Abstract
Complement-mediated bactericidal antibodies in serum confer protection against meningococcal disease. The minimum protective titer is estimated to be between 1:4 and 1:8 when measured by the Goldschneider assay performed with human complement, the assay used in the 1960s to establish the correlation between bactericidal antibodies and protection. A more recently described bactericidal assay standardized by an international consortium uses rabbit complement, which is known to augment bactericidal titers. To define a protective titer measured by the standardized assay, we compared bactericidal titers against serogroup C strains measured by this assay to titers measured by the assay described by Goldschneider et al. A titer of ≥1:128 measured by the standardized assay was needed to predict with ≥80% certainty a positive titer of ≥1:4 as measured by the Goldschneider assay. However, the majority of samples with titers of 1:4 measured by the Goldschneider assay had titers of <1:128 when measured by the standardized assay. Therefore, by the results of the standardized assay such persons would be falsely categorized as being susceptible to disease. In conclusion, high bactericidal titers measured with the standardized assay performed with rabbit complement are predictive of protection, but no threshold titer is both sensitive and specific for predicting a positive titer measured by the Goldschneider assay using human complement. Up to 10% of the U.S. adult population lacks intrinsic bactericidal activity against serogroup C strains in serum and can serve as complement donors. Therefore, use of the Goldschneider assay or an equivalent assay performed with human complement is preferred over assays that use rabbit complement.
Considerable data support a relationship between the presence of complement-mediated bactericidal antibodies in serum and protection from developing invasive meningococcal disease (reviewed by Frasch [4]). Therefore, there is a strong scientific rationale for inferring efficacy of a new meningococcal vaccine from immunogenicity data. However, this approach is based on the premise that the method used to assay bactericidal antibody yields clinically relevant titers that have been linked to supportive epidemiological and/or experimental studies.
In a seminal study on the role of bactericidal antibodies in serum and protection against meningococcal disease, Goldschneider et al. obtained baseline serum samples from unimmunized military recruits who were at high risk of acquiring serogroup C disease during 8 weeks of attending training camp (5). A baseline serum bactericidal titer of ≥1:4 was a strong predictor of protection. Subsequently, some experts recommended that a titer of ≥1:8 be used as the threshold to define protection (Carl Frasch, personal communication), reasoning that the higher titer provides a greater margin of safety in assessing protection. In general, a titer of ≥1:4 is accepted as the protective threshold titer in Europe (1), while a titer of ≥1:8 is used in the United States.
In Goldschneider's studies, the bactericidal antibody level in serum was measured using human complement (either endogenous complement or exogenous serum from a healthy adult who lacked intrinsic bactericidal activity) (5). However, sera from many healthy adults contain naturally acquired antibodies to the group-specific polysaccharide, lipopolysaccharide, or outer membrane proteins of meningococci. These antibodies can activate complement and evoke meningococcal bacteriolysis and interfere with the results of the assay. Therefore, it is necessary either to use serum from an untreated patient with agammaglobulinemia (rare in the population) or to screen sera from a number of healthy adults to find a suitable complement donor who lacks intrinsic bactericidal activity in serum. Some investigators have substituted infant rabbit sera as a complement source (reviewed in reference 15) because of potential difficulties in obtaining human complement. Infant rabbit sera that lack intrinsic bactericidal activity are readily available, and pooled infant rabbit serum can be prepared and shared among different laboratories, which furthers standardization of the bactericidal assay. For these reasons, rabbit serum was recommended as the source of exogenous complement in the bactericidal assay procedure developed by the Centers for Disease Control and Prevention and standardized in an international multilaboratory study (15). Rabbit serum was also recommended by the World Health Organization as a source of complement for assessing human bactericidal responses to meningococcal polysaccharide vaccines as part of biological standardization (11, 19, 20).
In 1983, Zollinger and Mandrell reported that the use of rabbit complement resulted in much higher bactericidal titers for serogroup B meningococcal strains than the use of human complement (21). This observation has been confirmed (14) and also reported for serogroup C strains (9). Since the principal study demonstrating a correlation between bactericidal activity in serum and protection against developing meningococcal disease used human complement in the assay (5), the clinical relevance of the higher bactericidal titers measured by the standardized assay with rabbit complement is unknown. It follows that a protective threshold of ≥1:4 or ≥1:8 established by the Goldschneider assay with human complement may be inappropriately low if bactericidal titers are measured by the standardized assay performed with rabbit complement. To date, there are no published comparisons of titers measured by the two assays.
In this study, we report the results of analysis of the bactericidal responses to meningococcal serogroup C strains in sera from vaccinated toddlers or older children as measured by the standardized bactericidal assay using rabbit complement or the Goldschneider assay using human complement.
MATERIALS AND METHODS
Serum samples.
The sera were obtained 1 month after immunization from 147 toddlers of 12 to 18 months of age who had been given a single dose of serogroup C conjugate vaccine (Menjugate; Chiron Vaccines, Siena, Italy) and from 152 children of 3 to 5 years of age who had been randomized to receive either a single dose of a combination meningococcal A, C, Y, and W-135 polysaccharide vaccine (Menomune; Aventis Pasteur, Swiftwater, Pa.) or a serogroup C conjugate vaccine (Menjugate). The collection was a convenience sample, with individual sera selected based on the availability of sufficient volumes for retesting.
Bactericidal assays.
The standardized assay was performed at the Manchester Public Health Laboratory, Public Health Laboratory Service (PHLS; England and Wales), and the Goldschneider assay was performed at Chiron Corporation (Emeryville, Calif.). At both laboratories, the test sera were heat inactivated (56°C for 30 min) to remove intrinsic complement activity.
To obtain a source of exogenous human complement for the Goldschneider assay, blood samples from 50 healthy adults residing in Northern California were screened as follows. The blood samples were allowed to clot at room temperature for 45 min and then incubated at 4°C for 40 min. The tubes were centrifuged at 3,400 rpm (2,490 × g) at 4°C, and the sera were separated and transferred to 1-ml storage tubes and stored frozen at −80°C. Sera collected by this method retain normal hemolytic complement activity for periods of 2 years or longer (data not shown). Aliquots of sera were screened for immunoglobulin G (IgG) and IgM anti-serogroup C capsule antibodies by enzyme-linked immunosorbent assay (ELISA) (8). Sera that were negative by ELISA were screened for the presence of intrinsic bactericidal activity in the bactericidal assay at 20 and 40% serum (final concentration in the reaction mixture), performed as previously described (14), with the exception that Gey's buffered salt solution containing 1% albumin was used instead of barbitol buffer. Five of the 50 sera screened (10%) had no detectable intrinsic bactericidal activity. For assaying bactericidal titers by the Goldschneider assay, sera from three complement donors were pooled on the day of the assay. The same three donors were used as the human complement source for all samples tested. When used as a complement source, this healthy human serum pool yielded comparable respective bactericidal titers for various heat-inactivated (56°C for 30 min) positive- and negative-control test sera to those obtained when serum from a patient with agammaglobulinemia was used (data not shown). The complement source for the standardized assay was from 3- to 4-week-old baby rabbits (Pelfreeze Biologics, Brown Deer, Wis.), and the complement was also qualified based on a lack of intrinsic bactericidal activity and yielded appropriate titers when used as a complement source with various positive- and negative-control heat-inactivated serum pools.
At both laboratories, test sera were assayed for bactericidal activity at a 1:4 starting dilution using serogroup C meningococcal strain C11 (also called 60E). Strain C11 was the prototype serogroup C test strain in the original study by Goldschneider et al. (5). To perform the standardized assay at the PHLS, the test organism was grown for 4 h on blood agar and was resuspended in Gey's buffered salt solution containing 0.5% bovine serum albumin. Bacterial killing in the final reaction vial was measured after 60 min of incubation at 37°C. Bactericidal titers were defined as the highest serum dilution giving a ≥50% decrease in CFU compared to the CFU measured at time zero. At the Chiron laboratory, the test organism for the Goldschneider assay was grown for 5 h on Mueller-Hinton chocolate agar and resuspended in Dulbecco's phosphate-buffered saline containing 0.5 mM Mg2+ and 1.0 mM Ca2+. Bacterial survival in the final reaction mixture was measured after 30 min of incubation at 37°C. The bactericidal titer was calculated from the following equation: percent survival = (CFU of sample well at 30 min/CFU with the complement control at 0 min) × 100. The bactericidal titer assigned was the 50% intercept when percent survival on the y axis was plotted versus reciprocal serum dilution on the x axis (log10 scale).
Anticapsular antibody concentrations.
IgG anticapsular antibody concentrations were measured by ELISA, performed as previously described using thiocyanate in the serum diluting buffer to dissociate low-avidity antibody from the solid-phase polysaccharide antigen (8). A similar ELISA, performed without thiocyanate in the diluting buffer, was used to measure IgM antibody concentrations using an alkaline phosphatase-conjugated murine monoclonal antibody specific for human IgM (clone no. HP6083; conjugation performed by American Qualex, San Clemente, Calif.). Data are expressed as ELISA units (EU) per milliliter. One EU is approximately 1 μg of antibody (8).
RESULTS
Geometric mean bactericidal titer as assessed by the different assays.
Table 1 summarizes the respective geometric mean bactericidal titers ± 95% confidence intervals (CI) stratified by study and vaccine, as measured by the Goldschneider and standardized assays. For subjects given the conjugate vaccine, the geometric mean titers measured by the standardized assay performed with rabbit complement were approximately 10-fold higher than those measured by the Goldschneider assay performed with human complement. Among children given the plain polysaccharide vaccine, the titers measured by the standardized assay were only threefold higher than those measured by the Goldschneider assay. These data imply that there are qualitative differences in the antibody populations elicited by the different vaccines and that these differences affect the respective performance of the two assays.
TABLE 1.
Summary of bactericidal antibody responses in serum
| Age group | Vaccine | No. of subjects | 1/geometric mean titer (95% CI)
|
|
|---|---|---|---|---|
| Goldschneider assay with human complement | Standard assay with rabbit complement | |||
| Toddlers (12–18 mo) | Conjugate | 147 | 13 (11–17) | 110 (80–152) |
| Children (3–5 yr) | Conjugate | 78 | 36 (25–52) | 486 (306–770) |
| Polysaccharide | 74 | 9 (6–14) | 28 (18–45) | |
Table 2 summarizes the distribution of the bactericidal titers measured by the standardized assay employing rabbit complement or the Goldschneider assay with human complement. Among the 299 postvaccination sera, a higher percentage had titers of <1:4 when measured with the Goldschneider assay (25%) compared to the standardized assay (5%; P < 0.01). One-third of the samples assayed by the standardized assay had titers of 1:512 or greater, compared to only 1.7% with the Goldschneider assay (P < 0.001). Thus, many children will appear to have bactericidal antibodies present in serum when assayed by the standardized assay who are negative when assayed by the Goldschneider assay, and a much higher proportion of immunized subjects will have very high titers if the standardized assay is used.
TABLE 2.
Summary of distribution of titers measured by the Goldschneider and standardized assays
| 1/bactericidal titer | No. of samples (%) with indicated titer by:
|
|
|---|---|---|
| Standardized assay with rabbit complement | Goldschneider assay with human complement | |
| <4 | 16 (5.4) | 75 (25.1) |
| 4 | 17 (5.7) | 36 (12.0) |
| 8 | 16 (5.4) | 45 (15.1) |
| 16 | 31 (10.4) | 43 (14.4) |
| 32 | 23 (7.7) | 36 (12.0) |
| 64 | 28 (9.4) | 37 (12.4) |
| 128 | 37 (12.4) | 13 (4.3) |
| 256 | 31 (10.4) | 9 (3.0) |
| 512 | 37 (12.4) | 3 (1.0) |
| >512 | 63 (21.1) | 2 (0.7) |
| 1/geometric mean titer | 116 | 16 |
Analysis of specificity and sensitivity of the standardized assay.
Using the traditional approach to assess sensitivity and specificity shown in Table 3, no threshold measured by the standardized assay with rabbit complement was both sensitive and specific (≥90%) compared to a protective titer of ≥1:4 or ≥1:8 by the Goldschneider assay performed with human complement.
TABLE 3.
Sensitivity and specificity of different titers measured by the standardized assay with rabbit complement compared to a protective titer measured by the reference Goldschneider assay
| 1/titer by standardized assay | Goldschneider titer of ≥1:4
|
Goldschneider titer of ≥1:8
|
||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| ≥32 | 85 | 61 | 91 | 58 |
| ≥64 | 78 | 73 | 86 | 68 |
| ≥128 | 69 | 83 | 78 | 81 |
| ≥256 | 54 | 87 | 63 | 88 |
| >512 | 41 | 92 | 49 | 94 |
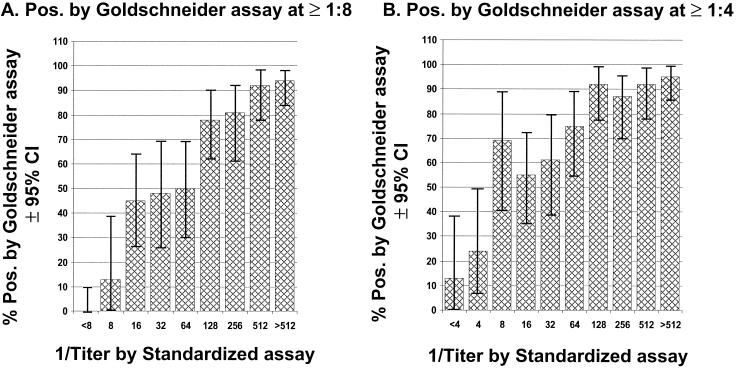
Figure 1 shows the percentages of sera, stratified by bactericidal titer against serogroup C measured by the standardized assay using rabbit complement, that were positive when retested with the Goldschneider assay. Panel A shows the results when a positive titer by the Goldschneider assay was defined as a titer of ≥1:8. Panel B shows the corresponding values when a positive titer by the Goldschneider assay was defined as a titer of ≥1:4. It was necessary to select a titer between 1:128 and 1:512 measured with the standardized assay in order for approximately 80 to 90% of samples to give a positive titer when measured with the Goldschneider assay. Less than half of the samples with lower titers, between 1:8 and 1:64 measured by the standardized assay, were positive when tested by the Goldschneider assay (defined as titers of 1:8 or higher). If a positive titer by the Goldschneider assay was defined as a titer of 1:4 or higher, approximately 27 to 64% of samples with titers between 1:8 and 1:64 by the standardized assay would be considered positive.
FIG. 1.
(A) Percentages of subjects (± 95% CI) stratified by bactericidal titer to serogroup C measured by the standardized protocol (15) using rabbit complement that were positive at titers of ≥1:8 when retested by the Goldschneider assay performed with human complement. (B) The same analysis is shown as in panel A, but a positive titer with the Goldschneider assay was defined as ≥1:4.
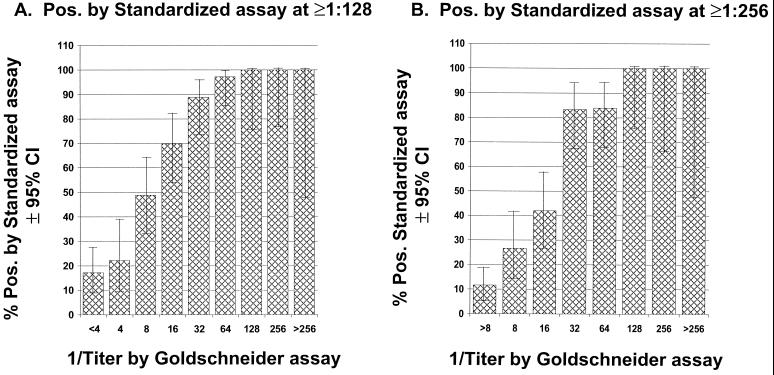
The use of high-threshold titers measured by the standardized assay with rabbit complement increases the specificity but results in many samples being classified as negative that would otherwise be considered protective when measured by the Goldschneider assay. Figure 2 illustrates the percentage of subjects with titers of ≥1:128 (panel A) or ≥1:256 (panel B) measured by the standardized assay when stratified by the titers measured with the Goldschneider assay. Only 8 of the 36 samples (24%) with titers between 1:4 and 1:7 when measured with the Goldschneider assay had titers of ≥128 by the standardized assay (panel A). Only 12 of 45 samples (24%) with titers between 1:8 and 1:15 measured with the Goldschneider assay had titers of ≥1:256 when assayed by the standardized assay (panel B).
FIG. 2.
Percentages of samples that were positive when tested by the standardized assay with rabbit complement according to the bactericidal titer measured by the Goldschneider assay performed with human complement. (A) Positive by the standardized assay is defined as 1:128 or greater; (B) positive by the standardized assay is defined as 1:256 or greater. A titer of 1:4 to 1:7 by the Goldschneider assay is shown as 1:4; a titer of 1:8 to 1:15 is shown as 1:8, etc.
Effect of Ig isotype.
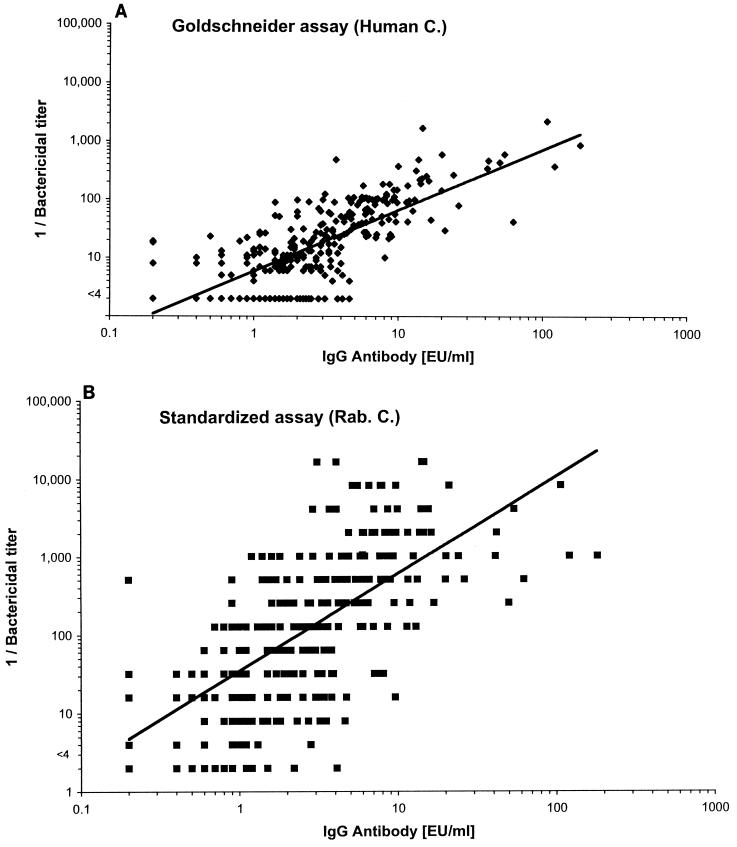
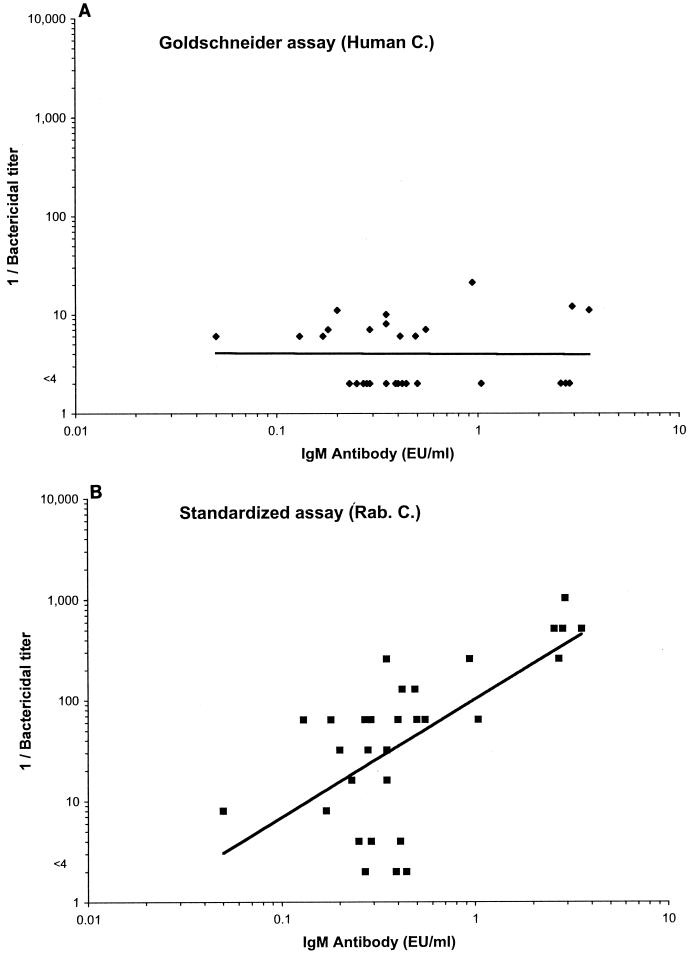
Figure 3 illustrates graphically the relationship between the bactericidal titers measured by the Goldschneider (panel A) and standardized (panel B) assays and the respective anti-serogroup C capsule IgG antibody concentrations measured by ELISA. The magnitude of the bactericidal antibody responses measured by both the standardized and Goldschneider assays parallels the IgG anticapsular antibody concentrations, although the distribution of points along the respective fitted curves appears closer with the Goldschneider assay. Figure 4 shows a similar analysis for IgM responses for a subset of sera with low but comparable IgG antibody concentrations (0.6 to 2.0 EU/ml, chosen to minimize the contribution of IgG antibody). For these sera, an increase in human IgM antibody levels did not result in higher bactericidal titers determined by the Goldschneider assay using human complement (panel A). In contrast, an increase in IgM antibody did result in higher bactericidal titers measured by the standardized assay performed with rabbit complement (panel B).
FIG. 3.
Relationship between the magnitude of the bactericidal antibody response to serogroup C measured by the Goldschneider and standardized assays and the respective anticapsular IgG antibody concentration measured by ELISA. (A) Goldschneider assay; (B) standardized assay. C., complement; Rab., rabbit.
FIG. 4.
Relationship between the magnitude of the bactericidal antibody response to serogroup C measured by the Goldschneider and standardized assays and the respective anticapsular IgM antibody concentration measured by ELISA. This analysis was performed on the subset of sera with low but comparable IgG antibody concentrations (0.6 to 2.0 EU/ml) to minimize the contribution of IgG antibody. (A) Goldschneider assay; (B) standardized assay. C., complement; Rab., rabbit.
DISCUSSION
Establishing a biologically relevant serologic surrogate for inferring protection against meningococcal disease is critical for vaccine development. The serologic results can be used by regulatory agencies as a basis for licensure of new meningococcal vaccines, by public health authorities for formulating rational recommendations on vaccine use, and by manufacturers for assessing the possible effects of manufacturing process changes on the efficacy of new or existing meningococcal vaccines. Fortunately, there is substantial evidence that bactericidal antibodies in serum confer protection against meningococcal disease, which permits the establishment of a serologic surrogate of protection. However, despite more than 25 years of research, controversies remain on the assay method of choice for the measurement of bactericidal antibodies.
The present study was designed to provide a direct comparison between titers measured by the current standardized bactericidal assay to those measured by the original Goldschneider assay. The results demonstrate unambiguously that use of the standardized assay with rabbit complement elevates bactericidal titers to meningococcal serogroup C in serum when compared to the respective titers measured by the Goldschneider assay with human complement (Tables 1 and 2 and Fig. 1). Although there are several small differences in the assay procedures, the largest factor contributing to the elevated titers measured by the standardized assay would appear to be the use of rabbit complement (1, 9, 14, 21). The augmentation of titers appears to be greatest for sera from subjects receiving conjugate vaccine (Table 1) and for sera containing IgM anticapsular antibodies (Fig. 4). With respect to the IgM results, a similar finding has been reported previously for monoclonal antibodies to the capsular polysaccharides of Neisseria meningitidis serogroup B and Haemophilus influenzae type b, for which the respective bactericidal activities of IgM antibodies assayed with rabbit complement were 30- to 1,000-fold higher than when assayed with human complement (14). In contrast, the bactericidal activity of an IgG2 monoclonal antibody to H. influenzae type b was not significantly different when assayed with human or rabbit complement. Augmentation of bactericidal titers by IgM antibodies is of particular concern because IgM responses may be transient and do not necessarily correlate with long-term protection. Also, the IgM responses are not predictive of immunological memory or affinity maturation. Therefore, bactericidal titers measured by the standardized assay with rabbit complement that disproportionately reflect IgM antibody may result in a misleading assessment of protective efficacy. Taken together, the current data, along with those previously published (9, 14, 21), indicate that bactericidal assays performed with rabbit complement can yield misleading results.
In the present study, a titer of ≥1:128 was needed in the standardized assay to predict with 80 to 90% certainty a positive titer defined as ≥1:4 or ≥1:8 when measured by the Goldschneider assay, the method that was used in the 1960s to establish a relationship between bactericidal activity in serum and protection against meningococcal disease (5). The critical problem in employing a titer of 1:128 measured by the standardized assay as the threshold for defining a protective titer is the lack of sensitivity (Table 3). The resulting false classification of susceptibility will be a particular problem in studies of serum collections where the magnitude of the antibody response is on average lower than that in the present serum collection. This situation is not uncommon for young children given plain meningococcal polysaccharide vaccine (12, 16) or for infants and toddlers given one dose of serogroup C conjugate vaccine (12, 13). Such an error could lead to the rejection of an otherwise effective new vaccine or to a perceived need for additional doses of a vaccine which in reality might be unnecessary.
To circumvent the lack of sensitivity when using a high-threshold titer to define protection as measured by the standardized assay, Borrow et al. propose two alternative serologic criteria for protection for persons with bactericidal titers between 1:8 and 1:64 (1). One is the demonstration of a fourfold or greater rise in titer, comparing postvaccination to prevaccination titers. The second is evidence of the induction of immunological memory upon subsequent exposure to a dose of plain meningococcal C polysaccharide. Although both of these criteria are plausible, to date there are no experimental or epidemiological data that provide direct evidence that these criteria reliably predict protection against meningococcal disease. The lack of direct supporting evidence is of particular concern since many persons with titers between 1:8 and 1:64 measured by the standardized assay will have subprotective titers if measured by the Goldschneider assay. Also, the ability to generate a memory antibody response may not be sufficient to confer a high level of protection against meningococcal disease, given its short incubation, abrupt onset, and intensity (3, 6). For example, in adults primed with meningococcal conjugate vaccine, it takes between 4 and 7 days after exposure to plain polysaccharide vaccine to generate a memory anti-serogroup C capsule antibody response (7).
The principal argument for using rabbit complement in the standardized assay is that it is difficult to obtain normal human serum that lacks intrinsic bactericidal antibodies. One approach to obtain human complement would be to absorb bactericidal antibodies from normal serum. However, preliminary studies indicate that absorption of human sera with whole bacterial cells or by antigen-specific affinity columns results in a substantial loss of complement activity, even when conditions are selected that should minimize complement activation (unpublished observations). This approach may not be necessary since, as described in Materials and Methods, of 50 sera screened from healthy adults residing in Northern California, 10% lacked intrinsic bactericidal antibodies and could serve as suitable complement sources for the bactericidal assay. Furthermore, we have bled individual donors repeatedly for periods of up to 5 years and have been able to obtain sufficient human complement to assay bactericidal antibody responses in sera from more than 1,000 individuals immunized during the development and licensure of a new meningococcal serogroup C conjugate vaccine in the United Kingdom. Other investigators also have shown that the use of human complement is feasible for assaying bactericidal antibody responses to serogroup B isolates in serum (2, 10, 17, 18).
In summary, the only scientifically proven correlate of protection against meningococcal disease is having a bactericidal titer in serum of 1:4 or higher when measured by the Goldschneider assay using human complement. The results of the present study confirm that bactericidal antibody titers measured by the standardized assay performed with rabbit complement are greatly augmented (1). By employing a sufficiently high threshold titer of ≥1:128 in the standardized assay it is possible to predict reliably that a serum considered positive will be positive when assayed by the Goldschneider assay with human complement. However, using such a high titer as the threshold of protection in the standardized assay results in the loss of sensitivity, particularly when the magnitude of the antibody response is modest, and risks falsely concluding that a person is susceptible to disease when he or she may actually be protected. Given these constraints, the best solution for predicting protection from studies of bactericidal activity is to measure titers by the assay described by Goldschneider et al. or by other assays shown to give comparable results.
ACKNOWLEDGMENTS
We thank Ray Borrow, PHLS Meningococcal Reference Unit, Manchester, United Kingdom, whose laboratory measured the bactericidal antibody titers by the standardized assay performed with rabbit complement; Lawrence H. Moulton, Departments of International Health and Biostatistics, The Johns Hopkins University School of Hygiene and Public Health, Baltimore, Md., for thoughtful review of the manuscript and helpful suggestions on presentation of the sensitivity and specificity analyses; and William Wacknov for invaluable technical assistance in the development and performance of the Goldschneider assay.
This work was supported in part by grants RO1 AI456464 and AI46464 from the National Institute of Allergy and Infectious Diseases, NIH (to D.M.G.).
REFERENCES
- 1.Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for the use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69:1568–1573. doi: 10.1128/IAI.69.3.1568-1573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Kleijn E D, de Groot R, Labadie J, Lafeber A B, van den Dobbelsteen G, van Alphen L, van Dijken H, Kuipers B, van Omme G W, Wala M, Juttmann R, Rumke H C. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine. 2000;18:1456–1466. doi: 10.1016/s0264-410x(99)00423-5. [DOI] [PubMed] [Google Scholar]
- 3.Edwards E A, Devine L F, Sengbusch G H, Ward H W. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis. 1977;9:105–110. doi: 10.3109/inf.1977.9.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 4.Frasch C E. Meningococcal vaccines: past, present and future. In: Cartwright K, editor. Meningococcal disease. New York, N.Y: John Wiley & Sons; 1995. pp. 245–283. [Google Scholar]
- 5.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granoff D M, Gupta R K, Belshe R B, Anderson E L. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–874. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 8.Granoff D M, Maslanka S E, Carlone G M, Plikaytis B D, Santos G F, Mokatrin A, Raff H V. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5:479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiss J M, Goroff D K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983;130:2882–2885. [PubMed] [Google Scholar]
- 10.Hoiby E A, Rosenqvist E, Froholm L O, Bjune G, Feiring B, Nokleby H, Ronnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 1991;14:147–155. [PubMed] [Google Scholar]
- 11.Jodar L, Cartwright K, Feavers I M. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A and C vaccines. Biologicals. 2000;28:193–197. doi: 10.1006/biol.2000.0253. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald N E, Halperin S A, Law B J, Forrest B, Danzig L E, Granoff D M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 13.MacLennan J M, Shackley F, Heath P T, Deeks J J, Flamank C, Herbert M, Griffiths H, Hatzmann E, Goilav C, Moxon E R. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA. 2000;283:2795–2801. doi: 10.1001/jama.283.21.2795. [DOI] [PubMed] [Google Scholar]
- 14.Mandrell R E, Azmi F H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly alpha 2→8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 15.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B, Harakeh H S, Dykes J K, Arhin F F, Devi S J, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C, Quataert S, Tai J Y, Carlone G M The Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslanka S E, Tappero J W, Plikaytis B D, Brumberg R S, Dykes J K, Gheesling L L, Donaldson K B, Schuchat A, Pullman J, Jones M, Bushmaker J, Carlone G M. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect Immun. 1998;66:2453–2459. doi: 10.1128/iai.66.6.2453-2459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters C C, Rumke H C, Sundermann L C, Rouppe van der Voort E M, Meulenbelt J, Schuller M, Kuipers A J, van der Ley P, Poolman J T. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–1015. doi: 10.1016/0264-410x(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 18.Tappero J W, Lagos R, Ballesteros A M, Plikaytis B, Williams D, Dykes J, Gheesling L L, Carlone G M, Hoiby E A, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman J T, Perkins B A. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–1527. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 19.Wong K H, Barrera O, Sutton A, May J, Hochstein D H, Robbins J D, Robbins J B, Parkman P D, Seligmann E B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5:197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Requirements for meningococcal polysaccharide vaccine (requirements for biological substances no. 23) WHO Tech Rep Ser. 1976;594:72–73. [Google Scholar]
- 21.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]