Abstract
Antigen-specific CD8+ T cells with cytotoxic activity are often critical in immune responses to infectious pathogens. To determine whether gamma interferon (IFN-γ) expression is a surrogate marker for cytotoxic T lymphocytes (CTL), human cytomegalovirus-specific CTL responses were correlated with CD8+ T-cell IFN-γ expression determined by cytokine flow cytometry. A strong positive correlation was observed between specific lysis of peptide-pulsed targets in a 51Cr release assay and frequencies of peptide-activated CD8+ T cells expressing IFN-γ at 6 h (r2 = 0.72) or 7 days (r2 = 0.91). Enumeration of responding cells expressing perforin, another marker associated with CTL, did not improve this correlation. These results demonstrate that IFN-γ expression can be a functional surrogate for identification of CTL precursor cells.
CD8+ T cells have the capacity to become activated, secrete cytokines and proliferate upon encounter with their cognate antigen presented by major histocompatibility complex (MHC) class I molecules (1). CD8+ T cells appear to be strongly associated with cytolytic activity, either by direct killing of antigen-bearing target cells by granule-mediated exocytosis or Fas-mediated cytotoxic mechanisms (14, 23, 25). In addition recent studies suggest that antigen-activated CD8+ T lymphocytes can eliminate or control viral infection by secretion of antiviral cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (16, 22), or in some instances may become functionally anergic (4, 24). IFN-γ production by CD8+ T cells can have both local and systemic consequences, whereas cytotoxins such as perforin are cytolytic for the cells that come in direct contact with the cytotoxic T lymphocytes (CTL) (7, 19).
A number of recent studies have shown a strong relationship between virus-specific cytotoxic activity and IFN-γ or perforin expression by CD8+ T cells (6, 9). A threshold of IFN-γ or tumor necrosis factor alpha production may be required for effective clearance of virus, as demonstrated in transgenic models where lower numbers of IFN-γ-producing CD8+ cells were unable to block replication of hepatitis B virus (8). Perforin, an effector protein that is stored in cytoplasmic granules and associated with cytolytic function, has long been used as a marker of cytolytic lymphocytes in vivo (14). Expression of perforin as examined by immunocytochemical staining has also been shown to correlate with cytolytic potential of CD8+ and CD4+ T-cell subpopulations in fresh peripheral blood mononuclear cells (PBMC) from infectious mononucleosis patients (17). By using in situ hybridization, immunohistochemistry, and flow cytometry techniques, perforin-positive cells have been detected in disease conditions such as rheumatoid arthritis (5), and tight regulation of perforin and IFN-γ expression has been demonstrated in pathogenic infections such as lymphocytic choriomeningitis virus (21). Although these studies suggest that IFN-γ and/or perforin expression in CD8+ T cells is associated with CTL activity, it has not been demonstrated at the single-cell level whether these markers are equally useful in identifying antigen-specific CTL precursors.
The recent development of cytokine flow cytometry (CFC) assays has provided a more quantitative approach to estimate the frequency of antigen-specific memory T cells and a means to characterize cytokine expression in individual cells (15, 18). In the present study we utilized CFC to correlate the frequency of CD8+ T cells expressing INF-γ and/or perforin with cytomegalovirus (CMV) peptide-induced CTL activity as measured by traditional 51Cr release assays of CD8+ T cells obtained from a number of HLA A2+ subjects against MHC-class-I-restricted peptide-loaded target cells.
Peptide-specific IFN-γ expression in CD8+ T cells correlates with peptide-specific CTL activity.
For flow cytometric detection of IFN-γ, CFC assays were performed using PBMC stimulated with peptides (10 μg/ml) and costimulatory monoclonal antibodies (MAbs) (CD28 and CD49d) as described previously (11, 26). The staining MAbs were typically FastImmune anti-IFN-γ fluorescein isothiocyanate (FITC), CD69 phycoerythrin (PE), or anti-perforin PE, CD3 peridinin chlorophyll protein, and CD8 allophycocyanin (BD Biosciences, Immunocytometry Systems [BDIS] San Jose, Calif.). For CTL assays, effector CD8+ cells were purified (>95%) from the 7-day peptide-stimulated culture of PBMC using immunomagnetic beads (Dynabeads M450 CD8; Dynal, Oslo, Norway). Target cells were either autologous B lymphoblastoid cell lines (B-LCL) or JY cells (an allogeneic HLA A2+ B-LCL) pulsed with peptides and labeled with 51Cr (NEN Life Science Products, Boston, Mass.) using the methods described previously (13). CTL were assayed for specific lysis of peptide-pulsed targets using a standard method (12).
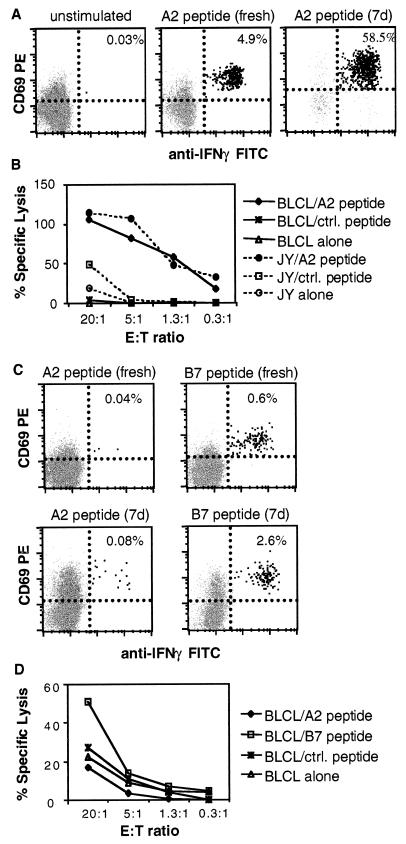
Figure 1A shows a representative CD8+ IFN-γ+ T-cell response to a 6-h stimulation with peptide on day 0 and on day 7 compared to unstimulated control. In this donor, the frequency of IFN-γ+ CD8+ T cells increased from 4.9% on day 0 to 58.5% after 7 days of activation with the peptide. As shown in Fig. 1B, the effector CD8+ T cells prepared from this donor were strongly cytolytic in a 51Cr release CTL assay against the peptide-loaded target cells (B-LCL and JY) compared to target cells loaded with a control peptide or not loaded with any peptide.
FIG. 1.
Representative cytokine and cytotoxicity responses to CMV peptides on day 0 and day 7 in one HLA A2+ donor. (A) CFC of CD3+ CD8+ lymphocytes from unstimulated PBMC (left panel), or PBMC stimulated with a pp65 HLA A2-restricted-peptide (amino acids 495 to 503, NLVPMVATV) for 6 h on day 0 (middle panel) or 7 days (right panel). (B) Four-hour 51Cr release assay of purified CD8+ lymphocytes after 7 days of peptide restimulation in the same donor as in panel A. Target cells were B-LCL or JY cells loaded with HLA A2-restricted peptide, control (ctrl.) peptide (MVATVGGGA), or no peptide. (C) Example of CFC responses to HLA-A2-restricted peptide (left panels) versus HLA B7-restricted peptide (pp65 amino acids 418 to 426, PRVTGGGAM) (right panels) in a second donor who was HLA A2+ B7+, stimulated for 6 h (top panels) or 7 days (lower panels). This donor, although HLA A2+, responded predominantly to the HLA B7-restricted epitope. (D) Four-hour 51Cr release assay of purified CD8+ lymphocytes after 7 days of HLA A2-restricted peptide stimulation in the same donor as in panel C. Target cells were B-LCL loaded with HLA A2-restricted or HLA B7-restricted peptide. Results correlated with those obtained by CFC in panel C.
Absence of IFN-γ+ T-cell response to HLA A2-restricted peptide correlates with lack of CTL activity.
We observed that 3 out of 12 CMV-seropositive and HLA A2-positive subjects were low responders in the day 0 CFC assay, and these remained low responders in both CFC and CTL assays on day 7 when stimulated with HLA A2-restricted peptide. Figure 1C depicts the results obtained for one such donor, who happened to show a strong response instead to an HLA B7-restricted peptide epitope. Effector CD8+ cells prepared from this donor also lysed target cells pulsed with HLA B7-restricted peptide but not HLA A2-restricted peptide or control peptide (Fig. 1D). The HLA B7-restricted killing was observed only at a higher effector-to-target cell (E:T) ratio (20:1) because the cells were restimulated with HLA A2-restricted peptide for 7 days, as opposed to the cognate HLA B7-restricted peptide.
IFN-γ expression is a functional surrogate marker for identifying CTL.
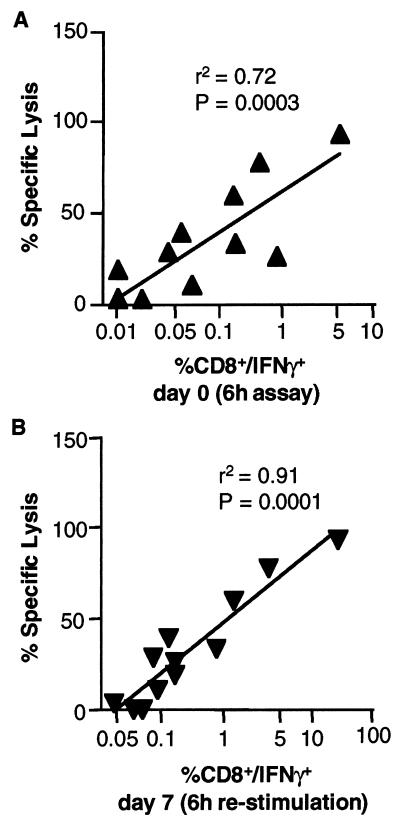
We observed a broad range of CD8+ T-cell responses to the dominant epitope of CMV among 12 HLA A2-positive and CMV-seropositive donors tested. To explore this further, the frequency data obtained by flow cytometry (IFN-γ expression) were correlated with percent specific lysis obtained in the 51Cr release cytotoxicity assay. Figure 2 demonstrates a significant positive correlation observed between CTL activity as measured by the 51Cr release assay (percent specific lysis; E:T of 20:1) and the frequency of CD8+ T cells expressing IFN-γ. The strongest correlation was observed between 51Cr release assay (day 7) and IFN-γ+ CD8+ T-cell frequencies on day 7 (Fig. 2B) (r2 = 0.91; P = 0.0001). Importantly, the frequency of IFN-γ-expressing cells measured on day 0 also correlated significantly with results of 51Cr release assay performed on day 7 (Fig. 2A) (r2 = 0.72; P = 0.0003).
FIG. 2.
Correlation between 6-h cytokine assay and 7-day 51Cr release assay in 12 donors. The statistical analysis was performed using GraphPad (San Diego, Calif.) software, assuming that the data are sampled from Gaussian populations with a two-tailed distribution. (A) Frequencies of CD8+ IFN-γ+ T lymphocytes following 6 h of activation with HLA A2 peptide on day 0 correlated with percent specific lysis of target cells in a 51Cr release assay performed after 7 days of in vitro stimulation of PBMC with HLA A2-restricted peptide. (B) Frequencies of CD8+ IFN-γ+ T cells obtained in the day 7 CFC assay (6-h restimulation) correlated with percent specific lysis of target cells in a 51Cr-release assay also done on day 7.
Perforin expression does not help identify CTL precursors.
Expression of perforin, which is considered to be a marker of cytolytic cells, was also examined as an additional marker to identify CTL precursors in activated cultures. Peptide-stimulated cells were stained intracellularly with PE-labeled anti-perforin MAb (BD PharMingen, San Diego, Calif.) in addition to anti-IFN-γ MAb to determine whether IFN-γ and perforin were coexpressed in antigen-activated CD8+ T cells. There was a weak positive correlation between coexpression of perforin with IFN-γ and cytolytic activity of CD8+ T cells (r2 = 0.49 and P = 0.0075 on day 0; r2 = 0.64 and P = 0.003 on day 7). This indicates that use of perforin expression as an additional marker for CTL does not add any significant value to the use of IFN-γ expression alone for the identification of CTL. Also, the total frequency of perforin-positive CD8+ T cells measured on day 0 did not correlate with CTL activity (r2 = 0.002; P = 0.89) (data not shown).
Correlation of tetramer staining and CFC.
The binding of MHC-class-I-restricted tetramer complexes to cognate epitope-specific T cells has been suggested as an alternate method to identify CTL precursor frequency (2). We performed surface staining of blood samples from HLA A2- positive and CMV-seropositive donors (n = 8) using MHC-class-I-restricted tetramer complexes containing the HLA A2-restricted peptide (pp65495–503). We observed significant positive correlation between the frequencies of tetramer-positive CD8+ T cells and IFN-γ+ CD8+ T cells (r2 = 0.92; P = 0.0001) (data not shown). Although tetramer-positive CD8+ T cells have often been associated with CTL, it cannot always be assumed that all T cells expressing T-cell receptor with specificity for the cognate epitope are in fact functionally competent. In a recent study, for example, Shankar et al. demonstrated that only 25% of tetramer-positive CD8+ cells from human immunodeficiency virus (HIV)-infected PBMC produced IFN-γ after stimulation with the relevant gag or reverse transcriptase peptide of HIV antigen, indicating an impaired function of HIV-specific CD8+ T cells in vivo (20). In another study, Lee et al. reported discordance between tetramer staining and T-cell function in metastatic melanoma disease (13). In our analysis, however, we could find no evidence for anergic cells at any significant level with samples obtained from healthy CMV-seropositive individuals. Thus, CD8+ T cells which were tetramer-positive also expressed IFN-γ when stimulated with cognate peptide.
Range of responses to the peptide epitope.
The range of frequencies of the peptide-specific IFN-γ-producing CD8+ T cells in HLA A2+, CMV-seropositive donors was 0.01 to 4.8% (n = 12) above unstimulated background after 5 to 6 h of stimulation on day 0. The unstimulated control backgrounds in the day 0 CFC assay for all the donors tested were in the range of 0 to 0.05%. The donors that exhibited detectable frequencies of IFN-γ+ CD8+ T cells in response to peptide stimulation on day 0 also demonstrated positive cytotoxicity responses on day 7 as measured by the 51Cr release assay. Frequencies of peptide-specific IFN-γ+ CD8+ T cells on day 7 were also increased in all such cases (see the example in Fig. 1). The unstimulated controls for the day 7 CFC assay were not performed, as the cells had already been stimulated with peptide and recombinant interleukin 2 for 7 days.
In contrast, the donors that exhibited very low frequencies of IFN-γ-producing cells (<0.05%) in response to HLA A2-restricted peptide stimulation on day 0 remained low responders in both CFC and CTL assays after 7 days of stimulation with peptide. Interestingly, CD8+ T cells from two such donors who were also HLA B7 positive responded to HLA B7-restricted peptide stimulation in both CFC (days 0 and 7) and CTL assays (see the examples in Fig. 1C and D). Although the cells were not activated with the HLA B7-restricted peptide for 7 days, the presence of recombinant interleukin 2 during this period maintained or expanded the HLA B7-restricted peptide-specific CD8+ cells in the culture.
The observation that a few donors did not respond to the HLA A2-restricted peptide in either CFC or CTL assays indicates that not all donors expressing a particular HLA allele will respond to an identified immunodominant epitope for that allele. The observed absence or diminished CD8+ T-cell response to HLA A2-restricted peptide compared to HLA B7-restricted peptide of CMV pp65 in two HLA A2+ B7+ donors (Fig. 1 and data not shown) suggests possible MHC-related immunodominance hierarchies similar to the one confirmed in murine influenza virus-specific CD8+ T-cell responses (3).
Conclusions.
The strong correlation observed between IFN-γ expression and cytolytic activity demonstrates that IFN-γ expression by CD8+ T cells identifies CTL effector cells, at least in the CMV system. It is particularly noteworthy that day 0 IFN-γ expression and day 7 CTL activity were still highly correlated. This suggests that the frequency of cells expressing IFN-γ obtained during the short-term 6-h CFC assay can be sufficient to predict CTL activity in longer-term cultures.
More complete analysis of T-cell responses (measured by IFN-γ expression) using mixtures of peptides spanning the complete immunodominant proteins of pathogens and autoimmune antigens, eliminating the obstacle of HLA restriction, has been proposed in a recent report (10). The observation that IFN-γ expression in short-term-activated CD8+ T cells identifies CTL precursors enables early quantitative detection of such cells and eliminates artifacts introduced in long-term cultures. Widespread use of this CFC assay to analyze CTL precursor activity at the single-cell level would provide more-accurate assessments of the CD8+ T-cell response in clinical settings.
Acknowledgments
We are thankful to H. Greenberg and X. He (Stanford University, Stanford, Calif.) for providing us with HLA-A2-restricted tetramer complex specific for the HCMV pp65 peptide.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. . (Erratum, 280:1821, 1998.) [PubMed] [Google Scholar]
- 3.Belz G T, Stevenson P G, Doherty P C. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ t cell response to influenza A viruses. J Immunol. 2000;165:2404–2409. doi: 10.4049/jimmunol.165.5.2404. [DOI] [PubMed] [Google Scholar]
- 4.Chai J G, Bartok I, Scott D, Dyson J, Lechler R. T:T antigen presentation by activated murine CD8+ T cells induces anergy and apoptosis. J Immunol. 1998;160:3655–3665. [PubMed] [Google Scholar]
- 5.Griffiths G M, Alpert S, Lambert E, McGuire J, Weissman I L. Perforin and granzyme A expression identifying cytolytic lymphocytes in rheumatoid arthritis. Proc Natl Acad Sci USA. 1992;89:549–553. doi: 10.1073/pnas.89.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 9.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 10.Kern F, Faulhaber N, Frommel C, Khatamzas E, Prosch S, Schonemann C, Kretzschmar I, Volkmer-Engert R, Volk H D, Reinke P. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Kern F, Surel I P, Brock C, Freistedt B, Radtke H, Scheffold A, Blasczyk R, Reinke P, Schneider-Mergener J, Radbruch A, Walden P, Volk H D. T-cell epitope mapping by flow cytometry. Nat Med. 1998;4:975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- 12.Kuzushima K, Hoshino Y, Fujii K, Yokoyama N, Fujita M, Kiyono T, Kimura H, Morishima T, Morishima Y, Tsurumi T. Rapid determination of Epstein-Barr virus-specific CD8(+) T-cell frequencies by flow cytometry. Blood. 1999;94:3094–3100. [PubMed] [Google Scholar]
- 13.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 14.Liu C C, Walsh C M, Young J D. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 15.Maino V C, Picker L J. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry. 1998;34:207–215. doi: 10.1002/(sici)1097-0320(19981015)34:5<207::aid-cyto1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 17.Nakata M, Kawasaki A, Azuma M, Tsuji K, Matsuda H, Shinkai Y, Yagita H, Okumura K. Expression of perforin and cytolytic potential of human peripheral blood lymphocyte subpopulations. Int Immunol. 1992;4:1049–1054. doi: 10.1093/intimm/4.9.1049. [DOI] [PubMed] [Google Scholar]
- 18.Nomura L E, Walker J M, Maecker H T. Optimization of whole blood antigen-specific cytokine assays for CD4(+) T cells. Cytometry. 2000;40:60–68. doi: 10.1002/(sici)1097-0320(20000501)40:1<60::aid-cyto8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 20.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 21.Slifka M K, Rodriguez F, Whitton J L. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 22.Slifka M K, Whitton J L. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–457. doi: 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- 23.Smyth M J, Trapani J A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 24.Sundstedt A, Hoiden I, Hansson J, Hedlund G, Kalland T, Dohlsten M. Superantigen-induced anergy in cytotoxic CD8+ T cells. J Immunol. 1995;154:6306–6313. [PubMed] [Google Scholar]
- 25.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 26.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Investig. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]