Abstract
Objective:
Neonatal neurodevelopmental follow-up clinic provides continued surveillance and assessment of high-risk premature infants. We hypothesized that attrition is associated with race and social factors.
Study Design:
We performed a retrospective cohort study of neonates born at 26-32 weeks gestation who were admitted to a level IV neonatal intensive care unit. Maternal and neonatal characteristics and follow-up attendance were collected. Statistical analysis was performed with significance set at p-value <0.05.
Results:
237 neonates met study criteria. There was a 62% loss to follow-up over 2 years. Factors associated with loss to follow-up included older gestational age, African American race, and maternal cigarette smoking. Protective factors included older maternal age, a neonatal diagnosis of bronchopulmonary dysplasia, and longer hospital length of stay.
Conclusion:
Social disparities negatively impact neonatal follow-up clinic attendance. Efforts to identify and target high-risk populations must be started during initial hospitalization before infants are lost to follow-up.
Keywords: neonatal intensive care unit, prematurity, neonatal follow-up, outcomes
1. INTRODUCTION
High-risk neonatal developmental follow-up provides an important transition of care from the inpatient to the outpatient environment1, 2, 3. These clinics specialize in complex neonates, make appropriate referrals to medical subspecialists, and offer specific anticipatory guidance to parents2, 4, 5, 6. Additionally, these clinics facilitate referrals to early intervention services, including speech, physical, and occupational therapy1, 2, 4. Early intervention services have a positive influence on both cognitive and motor outcomes during infancy, with cognitive benefits persisting into preschool age7. Neonatal developmental follow-up is supported by the American Academy of Pediatrics and is a recommended service that all level III and IV neonatal intensive care units (NICUs) should provide8.
Despite the recognized benefits of neonatal follow-up clinic, attendance has historically been challenging with high rates of attrition3, 4, 6, 9, 10, 11, 12. In a recent survey, many sites listed a first visit no-show rate between 10-30% with sustained rates of attrition between 10-70% on subsequent visits4. The reasons for loss to follow-up are multifactorial and include medical, sociodemographic, and maternal factors4. Infants with perceived “follow-up difficulty” have higher incidences of severe sensorimotor and cognitive disabilities, even after adjustment for perinatal and sociodemographic variables, and less access to early intervention services13, 14, 15, 16. Therefore, identifying patient specific factors that increase loss to follow-up is vital for targeting sustained attendance.
It is known nationally that many urban centers are racially and socioeconomically segregated17. There is growing evidence that residential segregation perpetuates health disparities through restricted access to quality health care18. There remains a relative gap in knowledge regarding factors driving loss to follow-up in our current healthcare system3, 10, 12, 19. We hypothesized that clinic attendance is associated with race and specific maternal social factors, such as insurance status, cigarette smoking, and home location by zip code. The objective of this study was to assess neonatal follow-up attendance and identify maternal and neonatal characteristics associated with attrition.
2. METHODS
Study design
We conducted a retrospective cohort study. Approval was granted through the Children’s Hospital of Wisconsin (CHW) Institutional Review Board with waiver of informed consent.
Study population
Neonates were identified who were born between 26-32 weeks gestation and admitted to a level IV NICU in Milwaukee, WI between 1/1/2015 and 7/31/2017. Neonates were excluded if they had any chromosomal anomalies, major congenital anomalies (i.e. congenital diaphragmatic hernia, gastroschisis, posterior urethral valves, etc.), if their family was a non-Wisconsin resident, or if they died during the study period.
Our standard practice is to refer all infants born <33 weeks gestation to neonatal follow-up clinic at time of discharge. Families select one of two locations for neonatal follow-up – CHW Main Campus (Milwaukee, WI) or CHW Fox Valley (Neenah, WI). The first appointment is scheduled by either the neonatal nurse practitioner, physician, or inpatient case manager at 3-6 months corrected gestational age (CGA). Families are then called by the clinic to verify timing and availability for the appointment. Prior to discharge, nursing staff reviews all appointments with the family. A neonatal nurse practitioner or physician also verbally addresses the importance of the visit in monitoring developmental progress.
Neonatal follow-up occurs at 3-6 months, 12 months, 18 months, and 24 months CGA. Each visit lasts approximately 1.5-2 hours. At each appointment, a physician within the clinic performs a physical exam focused on neuromotor assessment. A comprehensive team of therapists perform the Bayley Scales of Infant and Toddler Development, Third Edition. Speech, physical, and occupational therapy provide suggestions for the family for targeting developmental milestones. Referrals may be placed for continued early intervention services if a family is not already enrolled. Medical management of complex needs are taken care of by subspecialty services and/or a comprehensive special needs team at a separate visit.
If a family misses an appointment, they are notified via phone pending staff availability; a formal letter is sent to the address listed within the electronic health record.
Data collection
Maternal characteristics included multiple gestation, prenatal steroid administration (partial or complete), cesarean delivery, pre-eclampsia or hypertension, diabetes, race, age, parity, marital status, zip code, insurance type, cigarette smoking, and illegal drug use. Age was divided into 4 categories: <20 years, 20-29 years, 30-39 years, and >39 years old. Zip code was divided into quartiles based on median household income per year according to United States Census Bureau data collected from the American Community Survey 2017 5-year estimates (https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/). Quartiles were defined as follows: $0-29,999; $30,000-56,999; $57,000-83,999; and >$84,000. Insurance type was divided into 2 categories: Medicaid and private.
Neonatal characteristics included gender, gestational age, hospital length of stay, as well as common medical problems and therapies. These diagnoses or therapies included bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (grade III or IV), periventricular leukomalacia (on head ultrasound or brain magnetic resonance imaging), retinopathy of prematurity (requiring treatment with laser or Avastin), necrotizing enterocolitis, and feeding type at discharge. BPD was defined as a persistent oxygen requirement at 36 weeks CGA. Gestational age was divided into 3 categories: 26-28 weeks, 29-30 weeks, and 31-32 weeks. Feedings at discharge included formula only, breastmilk only, or a combination of formula and breastmilk.
Attendance relative to zip code was plotted utilizing Microsoft Excel 2016 3D-Maps. Reasons for loss to follow-up were collected from chart review and categorized.
Outcome measure
The primary outcome was neonatal follow-up attendance. Neonatal follow-up attendance was tracked at time of discharge (for initial referral placement), at 3-6 months, 12 months, 18 months, and 24 months CGA. Attendance was divided into 3 groups: 0, 1-2, and 3-4 visits attended over a 24-month period. All infants in the study had aged to 24 months CGA at time of data collection and should have been able to attend 4 appointments.
We considered infants lost to neonatal follow-up if they did not attend an appointment and were never subsequently evaluated despite recommendations for continued follow-up. This included infants who: 1) were never referred upon discharge from the NICU and 2) were referred upon discharge but due to scheduling conflicts were never evaluated in clinic. If an infant was discharged from neonatal follow-up clinic by 18 months due to continued progress, then we did not consider them lost to follow-up at subsequent appointment intervals.
Statistical analysis
Categorical data was reported as numbers and percentages. Discrete data was reported as medians with 25th and 75th percentiles. The Fisher exact test or the Chi-square test was used to compare categorical variables.
Variables found to be significantly associated with attendance in univariable analysis were further evaluated in multivariable analysis to account for confounding factors. Ordered logistic regression was performed. Variables were controlled for gestational age, multiple gestation, prenatal steroid administration, cesarean delivery, maternal race, maternal age, insurance type, maternal cigarette smoking, BPD, and feeding type at discharge. Odds ratios (OR) of risk factors were given with 95% confidence intervals (CI). Hospital length of stay and follow-up attendance were analyzed with Poisson regression, and incidence rate ratios (IRR) were given with 95% CI. A p-value <0.05 was considered statistically significant.
Kaplan-Meier survival analysis was completed to demonstrate follow-up attendance over the 24-month period.
Statistics were performed in STATA15 (College Station, TX).
3. RESULTS
Study population
During the study period, 420 infants between 26-32 weeks gestation were admitted. 237 infants were included in the study (gestational age- median, 30 weeks; birth weight- mean ± standard deviation, 1417±396 grams). A total of 183 infants were excluded: 125 with transfer at >3 days, 39 with major congenital or chromosomal anomalies, 11 with non-Wisconsin parents, and 8 who died in the NICU or within 6 months following discharge.
Socio-demographic and clinical characteristics are summarized in Table 1. Geographical distribution of study participants based upon number of visits attended is shown in Figure 1. Families who attended no appointments were centered around both medical campuses; those who attended 3-4 appointments were distributed more diffusely across the eastern part of the state.
Table 1.
Maternal and neonatal characteristics and neonatal follow-up attendance.
| All infants | Number of Follow-up Visits Attended |
p -value |
|||
|---|---|---|---|---|---|
| 0 | 1-2 | 3-4 | |||
| (n = 237) | (n = 65) | (n = 70) | (n = 102) | ||
| Male gender | 133 (56.1%) | 39 (60.0%) | 35 (50.0%) | 59 (57.8%) | 0.453 |
| Gestational age ** | 0.003 | ||||
| 26-28 weeks | 59 (24.9%) | 8 (12.3%) | 14 (20.0%) | 37 (36.3%) | |
| 29-30 weeks | 74 (31.2%) | 19 (29.2%) | 26 (37.1%) | 29 (28.4%) | |
| 31-32 weeks | 104 (43.9%) | 38 (58.5%) | 30 (42.9%) | 36 (35.3%) | |
| Multiple gestation ** | 80 (33.8%) | 11 (16.9%) | 28 (40.0%) | 41 (40.2%) | 0.003 |
| Prenatal steroid administration ** | 219 (92.4%) | 54 (83.1%) | 67 (95.7%) | 98 (96.1%) | 0.009 |
| Cesarean delivery * | 165 (69.6%) | 38 (58.5%) | 55 (78.6%) | 72 (70.6%) | 0.038 |
| Maternal pre-eclampsia or hypertension | 63 (26.6%) | 16 (24.6%) | 16 (22.9%) | 31 (30.4%) | 0.500 |
| Maternal diabetes | 18 (7.6%) | 6 (9.2%) | 3 (4.3%) | 9 (8.8%) | 0.590 |
| Maternal race * | 0.023 | ||||
| White | 141 (59.5%) | 28 (43.0%) | 43 (61.4%) | 70 (68.6%) | |
| African American | 70 (29.5%) | 29 (44.6%) | 21 (30.0%) | 20 (19.6%) | |
| Hispanic | 10 (4.2%) | 4 (6.2%) | 2 (2.9%) | 4 (3.9%) | |
| Other | 16 (6.8%) | 4 (6.2%) | 4 (5.7%) | 8 (7.9%) | |
| Maternal age *** | 0.000 | ||||
| <20 years | 10 (4.2%) | 7 (10.8%) | 2 (2.8%) | 1 (1.0%) | |
| 20-29 years | 103 (43.5%) | 36 (55.4%) | 37 (52.9%) | 30 (29.4%) | |
| 30-39 years | 119 (50.2%) | 20 (30.8%) | 31 (44.3%) | 68 (66.7%) | |
| >39 years | 5 (2.1%) | 2 (3.0%) | 0 (0.0%) | 3 (2.9%) | |
| Maternal parity | 0.073 | ||||
| Primigravida | 115 (48.5%) | 24 (36.9%) | 35 (50.0%) | 56 (54.9%) | |
| Multigravida | 121 (51.1%) | 40 (61.6%) | 35 (50.0%) | 46 (45.1%) | |
| Unknown | 1 (0.4%) | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) | |
| Marital status *** | 0.000 | ||||
| Single | 68 (28.7%) | 25 (38.5%) | 21 (30.0%) | 22 (21.6%) | |
| Married | 101 (42.6%) | 12 (18.5%) | 34 (48.6%) | 55 (53.9%) | |
| Unknown | 68 (28.7%) | 28 (43.0%) | 15 (21.4%) | 25 (24.5%) | |
| Median income quartiles *** | 0.000 | ||||
| <$30,000 | 13 (5.5%) | 6 (9.2%) | 2 (2.9%) | 5 (4.9%) | |
| $30,000-$56,999 | 127 (53.6%) | 45 (69.2%) | 40 (57.1%) | 42 (41.2%) | |
| $57,000-$83,999 | 69 (29.1%) | 14 (21.6%) | 20 (28.6%) | 35 (34.3%) | |
| >$83,999 | 28 (11.8%) | 0 (0.0%) | 8 (11.4%) | 20 (19.6%) | |
| Insurance type *** | 0.000 | ||||
| Medicaid | 118 (49.8%) | 50 (76.9%) | 31 (44.3%) | 37 (36.3%) | |
| Private | 119 (50.2%) | 15 (23.1%) | 39 (55.7%) | 65 (63.7%) | |
| Maternal cigarette smoking *** | 0.000 | ||||
| No | 186 (78.5%) | 39 (60.0%) | 59 (84.3%) | 88 (86.3%) | |
| Yes | 26 (11.0%) | 17 (26.2%) | 5 (7.1%) | 4 (3.9%) | |
| Unknown | 25 (10.5%) | 9 (13.8%) | 6 (8.6%) | 10 (9.8%) | |
| Illegal drug use | 0.256 | ||||
| No | 184 (77.6%) | 44 (67.7%) | 58 (82.8%) | 82 (80.4%) | |
| Yes | 28 (11.8%) | 12 (18.5%) | 6 (8.6%) | 10 (9.8%) | |
| Unknown | 25 (10.6%) | 9 (13.8%) | 6 (8.6%) | 10 (9.8%) | |
| Bronchopulmonary dysplasia *** | 62 (26.1%) | 9 (13.9%) | 13 (18.6%) | 40 (39.2%) | 0.000 |
| Intraventricular hemorrhage | 6 (2.5%) | 1 (1.5%) | 3 (4.3%) | 2 (2.0%) | 0.579 |
| Periventricular leukomalacia | 5 (2.1%) | 0 (0.0%) | 1 (1.4%) | 4 (3.9%) | 0.280 |
| Retinopathy of prematurity requiring treatment | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | 1.000 |
| Necrotizing enterocolitis | 11 (4.6%) | 4 (6.2%) | 3 (4.3%) | 4 (3.9%) | 0.799 |
| Feeding type at discharge ** | 0.002 | ||||
| Formula only | 88 (37.1%) | 36 (55.4%) | 24 (34.3%) | 28 (27.4%) | |
| Breastmilk only | 2 (0.9%) | 0 (0.0%) | 1 (1.4%) | 1 (1.0%) | |
| Formula and breastmilk | 147 (62.0%) | 29 (44.6%) | 45 (64.3%) | 73 (71.6%) | |
| Hospital length of stay, days, median (IQR) I | 41 (31-46) Reference |
46.5 (32-63) 1.01 (0.97-1.07) p = 0.48 |
55 (38-77) 1.24 (1.19-1.29)*** p = 0.000 |
||
IRR (95% CI)
p-value < 0.05
p-value < 0.01
p-value < 0.001
Figure 1.
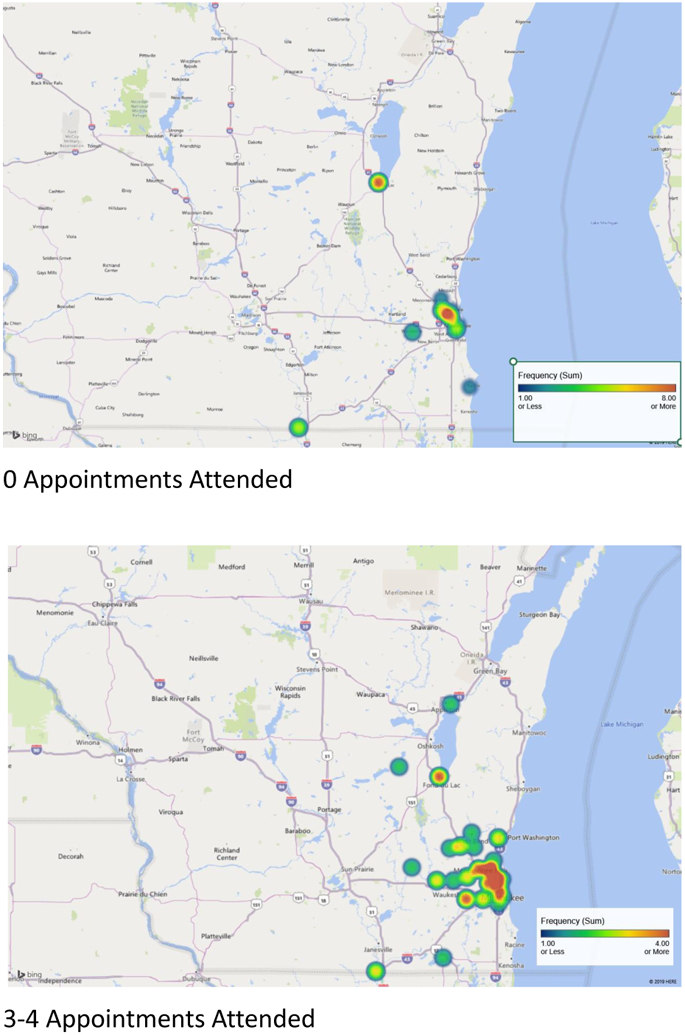
Geographical distribution of study participants based upon number of neonatal follow-up visits attended.
Neonatal follow-up clinic attendance
Follow-up attendance over a 2-year period is shown in Figure 2. Seven percent of infants were not referred to neonatal follow-up clinic at time of discharge. Attrition between appointments consistently decreased over time from 27% to 6%. By 24 months CGA, 62% of the cohort was lost to follow-up.
Figure 2.
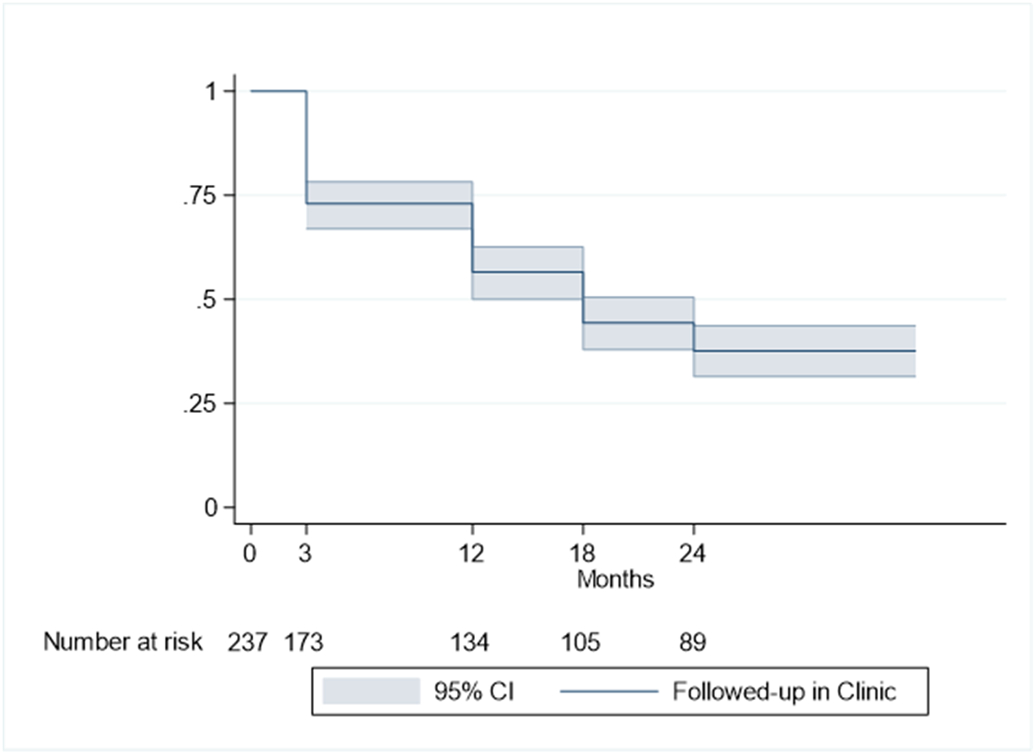
Kaplan-Meier survival curve of proportion of neonatal follow-up attendance over a 2-year period. X-axis represents month of scheduled follow-up and Y-axis represents proportion of follow-up attendance.
Analysis of factors associated with loss to follow-up
Multiple maternal and neonatal factors were associated with clinic attendance (Table 1). On multivariable analysis, factors associated with loss to neonatal follow-up were a gestational age of 31-32 weeks, maternal African American race, and maternal cigarette smoking (Table 2). Factors associated with attendance included prenatal steroid administration, maternal age between 30-39 years, and a neonatal diagnosis of BPD. Longer hospital length of stay was associated with increasing follow-up attendance.
Table 2.
Adjusted odds ratios showing association between neonatal follow-up attendance and maternal and neonatal characteristics.
| Adjusted I | p-value | |
|---|---|---|
| OR (95% CI) | ||
| Gestational Age | ||
| 26-28 weeks | Reference | |
| 29-30 weeks | 0.56 (0.24-1.29) | 0.175 |
| 31-32 weeks** | 0.30 (0.13-0.71) | 0.006 |
| Multiple gestation | 1.21 (0.68-2.15) | 0.525 |
| Prenatal steroid administration * | 3.85 (1.27-11.68) | 0.017 |
| Cesarean delivery | 0.87 (0.48-1.59) | 0.650 |
| Maternal race | ||
| White | Reference | |
| African American* | 0.39 (0.19-0.82) | 0.013 |
| Hispanic | 0.45 (0.11-1.78) | 0.255 |
| Other | 1.25 (0.40-3.91) | 0.696 |
| Maternal age | ||
| <20 years | 0.22 (0.05-1.07) | 0.061 |
| 20-29 years | Reference | |
| 30-39 years*** | 2.92 (1.62-5.25) | 0.000 |
| >39 years | 3.25 (0.31-34.37) | 0.328 |
| Insurance type | ||
| Medicaid | Reference | |
| Private | 0.82 (0.40-1.70) | 0.595 |
| Maternal cigarette smoking | ||
| No | Reference | |
| Yes*** | 0.13 (0.05-0.37) | 0.000 |
| Unknown | 1.30 (0.52-3.30) | 0.572 |
| Bronchopulmonary dysplasia ** | 2.82 (1.29-6.16) | 0.009 |
| Feeding type at discharge | 1.31 (0.95-1.79) | 0.096 |
Controlled for gestational age, multiple gestation, prenatal steroid administration, cesarean delivery, maternal race, maternal age, insurance type, maternal cigarette smoking, bronchopulmonary dysplasia, and feeding type at discharge
p-value < 0.05
p-value < 0.01
p-value < 0.001
Reasons for loss to follow-up were reviewed and categorized as follows: parent scheduling issues (76.2%); provider scheduling issues (18.4%); parent perception of appropriate development (3.4%); lack of insurance coverage (1.4%); and parent perception of alternate resources (0.6%). Parent scheduling issues included all no-shows and parent cancelation of appointments. Provider scheduling issues included all provider cancelations resulting in loss to neonatal follow-up as well as all situations in which no initial visit was scheduled.
4. DISCUSSION
Attrition to neonatal follow-up remains a major problem within our health system. We identified a 62% loss to neonatal follow-up over a 2-year time period. This single center, retrospective cohort study highlights that key social factors, such as maternal race, age, and cigarette smoking, as well as specific neonatal factors drive follow-up attendance.
Rates of neonatal follow-up attendance have been shown to vary significantly according to population demographics, study sample, and timing and frequency of follow-up3, 10, 11, 12, 20, 21. Patra et al. evaluated very low birth weight infants and showed that only 52-62% of infants attended their first follow-up visit, with subsequent attendance falling to 27-30% by 2 years2. Mas et al. studied infants <33 weeks and demonstrated that only 58% of infants were consistently followed-up at 20-28 months with 27% having never been evaluated12. Ballantyne et al. further showcased consistent non-attendance between clinic appointments of 16-26% 3. Our rate of attrition is in line with these previous studies, demonstrating that neonatal follow-up remains a substantial challenge in today’s landscape. Interestingly, attrition, contrary to being steady, decreased dramatically over consecutive appointments. We speculate that families who perceived consistent value in the appointment were more likely to attend over time.
We identified several potential drivers to loss to neonatal follow-up, including older gestational age, African American race, and maternal cigarette smoking, as well as multiple protective factors, including older maternal age, BPD, and longer hospital length of stay. It is well-recognized that specific social risk factors, such as young maternal age and non-white race, are associated with lower neonatal follow-up attendance6, 9. The racial bias we observed may have been impacted by the racial segregation within our city, with those living in certain areas having restricted access to resources22, 23, 24, 25. Milwaukee is a racially subdivided city, with its historical roots being greatly driven by highway construction26. Most of the inner-city African American population is located to the northwest, primarily in the poorest city zip codes, while the white population resides in the newer gentrified urban developments or city suburbs. Our hospital’s location in the suburbs potentially limits access to care for our most vulnerable inner-city members due to restricted public transportation. Surprisingly, socioeconomic status as represented by insurance status was not predictive of loss to follow-up. This is consistent with Catlett et al. who also found no significant difference in socioeconomic status between attendee and non-attendee families9. We speculate that our sample may underrepresent families of low socioeconomic status as multiple level III NICUs in our area provide care to our inner-city population, with those in the poorest zip codes located closer geographically to another health system.
We also demonstrated that older gestational age is associated with loss to neonatal follow-up. Older gestational age infants often have shorter lengths of stay and less complications related to prematurity. Families may perceive these infants as healthy and question their need for developmental follow-up, leading to poor attendance. This was consistent with our review of outpatient documentation, in which families canceled appointments if they perceived appropriate infant development. In addition, this is supported by our findings that infants with greater medical complexity, as inferred by a neonatal diagnosis of BPD and longer length of stay, were more likely to attend clinic. Other common neonatal co-morbidities, such as intraventricular hemorrhage and necrotizing enterocolitis were not significant in our study likely due to only a small number of infants being affected. Multiple other studies have demonstrated similar results to ours, emphasizing that infants with longer NICU stays1, multiple neonatal co-morbidities10, 12, 19, and those whose parents perceive them as “sick”27, 28 are more likely to attend neonatal follow-up.
Historically, greater distance from the clinic or hospital has been associated with loss to neonatal follow-up1, 28. We, in contrast, found that families who attended multiple appointments were geographically located across the state. This suggests that social factors may have played a greater role compared to distance from clinic in our study. Underlying patient characteristics as well as the location of our clinics may account for these differences.
Our reasons for loss to follow-up aligned with and expanded upon categories determined in previous studies2, 3. Most infants were lost to neonatal follow-up at ≤12 months CGA due to parent scheduling issues. This brings to question whether parents are truly recognizing the value of neonatal follow-up or if other subspecialty services are taking priority. Efforts should be made to educate families while they are still inpatient on the benefits of and importance of neonatal follow-up clinic. Other interventions can then be undertaken from a clinic perspective to increase attendance.
One of the main strengths of our study was our sample which included infants born from 26-32 weeks gestation who were followed longitudinally. Many of the previous studies reporting loss to neonatal follow-up focused only on the extremely premature or extremely low birth weight. The limitations of this study included its retrospective nature which resulted in missing information, such as maternal education or a comprehensive maternal history, as we only had access to the infant’s chart. Reasons for loss to follow-up were also unavailable unless there was specific telephone documentation in the chart, which limits our understanding of why families did not show to appointments. Finally, families that moved to other health systems during the duration of the study were also lost to follow-up due to inability to share medical records, which may have led to incomplete information regarding follow-up attendance.
Since identification of our high rate of attrition, our institution has taken measures to boost neonatal follow-up attendance. All families are now enrolled in MyChart® at time of discharge from the NICU. MyChart® is a secure on-line access portal that sends appointment reminders. Reminder phone calls are also provided within 48 hours of all scheduled appointments. Furthermore, we are routing pediatricians our developmental notes as well as sending them electronic messages for all families who do not show. The goal is for the pediatrician to meet with the family to assess development and re-refer to our clinic as needed. Finally, we will provide a developmental brochure to all families prior to discharge that introduces our clinic staff, reviews aspects of the neonatal follow-up visit, and explores the benefits of attendance.
In conclusion, our study confirms that loss to neonatal follow-up continues to be a substantial problem. We identified several potential drivers to loss to follow-up and categorized reasons for non-attendance. Future studies need to be undertaken to see how to best improve follow-up attendance and care for our most vulnerable population.
ACKNOWLEDGEMENTS
Statistical support was provided through the Division of Quantitative Health Sciences. This study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would like to thank the MCW Division of Neonatology and the members of the CHW Neonatal Neurocritical Care Program for their support.
FUNDING INFORMATION
This study was also supported by internal funding through MCW Department of Pediatrics.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Harmon SL, Conaway M, Sinkin RA, Blackman JA. Factors associated with neonatal intensive care follow-up appointment compliance. Clinical pediatrics 2013, 52(5): 389–396. [DOI] [PubMed] [Google Scholar]
- 2.Patra K, Greene M, Perez B, Silvestri JM. Neonatal high-risk follow-up clinics: how to improve attendance in very low birth weight infants. E-Journal of Neonatology Research 2014, 4(1): 3–13. [Google Scholar]
- 3.Ballantyne M, Stevens B, Guttmann A, Willan AR, Rosenbaum P. Transition to neonatal follow-up programs: is attendance a problem? J Perinat Neonatal Nurs 2012, 26(1): 90–98. [DOI] [PubMed] [Google Scholar]
- 4.Tang BG, Lee HC, Gray EE, Gould JB, Hintz SR. Programmatic and Administrative Barriers to High-Risk Infant Follow-Up Care. Am J Perinatol 2018, 35(10): 940–945. [DOI] [PubMed] [Google Scholar]
- 5.Dorling JS, Field DJ. Follow up of infants following discharge from the neonatal unit: structure and process. Early human development 2006, 82(3): 151–156. [DOI] [PubMed] [Google Scholar]
- 6.Nehra V, Pici M, Visintainer P, Kase JS. Indicators of compliance for developmental follow-up of infants discharged from a regional NICU. Journal of perinatal medicine 2009, 37(6): 677–681. [DOI] [PubMed] [Google Scholar]
- 7.Spittle A, Orton J, Anderson P, Boyd R, Doyle LW. Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments in preterm infants. The Cochrane database of systematic reviews 2012, 12: CD005495. [DOI] [PubMed] [Google Scholar]
- 8.Bockli K, Andrews B, Pellerite M, Meadow W. Trends and challenges in United States neonatal intensive care units follow-up clinics. Journal of perinatology : official journal of the California Perinatal Association 2014, 34(1): 71–74. [DOI] [PubMed] [Google Scholar]
- 9.Catlett AT, Thompson RJ Jr., Johndrow DA, Boshkoff MR. Risk status for dropping out of developmental followup for very low birth weight infants. Public Health Rep 1993, 108(5): 589–594. [PMC free article] [PubMed] [Google Scholar]
- 10.J LO, McGinley JL, Fox LM, Spittle AJ. Challenges of neurodevelopmental follow-up for extremely preterm infants at two years. Early human development 2015, 91(12): 689–694. [DOI] [PubMed] [Google Scholar]
- 11.Bruni R, Bahamonde LG, Gupta M, Findlay RD, Bean X. Long-Term Follow Up of NICU Graduates: Social Variables, not Clinical Problems, Determine Drop-Out Rates and Access to Health Care † 1215. Pediatric Research 1998, 43(4): 208–208. [Google Scholar]
- 12.Mas C, Gerardin P, Chirpaz E, Carbonnier M, Mussard C, Samperiz S, et al. Follow-up at two years of age and early predictors of non-compliance in a cohort of very preterm infants. Early human development 2017, 108: 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Tin W, Fritz S, Wariyar U, Hey E. Outcome of very preterm birth: children reviewed with ease at 2 years differ from those followed up with difficulty. Archives of disease in childhood Fetal and neonatal edition 1998, 79(2): F83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callanan C, Doyle L, Rickards A, Kelly E, Ford G, Davis N. Children followed with difficulty: how do they differ? J Paediatr Child Health 2001, 37(2): 152–156. [DOI] [PubMed] [Google Scholar]
- 15.Wolke D, Söhne B, Ohrt B, Riegel K. Follow-up of preterm children: important to document dropouts. The Lancet 1995, 345(8947): 447. [DOI] [PubMed] [Google Scholar]
- 16.Slater MA, Naqvi M, Andrew L, Haynes K. Neurodevelopment of monitored versus nonmonitored very low birth weight infants: the importance of family influences. J Dev Behav Pediatr 1987, 8(5): 278–285. [PubMed] [Google Scholar]
- 17.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports (Washington, DC : 1974) 2001, 116(5): 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017, 389(10077): 1453–1463. [DOI] [PubMed] [Google Scholar]
- 19.Kim NH, Youn YA, Cho SJ, Hwang JH, Kim EK, Kim EA, et al. The predictors for the non-compliance to follow-up among very low birth weight infants in the Korean neonatal network. PLoS One 2018, 13(10): e0204421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillen U, DeMauro S, Ma L, Zupancic J, Roberts R, Schmidt B, et al. Relationship between attrition and neurodevelopmental impairment rates in extremely preterm infants at 18 to 24 months: a systematic review. Arch Pediatr Adolesc Med 2012, 166(2): 178–184. [DOI] [PubMed] [Google Scholar]
- 21.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics 2004, 113(4): 781–789. [DOI] [PubMed] [Google Scholar]
- 22.Logan JR. The Persistence of Segregation in the 21(st) Century Metropolis. City Community 2013, 12(2): 10.1111/cico.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis E, McManus P, Magallanes N, Johnson S, Majnik A. Conquering racial disparities in perinatal outcomes. Clinics in perinatology 2014, 41(4): 847–875. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Dickinson K, Mandic CG, Willis E. Community matters for children's health. Pediatr Ann 2011, 40(3): 152–160. [DOI] [PubMed] [Google Scholar]
- 25.Byrd DR, Katcher ML, Peppard P, Durkin M, Remington PL. Infant Mortality: Explaining Black/White Disparities in Wisconsin. Maternal and Child Health Journal 2007, 11(4): 319–326. [DOI] [PubMed] [Google Scholar]
- 26.Chi G, Parisi D. Highway Expansion Effects on Urban Racial Redistribution in the Post—Civil Rights Period. Public Works Management & Policy 2011, 16(1): 40–58. [Google Scholar]
- 27.Ballantyne M, Benzies K, Rosenbaum P, Lodha A. Mothers' and health care providers' perspectives of the barriers and facilitators to attendance at Canadian neonatal follow-up programs. Child: Care, Health and Development 2015, 41(5): 722–733. [DOI] [PubMed] [Google Scholar]
- 28.Ballantyne M, Stevens B, Guttmann A, Willan AR, Rosenbaum P. Maternal and infant predictors of attendance at Neonatal Follow-Up programmes. Child Care Health Dev 2014, 40(2): 250–258. [DOI] [PubMed] [Google Scholar]


