 may be a potential multifunctional antifungal template compound for the control of C. albicans chronic infections [166]. C. albicans was also the topic in the study of Lara et al. (2015), where novel antifungal strategies targeting BFs were explored [59]. Notably, using microwave-assisted techniques, nanosized spherical silver nanoparticles (AgNPs), aiming to investigate their potential biological applications, were prepared by Lara et al. without the addition of contaminants [59]. A potent dose-dependent inhibitory effect of AgNPs on BF formation of C. albicans was demonstrated (IC50 of 0.089 ppm). Additionally, AgNPs were shown to be efficient when tested against pre-formed C. albicans BFs, resulting in an IC50 of 0.48 ppm while the cytotoxicity assay resulted in a CC50 of 7.03 ppm. Using SEM and TEM analyses, it was evidenced that treatments with AgNPs caused outer cell wall damage, absence of true hyphae, filamentation inhibition, and membrane permeabilization [59]. The same year (2015), Bahar et al. developed the arginine-tryptophan-arginine 2D-24 dendrimer peptide, which was found to be effective against P. aeruginosa normal planktonic and persister cells, and against P. aeruginosa BF cells [152]. Particularly, the second-generation peptide dendrimer (2D-24), with residues of arginine and tryptophan, was active both on planktonic and BF cells, and against the MDR isolates of P. aeruginosa PAO1 (ATCC BAA-47) and PDO300 (mutans mucA22 of P. aeruginosa PAO1) [152]. Collectively, 2D-24 displayed the same efficiency against both planktonic cells and BFs, thus establishing its capability to penetrate the BF’s mass and the alginate layer of mucoid isolates. Concentrations > 20 μM were sufficient to kill 80% of the planktonic and BF cells of PAO1 e PDO300 strains [152]. Further studies concerning combination therapies were carried out by Bahar et al., observing synergistic effects of 2D-24 with antibiotics such as ciprofloxacin (Cip), tobramycin (Tob), and carbenicillin (Car), all targeting the synthesis of DNA, proteins, and the cell wall [152]. The best result was obtained by combining 2D-24 with Tob. Additionally, in vitro studies on sheep erythrocytes and cocultures of PAO1 and human IB3-1 cells showed that 2D-24 exerted antibacterial effects at non-toxic concentrations on mammalian cells (25 µg/mL), thus providing encouraging evidence supporting the use of 2D-24 for chronic P. aeruginosa-borne infections [152]. Using a strategy similar to that used in 2013 by Lu et al. [164], Worley et al. described the synthesis of NO-releasing alkyl chain modified PAMAM dendrimers of the G1-G4 generations decorated with butyl or hexyl alkyl chains via a ring-opening reaction [167]. The resulting secondary amines were further reacted with N-diazenium-diolate NO donors, reaching an NO payload of ∼1.0 μmol/mg [167]. The bactericidal efficacy of these dendrimers was evaluated against BFs produced by isolates of P. aeruginosa, S. aureus, and MRSA. The anti-BF action of the dendrimers depended on the dendrimer generation, bacterial species, and alkyl chain length, with the most effective BF eradication occurring when antibacterial agents were capable of efficient BF infiltration. Regardless, the ability of dendrimers to release NO also helped the antibiofilm activity of those dendrimers incapable of effective BF penetration [167].
To better understand the binding mode of the tetravalent glycopeptide dendrimer (TGPD) GalAG2 to its target lectin LecA, the first crystal structures of LecA complexes with the divalent analog GalAG1 were obtained, revealing strong LecA binding and absence of lectin aggregation [168]. Secondly, a model of the chelate-bound GalAG27LecA complex was also obtained, rationalizing its unusually tight LecA binding (KD = 2.5 nM) and aggregation by lectin cross-linking [168]. By evaluating the BF inhibition with divalent LecA inhibitor (GalAG1), it was indicated that lectin accumulation is necessary for BF inhibition by GalAG2, thus establishing that multivalent glycoclusters represent a unique opportunity to control P. aeruginosa BFs [168]. The next year (2016), the same group of Worley, this time headed by Becklund, studied in more detail the modification of (G1) (PAMAM) dendrimers with several alkyl epoxides to generate propyl-, butyl-, hexyl-, octyl-, and dodecyl-functionalized dendrimers [169]. The resultant secondary amines were treated with NO to form N-diazeniumdiolate NO donor-modified dendrimer scaffolds (total NO ∼1 μmol/mg) [169]. In this study, the bactericidal action of the NO-releasing dendrimers was tested against both planktonic and BF cells of S. mutans, with the greatest efficiency observed with the increase in the alkyl chain length and at lower pH. Particularly, the best bactericidal efficacy was obtained at pH 6.4, probably due to the increase in the cationic scaffold surface charge, which promoted dendrimer–bacteria association and the ensuing membrane damage. For shorter propyl and butyl chain modifications, however, the increased antibacterial action at pH 6.4 was due to the faster NO-release kinetics from proton-labile N-diazeniumdiolate NO donors. Octyl- and dodecyl-modified PAMAM dendrimers proved to be the most effective in both eradicating S. mutans BFs with the help of NO release and displaying mitigated cytotoxicity [169].
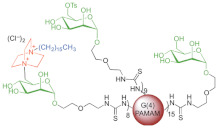
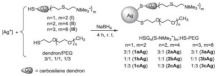
Inserting their studies in the research line concerning dendrimers that act as ligand inhibitors of LecA and/or LecB, Bergman et al. (2016) synthetized G3 and G4, analogous to GalAG2 and GalBG2, using a convergent synthetic technique and multivalent chloro-acetylcysteine (ClAc) thioether as the linker [170]. According to the reported results, G3 dendrimers showed an improved ability to link LecA with respect to the parent dendrimers G2 and were capable of exerting total inhibition of BF produced by P. aeruginosa, and causing its disaggregation [170]. The same year (2016), Michaud, Visini, Bergaman et al. focused on carrying out several changes in the structures of the earlier reported TGPDs acting as ligands of lectins LecB and/or LecA produced by P. aeruginosa to increase their BF inhibition activity [171]. As examples, they investigated heteroglycoclusters (Het1G2-Het8G2 and Het1G1Cys-Het8G1Cys), each having one pair of LecB-specific fucosyl groups and LecA-specific galactosyl groups, capable of simultaneously binding both lectins [171]. Interestingly, one of these ligands gave the first fully resolved crystal structure of the complex peptide dendrimer–LecB, providing a structural model for dendrimer–lectin interactions (PDB 5D2A) [171]. Furthermore, BF inhibition was increased by introducing additional cationic residues in these dendrimers. In a second approach, dendrimers with four copies of the natural LecB ligand (Lewisa) were prepared, which were able to strongly bind LecB and allowed higher BF inhibition effects. Finally, in this study, synergistic application of the previously reported dendrimer FD2 with the antibiotic tobramycin at sub-inhibitory concentrations of both compounds was carried out, demonstrating effective BF inhibition and dispersal [171]. The group of Scorciapino, this time headed by Batoni, in 2016, studied linear and dendrimer compounds derived from the previously reported lin-SB056 and den-SB0569 by changing the first two residues [KWKIRVRLSA-NH 2] of the original sequence [WKKIRVRLSA-NH 2] and obtained new compounds, namely lin-SB056-1 and den-SB056-1, which possessed an improved amphiphilic profile, and explored the effects of this modification [172]. Den-SB056-1 showed antibacterial effects against both Gram-negative and Gram-positive bacteria, based on the disruption of bacterial membranes. The results obtained against E. coli and S. aureus planktonic strains confirmed the added value of the dendrimer structure over the linear one, and the impact of the higher amphipathicity on enhancement of the peptide performances [172]. Unfortunately, while SB056 peptides exhibited enthralling antibiofilm properties mainly against sessile cells of S. epidermidis, SB056-1 showed reduced antibiofilm effects if compared with that of SB056 [172]. In a study of the same year by VanKoten et al. (2016), a highly cationic fourth-generation PAMAM dendrimer (G4-PAMAM), functionalized with 1-hexadecyl-azoniabicylo [2.2.2] octane (C16-DABCO), a quaternary ammonium compound known to have antibacterial activity, was synthetized [173]. According to the authors, the dendrimer activity depended on both the presence of mannose residues and the ammonium quaternary groups. The PAMAM dendrimer was active against both Gram-positive and Gram-negative species, especially Gram-positive isolates such as S. aureus and B. cereus [173]. Although the C16-DABCO-dendrimer did not show intrinsic antibiofilm effects, it was observed that membranes treated with 1 mg/mL C16-DABCO-dendrimer inhibited the formation of S. aureus BF, thus establishing its future use in pre-treating membranes and preventing BF formation [173]. Fuentes-Paniagua et al. focused on the antibacterial activity against S. aureus and E. coli of two types of cationic carbosilane (CBS) dendrimers and dendrons and their hemolytic properties [78]. SAR studies were carried out to evaluate the influence of the generation, type of peripheral groups near the cationic charges, core of dendrimers, and focal point of dendrons (-N3, -NH2, -OH) on the antibacterial activity of such compounds. These studies evidenced the importance of an adequate balance between the hydrophilic and lipophilic fragments of these molecules. The lowest hemolytic toxicity was registered for dendrimer systems with a sulfur atom close to the surface and when dendrons had a hydroxyl central point. One dendrimer and one dendron, both bearing a sulfur atom close to the surface, scored best in the activity–toxicity relationship analyses, and were chosen for resistance assays [78]. No changes in the inhibitory and bactericidal capacity were observed in the case of the dendron while only a slight increase in these values was noted for the dendrimer after 15 subculture cycles. Furthermore, these two compounds remained active towards different strains of resistant bacteria and prevented the formation of BF at concentrations over the minimum inhibitory concentration (MIC) [78]. In their study in 2019, Barrios-Gumiel and co-workers reported on the preparation of silver nanoparticles (AgNP) covered with cationic CBS and poly (ethylene glycol) (PEG), having antibacterial properties, antifouling effects, and improved biocompatibility due to the presence of PEG [174]. The new family of hetero-functionalized AgNPs was directly synthesized using silver precursor and cationic CBS and PEG ligands containing a thiol moiety. AgNPs were characterized by TEM, TGA, UV, 1H NMR, DLS, Z potential, and XRD. The antibacterial ability of these systems was evaluated against E. coli and S. aureus, evidencing that the effects obtained for PEGylated systems were slightly lower than those observed for non-PEGylated AgNPs, compensated for by the greater biocompatibility [174]. Furthermore, the authors tested one non-PEGylated and one PEGylated AgNP dendron for their tendency to induce resistance in a planktonic state. Both AgNP-based dendrons barely affected the minimum inhibitory concentration (MIC), whereas the reference antibiotics generated significant resistance [174]. Concerning the topic of the present paper, a relevant improvement in BF inhibition was achieved by dendronized AgNPs after PEGylation [174]. Heredero-Bermejo and Casanova focused on the discovery of dendrimers active against Candida spp., which is one of the most common fungal pathogens, the BFs of which, especially those by C. albicans, offer resistance mechanisms against most antifungal agents [60]. In their work of 2020, the authors carried out a study concerning the in vitro effects of different cationic CBS dendrimers against BF formation and mature BFs by both Colección Española de Cultivos Tipo (CECT) 1002 and clinical C. albicans strains [60]. One out of 14 dendritic molecules tested, BDSQ024 showed the highest activity with a minimum BF inhibitory concentration (MBIC) for BF formation and a minimum BF damaging concentration (MBDC) for existing BF of 16–32 and 16 mg/L, respectively [60]. Additionally, synergistic effects at non-cytotoxic concentrations were detected both with amphotericin (AmB) and caspofungin (CSF) [60]. Subsequently, the research group of Gómez-Casanova et al., using in vitro tests, evaluated the ability of three different generations of cationic CBS dendrons to inhibit the initial formation of C. albicans BF and disaggregate the mature BF [175]. Concerning their synthesis, the authors obtained highly water-soluble dendrons (ArCO2Gn(SNMe3I)m) starting from 4-phenylbutyric acid. The interaction with the cell membranes of the generated systems was found to be dependent on the hydrophilic/lipophilic balance (HLB), which increases with the number of the dendritic generation [175]. Although the compounds showed some toxicity, they showed good antifungal activity against C. albicans by inhibiting both the first formation of BF and causing its dispersal [175]. Furthermore, among all the tested dendrons, the second-generation sendron with four positive charges, ArCO2G2 (SNMe3I)4, was found to be the most effective in inhibiting the formation of BF, thus suggesting its hypothetical use as a disinfectant solution for nosocomial surfaces or as a lotion to treat skin infections [175]. Furthermore, ArCO2G2 (SNMe3I)4, when combined with ethylenediaminetetraacetic acid (EDTA) and silver nitrate (AgNO3), was able to work on a mature BF, deforming the cell wall and morphology of C. albicans [175]. Combination therapy by the association of conventional antibiotics with new compounds represents one of the main strategies used for countering infections by MDR pathogens that produce BFs. Since CBS dendrimers have been proven to be a promising solution for counteracting the formation of BFs, Fernandez et al. developed a new strategy to prevent the formation and/or disaggregation of S. aureus BF using a combination of a second-generation cationic CBS dendron with a maleimide group in the focal point and four positive charges MalG2(SNHMe2Cl)4 and levofloxacin (LEV) [176]. With the same purpose, LEV was also combined with a nanoconjugate, formed by a CBS dendron and a cell-penetrating peptide (gH625), called dendron-gH625 nanoconjugate (DPC). The data obtained by Fernandez et al. demonstrated that the combination therapy led to a greater anti-BF effect compared to the concentrations tested individually [176]. In particular, the highest percentages of inhibition were obtained from the combination of DPC-LEV, indicating it is a possible alternative option for BF-associated infections [176]. Moreover, Galdiero et al. (2020) [177] studied the antimicrobial and antibiofilm activities of an analogue of the peptide gH625 (namely gH625-M), a membranotropic peptide described to be a cell-penetrating peptide capable of interacting and disrupting the bilayers of membranes without pore formation. gH625-M was obtained by binding a sequence of lysine residues at the C-terminus, responsible for specific interactions with the negative charges of bacterial membranes [177]. The results obtained by Galdiero et al. showed that the gH625 peptide possessed low antibacterial activity against planktonic cells while it inhibited the initial BF formation of C. tropicalis, C. serratia, C. marcescens, and S. aureus and was also capable of disintegrating mature BFs formed on silicone surfaces [177]. Furthermore, Galdiero et al., exploiting combination therapy, developed a promising strategy to enhance the efficacy of conventional drugs against BFs by evaluating the possible synergism between gH625-M and commercial antifungal drugs such as amphotericin B (AmphB), fluconazole (FC), echinocandins, and 5 flucytosine (5-FLC) [177]. Among these combinations, gH625-M/FC and gH625-M/5-FLC proved to be effective against BF, unlike single use, even at high concentrations, of antifungals [177]. Very recently, the group of Quintana-Sanchez searched for new microbicide compounds against difficult-to-eradicate BF-forming bacteria, studying cationic multivalent dendrimers both as antibacterial agents and as carriers of active molecules [178]. Particularly, they estimated the antimicrobial activity of cationic CBS dendrimers, unmodified or modified with PEG residues, against planktonic and BF-forming P. aeruginosa colonies. This study revealed that the presence of PEG subverted the hydrophilic/hydrophobic balance, and reduced the antibacterial activity, as confirmed by different analytical techniques. On the other hand, the activity was improved by the combination of the CBS dendrimers with endolysin, a bacteriophage-encoded peptidoglycan hydrolase [178]. This enzyme, when used in the absence of the cationic CBS dendrimers, is ineffective against Gram-negative bacteria due to the protective outer membrane shield. On the contrary, the endolysin–CBS dendrimer combination enabled penetration through the membrane and then deterioration of the peptidoglycan layer, providing a synergic antimicrobial effect [178]. To provide readers with an eye-catching full vision of the reviewed scenario, the information reported in the main text is summarized in Table 6.
Table 6.
Antibiofilm dendrimers developed in the last fifteen years.
| Mechanism of Action | Type of Dendrimers | Target Pathogens |
Preventing BF Formation |
Hindering BF Development |
BF Dispersal | Joint Therapy | [Ref.] Year |
|---|---|---|---|---|---|---|---|
| Inhibition LecB | FD2 | P. aeruginosa |

|

|

|

|
[156] 2008 |
| 2G3 |

|

|

|

|
[157] 2009 | ||
| Membrane (Ms) Disruptors |
(RW) 4D | E. coli |

|

|

|

|
[158] 2009 |
| Inhibition LecB | D-FD2 | P. aeruginosa |

|

|

|

|
[159] 2011 |
| Inhibition LecA | GalAG2 and GalBG2 |

|

|

|

|
[160] 2011 | |
| Ms disruptors | (RW)4-NH2 | E. coli |

|

|

|
20 µM +0.5 µg/mL * 0% BF cells | [161] 2011 |
| (RW) 4D | E. coli HM22 |

|

|

|
20 µM +0.5 µg/mL * <10% BF cells | ||
| Ti-S(CaPO4)060-PEGPAMAMs Ti-S-060-PEGPAMAMs |
P. aeruginosa
S. aureus |

|

|

|

|
[162] 2011 | |
| SB056 | S. epidermidis P. aeruginosa |

|

|

|

|
[163] 2012 | |
| Ms disruptors Release of NO |
NO-releasing PAMAMs # | P. aeruginosa |

|

|

|

|
[164] 2013 |
| Inhibition LecA Inhibition LecB |
TGPDs | P. aeruginosa |

|

|

|

|
[165] 2013 |
| Membranolytic Apoptotic |
Cationic antifungal peptide dendrimers (9, 14) |
C. albicans |

|

|

|

|
[166] 2015 |
| Outer cell wall damage Absence of true hyphae Filamentation inhibition Ms permeabilization |
AgNPs | C. albicans |

|

|

|

|
[59] 2015 |
| Outer cell wall damage Ms permeabilization BF mass penetration |
2D-24 | P. aeruginosa |

|

|

|
Ciprofloxacin (Cip) 1
Tobramycin (Tob) **, 1 Carbenicillin (Car) 1 |
[152] 2015 |
| Ms disruptors Release of NO |
(G1–G4)-NO-releasing alkyl-PAMAMs # |
P. aeruginosa S. aureus MRSA |

|

|

|

|
[167] 2015 |
| Electrostatic interactions Ms disruptors Release of NO |
(G1)-NO-releasing alkyl PAMAMs # |
S. mutans |

|

|

|

|
[169] 2016 |
| Inhibition LecA | G3/G4 analogous of GalAG2 and GalBG2 | P. aeruginosa |

|

|

|

|
[170] 2016 |
| Inhibition LecA Inhibition LecB |
Het1G2-Het8G2 Het1G1Cys-Het8G1Cys Others |
P. aeruginosa |

|

|

|
Dendrimer FD2+Tob | [171] 2016 |
| Ms disruptors | den-SB056-1 | S. epidermidis |

|

|

|

|
[172] 2016 |
| C16-DABCO-G4-PAMAM |
S. aureus
B. cereus |
 § § |

|

|

|
[173] 2016 | |
| NH4+ carbosilane dendrimers and dendrons (CBSD) |
S. aureus CECT240 |

|

|

|

|
[78] 2016 | |
| Ms disruptor Ag release |
PEGylated-NH4+ CBSD-AgNPs |
S. aureus
E. coli |

|

|

|

|
[174] 2019 |
| Ms disruptor | BDSQ024 | C. albicans |

|

|

|
Amphotericin (AmphB) Caspofungin (CSF) |
[60] 2020 |
| ArCO2G2 (SNMe3I)4 | C. albicans |

|

|
 2
2
|
Ethylenediaminetetraacetic acid (EDTA) + silver nitrate (AgNO3) | [175] 2021 | |
| Interaction/disruption of the Ms bilayers | MalG2(SNHMe 2Cl)4
DPC |
S. aureus |
 2
2
|
 2
2
|

|
Levofloxacin (LEV) | [176] 2021 |
| Interaction/disruption of the Ms bilayers No pore formation |
gH625-M 3 |
C. tropicalis
C. serratia C. marcescens S. aureus |

|

|

|
AmphB 4
Fluconazole (FC) Echinocandins 4 5 Flucytosine (5-FLC) |
[177] 2020 |
| Interaction/disruption of the Ms bilayers | PEG/no-PEG cationic CBSD | P. aeruginosa |
 2
2
|
 2
2
|
 2
2
|
Endolysin | [178] 2022 |
* ofloxacin; ** best association; 1 target the synthesis of DNA, proteins, and cell wall; # NO payloads of ~1.0 μmol/mg;  = reduced with respect to SB056; § the treatment of membranes with 1 mg/mL causes complete inhibition of the growth of S. aureus BFs; 2 when in combination as in the column 7; 3 analogous of the cell penetratin peptide gH625; 4 not functioning; HM22 denotes a strain containing the hipA7 allele, which maps to the hipA gene in the antitoxin-toxin module HipBA. The expression of the hipA7 allele confers a 1000-fold higher frequency of persister cells formation; AgNPs = silver nanoparticles; MRSA = methicillin-resistant S. aureus;
= reduced with respect to SB056; § the treatment of membranes with 1 mg/mL causes complete inhibition of the growth of S. aureus BFs; 2 when in combination as in the column 7; 3 analogous of the cell penetratin peptide gH625; 4 not functioning; HM22 denotes a strain containing the hipA7 allele, which maps to the hipA gene in the antitoxin-toxin module HipBA. The expression of the hipA7 allele confers a 1000-fold higher frequency of persister cells formation; AgNPs = silver nanoparticles; MRSA = methicillin-resistant S. aureus;  = not reported;
= not reported;  = yes, active;
= yes, active;  = no, not active.
= no, not active.
3.3. Promising Areas for Modifying Dendrimer Matrices
As mentioned and confirmed by some case studies reported in the previous section, among cationic dendrimers that are also active on BFs, those with PAMAM, PPI, PEI, poly (lysine), and peptides matrices have been the most extensively prepared and evaluated [63]. Moreover, other structures, such as ammonium-terminated (AT) dendrimers, including AT phosphorous and carbosilane dendrimers, attracted the interest of scientists starting from 2015 [63]. Curiously, even though they were considered to be very attractive for biomedical applications as they are highly biodegradable and have low cytotoxicity, only very recently have polyester-based scaffolds, peripherally cationic for the presence of amino acids, been taken into consideration as novel antimicrobial and or bactericidal devices with very interesting results [4,63,68,69,70,71,72,155]. Regardless, concerning our knowledge, no dendrimer compound of this family has been tested as an antibiofilm agent so far. Particularly, these dendrimers, by presenting an uncharged hydrolysable matrix that is capable of balancing the density of charge on their surface better while retaining strong antibacterial activity, have reduced toxicity [63].
Within this category, peripherally amino acid-modified, polyester-based dendrimer scaffolds, obtained starting from 2,2-bis(hydroxymethyl)propanoic acid (bis-HMPA), as an AB2 monomer, have several features that make them particularly suitable as novel antimicrobial agents. The polyester-based uncharged matrices of these structures, while characterized by good biodegradability, due to the easy physiological hydrolysis, can be esterified with a high number of amino acids, supplying high multivalency, which has been demonstrated to be pivotal for exerting antibiofilm effects. Unfortunately, research in this field is still very limited and, as far as our knowledge is concerned, the literature presents only few studies, without any research on possible antibiofilm effects. In our opinion, this represents a good direction for implementing further studies on the development of new high-generation polyester dendrons and dendrimers peripherally decorated with amino acids to be assessed as antibiofilm agents or assessing the possible antibiofilm activity of those already reported as bactericidal agents.
4. Conclusions
Research on the discovery, design, and synthesis of new antimicrobial agents that are also active against BFs and ideally in all phases of its development and/or the development of effective antibiofilm strategies represents the daily challenge of microbiologists, pharmaceutics, organic chemists, pharmacologists, and all scientists who work for global health. The onset of resistance is a phenomenon that affects human health worldwide, but the form of resistance that pathogens develop by BF formation is a dramatic plague against which no current clinically approved drug works. BFs are responsible for the chronicization of BF-associated and biomaterial-associated infections (BAIs), especially those connected with nosocomial and healthcare settings, which translates to increased therapeutic costs and mortality. In this worrying scenario, a recent promising approach to counteract BAIs consists of the use of NAMPs as alternative compounds to existing no-longer-functioning antibiotics due to their mechanism of action as disruptors of the contact of bacterial membranes, which overcome bacterial resistance and limit diffusion. Unfortunately, due to their high costs of production, hemolytic toxicity, and, above all, fast in vivo inactivation by peptidases, their clinical use is still limited. In recent decades, NAMPs have inspired the synthesis of new cationic peptides also in the form of polymers and dendrimer nanoparticles (NPs) that merge the antimicrobial mechanism of NAMPs with the multivalence of polymers and dendrimers, and the nonpareil properties of NPs. While cationic polymer and copolymer NPs have been and are extensively studied for antimicrobial applications, including antibiofilm ones, although the first dendrimers were synthesized in the mid-1980s, and there are several biomedical uses, their use as antibacterial agents, as either drugs, surface coating agents, or drug-delivery systems, has only recently been recognized. In the last decade, dendrimers have mainly been synthesized for the treatment of infections caused by MDR pathogens and some of these have been shown to have an antibiofilm action, but their therapeutic use is still very distant. Here, aiming to incite further development of new antibacterial dendrimers capable of inhibiting the first BF formation and, hopefully, destroying mature BFs, we reviewed the cationic dendrimers developed to this end in the last fifteen years and provided a useful table collecting this information. From this study, it appears that in addition to dendrimer peptides, other forms of cationic dendrimers possess promising antibiofilm activities, being membranotropic molecules that are able to adhere to bacterial membranes and create damage and penetrate the BF biomass. Additionally, the present work evidences that a promising strategy using cationic dendrimers is represented by combination therapy, allowing the combination of conventional drugs with new compounds in order to create synergism following the interaction between the two molecules used. This strategy allows better antibiofilm activity than that obtained with the use of single drugs and the use of lower concentrations of the two ingredients, thus reducing their possible cytotoxicity and, in some cases, increasing the susceptibility of pathogens to antibiotics. We are confident that the extension of the knowledge about these promising but still little-explored materials is a successful approach for discovering new effective weapons for treating chronic infections and biomaterial-associated infections sustained by BF-producing MDR bacteria.
Author Contributions
The authors have contributed equally to this article. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vadivelu J. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Formatex Research Center; Copenhagen, Denmark: 2013. [Google Scholar]
- 2.Popęda M., Płuciennik E., Bednarek A.K. Proteins in cancer resistance. Postępy Hig. Med. Do’swiadczalnej. 2014;68:616–632. doi: 10.5604/17322693.1103268. [DOI] [PubMed] [Google Scholar]
- 3.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Alfei S., Caviglia D., Zorzoli A., Marimpietri D., Spallarossa A., Lusardi M., Zuccari G., Schito A.M. Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole. Biomedicines. 2022;10:907. doi: 10.3390/biomedicines10040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikaido H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 7.Loeffler J., Stevens D.A. Antifungal drug resistance. Clin. Infect. Dis. 2003;36((Suppl. 1)):S31–S41. doi: 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- 8.Rodero L., Mellado E., Rodriguez A.C., Salve A., Guelfand L., Cahn P., Cuenca-Estrella M., Davel G., Rodriguez-Tudela J.L. G484S Amino Acid Substitution in Lanosterol 14-α Demethylase (ERG11) Is Related to Fluconazole Resistance in a Recurrent Cryptococcus neoformans Clinical Isolate. Antimicrob. Agents Chemother. 2003;47:3653–3656. doi: 10.1128/AAC.47.11.3653-3656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard S.J., Arendrup M.C. Acquired Antifungal Drug Resistance in Aspergillus Fumigatus: Epidemiology and Detection. Med. Mycol. 2011;49:S90–S95. doi: 10.3109/13693786.2010.508469. [DOI] [PubMed] [Google Scholar]
- 10.Cuenca-Estrella M., Gomez-Lopez A., Mellado E., Buitrago M.J., Monzón A., Rodriguez-Tudela J.L. Scopulariopsis brevicaulis, a Fungal Pathogen Resistant to Broad-Spectrum Antifungal Agents. Antimicrob. Agents Chemother. 2003;47:2339–2341. doi: 10.1128/AAC.47.7.2339-2341.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lurain N.S., Chou S. Antiviral Drug Resistance of Human Cytomegalovirus. Clin. Microbiol. Rev. 2010;23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wutzler P. Antiviral Therapy of Herpes Simplex and Varicella-Zoster Virus Infections. Intervirology. 1997;40:343–356. doi: 10.1159/000150567. [DOI] [PubMed] [Google Scholar]
- 13.Cortez K.J., Maldarelli F. Clinical Management of HIV Drug Resistance. Viruses. 2011;3:347–378. doi: 10.3390/v3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurt A.C. The Epidemiology and Spread of Drug Resistant Human Influenza Viruses. Curr. Opin. Virol. 2014;8:22–29. doi: 10.1016/j.coviro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Suppiah J., Mohd Zain R., Haji Nawi S., Bahari N., Saat Z. Drug-Resistance Associated Mutations in Polymerase (P) Gene of Hepatitis B Virus Isolated from Malaysian HBV Carriers. Hepat. Mon. 2014;14:e13173. doi: 10.5812/hepatmon.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloland P.B., World Health Organization Anti-Infective Drug Resistance Surveillance and Containment Team. Drug Resistance in Malaria. 2001. [(accessed on 19 August 2022)]. Available online: https://apps.who.int/iris/handle/10665/66847.
- 17.Vanaerschot M., Dumetz F., Roy S., Ponte-Sucre A., Arevalo J., Dujardin J.-C. Treatment Failure in Leishmaniasis: Drug-Resistance or Another (Epi-) Phenotype? Expert Rev. Anti-Infect. Ther. 2014;12:937–946. doi: 10.1586/14787210.2014.916614. [DOI] [PubMed] [Google Scholar]
- 18.Mohapatra S. Drug Resistance in Leishmaniasis: Newer Developments. Trop. Parasitol. 2014;4:4–9. doi: 10.4103/2229-5070.129142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallon P.G., Doenhoff M.J. Drug-Resistant Schistosomiasis: Resistance to Praziquantel and Oxamniquine Induced in Schistosoma mansoni in Mice Is Drug Specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 20.Qi L., Cui J. A Schistosomiasis Model with Praziquantel Resistance. Discret. Dyn. Nat. Soc. 2013;2013:e945767. doi: 10.1155/2013/945767. [DOI] [Google Scholar]
- 21.Bansal D., Malla N., Mahajan R. Drug Resistance in Amoebiasis. Indian J. Med. Res. 2006;123:115–118. [PubMed] [Google Scholar]
- 22.Muzny C.A., Schwebke J.R. The Clinical Spectrum of Trichomonas Vaginalis Infection and Challenges to Management. Sex. Transm. Infect. 2013;89:423–425. doi: 10.1136/sextrans-2012-050893. [DOI] [PubMed] [Google Scholar]
- 23.McFadden D.C., Tomavo S., Berry E.A., Boothroyd J.C. Characterization of Cytochrome b from Toxoplasma gondii and Qo Domain Mutations as a Mechanism of Atovaquone-Resistance. Mol. Biochem. Parasitol. 2000;108:1–12. doi: 10.1016/S0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 24.Nagamune K., Moreno S.N.J., Sibley L.D. Artemisinin-Resistant Mutants of Toxoplasma gondii Have Altered Calcium Homeostasis. Antimicrob. Agents Chemother. 2007;51:3816–3823. doi: 10.1128/AAC.00582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doliwa C., Escotte-Binet S., Aubert D., Sauvage V., Velard F., Schmid A., Villena I. Sulfadiazine Resistance in Toxoplasma gondii: No Involvement of Overexpression or Polymorphisms in Genes of Therapeutic Targets and ABC Transporters. Parasite. 2013;20:19. doi: 10.1051/parasite/2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullman B. Multidrug Resistance and P-Glycoproteins in Parasitic Protozoa. J. Bioenerg. Biomembr. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg R.M. New Approaches for Understanding Mechanisms of Drug Resistance in Schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoodley P., Sauer K., Davies D.G., Costerton J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 29.Dincer S., Özdenefe M.S., Arkut A. Bacterial Biofilms. BoD—Books on Demand; Norderstedt, Germany: 2020. [Google Scholar]
- 30.Kostakioti M., Hadjifrangiskou M., Hultgren S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013;3:a010306. doi: 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjarnsholt T., Ciofu O., Molin S., Givskov M., Høiby N. Applying Insights from Biofilm Biology to Drug Development—Can a New Approach Be Developed? Nat. Rev. Drug Discov. 2013;12:791–808. doi: 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- 32.O’Toole G., Kaplan H.B., Kolter R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Cucarella C., Solano C., Valle J., Amorena B., Lasa Í., Penadés J.R. Bap, a Staphylococcus aureus Surface Protein Involved in Biofilm Formation. J. Bacteriol. 2001;183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branda S.S., Vik Å., Friedman L., Kolter R. Biofilms: The Matrix Revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Costerton W., Veeh R., Shirtliff M., Pasmore M., Post C., Ehrlich G. The Application of Biofilm Science to the Study and Control of Chronic Bacterial Infections. J. Clin. Invest. 2003;112:1466–1477. doi: 10.1172/JCI200320365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joo H.-S., Otto M. Molecular Basis of In Vivo Biofilm Formation by Bacterial Pathogens. Chem. Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasudevan R. Biofilms: Microbial cities of scientific significance. J. Microbiol. Exp. 2014;1:84–98. doi: 10.15406/jmen.2014.01.00014. [DOI] [Google Scholar]
- 38.Jamal M., Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Hussain T., Ali M., Rafiq M., Kamil M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Hathroubi S., Mekni M.A., Domenico P., Nguyen D., Jacques M. Biofilms: Microbial Shelters Against Antibiotics. Microb. Drug Resist. 2017;23:147–156. doi: 10.1089/mdr.2016.0087. [DOI] [PubMed] [Google Scholar]
- 40.Gebreyohannes G., Nyerere A., Bii C., Sbhatu D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon. 2019;5:e02192. doi: 10.1016/j.heliyon.2019.e02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 42.Costerton J.W., Cheng K.J., Geesey G.G., Ladd T.I., Nickel J.C., Dasgupta M., Marrie T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 43.Costerton J.W., Montanaro L., Arciola C.r. Bacterial Communications in Implant Infections: A Target for an Intelligence War. Int. J. Artif. Organs. 2007;30:757–763. doi: 10.1177/039139880703000903. [DOI] [PubMed] [Google Scholar]
- 44.Wolcott R.d., Rhoads D.d., Bennett M.e., Wolcott B.m., Gogokhia L., Costerton J.w., Dowd S.e. Chronic Wounds and the Medical Biofilm Paradigm. J. Wound Care. 2010;19:45–53. doi: 10.12968/jowc.2010.19.2.46966. [DOI] [PubMed] [Google Scholar]
- 45.James G.A., Swogger E., Wolcott R., deLancey Pulcini E., Secor P., Sestrich J., Costerton J.W., Stewart P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 46.Bjarnsholt T. The Role of Bacterial Biofilms in Chronic Infections. APMIS. 2013;121:1–58. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 47.Gjødsbøl K., Christensen J.J., Karlsmark T., Jørgensen B., Klein B.M., Krogfelt K.A. Multiple Bacterial Species Reside in Chronic Wounds: A Longitudinal Study. Int. Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyczak J.B., Cannon C.L., Pier G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 50.Oliver A., Cantón R., Campo P., Baquero F., Blázquez J. High Frequency of Hypermutable Pseudomonas aeruginosa in Cystic Fibrosis Lung Infection. Science. 2000;288:1251–1253. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 51.Maciá M.D., Blanquer D., Togores B., Sauleda J., Pérez J.L., Oliver A. Hypermutation Is a Key Factor in Development of Multiple-Antimicrobial Resistance in Pseudomonas aeruginosa Strains Causing Chronic Lung Infections. Antimicrob. Agents Chemother. 2005;49:3382–3386. doi: 10.1128/AAC.49.8.3382-3386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann M., Vaudaux P.E., Pittet D., Auckenthaler R., Lew P.D., Perdreau F.S., Peters G., Waldvogel F.A. Fibronectin, Fibrinogen, and Laminin Act as Mediators of Adherence of Clinical Staphylococcal Isolates to Foreign Material. J. Infect. Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 53.Nobile C.J., Mitchell A.P. Genetics and Genomics of Candida albicans Biofilm Formation. Cell. Microbiol. 2006;8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 54.Nobile C.J., Johnson A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gökmenoğlu C., Kara N.B., Beldüz M., Kamburoğlu A., Tosun İ., Sadik E., Kara C. Evaluation of Candida albicans Biofilm Formation on Various Parts of Implant Material Surfaces. Niger. J. Clin. Pract. 2018;21:33–37. doi: 10.4314/njcp.v21i1. [DOI] [PubMed] [Google Scholar]
- 56.Bouza E., Guinea J., Guembe M. The Role of Antifungals against Candida Biofilm in Catheter-Related Candidemia. Antibiotics. 2015;4:1–17. doi: 10.3390/antibiotics4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uppuluri P., Pierce C.G., López-Ribot J.L. Candida albicans Biofilm Formation and Its Clinical Consequences. Future Microbiol. 2009;4:1235–1237. doi: 10.2217/fmb.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gulati M., Nobile C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016;18:310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lara H.H., Romero-Urbina D.G., Pierce C., Lopez-Ribot J.L., Arellano-Jiménez M.J., Jose-Yacaman M. Effect of Silver Nanoparticles on Candida albicans Biofilms: An Ultrastructural Study. J. Nanobiotechnol. 2015;13:91. doi: 10.1186/s12951-015-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heredero-Bermejo I., Gómez-Casanova N., Quintana S., Soliveri J., de la Mata F.J., Pérez-Serrano J., Sánchez-Nieves J., Copa-Patiño J.L. In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida albicans Biofilms. Pharmaceutics. 2020;12:918. doi: 10.3390/pharmaceutics12100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Q., Zhang Y., Wang H., Brash J., Chen H. Anti-Fouling Bioactive Surfaces. Acta Biomater. 2011;7:1550–1557. doi: 10.1016/j.actbio.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 62.Wei T., Yu Q., Chen H. Antibacterial Coatings: Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019;8:1970007. doi: 10.1002/adhm.201970007. [DOI] [PubMed] [Google Scholar]
- 63.Alfei S., Schito A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials. 2020;10:2022. doi: 10.3390/nano10102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfei S., Grazia Signorello M., Schito A., Catena S., Turrini F. Reshaped as Polyester-Based Nanoparticles, Gallic Acid Inhibits Platelet Aggregation, Reactive Oxygen Species Production and Multi-Resistant Gram-Positive Bacteria with an Efficiency Never Obtained. Nanoscale Adv. 2019;1:4148–4157. doi: 10.1039/C9NA00441F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alfei S., Marengo B., Domenicotti C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants. 2020;9:50. doi: 10.3390/antiox9010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alfei S., Catena S., Turrini F. Biodegradable and Biocompatible Spherical Dendrimer Nanoparticles with a Gallic Acid Shell and a Double-Acting Strong Antioxidant Activity as Potential Device to Fight Diseases from “Oxidative Stress”. Drug Deliv. Transl. Res. 2020;10:259–270. doi: 10.1007/s13346-019-00681-8. [DOI] [PubMed] [Google Scholar]
- 67.Alfei S., Marengo B., Zuccari G., Turrini F., Domenicotti C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials. 2020;10:1243. doi: 10.3390/nano10061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alfei S., Brullo C., Caviglia D., Piatti G., Zorzoli A., Marimpietri D., Zuccari G., Schito A.M. Pyrazole-Based Water-Soluble Dendrimer Nanoparticles as a Potential New Agent against Staphylococci. Biomedicines. 2022;10:17. doi: 10.3390/biomedicines10010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schito A.M., Caviglia D., Piatti G., Zorzoli A., Marimpietri D., Zuccari G., Schito G.C., Alfei S. Efficacy of Ursolic Acid-Enriched Water-Soluble and Not Cytotoxic Nanoparticles against Enterococci. Pharmaceutics. 2021;13:1976. doi: 10.3390/pharmaceutics13111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schito A.M., Schito G.C., Alfei S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers. 2021;13:521. doi: 10.3390/polym13040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schito A.M., Alfei S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers. 2020;12:1818. doi: 10.3390/polym12081818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alfei S., Caviglia D., Piatti G., Zuccari G., Schito A.M. Bactericidal Activity of a Self-Biodegradable Lysine-Containing Dendrimer against Clinical Isolates of Acinetobacter Genus. Int. J. Mol. Sci. 2021;22:7274. doi: 10.3390/ijms22147274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polcyn P., Jurczak M., Rajnisz A., Solecka J., Urbanczyk-Lipkowska Z. Design of Antimicrobially Active Small Amphiphilic Peptide Dendrimers. Molecules. 2009;14:3881–3905. doi: 10.3390/molecules14103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janiszewska J., Sowińska M., Rajnisz A., Solecka J., Łącka I., Milewski S., Urbańczyk-Lipkowska Z. Novel Dendrimeric Lipopeptides with Antifungal Activity. Bioorg. Med. Chem. Lett. 2012;22:1388–1393. doi: 10.1016/j.bmcl.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 75.Winnicka K., Wroblewska M., Wieczorek P., Sacha P.T., Tryniszewska E. Hydrogel of Ketoconazole and PAMAM Dendrimers: Formulation and Antifungal Activity. Molecules. 2012;17:4612–4624. doi: 10.3390/molecules17044612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stolarska M., Gucwa K., Urbańczyk-Lipkowska Z., Andruszkiewicz R. Peptide Dendrimers as Antifungal Agents and Carriers for Potential Antifungal Agent—N3-(4-Methoxyfumaroyl)-(S)-2,3-Diaminopropanoic Acid—Synthesis and Antimicrobial Activity. J. Pept. Sci. 2020;26:e3226. doi: 10.1002/psc.3226. [DOI] [PubMed] [Google Scholar]
- 77.Gómez-Casanova N., Copa-Patiño J.L., Heredero-Bermejo I. Candida and Candidiasis. IntechOpen; London, UK: 2022. Novel Treatment Approach against Candida spp.: Evaluation of Antifungal and Antibiofilm In Vitro Activity of Dendritic Molecules. [DOI] [Google Scholar]
- 78.Fuentes-Paniagua E., Sánchez-Nieves J., Hernández-Ros J.M., Fernández-Ezequiel A., Soliveri J., Copa-Patiño J.L., Gómez R., de la Mata F.J. Structure–Activity Relationship Study of Cationic Carbosilane Dendritic Systems as Antibacterial Agents. RSC Adv. 2016;6:7022–7033. doi: 10.1039/C5RA25901K. [DOI] [Google Scholar]
- 79.Winnicka K., Sosnowska K., Wieczorek P., Sacha P.T., Tryniszewska E. Poly(Amidoamine) Dendrimers Increase Antifungal Activity of Clotrimazole. Biol. Pharm. Bull. 2011;34:1129–1133. doi: 10.1248/bpb.34.1129. [DOI] [PubMed] [Google Scholar]
- 80.Gorain B., Pandey M., Choudhury H., Jain G.K., Kesharwani P. Chapter 15—Dendrimer for Solubility Enhancement. In: Kesharwani P., editor. Dendrimer-Based Nanotherapeutics. Academic Press; Cambridge, MA, USA: 2021. pp. 273–283. [Google Scholar]
- 81.Verstraeten N., Braeken K., Debkumari B., Fauvart M., Fransaer J., Vermant J., Michiels J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008;16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Tolker-Nielsen T. Biofilm Development. Microbiol. Spectr. 2015;3:MB-0001-2014. doi: 10.1128/microbiolspec.MB-0001-2014. [DOI] [PubMed] [Google Scholar]
- 83.Gjermansen M., Nilsson M., Yang L., Tolker-Nielsen T. Characterization of Starvation-Induced Dispersion in Pseudomonas putida Biofilms: Genetic Elements and Molecular Mechanisms. Mol. Microbiol. 2010;75:815–826. doi: 10.1111/j.1365-2958.2009.06793.x. [DOI] [PubMed] [Google Scholar]
- 84.Tischler A.D., Camilli A. Cyclic Diguanylate (c-Di-GMP) Regulates Vibrio Cholerae Biofilm Formation. Mol. Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim B., Beyhan S., Meir J., Yildiz F.H. Cyclic-DiGMP Signal Transduction Systems in Vibrio cholerae: Modulation of Rugosity and Biofilm Formation. Mol. Microbiol. 2006;60:331–348. doi: 10.1111/j.1365-2958.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- 86.Hickman J.W., Tifrea D.F., Harwood C.S. A Chemosensory System That Regulates Biofilm Formation through Modulation of Cyclic Diguanylate Levels. Proc. Natl. Acad. Sci. USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kulesekara H., Lee V., Brencic A., Liberati N., Urbach J., Miyata S., Lee D.G., Neely A.N., Hyodo M., Hayakawa Y., et al. Analysis of Pseudomonas aeruginosa Diguanylate Cyclases and Phosphodiesterases Reveals a Role for Bis-(3′-5′)-Cyclic-GMP in Virulence. Proc. Natl. Acad. Sci. USA. 2006;103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrova O.E., Sauer K. A Novel Signaling Network Essential for Regulating Pseudomonas aeruginosa Biofilm Development. PLoS Pathog. 2009;5:e1000668. doi: 10.1371/journal.ppat.1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petrova O.E., Sauer K. SagS Contributes to the Motile-Sessile Switch and Acts in Concert with BfiSR to Enable Pseudomonas aeruginosa Biofilm Formation. J. Bacteriol. 2011;193:6614–6628. doi: 10.1128/JB.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colvin K.M., Irie Y., Tart C.S., Urbano R., Whitney J.C., Ryder C., Howell P.L., Wozniak D.J., Parsek M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S.-K., Lee J.-H. Biofilm Dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016;54:71–85. doi: 10.1007/s12275-016-5528-7. [DOI] [PubMed] [Google Scholar]
- 92.Harmsen M., Yang L., Pamp S.J., Tolker-Nielsen T. An Update on Pseudomonas aeruginosa Biofilm Formation, Tolerance, and Dispersal. FEMS Immunol. Med. Microbiol. 2010;59:253–268. doi: 10.1111/j.1574-695X.2010.00690.x. [DOI] [PubMed] [Google Scholar]
- 93.Fullagar J.L., Garner A.L., Struss A.K., Day J.A., Martin D.P., Yu J., Cai X., Janda K.D., Cohen S.M. Antagonism of a Zinc Metalloprotease Using a Unique Metal-Chelating Scaffold: Tropolones as Inhibitors of P. aeruginosa Elastase. Chem. Commun. 2013;49:3197–3199. doi: 10.1039/c3cc41191e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oglesby-Sherrouse A.G., Djapgne L., Nguyen A.T., Vasil A.I., Vasil M.L. The Complex Interplay of Iron, Biofilm Formation, and Mucoidy Affecting Antimicrobial Resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014;70:307–320. doi: 10.1111/2049-632X.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calfee M.W., Coleman J.P., Pesci E.C. Interference with Pseudomonas Quinolone Signal Synthesis Inhibits Virulence Factor Expression by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2001;98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryder C., Byrd M., Wozniak D.J. Role of Polysaccharides in Pseudomonas aeruginosa Biofilm Development. Curr. Opin. Microbiol. 2007;10:644–648. doi: 10.1016/j.mib.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jackson K.D., Starkey M., Kremer S., Parsek M.R., Wozniak D.J. Identification of Psl, a Locus Encoding a Potential Exopolysaccharide That Is Essential for Pseudomonas aeruginosa PAO1 Biofilm Formation. J. Bacteriol. 2004;186:4466–4475. doi: 10.1128/JB.186.14.4466-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Friedman L., Kolter R. Two Genetic Loci Produce Distinct Carbohydrate-Rich Structural Components of the Pseudomonas aeruginosa Biofilm Matrix. J. Bacteriol. 2004;186:4457–4465. doi: 10.1128/JB.186.14.4457-4465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang L., Hu Y., Liu Y., Zhang J., Ulstrup J., Molin S. Distinct Roles of Extracellular Polymeric Substances in Pseudomonas aeruginosa Biofilm Development. Environ. Microbiol. 2011;13:1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- 100.Yang L., Hengzhuang W., Wu H., Damkiær S., Jochumsen N., Song Z., Givskov M., Høiby N., Molin S. Polysaccharides Serve as Scaffold of Biofilms Formed by Mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2012;65:366–376. doi: 10.1111/j.1574-695X.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang S., Liu X., Liu H., Zhang L., Guo Y., Yu S., Wozniak D.J., Ma L.Z. The Exopolysaccharide Psl–EDNA Interaction Enables the Formation of a Biofilm Skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015;7:330–340. doi: 10.1111/1758-2229.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vasseur P., Vallet-Gely I., Soscia C., Genin S., Filloux A. The Pel Genes of the Pseudomonas aeruginosa PAK Strain Are Involved at Early and Late Stages of Biofilm Formation. Microbiology. 2005;151:985–997. doi: 10.1099/mic.0.27410-0. [DOI] [PubMed] [Google Scholar]
- 103.Colvin K.M., Gordon V.D., Murakami K., Borlee B.R., Wozniak D.J., Wong G.C.L., Parsek M.R. The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jennings L.K., Storek K.M., Ledvina H.E., Coulon C., Marmont L.S., Sadovskaya I., Secor P.R., Tseng B.S., Scian M., Filloux A., et al. Pel Is a Cationic Exopolysaccharide That Cross-Links Extracellular DNA in the Pseudomonas aeruginosa Biofilm Matrix. Proc. Natl. Acad. Sci. USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bagge N., Schuster M., Hentzer M., Ciofu O., Givskov M., Greenberg E.P., Høiby N. Pseudomonas aeruginosa Biofilms Exposed to Imipenem Exhibit Changes in Global Gene Expression and β-Lactamase and Alginate Production. Antimicrob. Agents Chemother. 2004;48:1175–1187. doi: 10.1128/AAC.48.4.1175-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 107.Allesen-Holm M., Barken K.B., Yang L., Klausen M., Webb J.S., Kjelleberg S., Molin S., Givskov M., Tolker-Nielsen T. A Characterization of DNA Release in Pseudomonas aeruginosa Cultures and Biofilms. Mol. Microbiol. 2006;59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 108.Mulcahy H., Charron-Mazenod L., Lewenza S. Pseudomonas aeruginosa Produces an Extracellular Deoxyribonuclease That Is Required for Utilization of DNA as a Nutrient Source. Environ. Microbiol. 2010;12:1621–1629. doi: 10.1111/j.1462-2920.2010.02208.x. [DOI] [PubMed] [Google Scholar]
- 109.Tseng B.S., Zhang W., Harrison J.J., Quach T.P., Song J.L., Penterman J., Singh P.K., Chopp D.L., Packman A.I., Parsek M.R. The Extracellular Matrix Protects Pseudomonas aeruginosa Biofilms by Limiting the Penetration of Tobramycin. Environ. Microbiol. 2013;15:2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drenkard E., Ausubel F.M. Pseudomonas Biofilm Formation and Antibiotic Resistance Are Linked to Phenotypic Variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 111.Bass J.I.F., Russo D.M., Gabelloni M.L., Geffner J.R., Giordano M., Catalano M., Zorreguieta Á., Trevani A.S. Extracellular DNA: A Major Proinflammatory Component of Pseudomonas aeruginosa Biofilms. J. Immunol. 2010;184:6386–6395. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 112.O’Toole G.A., Kolter R. Flagellar and Twitching Motility Are Necessary for Pseudomonas aeruginosa Biofilm Development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 113.Skerker J.M., Berg H.C. Direct Observation of Extension and Retraction of Type IV Pili. Proc. Natl. Acad. Sci. USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei Q., Ma L.Z. Biofilm Matrix and Its Regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2013;14:20983–21005. doi: 10.3390/ijms141020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagner V.E., Gillis R.J., Iglewski B.H. Transcriptome Analysis of Quorum-Sensing Regulation and Virulence Factor Expression in Pseudomonas aeruginosa. Vaccine. 2004;22:S15–S20. doi: 10.1016/j.vaccine.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 116.Lee J., Zhang L. The Hierarchy Quorum Sensing Network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wade D.S., Calfee M.W., Rocha E.R., Ling E.A., Engstrom E., Coleman J.P., Pesci E.C. Regulation of Pseudomonas Quinolone Signal Synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005;187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pamp S.J., Tolker-Nielsen T. Multiple Roles of Biosurfactants in Structural Biofilm Development by Pseudomonas aeruginosa. J. Bacteriol. 2007;189:2531–2539. doi: 10.1128/JB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Senturk S., Ulusoy S., Bosgelmez-Tinaz G., Yagci A. Quorum Sensing and Virulence of Pseudomonas aeruginosa during Urinary Tract Infections. J. Infect. Dev. Ctries. 2012;6:501–507. doi: 10.3855/jidc.2543. [DOI] [PubMed] [Google Scholar]
- 120.Jensen V., Löns D., Zaoui C., Bredenbruch F., Meissner A., Dieterich G., Münch R., Häussler S. RhlR Expression in Pseudomonas aeruginosa Is Modulated by the Pseudomonas Quinolone Signal via PhoB-Dependent and -Independent Pathways. J. Bacteriol. 2006;188:8601–8606. doi: 10.1128/JB.01378-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schafhauser J., Lepine F., McKay G., Ahlgren H.G., Khakimova M., Nguyen D. The Stringent Response Modulates 4-Hydroxy-2-Alkylquinoline Biosynthesis and Quorum-Sensing Hierarchy in Pseudomonas aeruginosa. J. Bacteriol. 2014;196:1641–1650. doi: 10.1128/JB.01086-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schuster M., Peter Greenberg E. A Network of Networks: Quorum-Sensing Gene Regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 123.Oglesby A.G., Farrow J.M., Lee J.-H., Tomaras A.P., Greenberg E.P., Pesci E.C., Vasil M.L. The Influence of Iron on Pseudomonas aeruginosa Physiology: A Regulatory Link Between Iron and Quorum Sensing*. J. Biol. Chem. 2008;283:15558–15567. doi: 10.1074/jbc.M707840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nadell C.D., Drescher K., Wingreen N.S., Bassler B.L. Extracellular Matrix Structure Governs Invasion Resistance in Bacterial Biofilms. ISME J. 2015;9:1700–1709. doi: 10.1038/ismej.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fleming D., Niese B., Redman W., Vanderpool E., Gordon V., Rumbaugh K.P. Contribution of Pseudomonas aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell. Infect. Microbiol. 2022;12:e835754. doi: 10.3389/fcimb.2022.835754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anderl J.N., Franklin M.J., Stewart P.S. Role of Antibiotic Penetration Limitation in Klebsiella pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000;44:1818–1824. doi: 10.1128/AAC.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bagge N., Hentzer M., Andersen J.B., Ciofu O., Givskov M., Høiby N. Dynamics and Spatial Distribution of β-Lactamase Expression in Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2004;48:1168–1174. doi: 10.1128/AAC.48.4.1168-1174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulcahy H., Charron-Mazenod L., Lewenza S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lewenza S. Extracellular DNA-Induced Antimicrobial Peptide Resistance Mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 2013;4:21. doi: 10.3389/fmicb.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johnson L., Mulcahy H., Kanevets U., Shi Y., Lewenza S. Surface-Localized Spermidine Protects the Pseudomonas aeruginosa Outer Membrane from Antibiotic Treatment and Oxidative Stress. J. Bacteriol. 2012;194:813–826. doi: 10.1128/JB.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keren I., Mulcahy L.R., Lewis K. Chapter Nineteen—Persister Eradication: Lessons from the World of Natural Products. In: Hopwood D.A., editor. Methods in Enzymology. Volume 517. Academic Press; Cambridge, MA, USA: 2012. pp. 387–406. Natural Product Biosynthesis by Microorganisms and Plants, Part C. [DOI] [PubMed] [Google Scholar]
- 132.Dörr T., Vulić M., Lewis K. Ciprofloxacin Causes Persister Formation by Inducing the TisB Toxin in Escherichia coli. PLoS Biol. 2010;8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen D., Joshi-Datar A., Lepine F., Bauerle E., Olakanmi O., Beer K., McKay G., Siehnel R., Schafhauser J., Wang Y., et al. Active Starvation Responses Mediate Antibiotic Tolerance in Biofilms and Nutrient-Limited Bacteria. Science. 2011;334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng Z., Stewart P.S. Growth Limitation of Staphylococcus Epidermidis in Biofilms Contributes to Rifampin Tolerance. Biofilms. 2004;1:31–35. doi: 10.1017/S1479050503001042. [DOI] [Google Scholar]
- 135.Pagès J.-M., James C.E., Winterhalter M. The Porin and the Permeating Antibiotic: A Selective Diffusion Barrier in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2008;6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 136.Gillis R.J., White K.G., Choi K.-H., Wagner V.E., Schweizer H.P., Iglewski B.H. Molecular Basis of Azithromycin-Resistant Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2005;49:3858–3867. doi: 10.1128/AAC.49.9.3858-3867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilton M., Charron-Mazenod L., Moore R., Lewenza S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015;60:544–553. doi: 10.1128/AAC.01650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoon S.S., Hennigan R.F., Hilliard G.M., Ochsner U.A., Parvatiyar K., Kamani M.C., Allen H.L., DeKievit T.R., Gardner P.R., Schwab U., et al. Pseudomonas aeruginosa Anaerobic Respiration in Biofilms: Relationships to Cystic Fibrosis Pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 139.Tolker-Nielsen T. Pseudomonas aeruginosa Biofilm Infections: From Molecular Biofilm Biology to New Treatment Possibilities. APMIS. 2014;122:1–51. doi: 10.1111/apm.12335. [DOI] [PubMed] [Google Scholar]
- 140.Burmølle M., Thomsen T.R., Fazli M., Dige I., Christensen L., Homøe P., Tvede M., Nyvad B., Tolker-Nielsen T., Givskov M., et al. Biofilms in Chronic Infections—A Matter of Opportunity—Monospecies Biofilms in Multispecies Infections. FEMS Immunol. Med. Microbiol. 2010;59:324–336. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 141.Eming S.A., Krieg T., Davidson J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 142.Pastar I., Nusbaum A.G., Gil J., Patel S.B., Chen J., Valdes J., Stojadinovic O., Plano L.R., Tomic-Canic M., Davis S.C. Interactions of Methicillin Resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in Polymicrobial Wound Infection. PLoS ONE. 2013;8:e56846. doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Banu A., Noorul Hassan M.M., Rajkumar J., Srinivasa S. Spectrum of Bacteria Associated with Diabetic Foot Ulcer and Biofilm Formation: A Prospective Study. Australas. Med. J. 2015;8:280–285. doi: 10.4066/AMJ.2015.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terreni M., Taccani M., Pregnolato M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules. 2021;26:2671. doi: 10.3390/molecules26092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melo M.N., Ferre R., Castanho M.A.R.B. Antimicrobial Peptides: Linking Partition, Activity and High Membrane-Bound Concentrations. Nat. Rev. Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 146.Ahmed T.A.E., Hammami R. Recent Insights into Structure–Function Relationships of Antimicrobial Peptides. J. Food Biochem. 2019;43:e12546. doi: 10.1111/jfbc.12546. [DOI] [PubMed] [Google Scholar]
- 147.Alfei S., Schito A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers. 2020;12:1195. doi: 10.3390/polym12051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen L., Harrison S.D. Cell-Penetrating Peptides in Drug Development: Enabling Intracellular Targets. Biochem. Soc. Trans. 2007;35:821–825. doi: 10.1042/BST0350821. [DOI] [PubMed] [Google Scholar]
- 149.Bechinger B., Gorr S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017;96:254–260. doi: 10.1177/0022034516679973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jain A., Duvvuri L.S., Farah S., Beyth N., Domb A.J., Khan W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014;3:1969–1985. doi: 10.1002/adhm.201400418. [DOI] [PubMed] [Google Scholar]
- 151.Mintzer M.A., Dane E.L., O’Toole G.A., Grinstaff M.W. Exploiting Dendrimer Multivalency to Combat Emerging and Re-Emerging Infectious Diseases. Mol. Pharm. 2012;9:342–354. doi: 10.1021/mp2005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bahar A.A., Liu Z., Totsingan F., Buitrago C., Kallenbach N., Ren D. Synthetic Dendrimeric Peptide Active against Biofilm and Persister Cells of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2015;99:8125–8135. doi: 10.1007/s00253-015-6645-7. [DOI] [PubMed] [Google Scholar]
- 153.Gibney K.A., Sovadinova I., Lopez A.I., Urban M., Ridgway Z., Caputo G.A., Kuroda K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012;12:1279–1289. doi: 10.1002/mabi.201200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stenström P., Hjorth E., Zhang Y., Andrén O.C.J., Guette-Marquet S., Schultzberg M., Malkoch M. Synthesis and In Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules. 2017;18:4323–4330. doi: 10.1021/acs.biomac.7b01364. [DOI] [PubMed] [Google Scholar]
- 155.Schito A.M., Piatti G., Caviglia D., Zuccari G., Zorzoli A., Marimpietri D., Alfei S. Bactericidal Activity of Non-Cytotoxic Cationic Nanoparticles against Clinically and Environmentally Relevant Pseudomonas Spp. Isolates. Pharmaceutics. 2021;13:1411. doi: 10.3390/pharmaceutics13091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johansson E.M.V., Crusz S.A., Kolomiets E., Buts L., Kadam R.U., Cacciarini M., Bartels K.-M., Diggle S.P., Cámara M., Williams P., et al. Inhibition and Dispersion of Pseudomonas aeruginosa Biofilms by Glycopeptide Dendrimers Targeting the Fucose-Specific Lectin LecB. Chem. Biol. 2008;15:1249–1257. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 157.Kolomiets E., Swiderska M.A., Kadam R.U., Johansson E.M.V., Jaeger K.-E., Darbre T., Reymond J.-L. Glycopeptide Dendrimers with High Affinity for the Fucose-Binding Lectin LecB from Pseudomonas aeruginosa. ChemMedChem. 2009;4:562–569. doi: 10.1002/cmdc.200800380. [DOI] [PubMed] [Google Scholar]
- 158.Hou S., Zhou C., Liu Z., Young A.W., Shi Z., Ren D., Kallenbach N.R. Antimicrobial dendrimer active against Escherichia coli biofilms. Bioorg. Med. Chem. Lett. 2009;19:5478–5481. doi: 10.1016/j.bmcl.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 159.Johansson E.M., Kadam R.U., Rispoli G., Crusz S.A., Bartels K.-M., Diggle S.P., Cámara M., Williams P., Jaeger K.-E., Darbre T., et al. Inhibition of Pseudomonas aeruginosa Biofilms with a Glycopeptide Dendrimer Containing D-Amino Acids. MedChemComm. 2011;2:418–420. doi: 10.1039/c0md00270d. [DOI] [Google Scholar]
- 160.Kadam R.U., Bergmann M., Hurley M., Garg D., Cacciarini M., Swiderska M.A., Nativi C., Sattler M., Smyth A.R., Williams P., et al. A Glycopeptide Dendrimer Inhibitor of the Galactose-Specific Lectin LecA and of Pseudomonas aeruginosa Biofilms. Angew. Chem. Int. Ed. 2011;50:10631–10635. doi: 10.1002/anie.201104342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen X., Zhang M., Zhou C., Kallenbach N.R., Ren D. Control of Bacterial Persister Cells by Trp/Arg-Containing Antimicrobial Peptides. Appl. Environ. Microbiol. 2011;77:4878–4885. doi: 10.1128/AEM.02440-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang L., Erasquin U.J., Zhao M., Ren L., Zhang M.Y., Cheng G.J., Wang Y., Cai C. Stability, Antimicrobial Activity, and Cytotoxicity of Poly(Amidoamine) Dendrimers on Titanium Substrates. ACS Appl. Mater. Interfaces. 2011;3:2885–2894. doi: 10.1021/am2004398. [DOI] [PubMed] [Google Scholar]
- 163.Scorciapino M.A., Pirri G., Vargiu A.V., Ruggerone P., Giuliani A., Casu M., Buerck J., Wadhwani P., Ulrich A.S., Rinaldi A.C. A Novel Dendrimeric Peptide with Antimicrobial Properties: Structure-Function Analysis of SB056. Biophys. J. 2012;102:1039–1048. doi: 10.1016/j.bpj.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu Y., Slomberg D.L., Shah A., Schoenfisch M.H. Nitric Oxide-Releasing Amphiphilic Poly(Amidoamine) (PAMAM) Dendrimers as Antibacterial Agents. Biomacromolecules. 2013;14:3589–3598. doi: 10.1021/bm400961r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reymond J.L., Bergmann M., Darbre T. Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors. Chem. Soc. Rev. 2013;42:4814–4822. doi: 10.1039/c3cs35504g. [DOI] [PubMed] [Google Scholar]
- 166.Zielinska P., Staniszewska M., Bondaryk M., Koronkiewicz M., Urbanczyk-Lipkowska Z. Design and Studies of Multiple Mechanism of Anti-Candida Activity of a New Potent Trp-Rich Peptide Dendrimers. Eur. J. Med. Chem. 2015;105:106–119. doi: 10.1016/j.ejmech.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 167.Worley B.V., Schilly K.M., Schoenfisch M.H. Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly(Amidoamine) Dendrimers. Mol. Pharm. 2015;12:1573–1583. doi: 10.1021/acs.molpharmaceut.5b00006. [DOI] [PubMed] [Google Scholar]
- 168.Visini R., Jin X., Bergmann M., Michaud G., Pertici F., Fu O., Pukin A., Branson T.R., Thies-Weesie D.M.E., Kemmink J., et al. Structural Insight into Multivalent Galactoside Binding to Pseudomonas aeruginosa Lectin LecA. ACS Chem. Biol. 2015;10:2455–2462. doi: 10.1021/acschembio.5b00302. [DOI] [PubMed] [Google Scholar]
- 169.Backlund C.J., Worley B.V., Schoenfisch M.H. Anti-Biofilm Action of Nitric Oxide-Releasing Alkyl-Modified Poly(Amidoamine) Dendrimers against Streptococcus Mutans. Acta Biomater. 2016;29:198–205. doi: 10.1016/j.actbio.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bergmann M., Michaud G., Visini R., Jin X., Gillon E., Stocker A., Imberty A., Darbre T., Reymond J.-L. Multivalency Effects on Pseudomonas aeruginosa Biofilm Inhibition and Dispersal by Glycopeptide Dendrimers Targeting Lectin LecA. Org. Biomol. Chem. 2016;14:138–148. doi: 10.1039/C5OB01682G. [DOI] [PubMed] [Google Scholar]
- 171.Michaud G., Visini R., Bergmann M., Salerno G., Bosco R., Gillon E., Richichi B., Nativi C., Imberty A., Stocker A., et al. Overcoming Antibiotic Resistance in Pseudomonas aeruginosa Biofilms Using Glycopeptide Dendrimers. Chem. Sci. 2016;7:166–182. doi: 10.1039/C5SC03635F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Batoni G., Casu M., Giuliani A., Luca V., Maisetta G., Mangoni M.L., Manzo G., Pintus M., Pirri G., Rinaldi A.C., et al. Rational Modification of a Dendrimeric Peptide with Antimicrobial Activity: Consequences on Membrane-Binding and Biological Properties. Amino Acids. 2016;48:887–900. doi: 10.1007/s00726-015-2136-5. [DOI] [PubMed] [Google Scholar]
- 173.Vankoten H.W., Dlakic W.M., Engel R., Cloninger M.J. Synthesis and Biological Activity of Highly Cationic Dendrimer Antibiotics. Mol. Pharm. 2016;13:3827–3834. doi: 10.1021/acs.molpharmaceut.6b00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barrios-Gumiel A., Sanchez-Nieves J., Pérez-Serrano J., Gómez R., de la Mata F.J. PEGylated AgNP Covered with Cationic Carbosilane Dendrons to Enhance Antibacterial and Inhibition of Biofilm Properties. Int. J. Pharm. 2019;569:118591. doi: 10.1016/j.ijpharm.2019.118591. [DOI] [PubMed] [Google Scholar]
- 175.Gómez-Casanova N., Lozano-Cruz T., Soliveri J., Gomez R., Ortega P., Copa-Patiño J.L., Heredero-Bermejo I. Eradication of Candida albicans Biofilm Viability: In Vitro Combination Therapy of Cationic Carbosilane Dendrons Derived from 4-Phenylbutyric Acid with AgNO3 and EDTA. J. Fungi. 2021;7:574. doi: 10.3390/jof7070574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fernandez J., Martin-Serrano Á., Gómez-Casanova N., Falanga A., Galdiero S., Javier de la Mata F., Heredero-Bermejo I., Ortega P. Effect of the Combination of Levofloxacin with Cationic Carbosilane Dendron and Peptide in the Prevention and Treatment of Staphylococcus aureus Biofilms. Polymers. 2021;13:2127. doi: 10.3390/polym13132127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Galdiero E., de Alteriis E., De Natale A., D’Alterio A., Siciliano A., Guida M., Lombardi L., Falanga A., Galdiero S. Eradication of Candida albicans Persister Cell Biofilm by the Membranotropic Peptide GH625. Sci. Rep. 2020;10:5780. doi: 10.1038/s41598-020-62746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Quintana-Sanchez S., Gómez-Casanova N., Sánchez-Nieves J., Gómez R., Rachuna J., Wasik S., Semaniak J., Maciejewska B., Drulis-Kawa Z., Ciepluch K., et al. The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. Aeruginosa Alone and in Combination with Phage-Derived Endolysin. Int. J. Mol. Sci. 2022;23:1873. doi: 10.3390/ijms23031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.