Abstract
Background
Obese women are at increased risk for complications during and after labor and delivery, including puerperal infection and cesarean delivery. Since labor induction has become increasingly common, it is crucial to find ways to decrease complication rates in this high-risk population.
Objective
To explore the effect of prophylactic antibiotics during labor induction of obese, nulliparous women on rates of cesarean delivery and puerperal infection, and to estimate the parameters needed to calculate the sample size for a larger, multicenter trial.
Study Design
In this randomized, placebo-controlled pilot trial, nulliparous patients with body mass index ≥30 were randomized to either prophylactic antibiotics (500 mg azithromycin for 1 dose, and 2 g cefazolin every 8 hours for up to 3 doses) or placebo, administered starting at the beginning of labor induction. Exclusion criteria were known fetal anomaly, fetal demise, multifetal gestation, ruptured membranes >12 hours, infection requiring antibiotics at start of labor induction, and/or allergy to azithromycin or beta-lactam antibiotics. The co-primary outcomes were rates of puerperal infection (composite of chorioamnionitis, endometritis, and/or cesarean wound infection) and cesarean delivery. Participants were followed for 30 days postpartum, and maternal and neonatal demographic and outcome data were collected. Proportions and ninety-five percent confidence limits were calculated for each of these outcomes.
Results
From 01/2019 to 05/2021, 101 patients were randomized in the class III stratum (1 patient who was randomized ultimately did not undergo labor induction). From 02/2020 to 05/2021, 38 and 47 patients were randomized in the class I and II strata, respectively (to assess the effect of obesity class on the outcomes expected to be impacted by antibiotic prophylaxis). In the antibiotics and placebo groups, respectively, rates of cesarean were 29.0% (95% confidence interval [CI] 19.8 to 38.3) vs 39.8% (95% CI 29.8 to 49.7), and puerperal infection occurred in 8.6% (95% CI 2.9 to 14.3) vs 9.7% (95% CI 3.7 to 15.7). In the subgroup with class III obesity, again in the antibiotics and placebo groups, respectively, rates of cesarean were 33.3% (95% CI 20.4 to 47.9) vs 46.0% (95% CI 32.2 to 59.8), and puerperal infection occurred in 7.8% (95% CI 0.5–15.2) vs 10.0% (95% CI 1.7 to 18.3). Note that this pilot study was not powered to detect differences of this magnitude but rather to estimate parameters.
Conclusions
Administration of prophylactic antibiotics during labor induction of obese nulliparous patients resulted in a 27% lower cesarean delivery rate overall and a 28% lower rate in class III obese patients. A larger trial is warranted to evaluate these differences.
Keywords: Obesity, obese, labor induction, cesarean, cesarean delivery rate, puerperal infection, chorioamnionitis, endometritis, wound infection, pilot study
Introduction
Approximately one-third of American women are obese prior to becoming pregnant.1 Obesity, defined as a body mass index (BMI) ≥30 kg/m2, increases the risk of pregnancy complications including hypertensive disorders, gestational diabetes, macrosomia, puerperal infection, cesarean delivery, and stillbirth.2–6 Risk of complications rises with increasing BMI,7 and women with class III obesity (BMI ≥40) have a risk of stillbirth that increases more sharply with advancing gestational age compared to women with lesser obesity.8
Consequently, since June 2014, the Section of Maternal-Fetal Medicine at our institution has had a policy of delivering women at 39 weeks for the indication of class III obesity. We recently evaluated this practice change in a before-and-after cohort study and found that the policy did not affect the overall cesarean rate among women with class III obesity or rates of maternal or neonatal morbidity.9 In addition, a large randomized controlled trial of labor induction at 39 weeks versus expectant management until 41 weeks in low-risk nulliparous (including both lean and obese) women was published in 2018 and demonstrated that labor induction did not adversely affect neonatal morbidity but reduced the cesarean delivery and maternal morbidity rates.10 Therefore, we anticipate that the rate of labor inductions will continue to increase.
Obese women undergoing induction of labor have an increased rate of puerperal infection (chorioamnionitis, endometritis, and/or cesarean wound infection).3 Furthermore, chorioamnionitis, even if subclinical, is associated with an increased likelihood of cesarean delivery due to labor dystocia.11 Both subclinical and clinical chorioamnionitis have been associated with decreased uterine contractility, which is a likely mechanism for the higher rate of labor abnormalities associated with these conditions12,13. In light of the above information, it is important to identify interventions to decrease the rates of infection and cesarean delivery in obese women undergoing labor induction.
Antibiotic prophylaxis is standard practice before cesarean delivery, since it results in lower rates of post-cesarean infection including endometritis and wound infection.14,15 In contrast to cesarean delivery, antibiotic prophylaxis is not standard during labor inductions except as it relates to prevention of early-onset neonatal infection with group B streptococci (GBS).16 That prophylaxis is with a narrow-spectrum agent (penicillin or ampicillin). There is a clear benefit of giving pre-operative antibiotic prophylaxis for patients undergoing cesarean delivery and also evidence for a reduction in surgical site infection in obese women receiving post-operative antibiotic prophylaxis.17 Therefore, we speculate that giving prophylactic antibiotics during labor induction will lower the rates of both puerperal infection and cesarean delivery (by reducing clinical and subclinical chorioamnionitis). Specifically, we hypothesize that antibiotic prophylaxis given at the start of labor induction in nulliparous women with obesity may reduce the rates of cesarean delivery and puerperal infection in this high-risk group. We conducted a pilot randomized controlled trial to explore the impact of prophylactic antibiotic administration during labor induction and to estimate the parameters necessary to calculate the sample size for a next-step, large, multicenter trial to test this hypothesis.
Materials and Methods
This was a randomized, placebo-controlled, triple-blind pilot trial exploring the effect of prophylactic antibiotics during labor induction of nulliparous, obese women. We enrolled women presenting for labor induction at our institution. Each participant gave written, informed consent prior to enrollment. Inclusion criteria were BMI ≥30 kg/m2, no prior deliveries at or beyond 20 weeks gestation, gestational age 37 weeks or greater, age 15–45 years, and undergoing labor induction. Exclusion criteria were fetal death prior to labor induction, known fetal anomaly, multifetal gestation, ruptured membranes for more than 12 hours, chorioamnionitis or other infection requiring antibiotics at the start of the labor induction, previous myometrial surgery, and/or allergy to azithromycin or beta-lactam antibiotics. We initially enrolled women with class III obesity (BMI ≥40 kg/m2) only. However, after 13 months of enrollment we expanded to women with class I and II obesity, randomized in separate strata, in order to assess whether the outcomes expected to be impacted by prophylactic antibiotics differed with obesity class.
Women were randomized 1:1 to receive either cefazolin 2 grams intravenously at the start of the labor induction and every 8 hours thereafter for a maximum of three doses plus azithromycin 500 mg intravenously once at the start of the labor induction, or like placebos of each drug. Regardless of assigned group, patients in whom intrapartum antibiotic prophylaxis against early-onset neonatal group B streptococci (GBS) was indicated received penicillin G 5 million units intravenously then 3 million units intravenously every 4 hours until delivery. Per our institutional guideline, patients in whom intrapartum cesarean delivery was indicated received cefazolin 2 grams intravenously and azithromycin 500 mg intravenously within 60 minutes prior to skin incision, regardless of their assigned study group. Patients with an intrapartum diagnosis of chorioamnionitis were treated with ampicillin 2 grams intravenously every 6 hours and gentamicin (dose based on weight) every 8 hours. If they delivered vaginally, both of those drugs were discontinued after the first postpartum dose. If they underwent cesarean delivery, clindamycin 900 mg intravenously every 8 hours was started, and all three drugs were continued for 24–48 hours postpartum. These antibiotic drugs (given for intrapartum antibiotic prophylaxis against early-onset neonatal infection with GBS, prophylaxis prior to cesarean delivery, and/or to treat chorioamnionitis) were in addition to receiving the assigned study drugs or placebo. Patients with chorioamnionitis who underwent cesarean delivery also received prophylactic azithromycin 500 mg intravenously at the time of cesarean.
Randomization was stratified by obesity class. The allocation tables for the randomization sequences were computer-generated in randomly chosen blocks of size 4 and 6 and provided to the investigational pharmacist, who prepared and delivered the treatment packets in the randomly assigned order. The placebo consisted of normal saline. The treatment packets were packaged with opaque coverings so that they were identical in appearance to the treatment team. Investigators, clinical personnel including obstetrical providers and nursing staff, patients, and study team members responsible for assessing outcomes were blinded to allocation, and only the investigational pharmacist was aware of the treatment allocation.
All study participants received usual care per our local institutional guidelines for labor and delivery management and postpartum care. Method of labor induction was at the discretion of the labor and delivery attending physician, however all providers delivering at our hospital follow our institutional clinical guideline regarding labor induction. There were no significant practice changes in labor induction or management of medical comorbidities in pregnancy, including delivery timing, over the course of enrollment. Study outcomes were abstracted from the medical record by trained research staff.
The co-primary outcomes were cesarean delivery rate and puerperal infection rate (defined as a composite of chorioamnionitis, endometritis, and/or cesarean wound infection). Chorioamnionitis was defined clinically as 1) a maternal temperature of 38.0 degrees Celsius or higher plus one or more of the following: maternal heart rate over 100, baseline fetal heart rate over 160, fundal tenderness, purulent amniotic fluid, and/or 2) antibiotics administered for that indication. Endometritis was defined clinically as 1) a maternal temperature of 38.0 degrees Celsius or higher plus uterine tenderness and/or foul-smelling lochia and/or 2) antibiotics administered for that indication within 30 days after delivery. Cesarean wound infection was defined according to the Centers for Disease Control and Prevention’s National Healthcare Safety Network definitions for surgical site infections if occurring within 30 days after delivery.15 The secondary outcomes were chorioamnionitis, endometritis, and wound infection as individual measures; postpartum hemorrhage; blood transfusion; intensive care unit (ICU) admission; maternal hospital readmission within 30 days after delivery; and a composite of neonatal complications (neonatal ICU [NICU] admission, respiratory distress syndrome, intubation within the first 72 hours of life, hyperbilirubinemia requiring phototherapy, suspected or confirmed sepsis, body fluid culture with organisms identified, necrotizing enterocolitis, periventricular leukomalacia, intraventricular hemorrhage grade III or higher, and/or neonatal death). The above outcomes were ascertained for each subject and neonate via manual review of the electronic medical record by trained research nurses.
Participants were followed for 30 days after delivery, and at that time were contacted by phone call from the research team to ascertain whether any additional complications had occurred after hospital discharge. Participants’ electronic medical records were also reviewed by the research team to capture information related to the primary and secondary study outcomes. If a participant reported seeking care at an outside facility after discharge from the birth hospitalization, records were obtained from the outside facility and reviewed by the research team.
No a priori sample size calculation was performed, since this was a pilot trial designed to estimate the parameters necessary to calculate the sample size for a large, multicenter trial. For the overall group, and for each of the three obesity classes separately, we estimated the frequency of the primary outcomes in the placebo and active drug groups. Proportions and ninety-five percent confidence limits were calculated for each of these outcomes.. We did not stratify the randomization by whether or not prophylaxis against early-onset neonatal infection with group B streptococcus (GBS) was indicated at the beginning of the labor induction (due to known colonization). However, we did perform a pre-specified secondary analysis stratifying by whether GBS prophylaxis was received.
This study was approved by the Institutional Review Board at our institution on 12/12/18. The trial information was registered on clinicaltrials.gov prior to beginning enrollment (identifier NCT03801252). Date of registration: 1/11/19; date of initial participant enrollment: 1/29/19; clinical trial identification number: NCT03801252; URL of the registration site: https://clinicaltrials.gov/ct2/show/NCT03801252?term=NCT03801252&draw=2&rank=1
Results
From January 2019 to May 2021, 101 patients were randomized in the class III stratum. From February 2020 to May 2021, 38 and 47 patients were randomized in the class I and II strata, respectively (to assess whether obesity class had an effect on the outcomes expected to be impacted by prophylactic antibiotics). Total enrollment was 186 women, 93 in the placebo group and 93 in the treatment group (Figure). Two patients were randomized but did not receive the study intervention (one participant ultimately underwent primary cesarean delivery due to suspected fetal macrosomia prior to the start of labor induction, and one participant was excluded after randomization due to discovery of an infection requiring antibiotic treatment [Chlamydia cervicitis] prior to the start of her labor induction). One participant presented for labor induction and received the study intervention, however over the course of her admission was found to be in spontaneous labor and never required induction. All three of these participants were included in the intention-to-treat analysis. Baseline characteristics of the subjects are shown in Table 1.
Figure.
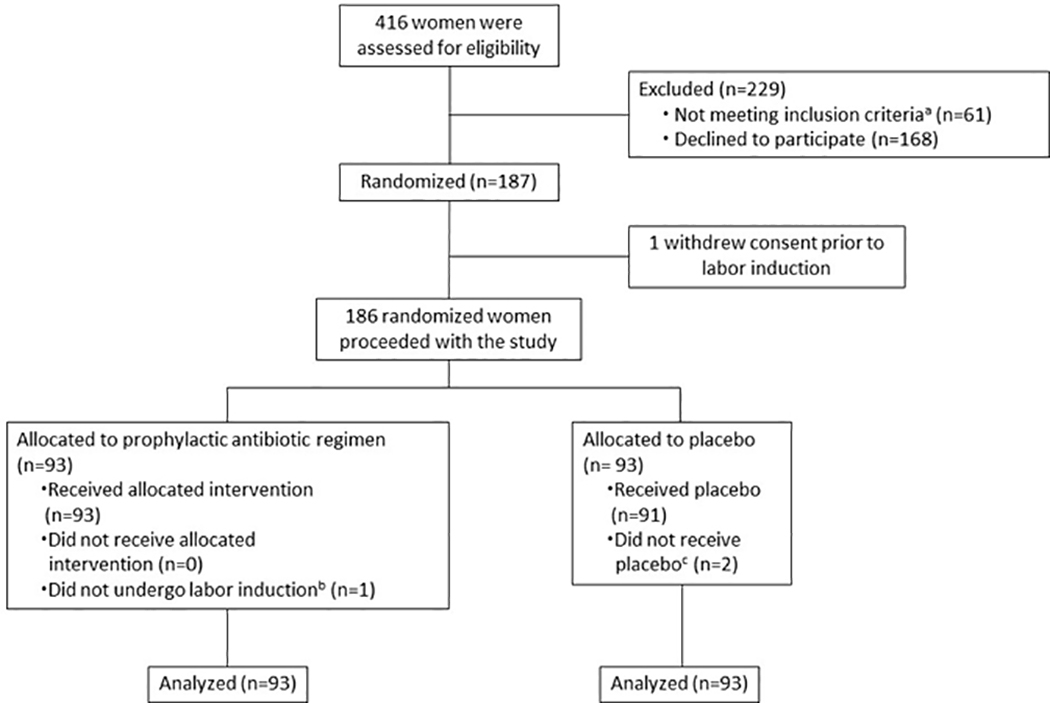
CONSORT (Consolidated Standards of Reporting Trials) enrollment flow diagram. This diagram shows the number of patients approached for the study, the number who declined, the number who were enrolled and randomized, and the number of women in each treatment group.
a30 had an allergy to one or more of the study medications, 8 underwent pre-labor cesarean delivery, 6 had a major fetal anomaly, 5 were minor patients without parent consent, 5 had infection requiring antibiotic therapy, 3 had ruptured membranes >12 hours, 3 were preterm, and 1 was incarcerated
bOne participant presented for induction of labor and received study drug, but over the course of her admission was found to be in spontaneous labor and did not undergo labor induction
cTwo participants allocated to the placebo group did not receive the placebo: One participant was randomized but ultimately did not undergo labor induction (delivered via primary cesarean due to concern for fetal macrosomia); one participant was excluded after randomization due to discovery of an infection requiring antibiotic treatment (chlamydia) prior to the start of her labor induction
Table 1.
Patient baseline characteristics in a pilot randomized controlled trial of nulliparous patients with BMI ≥30 randomized to prophylactic antibiotics during labor induction or placebo.
| Prophylactic Antibiotics n=93 |
Placebo n=93 |
|
|---|---|---|
|
| ||
| Age (years) | 26.9 ± 5.6 | 25.3 ± 5.2 |
| Gestational age (days) | 271.4 ± 8.4 | 271.7 ± 6.2 |
| Race/ethnicity | ||
| White | 57 (61.3) | 46 (44.7) |
| Black | 7 (7.5) | 9 (9.7) |
| Hispanic | 16 (17.2) | 18 (19.4) |
| Asian | 4 (4.3) | 2 (2.1) |
| Native American | 7 (7.5) | 11 (11.8) |
| Other | 2 (2.2) | 5 (5.4) |
| Not reported | 0 | 2 (2.2) |
| Obesity Class | ||
| Class I (BMI 30–34) | 19 (20.4) | 19 (20.4) |
| Class II (BMI 35–39) | 23 (24.7) | 24 (25.8) |
| Class III (BMI ≥40) | 51 (54.8) | 50 (53.8) |
| Chronic hypertension | 9 (9.7) | 8 (8.6) |
| Gestational hypertension | 32 (34.4) | 18 (19.4) |
| Preeclampsia | 19 (20.4) | 22 (23.7) |
| Diabetes | ||
| Gestational | 10 (10.8) | 11 (11.8) |
| Pre-gestational | 8 (8.6) | 5 (5.4) |
| Fetal growth restriction | 1 (1.1) | 3 (3.2) |
Data are presented as mean ± standard deviation or n (%)
Table 2 shows the co-primary outcome data overall and stratified by obesity class. In the overall groups, rates of cesarean delivery (29.0% [95% CI 19.8 to 38.3] vs 39.8% [95% CI 29.8 to 49.7]) and puerperal infection (8.6% [95% CI 2.9 to 14.3] vs 9.7% [95% CI 3.7 to 15.7]) were numerically lower in the group that received prophylactic antibiotics during labor induction. Patterns were similar for both cesarean delivery and puerperal infection in the various obesity classes, with lower rates of cesarean delivery in the active drugs group and similar rates of puerperal infection between the active drugs and placebo groups, with the exception of cesarean rate in the class II obesity group, which was similar between the active drug and placebo groups. Note that this pilot study was not powered to detect differences of this magnitude but rather to estimate parameters. When the results were stratified by intrapartum antibiotic prophylaxis for GBS, the treatment-related reduction in cesarean deliveries was observed only in patients who did not receive intrapartum antibiotic prophylaxis against early-onset neonatal GBS infection (Table 3).
Table 2.
Intent-to-treat analysis of primary outcomes among women randomized to prophylactic antibiotic treatment and placebo, stratified by obesity class.
| Prophylactic Antibiotics n=93 |
Placebo n=93 |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | % | 95% CI | n | % | 95% CI | |
|
| ||||||
| Cesarean Delivery | 27 | 29.0 | (19.8, 38.3) | 37 | 39.8 | (29.8, 49.7) |
| Puerperal Infection | 8 | 8.6 | (2.9, 14.3) | 9 | 9.7 | (3.7, 15.7) |
| Class I Obesity | n=19 | n=19 | ||||
| Cesarean Delivery | 4 | 21.1 | (2.7, 39.4) | 7 | 36.8 | (15.2, 58.5) |
| Puerperal Infection | 2 | 10.5 | (0.0, 24.3) | 1 | 5.3 | (0.0, 15.3) |
| Class II Obesity | n=23 | n=24 | ||||
| Cesarean Delivery | 6 | 26.1 | (8.1, 44.0) | 7 | 29.2 | (11.0, 47.4) |
| Puerperal Infection | 2 | 8.7 | (0.0, 20.2) | 3 | 12.5 | (0.0, 25.7) |
| Class III Obesity | n=51 | n=50 | ||||
| Cesarean Delivery | 17 | 33.3 | (20.4, 47.9) | 23 | 46.0 | (32.2, 59.8) |
| Puerperal Infection | 4 | 7.8 | (0.5, 15.2) | 5 | 10.0 | (1.7, 18.3) |
CI = Confidence interval
Table 3.
Intent to treat analyses stratified by GBS status and GBS prophylaxis.
| Outcomes Stratified by GBS Prophylaxis | Prophylactic Antibiotic Study Regimen n=93 n (%) |
Placebo n=93 n (%) |
|---|---|---|
|
| ||
| Yes (n=42, 22.5%) | n=22 | n=20 |
| Cesarean delivery | 8 (36.4) | 7 (35.0) |
| Puerperal Infection | 2 (9.1) | 0 |
| No (n=144, 77.4%) | n=71 | n=73 |
| Cesarean delivery | 19 (26.8) | 30 (41.1) |
| Puerperal Infection | 6 (8.5) | 9 (12.3) |
Data are presented as n(%)
Secondary maternal and neonatal outcomes are presented in Table 4. Individual rates of chorioamnionitis, endometritis and wound infection were similar between the antibiotics and placebo groups, as were rates of other secondary outcomes. The most common indications for cesarean delivery in both groups were non-reassuring fetal status (44.4% in the antibiotic group and 37.8% in the placebo group) and failed induction (40.7% in the antibiotic group and 29.7% in the placebo group). Of the 27 cesarean deliveries in the antibiotics group, three (11.1%) were due to active phase or second stage arrest of labor, while 10 (27.0%) of the 37 cesarean deliveries in the placebo group were due to one of those two indications. The neonatal composite outcome occurred in 53.8% of the antibiotic group vs 45.2% of the placebo group.
Table 4.
Intent-to-treat analysis of secondary outcomes among women randomized to prophylactic antibiotic treatment or placebo.
| Prophylactic Antibiotics (n=93) |
Placebo (n=93) |
|
|---|---|---|
|
| ||
| Maternal Outcomes | ||
| Chorioamnionitis | 5 (5.4) | 5 (5.4) |
| Endometritis | 1 (1.1) | 2 (2.2) |
| Wound infection | 2 (2.2) | 2 (2.2) |
| Postpartum hemorrhage | 16 (17.2) | 16 (17.2) |
| Blood transfusion | 4 (4.3) | 4 (4.3) |
| Intensive care unit admission | 1 (1.1) | 0 |
| Maternal hospital readmission within 30 days after delivery | 1 (1.1) | 2 (2.2) |
| Primary indications for cesarean delivery | n=27 | n=37 |
| Non-reassuring fetal status | 12 (44.4) | 14 (37.8) |
| Failed induction | 11 (40.7) | 11 (29.7) |
| Active phase arrest | 2 (7.4) | 6 (16.2) |
| 2nd stage arrest | 1 (3.7) | 4 (10.8) |
| Other | 1 (3.7) | 2 (5.4) |
| Neonatal Outcomes | ||
| Neonatal complications composite | 50 (53.8) | 42 (45.2) |
| NICU admission | 11 (11.8) | 10 (10.8) |
| Respiratory distress syndrome | 8 (8.6) | 7 (7.5) |
| Intubation required within first 72 hours | 1 (1.1) | 3 (3.2) |
| Hyperbilirubinemia requiring phototherapy | 41 (44.1) | 37 (39.8) |
| Suspected or confirmed sepsis | 11 (11.8) | 8 (8.6) |
| Culture with organisms identified | 0 | 0 |
| Necrotizing enterocolitis | 0 | 0 |
| Periventricular leukomalacia | 0 | 0 |
| Intraventricular hemorrhage grade III or higher | 0 | 0 |
| Neonatal death | 0 | 0 |
Data are presented as n (%)
In addition to the intention-to-treat analyses presented above, we also performed per-protocol analyses of the primary and secondary outcomes, which yielded similar results as the intention-to-treat analyses (Supplemental Tables 1 and 2).
There was one subject in the antibiotics group with an adverse reaction (vomiting) within 24 hours of first administration of the study drug, and none in the placebo group.
Discussion
Principal Findings
We found that women who received antibiotic prophylaxis during labor induction had a 27% reduction in the rate of cesarean delivery. Though this pilot trial was not powered to detect statistically significant changes in the co-primary outcomes, we think the magnitude of this difference is clinically meaningful and should be evaluated in a next-step larger trial. When examining the obesity classes individually as subgroups, we found that cesarean delivery was lower in the antibiotic group than the placebo group for all three obesity classes. We did not note clinically significant differences in puerperal infection rates in the group receiving antibiotics compared to those receiving placebo.
Results in the Context of What is Known
There are other studies examining intrapartum antibiotic prophylaxis in the obstetrical literature. However, the vast majority of these focus on prophylaxis for GBS, which is now routine practice due to its ability to decrease rates of neonatal sepsis from GBS.16
There are data regarding antibiotic prophylaxis during the postpartum period. One trial showed that giving antibiotic prophylaxis for 48 hours postpartum in women with obesity (class I, II and III) who underwent cesarean delivery was associated with a decreased risk of surgical site infection – 15.4% in the placebo group vs 6.4% in the group receiving antibiotics, relative risk 0.41 (95% confidence interval 0.22–0.71).17 However, usual practice today is to give antibiotic prophylaxis before cesarean delivery in order to decrease rates of surgical site infection and endometritis.14,15
One other study evaluating antibiotic prophylaxis during labor was recently published by Subramaniam et al and evaluated the effect of antibiotic prophylaxis given to laboring women in Cameroon.18 In that study, women who were either laboring for 18 hours or more or had rupture of membranes for 8 hours or more were randomized to either azithromycin, azithromycin plus amoxicillin, or placebo. The study did not find a significant reduction in maternal peripartum or neonatal infection or death. In addition, rates of cesarean delivery in that trial were similar between groups--31.6% (azithromycin group) vs 34% (azithromycin plus amoxicillin group) vs 34% (placebo group). Our trial differs from the study by Subramaniam and colleagues in several ways. In particular, our design resulted in antibiotics being started earlier during the induction process, which may explain why we saw a lower rate of cesarean delivery in the group receiving antibiotics.
Clinical Implications
Our study findings have several potential future clinical implications. Since the study was a pilot trial, further evaluation of this intervention is warranted before introduction into clinical practice. However, it is intriguing that our results illustrate a potential new intervention for lowering the rate of cesarean delivery in obese women. We noted a 27% reduction in the overall cesarean rate with prophylactic antibiotics and a 28% reduction in class III obese women who received antibiotics. The mechanism for this reduction is unclear, but we speculate that, given the well-established association of chorioamnionitis with dysfunctional labor,19 our prophylactic antibiotic regimen may have achieved this effect by preventing some degree of subclinical chorioamnionitis from exerting detrimental effects on the labor course. In fact, the numerically lower rate of cesarean due to active phase or second stage arrest in the antibiotics group supports this speculation. Although we did not identify an a priori value to define a clinically meaningful difference in the co-primary outcomes, given the fact that 31.8% of the 3,613,647 births in the United States in 2020 were cesarean deliveries, a 27% reduction noted in that rate would mean 310,146 fewer cesarean deliveries annually20.
We did find an important distinction in the patients who received intrapartum antibiotic prophylaxis for GBS versus those who did not. Most of the effect of the study intervention appeared to be in women who did not receive intrapartum antibiotic prophylaxis for GBS. In the women who did receive GBS prophylaxis, cesarean rates were similar between the active drug and placebo groups. Therefore, it will be important to consider this factor in the design of the planned future next-step trial. We speculate that our study drug regimen and GBS prophylaxis may work in similar ways, i.e. via reduction of clinical and subclinical chorioamnionitis. However, this theory does not explain why patients who received GBS prophylaxis along with the study drug regimen did not have a cesarean rate similar to those who received our study drug regimen alone. Further investigation is needed into the mechanism of action of our prophylactic antibiotic regimen in reducing cesarean delivery rate.
Regarding neonatal outcomes, we found a numerically higher rate of suspected or confirmed sepsis in the neonates of women who received prophylactic antibiotics during labor induction. This result is counterintuitive, and the reason for this finding is unclear, though it may be due to the fact that the pediatricians used maternal antibiotic administration during labor as a risk factor for suspecting neonatal infection. This outcome should be further investigated as a safety measure in future studies.
Given that this is a pilot study, these results should not be used to alter clinical practice without further data from a large, adequately-powered trial to support the conclusions.
Research Implications
As stated above, our findings from this pilot study should be further explored with a large-scale multi-center clinical trial. Since the effect of the intervention appears most pronounced in women who did not receive intrapartum antibiotic prophylaxis for GBS, consideration should be given to powering a future study for the primary outcome of cesarean delivery in the subset of women not receiving GBS prophylaxis.
Strengths and Limitations
Our study has several strengths. The study was conducted at an academic tertiary care center, where it is routine practice to induce women at 39 weeks for the indication of class III obesity and where care is standardized based on locally developed clinical guidelines. The study was designed to reduce the risk for bias by randomly assigning participants to either the treatment or control group and by using a placebo for the control group. Participants, obstetrical providers, and nursing staff were blinded to the group assignment. The biggest limitation of our study is the relatively small sample size. However, we wanted to conduct a pilot study to examine preliminary evidence prior to devoting the significant resources required to conduct an adequately powered large-scale clinical trial. In addition, we did not assess placental pathology as a part of our study, but this may be useful information to gain in a future, large-scale study as a way to explore our theory that prophylactic antibiotics work to decrease the cesarean rate by lowering rates of subclinical chorioamnionitis. Lastly, of late there has been increased attention to the potential effects of early antibiotic exposure on the development of the neonatal microbiome.21 In this pilot study we did not assess the effect of our intrapartum antibiotic regimen on the neonatal microbiome, but this would be important to address in a future, larger study.
Conclusions
Our pilot data suggest that prophylactic antibiotic administration during labor inductions of obese, nulliparous women may reduce the rate of cesarean delivery. A larger trial, powered to detect clinically significant differences in that primary outcome, is warranted.
Supplementary Material
AJOG at a Glance.
A. Why was the study conducted?
Rates of both obesity and labor induction are increasing in the pregnant population.
Obesity is associated with increased risk for labor- and delivery-related complications including puerperal infection and cesarean delivery.
It is important to find interventions to lower the rates of these adverse outcomes in women with obesity.
B. What are the key findings?
In this pilot trial, prophylactic antibiotic administration during labor induction of obese, nulliparous women was associated with a numerically lower rate of cesarean delivery.
C. What does this study add to what is already known?
Intrapartum prophylactic antibiotics during labor induction in obese, nulliparous women may provide a means to lower the rate of cesarean delivery in this group, and this intervention should be evaluated in a larger multicenter trial.
Financial support:
Support was provided by a University of Oklahoma College of Medicine Alumni Association grant and the National Institute of General Medical Sciences (U54GM104938). The funding sources had no role in: study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the article for publication.
This study was registered on clinicaltrials.gov prior to beginning enrollment.
Date of registration: 1/11/19
Date of initial participant enrollment: 1/29/19
Clinical trial identification number: NCT03801252
URL of the registration site: https://clinicaltrials.gov/ct2/show/NCT03801252?term=NCT03801252&draw=2&rank=1
Footnotes
The authors report no conflicts of interest.
Condensation:
Intrapartum prophylactic antibiotics during labor induction of nulliparous women with obesity may be associated with a lower cesarean delivery rate
References
- 1.Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016–2019. NCHS Data Brief, no 392. Hyattsville, MD: National Center for Health Statistics. 2020. [PubMed] [Google Scholar]
- 2.Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1997; 177:1003–10. [DOI] [PubMed] [Google Scholar]
- 3.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol 2004; 190:1091–7. [DOI] [PubMed] [Google Scholar]
- 4.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henrikson TB, and Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol 2005; 106:250–9. [DOI] [PubMed] [Google Scholar]
- 5.Huang DY, Usher RH, Kramer MS, Yang H, Morin L, and Fretts RC. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol 2000; 95:215–21. [DOI] [PubMed] [Google Scholar]
- 6.Magann EF, Doherty DA, Chauhan SP, Klimpel JM, Huff SD, and Morrison JC. Pregnancy, obesity, gestational weight gain, and parity as predictors of peripartum complications. Arch Gynecol Obstet, 2011; 284:827–36. [DOI] [PubMed] [Google Scholar]
- 7.Salihu HM, Dunlop AL, Headayatzadeh M, Alio AP, Kirby RS, and Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol 2007; 110:552–7. [DOI] [PubMed] [Google Scholar]
- 8.Yao R, Ananth CV, Park BY, Pereira L, and Plante LA; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol 2014; 210:457 e1–9. [DOI] [PubMed] [Google Scholar]
- 9.Pierce S, Maxted M, Peck JD, et al. Impact of a policy to deliver at 39 weeks for the indication of class III obesity. Obesity 2020;28(3):563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 2018; 379:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards RK. Chorioamnionitis and labor. Obstet Gynecol Clin N Am 2005; 32:287–96. [DOI] [PubMed] [Google Scholar]
- 12.Silver RS, Gibbs RS, and Castillo M. Effect of amniotic fluid bacteria on the course of labor in nulliparous women at term. Obstet Gynecol. 1986;68(587–592). [PubMed] [Google Scholar]
- 13.Duff P, Sanders R, and Gibbs RS. The course of labor in term patients with chorioamnionitis. Am J Obstet Gynecol. 1983;147(391–395). [DOI] [PubMed] [Google Scholar]
- 14.Tita ATN, Szychowski JM, Boggess K, et al. and the C/SOAP trial consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med 2016; 375:1231–41.27682034 [Google Scholar]
- 15.Smaill FM and Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014. October;(10):CD007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verani JR, McGee L, and Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59(RR-10):1–36. [PubMed] [Google Scholar]
- 17.Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA 2017; 318(11):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol 2021;138(5):703–713. [DOI] [PubMed] [Google Scholar]
- 19.Tita ATN and Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010;37(2):339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterman M, Hamilton B, Martin J, Driscoll A, and Valenzuela C. Births: Final data for 2020. Natl Vital Stat Rep. 2022. February;70(17). [PubMed] [Google Scholar]
- 21.Corvaglia L, Tonti G, Martini S, et al. Influence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr 2016;62(2):304–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


