Abstract
Rhabdomyolysis is a condition in which muscle breaks down potentially leading to renal dysfunction, and often occurs secondary to a precipitating factor. Viral or bacterial infections are common precipitants for initiating rhabdomyolysis. Recently, healthcare systems across the world have been challenged by a pandemic of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causing ‘coronavirus disease 2019’ (COVID-19) disease. SARS-CoV-2 infection is recognized to cause respiratory and cardiovascular compromise, thromboembolic events, and acute kidney injury (AKI); however, it is not known whether it can precipitate rhabdomyolysis, with only a limited number of cases of SARS-CoV-2 infection preceding rhabdomyolysis reported to date. Here, we report the case of a 64-year-old woman who developed rhabdomyolysis shortly after SARS-CoV-2 infection and COVID-19. She initially presented with muscular pain, a creatine kinase level of 119,301 IU/L, and a mild rise in her creatinine level to 92 µmol/L, but successfully recovered with intravenous fluid support. We also review the literature to summarise previously reported cases of rhabdomyolysis precipitated by SARS-CoV-2, highlighting the need to consider this diagnosis in patients presenting with SARS-CoV-2 and myalgia.
Keywords: rhabdomyolysis, viral-induced rhabdomyolysis, COVID-19 disease, SARS-CoV-2 infection
1. Introduction
Since its first emergence in 2019, SARS-CoV-2 infection causing COVID-19 disease has been associated with numerous complications including respiratory and cardiovascular compromise, thromboembolic events, and acute kidney injury (AKI) [1,2]. The incidence of rhabdomyolysis after COVID-19 is not known, and this association has only been described in a few case reports to date. Viral infections are a known precipitant of rhabdomyolysis [3], and myalgia is a common presenting feature of both COVID-19 and rhabdomyolysis. Here, we present a case of rhabdomyolysis in a patient with COVID-19 illness highlighting the need to consider this diagnosis in patients presenting with both COVID-19 and myalgia.
2. Case Presentation
A 64-year-old Afro-Caribbean woman with a background history of hypertension and previous infrequent episodes of viral-induced rhabdomyolysis presented to the emergency department with bilateral thigh pain and dark urine for one day. She reported a 5-day history of cough, lethargy, reduced appetite, and taste disturbance. She had tested positive for COVID-19 infection on lateral flow test (variant not specified) on the day of her admission. She had declined vaccination for SARS-CoV-2.
On further assessment, the patient denied nausea, vomiting, or any gastrointestinal, urinary, or neurological symptoms. She did not report any fevers and was apyrexial on admission. On physical examination, the patient has a blood pressure of 139/88 mmHg, a pulse rate of 88 beats per minute, and a respiratory rate of 16 breaths per minute. Her pulse oximetry reading reported an oxygen saturation of 97% on room air. She was alert and had normal vesicular breathing on pulmonary examination and the remainder of her systems examinations were unremarkable.
3. Background History and Previous Presentations of Rhabdomyolysis
Our patient had a history of infrequent episodes of rhabdomyolysis in the past on five occasions, all of which were triggered by viral infections. The first was in 1987 which was triggered by a suspected viral upper respiratory tract infection (URTI), although the specific organism was not identified. Following this she was noted to have had a normal muscle biopsy. The second episode was in 2000 also preceded by a suspected viral URTI. The third episode in 2013 was attributed to a presumed viral URTI as well as dental infection. The fourth episode was in 2018 triggered by the Influenza virus causing an URTI; she had mild muscle ache however she did not seek medical attention. The fifth episode in 2019 was also triggered by the Influenza virus causing an URTI. This patient had two additional viral infections (2015 and 2016) but experienced no muscle pain or neurological sequelae and did not seek medical attention.
During the episode of rhabdomyolysis in 2016, her creatine kinase (CK) level was 205 IU/L (Reference Range; RR 25–200 IU/L), erythrocyte sedimentation rate (ESR) was 51 mm/h (RR 5.0–15 mm/h) and C-reactive protein (CRP) was 10 mg/L (RR 0–5 mg/L). Her antinuclear antibody (ANA) was borderline positive (1:160) with a homogenous pattern. antineutrophil cytoplasmic antibody (ANCA), extractable nuclear antigen (ENA) and myositis immunoblot were all negative. In 2017, DNA samples analysed in Sheffield for extended rhabdomyolysis gene panel testing as well as molecular analysis, did not reveal any significant genetic predisposition variants. In 2019, her CK levels reached a peak at 36,320 IU/L during an episode of mild rhabdomyolysis triggered by a viral infection. Further tests including a non-ischemic forearm test, Pompe disease tests, and urinary organic acid, were all within normal limits.
4. Investigations
During the admission, blood test results showed an initial creatine kinase level of 119,301 IU/L (RR 25–200 IU/L). Her creatinine level was 92 µmol/L (RR 45–84 µmol/L) with an estimated glomerular filtration rate (eGFR) of 82 mL/min/1.73 m2 (RR > 90 mL/min/1.73 m2). Her platelet count was mildly reduced at 114 × 109/L (RR 150–450 × 109/L). Her serum ferritin level was within the reference range at 246 µg/L (RR 24–307 µg/L), serum vitamin B12 level was mildly elevated at 804 ng/L (RR 200–800 ng/L) and folate was elevated at 14 µg/L (RR 1.8–9 µg/L). Pertinent blood test results following the day of admission (Day 0) are presented in Table 1 and Figure 1 below.
Table 1.
Blood test results during admission and at follow up. Day 0 indicates day of presentation and admission to hospital.
| Date | Hb (g/L) | WCC (×109/L) | Creatinine (µmol/L) |
eGFR (ml/min/1.73 m2) |
CRP (mg/L) |
Platelets (×109/L) | Creatine Kinase (IU/L) |
|---|---|---|---|---|---|---|---|
| Day 0 | 137 | 4.6 | 92 | 57 | - | 114 | 119,301 |
| Day 1 | 123 | 3.2 | 68 | 82 | 11.9 | 72 | 114,965 |
| Day 2 | 125 | 3.5 | 67 | 83 | 8.4 | 68 | 71,303 |
| Day 3 | 128 | 4.2 | 66 | 85 | 10.3 | 79 | 39,540 |
| Day 4 | 116 | 3.8 | 66 | 85 | 8.7 | 106 | 21,344 |
| Day 10 | 126 | 5.4 | 71 | 78 | - | 314 | 1709 |
| Day 41 | - | - | 68 | 82 | - | - | 195 |
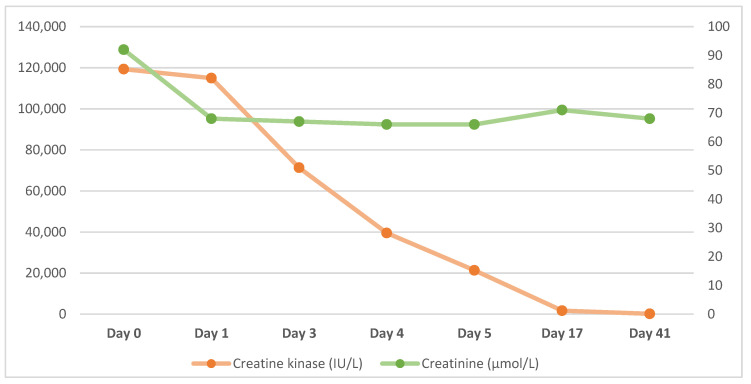
Figure 1.
Creatine kinase (left axis) and creatinine levels (right axis) during admission and at follow up.
Creatine kinase levels were highest on admission at 119,301 IU/L and reduced significantly within five days to 20,000 IU/L. At two weeks follow-up her CK levels had returned to within the reference range. With regard to her renal function, her creatinine was mildly elevated on admission but did not deteriorate following treatment with intravenous fluid support.
We undertook a full renal screen: Immunoglobulin testing revealed levels of IgA 1.22 g/L (RR 0.80–4.00 g/L), IgG 10.9 g/L (RR 6.0–16 g/L), IgM 0.27 g/L (RR 0.50–2.00 g/L). Serum free light chains were within normal limits; kappa light chains were 17.8 mg/L (RR 3.3–19.4 mg/L), lambda light chains were 15 mg/L (RR 5.71–26.3 mg/L), and kappa to lambda ratio was 1.19 (RR 0.26–1.65). Protein electrophoresis was normal. Rheumatoid factor was undetectable (RR < 0.20 IU/L). Autoantibody serology testing was negative for ANA, ANCA, serine proteinase 3 antibody (PR3), and myeloperoxidase (MPO). Viral serology indicating active infection with cytomegalovirus (CMV), Epstein–Barr virus (EBV), human immunodeficiency virus (HIV), Hepatitis A, Hepatitis B, Hepatitis C, and parvovirus were all not detected.
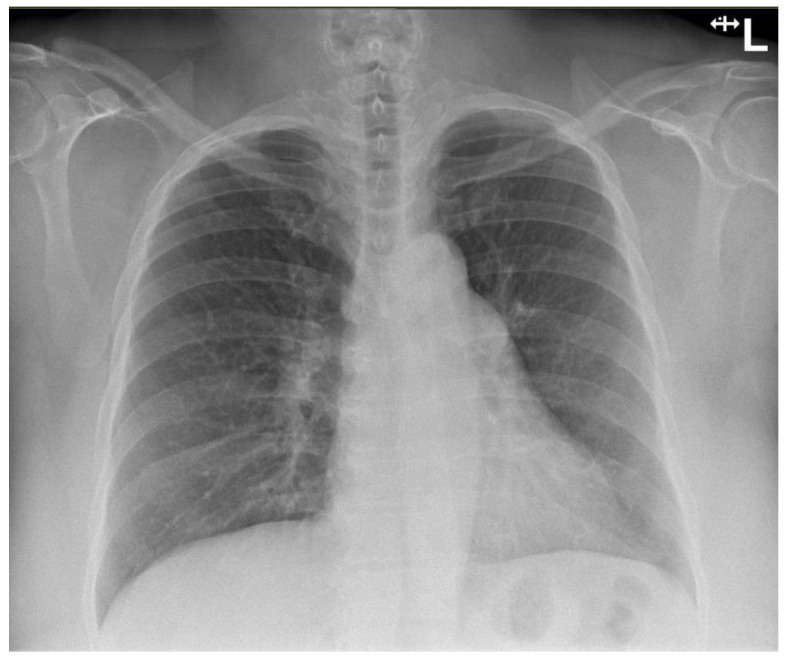
Urine dip showed blood (3+), protein (3+), leukocytes negative, nitrites negative, ketones negative and pH 5. Chest X-ray on admission showed clear lungs and pleural spaces (Figure 2).
Figure 2.
Normal chest X-ray radiograph on admission.
The patient did not exhibit any radiographic evidence of COVID-19 disease and had presented with mild respiratory symptoms only, mainly cough.
5. Treatment
The patient was admitted to a medical ward where she received treatment with continuous intravenous (IV) fluids with 4 L per day. She was also treated with 40 mg of enoxaparin subcutaneously for prophylaxis of venous thrombosis. During her admission she also received calcium-vitamin D (Adcal D3) tablets for hypocalcaemia, potassium bicarbonate-potassium chloride (Sando-K) tablets for hypokalaemia, and sodium dihydrogen phosphate (Sando-Phos) tablets for hypophosphatemia. In addition, she received paracetamol for analgesia, and a phosphate enema for constipation. Her regular medications, including losartan and indapamide, were held during admission to prevent kidney dysfunction.
6. Outcome and Follow-Up
The patient’s clinical condition remained stable, and her levels of creatine kinase decreased on daily monitoring without any suggestion of abnormal renal function. As her CK continued to fall to 33,000 IU/L by the fifth day of her admission, she was discharged and asked to continue good hydration with oral fluid intake. She had follow-up at 9 days following discharge to confirm that her creatine kinase levels continued to decrease (CK 1709 IU/L). She was also followed up in the neuromuscular clinic 1 month following admission, when was noted to have some mild residual proximal muscle weakness in her left leg.
7. Discussion
Rhabdomyolysis is a known complication of viral and bacterial infections [3]. It is characterized by the breakdown of skeletal muscle leading to the release of muscular components into the blood; including myoglobin, creatine kinase and lactate dehydrogenase (LDH) [3]. Rhabdomyolysis presents on a spectrum of illness severity ranging from being asymptomatic to life-threatening, and can be associated with electrolyte derangement, impairment of renal function, and rarely disseminated intravascular coagulation (DIC) [4]. It is commonly precipitated by direct injury or trauma; however, it can also be caused by drugs, toxins, infections, metabolic or genetic conditions, muscle ischemia, or hyperthermic states [4]. It is thought that viral-induced rhabdomyolysis occurs due to viral invasion of muscle tissue, toxin-induced damage, innate inflammatory responses, or all of these mechanisms in combination [3]. Patients often present with muscle pain or weakness, swelling, tea-coloured urine, and have significantly elevated levels of creatine kinase [5].
7.1. Risk Factors for Rhabdomyolysis in COVID-19 Illness
Rhabdomyolysis has been associated with COVID-19 infection in previous reports [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], which we have summarised in Table 2. Although there is limited information with regard to the association between SARS-CoV-2 infection and rhabdomyolysis, there seems to be no direct correlation between the severity of COVID-19 illness and the incidence of rhabdomyolysis. Studies from early in the pandemic suggested an association between rhabdomyolysis after COVID-19 illness and an increased risk of morbidity, ICU admission, and in-hospital mortality [23]. However, this could simply reflect rhabdomyolysis being more common in patients who are critically unwell with longer hospital stays [24,25]. A study including 140 patients with rhabdomyolysis and COVID-19 found that there was an increased risk of requiring renal replacement therapy and increased risk of death [26]; however, the prognosis is favourable amongst patients with normal renal function such as in our case.
Table 2.
Summary of case reports in the literature describing rhabdomyolysis in COVID-19.
| Age (Years) |
Gender | Ethnicity | Background | Level of Care | Peak CK Level (IU/L) | AKI | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Valente-Acosta 2020 [6] | 71 | Male | Unknown | BPH, smoker | Ward | 8720 | Yes | Discharged |
| Taxbro 2020 [7] | 38 | Male | Unknown | T2DM, gout, mild obesity | ICU | N/A | Yes | Discharged |
| Fujita 2021 [8] | 19 | Female | Unknown | None | Ward | 55,613 | No | Discharged |
| Jin & Tong 2020 [9] | 60 | Male | Unknown | Unknown | Ward | 11,842 | No | Discharged |
| Suwanwongse & Shabarek 2020 [10] | 88 | Male | Unknown | HTN, CKD, HF, BPH, OA, Cognitive impairment | Ward | 13,581 | Yes | Discharged |
| Uysal 2020 [11] | 60 | Male | Unknown | None | Ward | 4267 | No | Discharged |
| Singh 2020 [12]—Case 1 | 67 | Male | Unknown | HTN | ICU | 19,773 | Yes | Died |
| Singh 2020 [12]—Case 2 | 39 | Male | Unknown | HTN | ICU | 4330 | Yes | Died |
| Singh 2020 [12]—Case 3 | 43 | Male | Unknown | ESRF | Ward | 9793 | Unknown | Died |
| Singh 2020 [12]—Case 4 | 70 | Male | Unknown | None | ICU | 5008 | Unknown | Died |
| Khosla 2020 [13]—Case 1 | 65 | Male | Black | HTN, OSA hyperlipidaemia, | ICU | 7854 | Yes | Died |
| Khosla 2020 [13]—Case 2 | 78 | Male | White | T2DM, HTN, hyperlipidaemia, HF, MVR, CABG | ICU | >22,000 | Yes | Died |
| Khosla 2020 [13]—Case 3 | 67 | Male | Black | T2DM, HTN, hyperlipidaemia CKD, hemicolectomy | Dialysis | 6164 | Yes | Discharged |
| Khosla 2020 [13]—Case 4 | 58 | Male | Black | T2DM, HTN, hyperlipidaemia | Ward | 4625 | Yes | Discharged |
| Khosla 2020 [13]—Case 5 | 64 | Male | Black | T2DM, HTN, HIV | Ward | 3135 | No | Discharged |
| Rivas-Garcia 2020 [14] | 78 | Male | White | HTN, T2DM | Ward | 22,511 | Yes | Discharged |
| Hussein 2020 [15] | 38 | Male | Unknown | Obesity | ICU | 33,000 | Yes | Discharged |
| Alrubaye & Choudhury 2020 [16] | 35 | Female | Unknown | None | Ward | 71,000 | Unknown | Discharged |
| Buckholz 2020 [17]—Case 1 | 43 | Male | Unknown | None | ICU | 75,240 | Yes | Discharged |
| Buckholz 2020 [17]—Case 2 | 37 | Male | Unknown | None | ICU | 82,960 | Yes | Discharged |
| Buckholz 2020 [17]—Case 3 | 75 | Male | Unknown | DVT | Ward | 3638 | Yes | Discharged |
| Buckholz 2020 [17]—Case 4 | 59 | Male | Unknown | None | ICU | 8310 | No | Died |
| Buckholz 2020 [17]—Case 5 | 66 | Male | Unknown | HTN | ICU | 10,100 | No | Discharged |
| Buckholz 2020 [17]—Case 6 | 70 | Female | Unknown | MM, CKD | ICU | 406,300 | Yes | Died |
| Solis 2020 [18] | 46 | Male | Unknown | CML | Unknown | 400,000 | Yes | Died |
| Chedid 2020 [19] | 51 | Male | Unknown | HTN, T2DM, CKD | Unknown | 464,000 | Yes | Discharged on HD |
| Byler 2021 [20] | 67 | Female | Unknown | HTN, T2DM, Hyperlipidaemia | ICU | 15,085 | Yes | Discharged on HD |
| Singh 2020 [21]—Case 1 | 54 | Male | Hispanic | Asthma, DM, HTN, obesity | Unknown | 7337 | Unknown | Died |
| Singh 2020 [21]—Case 2 | 54 | Male | White | None | Unknown | 3068 | Unknown | Discharged |
| Singh 2020 [21]—Case 3 | 34 | Male | White | Obesity, prediabetes | Unknown | 5454 | Unknown | Died |
| Singh 2020 [21]—Case 4 | 71 | Male | Black | HTN, seizures schizophrenia, | Unknown | 10,247 | Unknown | Died |
| Singh 2020 [21]—Case 5 | 88 | Male | White | Diabetes, HTN | Unknown | 2628 | Unknown | Died |
| Singh 2020 [21]—Case 6 | 56 | Male | Hispanic | HTN, prediabetes | Unknown | 5388 | Unknown | Discharged |
| Singh 2020 [21]—Case 7 | 57 | Male | Hispanic | None | Unknown | 37,524 | Unknown | Died |
| Singh 2020 [21]—Case 8 | 64 | Male | White | None | Unknown | 6435 | Unknown | Died |
| Singh 2020 [21]—Case 9 | 36 | Male | White | None | Unknown | 5531 | Unknown | Died |
| Singh 2020 [21]—Case 10 | 39 | Male | Black | HTN | Unknown | 4330 | Unknown | Died |
| Shanbhag 2020 [22] | 19 | Male | Black | Anxiety, previous influenza-associated rhabdomyolysis | Unknown | 694,200 | No | Discharged |
AC = Afro-Caribbean, BPH = benign prostatic hyperplasia; T2DM = Type 2 diabetes mellitus; ICU = intensive care unit; HTN = hypertension; CKD = chronic kidney disease; HF = heart failure; OA = osteoarthritis; ESRF = end-stage renal failure; OSA = obstructive sleep apnoea; MVR = mitral valve repair; CABG = coronary artery bypass graft; HIV = Human Immunodeficiency Virus; DVT = deep vein thrombosis; MM = multiple myeloma; CML = chronic myeloid leukaemia; Haemodialysis = HD.
In our summary of reported cases, 34 of 38 (87%) were male, with an average age of 55.9 years, 7 of the 17 cases with known ethnicity were Black, and presented with an average CK level of 70,250 IU/L. Notably, 17 of the 38 cases (44.7%) died and 2 of the remaining survivors required haemodialysis on discharge. Thus, it is possible that rhabdomyolysis following SARS-CoV-2 could occur more commonly in patients with an increased risk of death.
It is unclear why some patients develop rhabdomyolysis whereas others do not, and risk factors for rhabdomyolysis in this context have not been clearly defined. Rhabdomyolysis after SARS-CoV-2 infection has been most commonly documented among older males [21]. However, there does not appear to be any correlation with ethnicity or medical background in our summary of published case reports (Table 2).
7.2. Viral-Induced Rhabdomyolysis
Our patient has had previous episodes of rhabdomyolysis in the past, precipitated by viral infection. However, these have been infrequent with four episodes over four decades. Nevertheless, she may well be predisposed to rhabdomyolysis in response to viral infection. Nonetheless, despite thorough investigation, to date, no predisposing factor has been identified in this patient.
This phenomenon of recurrent rhabdomyolysis has been observed in another case of a young Afro-Caribbean male [22] with significantly raised CK (>100,000 IU/L) who was also investigated for a metabolic myopathy; however, they too were unable identify the aetiology of recurrent viral-induced rhabdomyolysis.
7.3. Rhabdomyolysis and COVID-19 Vaccination
Data regarding vaccine-induced rhabdomyolysis is limited however some studies have described cases of rhabdomyolysis related to mRNA COVID-19 vaccination including Pfizer-BioNTech and Moderna vaccines [27,28,29,30,31]. These patients presented with muscle pain and fatigue up to two weeks after vaccination. One particular study identified a patient who developed rhabdomyolysis following COVID-19 infection due to a ryanodine receptor 1 (RYR1) gene mutation, which is known to increase susceptibility to rhabdomyolysis [32].
A study investigated the association between COVID-19 vaccination and rhabdomyolysis using the Vaccine Adverse Event Reporting System (VAERS) and found that rhabdomyolysis following COVID-19 vaccination was more frequently reported compared to rhabdomyolysis following all other vaccinations; however, the rates remained within the expected incidence range for the general population [33]. They concluded that there is no significant increase in risk of rhabdomyolysis following COVID-19 vaccination. Furthermore, rates of rhabdomyolysis were not found to differ between different vaccines including Pfizer, Moderna, or Johnson & Johnson. Vaccine-induced rhabdomyolysis has been observed with other viral infections including H1N1 and influenza [34,35,36]. It was postulated that patients on statin therapy for hyperlipidaemia are predisposed to rhabdomyolysis and that the influenza vaccine had triggered its onset, which may also be the case for COVID-19 vaccine-related rhabdomyolysis. Nevertheless, our patient did not receive the SARS-CoV-2 vaccination and as such, this was not a factor.
7.4. “Long COVID” and Musculoskeletal Involvement
The musculoskeletal system is commonly affected by COVID-19 infection and causes symptoms including myalgia, muscle weakness, and arthralgia. Such symptoms tend to disappear as the patients recover from COVID-19 illness however they have been found to persist past the acute phase of the infection [37,38]. “Long COVID” is the term used to describe the persistence of COVID-19 symptoms for longer than four weeks after the acute phase has subsided and has been documented among patients who recovered from both mild and severe forms of COVID-19 infection. It is thought that “Long-COVID” is caused by a persistent pro-inflammatory state and can therefore exacerbate muscle pain leading to prolonged intolerance of physical activity [39].
In one report, a young female suffering from “Long-COVID” with persistent muscle weakness and exercise intolerance was investigated with muscle biopsy, which was found to be suggestive of critical illness myopathy (CIM) despite having a relatively mild acute COVID-19 infection [40]. However, there are no documented reports of an association between “Long-COVID” and rhabdomyolysis.
8. Conclusions
We report the case of a patient with rhabdomyolysis as a complication of COVID-19 illness associated with myalgia, fatigue, and significantly elevated CK levels. This 64-year-old woman had SARS-CoV-2 infection with mild symptoms and successful recovery with intravenous fluid support with no impairment of renal function. There have been reports to suggest that vaccination against SARS-CoV-2 could also precipitate rhabdomyolysis in some patients; however, this has not been proven to be a common adverse effect of vaccination and equally it is also possible that vaccination could reduce complication rates after SARS-CoV-2 infection. Finally, COVID-19 illness can lead to long-term muscle pain and weakness; however, it has not been reported to be specifically associated with rhabdomyolysis. Thus, our case report suggests that rhabdomyolysis should be considered in patients presenting with SARS-CoV-2 infection, especially in the presence of myalgia, or if there is urinary discoloration.
Acknowledgments
We would like to acknowledge all of the healthcare professionals at Charing Cross Hospital involved in this patient’s care.
Author Contributions
All authors have contributed to the conception of this report, analysis, interpretation of data and drafting the manuscript. A.A. and M.B. edited and finalized the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper, prior to the development of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
A.A. is funded by an NIHR Clinician Scientist Award.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020;20:493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres P.A., Helmstetter J.A., Kaye A.M., Kaye A.D. Rhabdomyolysis: Pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Huerta-Alardín A.L., Varon J., Marik P.E. Bench-to-bedside review: Rhabdomyolysis—An overview for clinicians. Crit. Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan F.Y. Rhabdomyolysis: A review of the literature. Neth. J. Med. 2009;67:272–283. [PubMed] [Google Scholar]
- 6.Valente-Acosta B., Moreno-Sanchez F., Fueyo-Rodriguez O., Palomar-Lever A. Rhabdomyolysis as an initial presentation in a patient diagnosed with COVID-19. BMJ Case Rep. 2020;13:e236719. doi: 10.1136/bcr-2020-236719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taxbro K., Kahlow H., Wulcan H., Fornarve A. Rhabdomyolysis and acute kidney injury in severe COVID-19 infection. BMJ Case Rep. 2020;13:e237616. doi: 10.1136/bcr-2020-237616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita K., Kanai O., Nanba K., Esaka N., Hata H., Seta K., Odagaki T. Acute rhabdomyolysis in a young woman with moderate COVID-19. IDCases. 2021;25:e01212. doi: 10.1016/j.idcr.2021.e01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020;26:1618. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suwanwongse K., Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12:e7561. doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uysal B.B., Ikitimur H., Yavuzer S., Islamoglu M.S., Cengiz M. Case report: A COVID-19 patient presenting with mild rhabdomyolysis. Am. J. Trop. Med. Hyg. 2020;103:847. doi: 10.4269/ajtmh.20-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh B., Kaur P., Mechineni A., Maroules M. Rhabdomyolysis in COVID-19: Report of four cases. Cureus. 2020;12:e10686. doi: 10.7759/cureus.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosla S.G., Nylen E.S., Khosla R. Rhabdomyolysis in Patients Hospitalized With COVID-19 Infection: Five Case Series. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620984603. doi: 10.1177/2324709620984603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas-García S., Bernal J., Bachiller-Corral J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology. 2020;59:2174–2176. doi: 10.1093/rheumatology/keaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain R., Corcuera-Solano I., Dayan E., Jacobi A.H., Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: A case report. Radiol. Case Rep. 2020;15:1633–1637. doi: 10.1016/j.radcr.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alrubaye R., Choudhary H. Severe rhabdomyolysis in a 35-year-old woman with COVID-19 due to SARS-CoV-2 infection: A case report. Am. J. Case Rep. 2020;21:e926733-1. doi: 10.12659/AJCR.926733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckholz A.P., Kaplan A., Rosenblatt R.E., Wan D. Clinical characteristics, diagnosis, and outcomes of 6 patients with COVID-19 infection and rhabdomyolysis. Mayo Clin. Proc. 2020;95:2557–2559. doi: 10.1016/j.mayocp.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solís J.G., Pineda A.E., Minutti P.A., Sánchez A.A. Case report: Rhabdomyolysis in a patient with COVID-19: A proposed diagnostic-therapeutic algorithm. Am. J. Trop. Med. Hyg. 2020;103:1158. doi: 10.4269/ajtmh.20-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chedid N.R., Udit S., Solhjou Z., Patanwala M.Y., Sheridan A.M., Barkoudah E. COVID-19 and rhabdomyolysis. J. Gen. Intern. Med. 2020;35:3087–3090. doi: 10.1007/s11606-020-06039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byler J., Harrison R., Fell L.L. Rhabdomyolysis following recovery from severe COVID-19: A case report. Am. J. Case Rep. 2021;22:e931616-1. doi: 10.12659/AJCR.931616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh B., Kaur P., Reid R.-J.R. Case Reports: Rhabdomyolysis associated with COVID-19. Am. Fam. Physician. 2020;102:645–648. [PubMed] [Google Scholar]
- 22.Shanbhag A., Manaktala P.S., Rizvi H., Frey K., Narayanan R. COVID-19 presenting as severe rhabdomyolysis with normal renal function. Cureus. 2020;12:e9556. doi: 10.7759/cureus.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng Y., Ma Q., Du Y.-S., Peng N., Yang T., Zhang S.-Y., Wu F.-F., Lin H.-L., Su L. Rhabdomyolysis is associated with in-hospital mortality in patients with COVID-19. Shock. 2021;56:360. doi: 10.1097/SHK.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rosa A., Verrengia E.P., Merlo I., Rea F., Siciliano G., Corrao G., Prelle A. Muscle manifestations and CK levels in COVID infection: Results of a large cohort of patients inside a Pandemic COVID-19 Area. Acta Myol. 2021;40:1–7. doi: 10.1016/j.jns.2021.119838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokhtari A.K., Maurer L.R., Christensen M.A., El Moheb M., Naar L., Alser O., Gaitanidis A., Langeveld K., Kapoen C., Breen K. Rhabdomyolysis in Severe COVID-19: Male Sex, High Body Mass Index, and Prone Positioning Confer High Risk. J. Surg. Res. 2021;266:35–43. doi: 10.1016/j.jss.2021.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroun M.W., Dieiev V., Kang J., Barbi M., Nia S.F.M., Gabr M., Eman G., Kajita G., Swedish K. Rhabdomyolysis in COVID-19 patients: A retrospective observational study. Cureus. 2021;13:e12552. doi: 10.7759/cureus.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack M., Nichols L., Guerrero D.M. Rhabdomyolysis Secondary to COVID-19 Vaccination. Cureus. 2021;13:e15004. doi: 10.7759/cureus.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassar M., Chung H., Dhayaparan Y., Nyein A., Acevedo B.J., Chicos C., Zheng D., Barras M., Mohamed M., Alfishawy M., et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab. Syndr. 2021;15:102170. doi: 10.1016/j.dsx.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirillo E., Esposito C., Giardino G., Azan G., Fecarotta S., Pittaluga S., Ruggiero L., Barretta F., Frisso G., Notarangelo L.D., et al. Case Report: Severe Rhabdomyolysis and Multiorgan Failure after ChAdOx1 nCoV-19 Vaccination. Front. Immunol. 2022;13:845496. doi: 10.3389/fimmu.2022.845496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajmera K.M. Fatal case of rhabdomyolysis post-COVID-19 vaccine. Infect. Drug Resist. 2021;14:3929. doi: 10.2147/IDR.S331362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unger K., Ponte C.D., Anderson D. A Possible Case of COVID-19 Booster Vaccine–Associated Rhabdomyolysis and Acute Kidney Injury. J. Pharm. Technol. 2022;38:247–250. doi: 10.1177/87551225221093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salter B., Jessome M., Tarnopolsky M., Yousuf H. Possible association between rhabdomyolysis and mRNA SARS-CoV-2 vaccination in a patient with RYR1 gene mutation. Can. Med. Assoc. J. 2022;194:E252. doi: 10.1503/cmaj.211856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandava K., Jaffry M., Rosario S., Jedidi K., Souayah N. Association between COVID-19 Vaccination and Rhabdomyolysis in Adults: A Vaccine Adverse Event Reporting System (VAERS) Study (S18.006) Neurology. 2022;98((Suppl. 18)):1265. [Google Scholar]
- 34.Callado R.B., Carneiro T.G.P., da Cunha Parahyba C.C., de Alcantara Lima N., da Silva Junior G.B., de Francesco Daher E. Rhabdomyolysis secondary to influenza A H1N1 vaccine resulting in acute kidney injury. Travel Med. Infect. Dis. 2013;11:130–133. doi: 10.1016/j.tmaid.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Raman K.S., Chandrasekar T., Reeve R., Roberts M., Kalra P. Influenza vaccine-induced rhabdomyolysis leading to acute renal transplant dysfunction. Nephrol. Dial. Transplant. 2006;21:530–531. doi: 10.1093/ndt/gfi195. [DOI] [PubMed] [Google Scholar]
- 36.Plotkin E., Bernheim J., Ben-Chetrit S., Mor A., Korzets Z.E. Influenza vaccine—A possible trigger of rhabdomyolysis induced acute renal failure due to the combined use of cerivastatin and bezafibrate. Nephrol. Dial. Transplant. 2000;15:740–741. doi: 10.1093/ndt/15.5.740. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs L.G., Gourna Paleoudis E., Lesky-Di Bari D., Nyirenda T., Friedman T., Gupta A., Rasouli L., Zetkulic M., Balani B., Ogedegbe C. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE. 2020;15:e0243882. doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagarra-Romero L., Viñas-Barros A. COVID-19: Short and long-term effects of hospitalization on muscular weakness in the elderly. Int. J. Environ. Res. Public Health. 2020;17:8715. doi: 10.3390/ijerph17238715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dos Santos P.K., Sigoli E., Bragança L.J., Cornachione A.S. The Musculoskeletal Involvement after Mild to Moderate COVID-19 Infection. Front. Physiol. 2022;13:813924. doi: 10.3389/fphys.2022.813924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez B., Nansoz S., Cameron D.R., Z’Graggen W.J. Is myopathy part of Long-COVID? Clin. Neurophysiol. 2021;132:1241–1242. doi: 10.1016/j.clinph.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.