Abstract
Acute febrile illness (AFI) is a morbid condition with a sudden onset of fever with at least seven days of evolution, where no signs or symptoms related to an apparent infection have been identified. In Latin America, a high proportion of disease is typically due to malaria and arboviruses. However, among the infectious etiologies, tick-borne diseases (TBDs) should also be considered, especially in areas where people come into direct contact with these arthropods. This study aims to describe the etiology and epidemiology related to tick-borne agents in patients with AFI and the tick’s natural infection by agents of TBD in the rural tropical Magdalena Medio region in Colombia, and explore the factors associated with the presence of Coxiella burnetii infection. We conduct a cohort study enrolling 271 patients with AFI to detect the bacteria of the genera Anaplasma, Ehrlichia, Coxiella, Rickettsia, Borrelia, and Francisella through molecular techniques, and additionally evaluate the presence of IgG antibodies with commercially available kits. We also conduct tick collection in the patient’s households or workplaces for the molecular screening of the same bacterial genera. Seropositivity to IgG antibodies was obtained for all the bacteria analyzed, with Francisella being the most common at 39.5% (107/271), followed by R. rickettsii at 31.4% (85/271), Ehrlichia at 26.9% (73/271), R. typhi at 15.5% (42/271), Anaplasma at 14.4% (39/271), and Borrelia at 6.6% (18/271). However, these bacteria were not detected by the molecular techniques used. Coxiella burnetii infection was detected in 39.5% of the patients: 49.5% only by phase I and II IgG antibodies, 33.6% only by real-time PCR, and 16.8% had a concordant positive result for both techniques. A total of 191 adult ticks, 111 females and 80 males, were collected and identified as Rhipicephalus sanguineus s.l. and Rhipicephalus microplus. In the 169 adult ticks in which natural infection was evaluated, Ehrlichia spp. was detected in 21.3% (36/169), Coxiella spp. in 11.8% (20/169), and Anaplasma spp. in 4.7% (8/169). In conclusion, we identified the prior exposition to Francisella, Anaplasma, Ehrlichia, Rickettsia, Borrelia, and Coxiella in patients through serological tests. We also detected the infection of C. burnetii using molecular techniques. In the ticks, we identified bacteria of the genera Coxiella, Anaplasma, and Ehrlichia. These results suggest the importance of these zoonotic agents as possible causes of AFI in this region.
Keywords: bacterial zoonoses, molecular diagnostic techniques, serologic tests, Coxiella
1. Introduction
Acute febrile illness (AFI) or acute undifferentiated fever is defined as a morbid state with the sudden onset of fever within less than seven days of evolution [1]. The differential etiological diagnosis for AFI is particularly challenging in tropical regions due to environmental conditions, socio-economic factors, and limited resources [2]. In addition, the great diversity of possible infectious etiologies may confound healthcare providers because most patients present non-specific symptoms, such as fever, fatigue, headache, and muscle ache [3]; hence, diagnosis relies on the index of suspicion and performing the appropriate diagnostic tests.
Zoonotic diseases are widespread among the infectious etiologies for AFI, including tick-borne diseases (TBDs). More than six out of every ten known infectious diseases can be spread from animals, and three out of every four new or emerging infectious diseases in people originate from animals [4]. The frequency of emerging vector-borne zoonoses has significantly increased during the last ten years [5]. Ticks, along with mosquitoes, are recognized as the main arthropod vectors of microorganisms transmitted to humans and domestic animals globally [6]. They are responsible for the transmission of many infectious agents: bacteria (Borrelia, Anaplasma), viruses (tick-borne encephalitis), and even parasites (Babesia, Theileria) [7].
Humans are accidental hosts in these diseases associated with ticks, and transmission usually occurs through hematophagous bites [8]. The pathogen may persist for a long time in ticks because it can be transmitted from stage to stage (trans-stadial transmission), from females to eggs (vertical transmission), and from tick to tick via the host (horizontal transmission), depending on the pathogen [9]. In a study published in 2022, 529 ticks of 7 different species (Ixodes ricinus, Haemaphysalis concinna, Haemaphysalis punctata, Dermacentor marginatus, Haemaphysalis inermis, Dermacentor reticulatus, and Rhipicephalus bursa) from North-Western Spain were found to be infected with Rickettsia spp., Anaplasma phagocytophilum, piroplasms, Borrelia burgdorferi s.l., and C. burnetii through specific PCR [10], proving the importance of the vectors in transmitting these microorganisms from animals to humans.
Additionally, a study in 2016 performed on 314 ticks and 626 blood samples from the Netherlands reported that half of the ticks removed from humans tested positive for Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, Rickettsia monacensis, Borrelia miyamotoi, and several Babesia species. In 16 blood samples, DNA was detected from Candidatus Neoehrlichia mikurensis, Anaplasma phagocytophilum, Babesia divergens, Borrelia miyamotoi, and Borrelia burgdorferi s. l. [11]. At present, the diversity of causes behind severe AFI is considered a leading cause of preventable deaths in low- and middle-income countries.
Additionally, it has been reported that most fatal cases of AFI with an infectious etiology could have been treated earlier and diagnosed adequately [12]. However, limited microbiological and epidemiological studies evaluate the transmission dynamics of these pathogens in Colombian agricultural tropical areas. This study aims to describe the etiology and epidemiology related to tick-borne agents in patients with AFI and the tick’s natural infection by agents of TBD in the rural, tropical Magdalena Medio region in Colombia. In addition, the study aims to explore the factors associated with C. burnetii infection.
2. Results
2.1. Demographic Characteristics of the Patients
A total of 271 patients with acute febrile syndrome were enrolled in the study. Most patients were male (64.9%), 81.9% residing in Puerto Berrio, and 54.6% in rural areas. In addition, 60.1% reported having pets, with dogs (59.8%) and cats (57.6%) being the most common companion animals in the participants’ residences. Additionally, 91.9% consumed products derived from raw milk, such as cheese or butter, and 87.8% reported being bitten by ticks.
2.2. Patients Follow-Ups
Of the 271 patients, 92 accepted the collection of a second sample 2 to 4 weeks following enrollment, and 121 received a telephone follow-up session at 6 months.
In the first follow-up session, 3.3% (3/92) reported being diagnosed with malaria and the same percentage with dengue. Among the other most reported conditions, we observed headache and general malaise in 18.5% (17/92) of the patients. For C. burnetii, 22.8% (21/92) tested positive by qPCR, and only 9.5% (2/21) remained positive. In serology, 31.5% (29/92) presented phase I and/or II IgG antibodies, with 65.5% (19/29) presenting as positive in the second sample.
At the telephone call at 6 months, 39.5% (47/121) reported new consultations to the hospital for similar symptoms, 60.5% (72/121) reported headaches, 51.3% (61/121) fatigue, and 42.9% (51/121) fever. Furthermore, 2.5% (3/121) died.
2.3. Serological Evidence of Previous Exposure and Molecular Detection
Seropositivity to IgG antibodies was obtained for all the bacteria analyzed, with the Francisella genera being the most common at 39.5% (107/271), followed by R. rickettsii at 31.4% (85/271), Ehrlichia genera at 26.9% (73/271), R. typhi at 15.5% (42/271), Anaplasma at 14.4% (39/271), and Borrelia genera at 6.6% (18/271). The municipality of residence; the presence of cats, hens, sheep, and horses in the residence; and direct contact with animals in the workplace, among others, were identified as possible associated factors to previous exposure to these bacteria (Table S1). None of these bacteria were detected by the molecular techniques used in the current study.
In the Coxiella burnetii test, this microorganism was detected in 39.5% of the patients: 49.5% only by phase I and II IgG antibodies, 33.6% only by real-time PCR, and 16.8% had a concordant positive result using both techniques. This microorganism was detected in 40.3% of males vs. 37.9% of females, in addition to 50% of the people who reported being veterinarians or farmers and 61.9% of the patients who reported abortions of animals at work (Table 1).
Table 1.
Demographic and epidemiological characteristics describing patients with acute febrile syndrome according to a positive result in any test used to detect Coxiella burnetii.
| Demographic/Epidemiological Characteristics | n/N (%) 1 | p-Value |
|---|---|---|
| Sex | ||
| Male | 71/176 (40.3) | 0.694 |
| Female | 36/95 (37.9) | |
| Municipality of residence | ||
| Puerto Berrio | 82/222 (36.9) | 0.437 |
| Cimitarra | 8/16 (50.0) | |
| Maceo | 8/14 (57.1) | |
| Puerto Nare | 4/7 (57.1) | |
| Others | 5/12 (41.7) | |
| Place of enrollment | ||
| Consultation | 11/21 (52.4) | 0.29 |
| Emergency | 68/186 (36.6) | |
| Hospitalization | 28/64 (43.8) | |
| Place of residence | ||
| Rural | 56/148 (37.8) | 0.543 |
| Urban | 51/123 (41.5) | |
| Workplace | ||
| No work | 8/15 (53.3) | 0.415 |
| Rural | 44/121 (36.4) | |
| Urban | 55/135 (40.7) | |
| Type of health insurance | ||
| Contributory | 24/95 (25.3) | 0.002 |
| Subsidized | 62/128 (48.4) | |
| Special or exception | 21/48 (43.8) | |
| Occupation | ||
| Veterinarian/farmer | 11/22 (50.0) | 0.082 |
| Agriculturist | 4/9 (44.4) | |
| Health personnel | 0/6 (.0) | |
| Military or police | 20/41 (48.8) | |
| Other | 72/193 (37.3) | |
| Cats in residence and at work | ||
| Yes | 65/156 (41.7) | 0.392 |
| No | 42/115 (36.5) | |
| Dogs in residence and at work | ||
| Yes | 67/162 (41.4) | 0.442 |
| No | 40/109 (36.7) | |
| Cows in residence and at work | ||
| Yes | 28/64 (43.8) | 0.400 |
| No | 78/206 (37.9) | |
| Pigs in residence and at work | ||
| Yes | 17/35 (48.6) | 0.239 |
| No | 90/236 (38.1) | |
| Goats in residence and at work | ||
| Yes | 3/9 (33.3) | 1.000 |
| No | 104/262 (39.7) | |
| Sheep in residence and at work | ||
| Yes | 2/8 (25.0) | 0.487 |
| No | 104/262 (39.7) | |
| Chickens in residence and at work | ||
| Yes | 38/86 (44.2) | 0.280 |
| No | 69/185 (37.3) | |
| Horses in residence and at work | ||
| Yes | 26/65 (40.0) | 0.922 |
| No | 81/206 (39.3) | |
| Do pets sleep indoors? | ||
| Yes | 49/111 (44.1) | 0.191 |
| No | 58/160 (36.3) | |
| Has any animal had an abortion at the residence? | ||
| Yes | 7/19 (36.8) | 0.807 |
| No | 100/252 (39.7) | |
| Has any animal had an abortion at work? | ||
| Yes | 13/21 (61.9) | 0.029 |
| No | 94/250 (37.6) | |
| Exposed to animal births in the last six months | ||
| Yes | 18/43 (41.9) | 0.728 |
| No | 89/228 (39.0) | |
| Consume boiled or potable water | ||
| Yes | 47/128 (36.7) | 0.353 |
| No | 60/142 (42.3) | |
| Hand washing before eating or preparing food | ||
| Yes | 92/219 (42.0) | 0.081 |
| No | 15/52 (28.8) | |
| Raw milk consumption | ||
| Yes | 17/42 (40.5) | 0.886 |
| No | 90/229 (39.3) | |
| Raw meat consumption or three quarters | ||
| Yes | 16/40 (40.0) | 0.942 |
| No | 91/231 (39.4) | |
| Preparation of products derived from raw milk | ||
| Yes | 21/60 (35.0) | 0.421 |
| No | 86/211 (40.8) | |
| Consumption of raw milk derivatives | ||
| Yes | 98/249 (39.4) | 0.887 |
| No | 9/22 (40.9) | |
| Has a history of visiting farms with animals in the last two months? | ||
| Yes | 42/118 (35.6) | 0.250 |
| No | 65/153 (42.5) | |
| Another family member is presenting AFI | ||
| Yes | 28/83 (33.7) | 0.198 |
| No | 79/188 (42.0) | |
| Has a tick-bitten history of ever? | ||
| Yes | 94/238 (39.5) | 0.991 |
| No | 13/33 (39.4) | |
| Ticks or immature forms of ticks in residence or at work | ||
| Yes | 94/238 (39.5) | 0.991 |
| No | 13/33 (39.4) | |
| Blood transfusion | ||
| Yes | 6/15 (40.0) | 0.952 |
| No | 100/255 (39.2) | |
| Travel in the last six months | ||
| Yes | 54/142 (38.0) | 0.607 |
| No | 53/129 (41.1) | |
| Direct contact with animals at work | ||
| Yes | 26/59 (44.1) | 0.415 |
| No | 81/212 (38.2) | |
1 The real-time PCR results performed to detect C. burnetii, plus the serologic detection of phase I and II IgG antibodies defined as the outcome variable.
Among the most reported clinical signs and symptoms in these patients were fatigue in 86% (92/107), chills in 89.7% (96/107), and headache in 90.7% (97/107) (Table 2).
Table 2.
Clinical characteristics of the patients according to the results of tests for the detection of Coxiella burnetii.
| Clinical Features | Results of Tests for the Detection of C. burnetii | ||||
|---|---|---|---|---|---|
| Negative N = 164 | PCR+ Serology+ N = 18 |
PCR+ Serology− N = 36 |
PCR- Serology+ N = 53 |
p-Value | |
| n % | n % | n % | n % | ||
| Shaking chills | 155 (94.5) | 16 (88.9) | 33 (91.7) | 47 (88.7) | 0.555 |
| Profuse sweating | 110 (67.1) | 11 (61.1) | 22 (62.9) | 27 (50.9) | 0.212 |
| Sickness | 116 (70.7) | 10 (55.6) | 25 (69.4) | 37 (71.2) | 0.607 |
| Fatigue | 148 (90.2) | 17 (94.4) | 31 (86.1) | 44 (83.0) | 0.325 |
| Anorexia | 142 (86.6) | 16 (88.9) | 32 (88.9) | 41 (77.4) | 0.331 |
| Myalgia | 140 (85.4) | 16 (88.9) | 30 (83.3) | 45 (84.9) | 0.960 |
| Arthralgia | 128 (78.0) | 14 (77.8) | 27 (75.0) | 35 (66.0) | 0.366 |
| Headache | 149 (90.9) | 18 (100.0) | 33 (91.7) | 46 (86.8) | 0.271 |
| Diarrhea | 55 (33.5) | 4 (22.2) | 12 (33.3) | 23 (43.4) | 0.371 |
| Nauseous | 75 (45.7) | 12 (66.7) | 19 (52.8) | 23 (43.4) | 0.306 |
| Conjunctivitis | 98 (59.8) | 12 (66.7) | 19 (52.8) | 27 (50.9) | 0.528 |
| Maculopapular rash | 43 (26.4) | 3 (16.7) | 14 (38.9) | 15 (28.8) | 0.321 |
| Lymphadenopathy: cervical | 10 (6.1) | 0 (.0) | 0 (.0) | 1 (1.9) | 0.105 |
| Lymphadenopathy: axillary | 5 (3.0) | 1 (5.6) | 2 (5.6) | 4 (7.5) | 0.685 |
| Lymphadenopathy: inguinal | 10 (6.1) | 0 (.0) | 3 (8.3) | 5 (9.4) | 0.428 |
| Lymphadenopathy: epitrochlear | 2 (1.2) | 0 (.0) | 0 (.0) | 0 (.0) | 0.762 |
| Facial paralysis | 5 (3.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0.200 |
| Cough | 57 (34.8) | 2 (11.1) | 11 (30.6) | 15 (28.3) | 0.208 |
| Expectoration | 15 (9.1) | 2 (11.1) | 4 (11.1) | 6 (11.3) | 0.959 |
| Dyspnea | 26 (15.9) | 4 (22.2) | 7 (19.4) | 7 (13.2) | 0.772 |
| Nasal congestion | 15 (9.1) | 5 (27.8) | 5 (13.9) | 6 (11.3) | 0.252 |
| Rhinorrhea | 13 (7.9) | 3 (16.7) | 2 (5.6) | 4 (7.5) | 0.677 |
| Physical exam | |||||
| Choluria | 63 (38.4) | 4 (22.2) | 13 (36.1) | 15 (28.3) | 0.363 |
| Jaundice | 7 (4.3) | 2 (11.1) | 2 (5.6) | 3 (5.7) | 0.784 |
| Right-upper-quadrant pain | 60 (36.6) | 8 (44.4) | 13 (36.1) | 9 (17.0) | 0.040 |
| Pruritus | 22 (13.4) | 2 (11.1) | 8 (22.2) | 2 (3.8) | 0.065 |
| Retro eye pain | 41 (25.2) | 9 (50.0) | 9 (25.0) | 13 (24.5) | 0.146 |
| Hepatomegaly | 2 (1.2) | 0 (.0) | 0 (.0) | 1 (1.9) | 1.000 |
| Splenomegaly | 2 (1.2) | 0 (.0) | 0 (.0) | 2 (3.8) | 0.508 |
| Exanthema | 28 (17.1) | 2 (11.1) | 11 (30.6) | 10 (18.9) | 0.233 |
| Petechiae | 12 (7.3) | 2 (11.1) | 3 (8.6) | 4 (7.5) | 0.979 |
| Ecchymosis | 3 (1.8) | 0 (.0) | 0 (.0) | 1 (1.9) | 0.884 |
| Bleeding | 7 (4.3) | 0 (.0) | 3 (8.3) | 0 (.0) | 0.073 |
| Conjunctivitis | 51 (31.1) | 9 (50.0) | 10 (27.8) | 14 (26.4) | 0.292 |
| Clinical examination | |||||
| Jaundice | 3 (1.8) | 0 (.0) | 0 (.0) | 3 (5.7) | 0.230 |
| Choluria | 29 (17.7) | 0 (.0) | 5 (13.9) | 7 (13.2) | 0.237 |
| Right-upper-quadrant pain | 52 (31.7) | 4 (22.2) | 11 (30.6) | 8 (15.1) | 0.115 |
| Altered consciousness | 1 (.6) | 0 (.0) | 0 (.0) | 2 (3.8) | 0.201 |
2.4. Tick Data
Fifteen homes and one workplace of the patients who agreed to participate in the entomological screening were visited. A total of 61 people and 138 domestic animals were examined by the visual inspection technique. A total of 191 adult ticks, 111 females and 80 males, were collected. Thirteen canines (Canis lupus familiaris) and two bovines (Holstein) were infested with ticks. Then, the collected ticks were identified as Rhipicephalus sanguineus s.l. (93 females and 80 males), all collected from domestic dogs in seven AFI patient residences and one from a workplace. Rhipicephalus microplus (18 females) were collected from two bovines sampled in a patient’s house (Table 3).
Table 3.
Morphological and molecular identifications of ticks, frequency of infestation, and natural infection by molecular detection of ticks.
| Site of Sampling | Sampled Animals 1/Sampling of Immature Stages 2 | Immature Stages Obtained 3 N = 95 n (%) |
Sampled Animals N = 138 n/N (%) | Infested Animals N = 16 n (%) |
Tick Identification 4 | Detected Microorganisms | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
R. sanguineus s.l. N = 173 n (%) |
R. microplus N = 18 n (%) |
Ehrlichia n (%) |
Anaplasma n (%) |
Coxiella n (%) |
||||||
| 10015 | CFFW/Not | 0 (0.0) | 47 (34.1) | Canines | 2 (12.5) | 23 (13.3) | 0 (0.0) | 9 (39.1) | 0 (0.0) | 0 (0.0) |
| 10027 | CFFW/Not | 0 (0.0) | 5 (3.6) | Canines | 2 (12.5) | 41 (23.7) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 5 (12.2) |
| 10027 5 | C/Yes | 0 (0.0) | 2 (1.4) | Canines | 1 (6.3) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10020 | CF/Not | 0 (0.0) | 8 (5.8) | Canines | 3 (18.6) | 52 (30.1) | 0 (0.0) | 15 (28.9) | 1 (1.9) | 9 (17.3) |
| 10001 | CBEFFW/Yes | 95 (100) | 10 (7.2) | Canines | 1 (6.3) | 26 (15.0) | 0 (0.0) | 4 (15.4) | 1 (3.8) | 3 (11.5) |
| 10083 | C/Not | 0 (0.0) | 2 (1.4) | Canines | 1 (6.3) | 4 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10198 | CEFFW/Not | 0 (0.0) | 10 (7.2) | Canines | 2 (12.5) | 8 (4.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) |
| 10227 | CBFW/Yes | 0 (0.0) | 18 (13) | Canines | 2 (12.5) | 18 (10.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) |
| 0 (0.0) | Bovines | 2 (12.5) | 0 (0.0) | 18 (100) | 7 (38.9) | 6 (33.3) | 0 (0.0) | |||
| 10094 | /Not | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10082 | C/Not | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10131 | C/Not | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10163 | /Not | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10205 | FW/Not | 0 (0.0) | 16 (11.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10242 | CF/Not | 0 (0.0) | 3 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10243 | CFW/Not | 0 (0.0) | 12 (8.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 10263 | CF/Not | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
1 Sampled animals: C: canines, B: bovines, E: equines, F: felines, FW: fowls. 2 Sampling at immature stages using the dragging technique. 3 Only larvae were obtained using the dragging technique. 4 Morphological identifications verified with the COI molecular marker. 5 Workplace.
A total 3 of out of the 16 sites included in the entomological screening had green areas, gardens, or paddocks to perform the dragging technique; those 6 areas were examined, and we obtained a positive result in 1. Ninety-five larvae of ticks were obtained, while nymphs were not found using the dragging method.
Genomic DNA was extracted from the complete body of 169 adult ticks; 16 adult ticks were stored in ethanol to support species identification, and 6 voucher specimens were deposited in the entomological collection of Tecnológico de Antioquia-CETdeA Colombia. The molecular confirmation of tick species based on the COI gene analysis correlated with the previous identification by morphological keys identifying the ticks as Rhipicephalus sanguineus sensu lato ‘tropical lineage’ and Rhipicephalus microplus. In the 169 adult ticks in which natural infection was evaluated, the bacteria of the genus Ehrlichia were detected in 21.3% (36/169), Coxiella in 11.8% (20/169), and Anaplasma in 4.7% (8/169). In the ticks identified as R. sanguineus s.l., these four genera were observed, while in those identified as R. microplus, only Ehrlichia and Anaplasma spp. were identified (Table 3). By the BLASTn analysis of the sequences obtained from these bacteria, one had an identity percentage range of 99–100% with uncultured Ehrlichia sp. (GenBank® code: OM47536), one with Anaplasma marginale (GenBank® code: OM475362), and two with Anaplasma platys (GenBank® codes: OM475363 and OM475364).
2.5. Molecular Phylogenetic Analysis of 16s rRNA Sequences of the Coxiella Genus
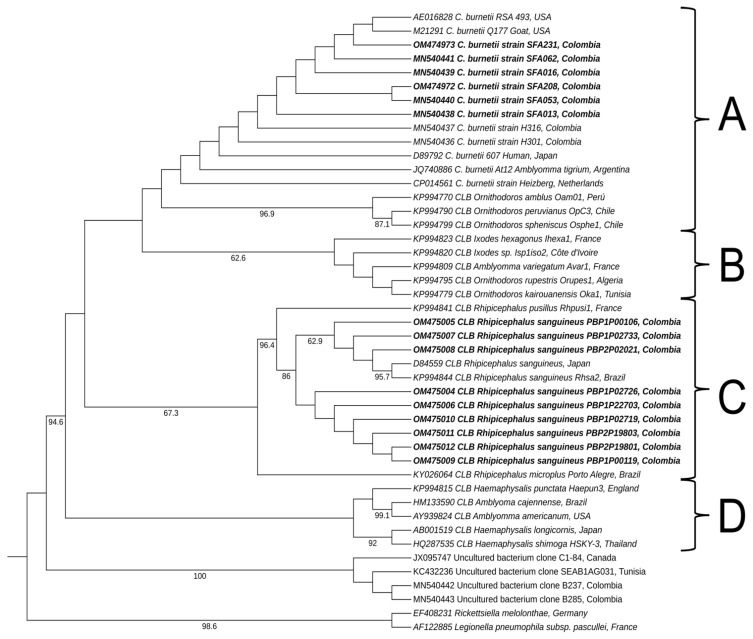
BLASTn revealed that the sequences belonging to samples collected from six patients had high identity percentages (99–100%) of C. burnetii (GenBank® codes: MN540438, MN540439, MN540440, MN540441, OM474972, and OM474973) and were grouped within A clade in the phylogeny analysis. In this group, this species had been previously described within the Coxiella genus [13]. The sequences collected from nine positive ticks showed the highest identity percentages, ranging from 98% to 100%, with Coxiella-like endosymbiont from Rhipicephalus sanguineus (GenBank® codes: OM475004, OM475005OM475006, OM475007, OM475008, OM475009, OM475010, OM475011, and OM475012). Consistent with the molecular phylogeny analysis results, the sequences obtained from ticks were grouped in clade C (Figure 1).
Figure 1.
Phylogenetic tree constructed using maximum-likelihood based on partial sequences of 16S rRNA for the Coxiella genus. All four clades previously reported for the Coxiella genus [13] are labeled with letters A to D. The C. burnetii group is included within clade A. The labels for each reference sequence include GenBank accession numbers and specify the country of origin. Its branches have numbers indicating bootstrap support (out of 1000 repetitions), ranging from 60% to 100%. The sequences obtained for the present study are displayed in a bold font. CLB: Coxiella-like bacteria.
2.6. Factors Associated with C. burnetii Detected in Patients with AFI
In the molecular screening of patients with AFI, C. burnetii was the only bacterium detected, so this study explored the possible factors associated with C. burnetii infection. The factors associated with an increase in C. burnetii detection frequency were type of health insurance, any animal that had abortions in the workplace, and pets allowed to sleep inside the residence. Those with lower frequencies identified similar symptoms in family members and the presence of chills (Table 4).
Table 4.
A multivariate model of variables associated with C. burnetii.
| Features | cPR 1 (95% CI) | p-value | aPR 2 (95% CI) | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Social security | ||||
| Contributory | Reference | - | Reference | - |
| Subsidized | 1.91 (1.57–2.32) | 0.000 | 2.08 (1.67–2.59) | 0.000 |
| Special or exception | 1.73 (1.39–2.14) | 0.000 | 2.06 (1.61–2.63) | 0.000 |
| Epidemiological | ||||
| Abortions at work | 1.64 (1.35–1.99) | 0.000 | 1.81 (1.44–2.28) | 0.000 |
| Pets sleep in the house | 1.21 (1.02–1.44) | 0.022 | 1.45 (1.24–1.71) | 0.000 |
| Symptoms in family members | 0.80 (0.70–0.92) | 0.022 | 0.78 (0.65–0.94) | 0.010 |
| Clinical | ||||
| Sweating | 0.76 (0.72–0.80) | 0.000 | 0.79 (0.69–0.91) | 0.001 |
| Chill | 0.69 (0.51–0.94) | 0.018 | 0.75 (0.63–0.89) | 0.001 |
| Congestion | 1.36 (0.96–1.91) | 0.078 | 1.66 (1.24–2.23) | 0.001 |
| Arthralgia | 0.80 (0.67–0.95) | 0.013 | 0.75 (0.63–0.91) | 0.003 |
| Jaundice | 1.28 (0.99–1.66) | 0.058 | 1.58 (1.02–2.44) | 0.037 |
| Petechiae | 1.10 (0.78–1.53) | 0.575 | 1.32 (0.98–1.77) | 0.061 |
| Choluria (physical exam) | 0.70 (0.57–0.86) | 0.001 | 0.70 (0.29–0.38) | 0.002 |
1 Crude prevalence ratio; 2 adjusted prevalence ratio.
3. Discussion
In the current study, the IgG serology results identify prior exposure to all tick-borne agents studied among patients presenting with AFI in the Magdalena Medio region. However, molecular detection was observed only for the bacteria C. burnetii, suggesting it plays a role as a potential etiological agent of AFI in the Colombian Magdalena Medio region. We previously determined that C. burnetii infection was confirmed in livestock farmers from this region [14].
Regarding the other bacteria evaluated, 39.5% were positive for IgG antibodies against F. tularensis, which is high compared to other countries. A cross-sectional study conducted in Jordan identified, through ELISA, this microorganism in 7.7% of the 828 people evaluated. However, no convalescent-phase serum was analyzed, and the people did not present clinical symptoms of the disease [15]. Furthermore, in this same study, it was reported that those owning a small ruminant had 1.86 (95% CI: 1.02–3.40) greater odds for seropositivity than individuals who did not own a small ruminant, and those practicing horticulture had 2.10 (95% CI: 1.20–3.66) greater odds for seropositivity than individuals who did not practice horticulture [15]. Although we did not perform multivariate analyses as we could not confirm or exclude acute infection by these bacteria, the municipality of residence of the patients and animal abortions might be associated with the presence of this microorganism (Table S1).
Q fever is among the global priority bacterial zoonoses, including anthrax, brucellosis, leptospirosis, plague, salmonellosis, and zoonotic tuberculosis. However, it is still ignored and under-reported in most of the world [16]. C. burnetii causes Q fever, a zoonosis prevalent in all countries except New Zealand [17]. The bacterium’s hosts are broad, mainly including domestic and wild mammals, birds, and arthropods [18,19]. However, domestic ruminants, primarily cattle, sheep, and goats, are considered as essential reservoirs and transmission sources for humans [20].
One of the associated factors for detecting C. burnetii is that any animal that has had an abortion in the patient’s workplace (aPR: 1.81, 95% CI: 1.44–2.28). This bacterium can be recovered and identified in the placentas of infected animals, both from abortions and natural births [21]. According to the previous studies, the estimated bacterial load in an infected animal’s placenta can be as high as 109 bacterial cells per gram of tissue [22]. As the estimated infectious dose through the inhalation of aerosols is slightly higher than one bacterial cell [23], people’s exposure to these highly infectious animal products represents an increased risk of exposure and transmission of this bacterium. Furthermore, suppose several herds are simultaneously affected in a wide geographic area; in that case, it can increase abortion rates in animals, increasing the probability of infection and the occurrence of human outbreaks [24].
Detecting C. burnetii in symptomatic and asymptomatic people is vital because there is no single management strategy. In countries where reporting this microorganism is mandatory, acute Q fever is defined by clinical symptoms and laboratory criteria [25]. This situation leads to underestimating primary infection cases by C. burnetii, without monitoring asymptomatic people, possibly increasing complications that are not related to the severity of the primary infection, but mainly to immunology factors [25]. Additionally, although this infection during pregnancy is frequently asymptomatic and underreported as it is not part of the screening tests of prenatal care, it can lead to severe obstetric complications, such as fetal death and malformations [26].
Regarding the clinical signs and symptoms identified in the present study, it is known that acute Q fever usually presents as a flu-like illness, with a sudden onset of high fever, which can last for more than 15 days and is frequently associated with myalgia and headaches [17,27]. In a study that followed-up 85 patients with the acute form of this disease, the main symptoms were fever, fatigue, headache, cough, myalgias, and arthralgias [28]. As it can be observed, this clinical presentation is non-specific and misleading, so physicians should request the specific detection of this microorganism in patients with acute febrile syndrome who present risk factors, such as direct contact with animals and a history of tick bites either in their residence or at work.
Goats and sheep are frequently identified as a source of Q-fever outbreaks in humans [29,30], as was the case in the Q-fever outbreak in the Netherlands in 2007–2010, where more than 4500 people were infected [31]. These infected animals can release high levels of C. burnetii into the environment during parturition. Other documented routes of elimination include raw milk, feces, urine, and saliva [32,33]. Previous studies have shown that cattle, goats, and horses can be potential reservoirs for C. burnetii and play an essential role in transmitting the infection. Specifically, a study conducted in Korea, where 592 blood samples were collected from animals, identified this microorganism in 22.7% of goats, 16.4% of dairy cattle, 15.2% of beef cattle, 6.0% of Boer goats, and 5.2% of horses [34], suggesting that these mammals may play an important role as reservoirs for this microorganism. Moreover, other studies have described C. burnetii seropositive individuals associated with rural residences, with an alleged relationship with the livestock number, finding the bacteria in 4.8% of the samples evaluated [35].
The clinical manifestations of Q fever are non-specific so the disease can be confused with other conditions, such as those caused by the influenza virus, dengue fever, malaria, leptospirosis, and hantavirus, among others. In 2016, Cortés et al. reviewed the most frequent diseases associated with the acute febrile syndrome to guide the general practitioner and specialist in providing a reasonable and rational approach to this syndrome [1]. Based on our results, we considered it essential to include C. burnetii in the list of differential diagnoses for people with acute febrile diseases, mainly in patients with risk factors for acquiring the infection.
We confirmed the usefulness of the COI gene sequence for the molecular identification of ticks by demonstrating that the morphological and molecular identifications of adult ticks of the species Rhipicephalus sanguineus s.l. and Rhipicephalus microplus were 100% congruent, confirming that the COI gene is one of the most valuable and informative markers for the identification of ticks [36,37,38]. The DNA of Ehrlichia spp. (16.7%), Anaplasma spp. (1.2%), and Coxiella spp. (11.6%) were detected in ticks of the species R. sanguineus s.l. from domestic dogs; Ehrlichia (38.8%) and Anaplasma (33.3%) were detected in ticks of the genus R. microplus recovered from the cattle of one participant. These results are congruent with the work of other authors on these tick species around the world [39,40] and in South America [41,42,43]. Additionally, these species are anthropophilic. In a study performed in Greece, 537 ticks were removed from humans by medical staff; all ticks belonged to three genera in the Ixodidae family: Rhipicephalus (469/519, 90.4%) with four species (R. sanguineus, R. turanicus, R. bursa, and R. [Boophilus] annulatus), Hyalomma (39/519, 7.51%) with three species (H. marginatum, H. rufipes, and H. anatolicum), and Ixodes (11/519, 2.12%) with two species (I. rinicus and I. gibbosus) [44]. This study suggested that humans that were in constantly around animals were at risk of becoming infected with TBD.
As a limitation of the present study, a second sample was only obtained from 33.95% of the patients (92/271), so they could not be analyzed as diagnostic tests. However, a serological fingerprint was detected in the patients from this area, which makes it essential to conduct studies that search for these microorganisms as causes of disease. This study presented an exploratory approach to the potential risk factors associated with C. burnetii infection; therefore, further studies must evaluate and confirm these findings.
Finally, conducting epidemiological studies that help to detect, through diagnostic techniques, the presence of zoonotic bacteria and specifically C. burnetii in biological samples, such as milk, vaginal swabs, and meat, among others, from animal hosts, such as goats, sheep, horses, and chickens, in addition to evaluating natural infections caused by these microorganisms in the adult and immature forms of ticks found in these animals, will help us to encourage health promotion and prevention strategies, including food safety in populations that depend on these types of production systems, and thus mitigate the impact of both on health and the economy that these microorganisms can affect in Colombia.
4. Materials and Methods
4.1. Area of Study, Patients, and Data Collection
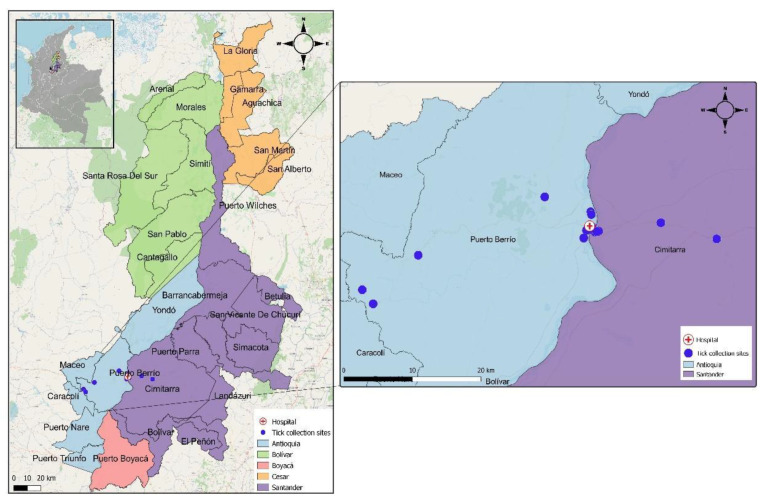
This cohort study enrolled patients who presented themselves for outpatient consultations, emergency visits, or hospitalizations in a hospital of the second level of complexity in the Magdalena Medio region between November 2018 and January 2020. This healthcare institution receives patients from various regional municipalities, such as Puerto Triunfo, Puerto Nare, Caracolí, San Roque, Maceo, and San José del Nus. It has an average monthly care rate of 2500 to 3000 patients in outpatient, 2700 in emergency, and 450 in hospitalization services [45]. It is located in the municipality of Puerto Berrio in Magdalena Medio (Antioquia, Colombia), where the temperature ranges from 32 to 43 °C, relative humidity is 53%, and the altitude ranges from 125 to 150 m.a.s.l. (Figure 2) [46].
Figure 2.
The geographic location of the 16 sites where tick collection was performed and the hospital of the second level of complexity from the Magdalena Medio region.
A Total of 271 patients over 18 years of age were recruited who agreed to participate in the study and signed the informed consent and presented fever (oral temperature ≥ 38) lasting less than 2 weeks and 1 or more of the following symptoms: rash or hemorrhage, or lower respiratory tract infection, or jaundice or lymphadenopathy.
Patients were excluded if they had HIV category C (any opportunistic infection) or 3 (≤200 CD4 cells), consumed antibiotics for more than 72 h in the last 8 days, received treatment with steroids (prednisone ≥ 0.3 mg/kg/day for 3 weeks or more, or ≥1 mg/kg/day for ≥7 days) or cytostatics (except methotrexate at low doses: ≤15 mg/week), known neoplasms (except basal cell and thyroid carcinomas), granulocytopenia < 500 cells/mm3, a cough or expectoration for more than 15 days, kidney or liver failure, a history of trauma or previous surgery in the last 6 months, fever attributed to antibiotics, diarrhea as an initial and primary symptom, snakebites or acute poisoning, rhinitis, sinusitis, otitis, tonsillitis, or exclusive symptomatology of the upper respiratory tract.
At baseline, a structured questionnaire administered by the field team collected the sociodemographic characteristics, and the epidemiological and clinical aspects of each participant, including tick, animal, and food exposures in the workplace or at home, and other personal and environmental risk factors that were reported to be associated with TBD. In addition, a blood sample was obtained for acute serology and molecular diagnosis.
Each participant underwent a first follow-up visit between 2 to 4 weeks following enrollment for information about the evolution of the symptoms, and a second blood sample for molecular detection was collected. Additionally, each patient received a follow-up call at six months and was asked about recurrent febrile episodes, symptoms, and clinical diagnoses since the enrollment.
4.1.1. Blood Sample Collection
Venous blood was obtained from each participant using a vacuum system. The samples were preserved and transported to the laboratory using sterile vacuum tubes, anticoagulant (for DNA extraction), and without anticoagulant (for serum separation).
4.1.2. Molecular Detection and Serological Antibody IgG Analysis
Genomic DNA was extracted from each participant’s blood sample using QIAmp DNeasy Blood & Tissue (Qiagen, Valencia, CA, USA). The concentration obtained was evaluated in a Nanodrop 2000 (Thermo Scientific, Waltham, MA USA), and the DNA was stored at −20 °C until processing. All genomic DNA samples obtained from patients underwent a quality check (absence of amplification inhibitors) with real-time PCR amplification of the GAPDH enzyme gene using the primers GAPDH_F: 5′- TGG GTG TGA ACC ATG AGA AG -3′ and GAPDH_R: 5′- GCT AAG CAG TTG GTG GTG C -3′ [47]. The primers and probes for the molecular identification of the bacterial genera under study were used according to the conditions previously described (Table S2) [48,49,50,51,52,53]. Commercially available DNA controls Amplirun® Rickettsia conorii, Amplirun® Borrelia burgdorferi, Amplirun® Francisella tularensis, and Amplirun® Coxiella burnetii were used as positive controls; additionally, genomic DNA extracted from the Am13Vi-la strain of Anaplasma marginale provided by the Germplasm Bank of the Colombian Agricultural Research Corporation (AGROSAVIA), and genomic DNA from Coxiella burnetii, Rickettsia rickettsii, and Ehrlichia canis strains supplied by the Instituto de Investigaciones Biológicas del Trópico de la Universidad de Córdoba were used as positive controls according to each protocol. Milli-Q® Type I (Merck, Darmstadt, Germany) water was used as a negative control.
The presence of IgG antibodies was determined through commercially available kits to test previous exposure (Table S2). The selected serological kits were implemented following the recommendations of the Centers for Disease Control and Prevention (CDC) for the detection of rickettsiosis (Rickettsia spp.), anaplasmosis (Anaplasma spp.), ehrlichiosis (Ehrlichia spp.) [54], Q fever (Coxiella burnetii) [25], borreliosis (Borrelia burgdorferi) [55], and tularemia (Francisella tularensis) [56].
4.2. Field Collection of Ticks and Species Identification
Tick collection was conducted in the patients’ homes or work environments, with a history of tick bites and positive results from molecular screening for C. burnetii to identify tick species distributed in the zone. We evaluated the natural infection of ticks by the microorganisms under study.
The specimens collected in this study were approved by the National Environmental Licensing Authority (ANLA, Spanish acronym): Collection Framework Agreement granted to Universidad Pontificia Bolivariana through resolution 744 - 26 July 2016. Preserved voucher specimens were deposited in the entomological collection Tecnologico de Antioquia-CETdeA (Medellín, Colombia). The dataset was uploaded to SiB Colombia’s data portal (https://sibcolombia.net/, accessed on 23 June 2021). Tick tracking was performed by physically inspecting the patient’s house (or the workplace of a patient dedicated to livestock), environment, and domestic animals present. The fieldwork was performed through intra-house collection.
Additionally, a peri-domiciliary collection was performed on a perimeter of 10 m around the house [57]. The collection of adult ticks was conducted by the visual inspection of an animal’s tail, loin, neck, and chest, and the extraction by hand of the adult ticks with a size larger than or equal to 3 mm [58,59,60]. Adult ticks were collected from domestic animals from December 2018 to February 2019, and individually stored in bottles with 95% ethanol [61]. The taxonomic identification of adult ticks was performed by evaluating each specimen using taxonomic keys based on the morphology described in the literature for genus and species [62,63,64]. Genomic DNA was extracted from each adult tick using the previously described protocol [65]. The molecular typing of a subset of the collected adult specimens was performed to confirm the morphological identification. Each adult-tick DNA sample was amplified by PCR using specific primers for a fragment of the cytochrome oxidase I (COI) gene (655–680 bp) with the primers LCO1490: 5′- GGT CAA CAA ATC ATA AAG ATA TTG G -3′ and HCO2198: 5′- TAA ACT TCA GGG TGA CCA AAA AAT CA -3′ [66], and COI sequences were bidirectionally obtained.
A white flannel (1 m2) was horizontally dragged through patios, gardens, and paddocks to gather non-parasitic tick forms. The attached ticks were removed from the flannel with a lint brush and placed in resealable bags with silica gel [67] until they were counted under a stereomicroscope (Nikon SMZ1270, Tokyo, Japan).
Molecular Detection of Natural TBD Infection in Ticks
For the molecular detection of natural infection by the bacteria of the Anaplasma, Ehrlichia, Coxiella, Rickettsia, Borrelia, and Francisella genera, the same protocols and primers used for molecular detection in patients with acute febrile syndrome were used to test all ticks’ DNA genomic samples (Table S2).
4.3. Data Analysis
4.3.1. Molecular Phylogenetic Analysis for Coxiella Genus
The DNA sequences we obtained were edited, assembled, and aligned using the Geneious® 9.1.2 program (Wellesley St, Auckland, New Zealand). For the molecular phylogenetic analysis, samples that were positive for the bacterium of the Coxiella genus, obtained through nested PCR (Table S2) [68], were used. This analysis was conducted based on the maximum-likelihood method and the PhyML plugin, available via the Geneious® software [69]. During these analyses, the general time-reversible model was used, with gamma distribution (G) and the proportion of invariable sites (I) selected by the jModelTest 2.1 tool [70]. The robustness of the phylogenetic tree was estimated using bootstrap analysis with 1000 copies. Legionella pneumophila subsp. pascullei and Rickettsiella melolonthae were included as external groups. Tree appearance was edited using the Interactive Tree Of Life (iTOL) version 4.4.2 [71].
4.3.2. Tick Data
The morphological tick-species identification was complemented with molecular analyses performed in the COI species database in the Barcode of Life Data System (BOLD)v4 using the following options: animal identification (COI) and species level barcode records.
The absolute and relative frequencies of tick adult species observed in each host were calculated (Microsoft Excel, Microsoft Corporation, Redmond, USA). Furthermore, each sample collection site counted the number of non-parasitic tick forms (larvae and nymphs) obtained in the gardens and paddocks.
4.3.3. Descriptive and Inferential Statistical Analysis of Associated Factors
The real-time PCR performed to detect C. burnetii plus the serologic detection of phase I and II IgG antibodies were defined as the outcome variables. When analyzing the factors associated with C. burnetii infection, the crude prevalence ratio (cPR) and adjusted prevalence ratio (aPR) and their corresponding confidence intervals (CIs) at 95% were estimated with a generalized linear model with a Poisson distribution and logarithmic link, with adjustment by cluster with the variable municipality of residence in Stata® software (Lakeway Drive, TX, USA). The variables were included in the multivariate model because they met the statistical criterion of a p-value of < 0.25 after performing bivariate analysis or because they played an essential role in disease transmission or were risk factors.
Relative and absolute frequencies were calculated for qualitative variables using SPSS® statistical software (SPSS® Inc., Armonk, NY, USA).
5. Conclusions
In this study, we identified through serological tests the previous exposition to Francisella, Rickettsia spp., Ehrlichia spp., Anaplasma spp., and Borrelia spp. Additionally, C. burnetii was identified in 39.5% of the patients either by serology or molecular techniques, considering the type of health insurance, any animal that had an abortion in the workplace, and pets allowed to sleep inside the residence as the factors associated with the infection. The ticks collected were identified as Rhipicephalus sanguineus s.l. and Rhipicephalus microplus and the bacteria of the genus Ehrlichia spp., Coxiella spp., and Anaplasma spp. by PCR. The phylogenetic tree constructed with sequences of 16S rRNA for the Coxiella genus showed that the sequences obtained from the patients were C. burnetii and from ticks were Coxiella-like endosymbionts from Rhipicephalus sanguineus. This project integrated human- and animal-health elements, showing the importance of these microorganisms and the challenges of confirming the etiology of AFIs, and suggested that C. burnetii should be considered as a potential etiology of AFIs in this region.
Acknowledgments
We thank the healthcare institution, Secretaria de Salud de Puerto Berrio, and the Consejo Departamental de Zoonosis de la Dirección de Salud Ambiental y Factores de Riesgo de la Gobernación de Antioquia for their support in the development of this research. We thank researchers Salim Mattar Velilla and Jorge Luis Miranda Regino from the Instituto de Investigaciones Biológicas del Trópico of Universidad de Córdoba for donating the C. burnetii, R. rickettsii, and Ehrlichia canis DNA used as positive controls in all the DNA amplification reactions performed in the present study, and to the Germoplasm Bank of the Colombian Agricultural Research Corporation (AGROSAVIA) for the A. marginale control. Additionally, we thank Kathleen L. Chavarría from grand SGR (Sistema General de Regalías) BPIN 2020000100131, who supported the statistical analysis of this research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11101090/s1, Table S1: Demographic and epidemiological characteristics according to the serological results of the bacteria studied; Table S2: Selected serology kits for the detection of antibody anti- IgG and primers for the molecular detection of microorganisms of the genera Rickettsia, Anaplasma, Borrelia, Ehrlichia, Coxiella, and Francisella.
Author Contributions
Z.V.R., L.L., Y.K. and L.A.G., conceived the study design and analyzed and interpreted the data. W.M., L.L.-M., M.A.C., N.O., V.C. and R.C., collected the data and conducted the experiments. R.C., W.M., L.L. and L.A.G. structured and drafted the original manuscript. L.L.-M., M.A.C., N.O., V.C., Y.K. and Z.V.R. provided considerable input for this manuscript’s structure, discussion, and review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics Committee on Health Research of the Universidad Pontificia Bolivariana (16 May 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available upon request from the corresponding author. C. burnetii sequences MN540438, MN540439, MN540440, MN540441, OM474972, and OM474973; CLB from Rhipicephalus sanguineus sequences OM475004, OM475005, OM475006, OM475007, OM475008, OM475009, OM475010, OM475011, and OM475012; Anaplasma platys sequences OM475363 and OM475364; Anaplasma marginale sequence OM475362; and uncultured Ehrlichia sp. sequence OM475361.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Minciencias (Ministerio de Ciencia Tecnología e Innovación), Colombia, Contract 692-2017 and CIDI UPB grant number 020C-01/18-65. Statistical analysis of this research was supported by grand SGR (Sistema General de Regalías) BPIN 2020000100131.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cortés J.A., Romero-Moreno L.F., Aguirre-León C.A., Pinzón-Lozano L., Cuervo S.I. Enfoque Clínico Del Síndrome Febril Agudo En Colombia. Infectio. 2017;21:39–50. doi: 10.22354/in.v21i1.640. [DOI] [Google Scholar]
- 2.Moreira J., Bressan C.S., Brasil P., Siqueira A.M. Epidemiology of Acute Febrile Illness in Latin America. Clin. Microbiol. Infect. 2018;24:827–835. doi: 10.1016/j.cmi.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasper M.R., Blair P.J., Touch S., Sokhal B., Yasuda C.Y., Williams M., Richards A.L., Burgess T.H., Wierzba T.F., Putnam S.D. Infectious Etiologies of Acute Febrile Illness among Patients Seeking Health Care in South-Central Cambodia. Am. J. Trop. Med. Hyg. 2012;86:246–253. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoonotic Diseases|One Health|CDC. [(accessed on 28 April 2021)]; Available online: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html.
- 5.Wikel S.K. Ticks and Tick-Borne Infections: Complex Ecology, Agents, and Host Interactions. Vet. Sci. 2018;5:60. doi: 10.3390/vetsci5020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colwell D.D., Dantas-Torres F., Otranto D. Vector-Borne Parasitic Zoonoses: Emerging Scenarios and New Perspectives. Vet. Parasitol. 2011;182:14–21. doi: 10.1016/j.vetpar.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.McDaniel C.J., Cardwell D.M., Moeller R.B., Gray G.C. Humans and Cattle: A Review of Bovine Zoonoses. Vector Borne Zoonotic Dis. 2014;14:1–19. doi: 10.1089/vbz.2012.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulanger N., Boyer P., Talagrand-Reboul E., Hansmann Y. Ticks and Tick-Borne Diseases. Med. Mal. Infect. 2019;49:87–97. doi: 10.1016/j.medmal.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Randolph S.E., Gern L., Nuttall P.A. Co-Feeding Ticks: Epidemiological Significance for Tick-Borne Pathogen Transmission. Parasitol. Today. 1996;12:472–479. doi: 10.1016/S0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- 10.Del Cerro A., Oleaga A., Somoano A., Barandika J.F., García-Pérez A.L., Espí A. Molecular Identification of Tick-Borne Pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and Piroplasms) in Questing and Feeding Hard Ticks from North-Western Spain. Ticks Tick-borne Dis. 2022;13:101961. doi: 10.1016/j.ttbdis.2022.101961. [DOI] [PubMed] [Google Scholar]
- 11.Jahfari S., Hofhuis A., Fonville M., van der Giessen J., van Pelt W., Sprong H. Molecular Detection of Tick-Borne Pathogens in Humans with Tick Bites and Erythema Migrans, in the Netherlands. PLoS Negl. Trop. Dis. 2016;10:e0005042. doi: 10.1371/journal.pntd.0005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FIND Acute Febrile Syndrome Strategy. [(accessed on 25 May 2021)]; Available online: https://www.gov.uk/research-for-development-outputs/find-acute-febrile-syndrome-strategy.
- 13.Duron O., Noël V., McCoy K.D., Bonazzi M., Sidi-Boumedine K., Morel O., Vavre F., Zenner L., Jourdain E., Durand P., et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015;11:e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera Orrego R., Ríos-Osorio L.A., Keynan Y., Rueda Z.V., Gutiérrez L.A. Molecular Detection of Coxiella burnetii in Livestock Farmers and Cattle from Magdalena Medio in Antioquia, Colombia. PLoS ONE. 2020;15:e0234360. doi: 10.1371/journal.pone.0234360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obaidat M.M., Malania L., Bani Salman A.E., Arner R.J., Roess A.A. Seroepidemiology, Spatial Distribution, and Risk Factors of Francisella tularensis in Jordan. Am. J. Trop Med. Hyg. 2020;103:659–664. doi: 10.4269/ajtmh.19-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salyer S.J., Silver R., Simone K., Barton Behravesh C. Prioritizing Zoonoses for Global Health Capacity Building-Themes from One Health Zoonotic Disease Workshops in 7 Countries, 2014–2016. Emerg. Infect. Dis. 2017;23 doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldin C., Mélenotte C., Mediannikov O., Ghigo E., Million M., Edouard S., Mege J.-L., Maurin M., Raoult D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017;30:115–190. doi: 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper A., Stephens J., Ketheesan N., Govan B. Detection of Coxiella burnetii DNA in Wildlife and Ticks in Northern Queensland, Australia. Vector Borne Zoonotic Dis. 2013;13:12–16. doi: 10.1089/vbz.2011.0853. [DOI] [PubMed] [Google Scholar]
- 19.González-Barrio D., Hagen F., Tilburg J.J.H.C., Ruiz-Fons F. Coxiella burnetii Genotypes in Iberian Wildlife. Microb. Ecol. 2016;72:890–897. doi: 10.1007/s00248-016-0786-9. [DOI] [PubMed] [Google Scholar]
- 20.Guatteo R., Beaudeau F., Joly A., Seegers H. Coxiella burnetii Shedding by Dairy Cows. Vet. Res. 2007;38:849–860. doi: 10.1051/vetres:2007038. [DOI] [PubMed] [Google Scholar]
- 21.Roest H.-J., van Gelderen B., Dinkla A., Frangoulidis D., van Zijderveld F., Rebel J., van Keulen L. Q Fever in Pregnant Goats: Pathogenesis and Excretion of Coxiella burnetii. PLoS ONE. 2012;7:e48949. doi: 10.1371/journal.pone.0048949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arricau-Bouvery N., Souriau A., Lechopier P., Rodolakis A. Excretion of Coxiella burnetii during an Experimental Infection of Pregnant Goats with an Abortive Goat Strain CbC1. Ann. N. Y. Acad. Sci. 2003;990:524–526. doi: 10.1111/j.1749-6632.2003.tb07422.x. [DOI] [PubMed] [Google Scholar]
- 23.Brooke R.J., Kretzschmar M.E.E., Mutters N.T., Teunis P.F. Human Dose Response Relation for Airborne Exposure to Coxiella burnetii. BMC Infect. Dis. 2013;13:488. doi: 10.1186/1471-2334-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiev M., Afonso A., Neubauer H., Needham H., Thiery R., Rodolakis A., Roest H., Stark K., Stegeman J., Vellema P., et al. Q Fever in Humans and Farm Animals in Four European Countries, 1982 to 2010. Euro. Surveill. 2013;18:20407. doi: 10.2807/ese.18.08.20407-en. [DOI] [PubMed] [Google Scholar]
- 25.Anderson A., Bijlmer H., Fournier P.-E., Graves S., Hartzell J., Kersh G.J., Limonard G., Marrie T.J., Massung R.F., McQuiston J.H., et al. Diagnosis and Management of Q Fever--United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 26.Million M., Roblot F., Carles D., D’Amato F., Protopopescu C., Carrieri M.P., Raoult D. Reevaluation of the Risk of Fetal Death and Malformation after Q Fever. Clin. Infect. Dis. 2014;59:256–260. doi: 10.1093/cid/ciu259. [DOI] [PubMed] [Google Scholar]
- 27.Schneeberger P.M., Wintenberger C., van der Hoek W., Stahl J.P. Q Fever in the Netherlands - 2007-2010: What We Learned from the Largest Outbreak Ever. Med. Mal. Infect. 2014;44:339–353. doi: 10.1016/j.medmal.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Limonard G.J.M., Nabuurs-Franssen M.H., Weers-Pothoff G., Wijkmans C., Besselink R., Horrevorts A.M., Schneeberger P.M., Groot C.A.R. One-Year Follow-up of Patients of the Ongoing Dutch Q Fever Outbreak: Clinical, Serological and Echocardiographic Findings. Infection. 2010;38:471–477. doi: 10.1007/s15010-010-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatchette T.F., Hudson R.C., Schlech W.F., Campbell N.A., Hatchette J.E., Ratnam S., Raoult D., Donovan C., Marrie T.J. Goat-Associated Q Fever: A New Disease in Newfoundland. Emerg. Infect. Dis. 2001;7:413–419. doi: 10.3201/eid0703.017308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennette E.H., Clark W.H., Dean B.H. Sheep and Goats in the Epidemiology of Q Fever in Northern California. Am. J. Trop Med. Hyg. 1949;29:527–541. doi: 10.4269/ajtmh.1949.s1-29.527. [DOI] [PubMed] [Google Scholar]
- 31.Dijkstra F., van der Hoek W., Wijers N., Schimmer B., Rietveld A., Wijkmans C.J., Vellema P., Schneeberger P.M. The 2007–2010 Q Fever Epidemic in The Netherlands: Characteristics of Notified Acute Q Fever Patients and the Association with Dairy Goat Farming. FEMS Immunol. Med. Microbiol. 2012;64:3–12. doi: 10.1111/j.1574-695X.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 32.Arricau Bouvery N., Souriau A., Lechopier P., Rodolakis A. Experimental Coxiella burnetii Infection in Pregnant Goats: Excretion Routes. Vet. Res. 2003;34:423–433. doi: 10.1051/vetres:2003017. [DOI] [PubMed] [Google Scholar]
- 33.Rodolakis A., Berri M., Héchard C., Caudron C., Souriau A., Bodier C.C., Blanchard B., Camuset P., Devillechaise P., Natorp J.C., et al. Comparison of Coxiella burnetii Shedding in Milk of Dairy Bovine, Caprine, and Ovine Herds. J. Dairy Sci. 2007;90:5352–5360. doi: 10.3168/jds.2006-815. [DOI] [PubMed] [Google Scholar]
- 34.Cho H.-C., Hwang S., Kim E.-M., Park Y.-J., Shin S.-U., Jang D.-H., Chae J.-S., Choi K.-S. Prevalence and Molecular Characterization of Coxiella burnetii in Cattle, Goats, and Horses in the Republic of Korea. Vector-Borne Zoonotic Dis. 2021;21:502–508. doi: 10.1089/vbz.2020.2764. [DOI] [PubMed] [Google Scholar]
- 35.Meurer I.R., Silva M.R., Silva M.V.F., de Lima Duré A.Í., Adelino T.É.R., da Costa A.V.B., Vanelli C.P., de Paula Souza e Guimarães R.J., Rozental T., de Lemos E.R.S., et al. Seroprevalence Estimate and Risk Factors for Coxiella burnetii Infections among Humans in a Highly Urbanised Brazilian State. Trans. R. Soc. Trop. Med. Hyg. 2021 doi: 10.1093/trstmh/trab113. [DOI] [PubMed] [Google Scholar]
- 36.Paternina L.E., Verbel-Vergara D., Bejarano E.E. Comparison of 16S and COX1 Genes Mitochondrial Regions and Their Usefulness for Genetic Analysis of Ticks (Acari: Ixodidae) Biomedica. 2016;36:295–302. doi: 10.7705/biomedica.v36i2.3116. [DOI] [PubMed] [Google Scholar]
- 37.Lv J., Wu S., Zhang Y., Chen Y., Feng C., Yuan X., Jia G., Deng J., Wang C., Wang Q., et al. Assessment of Four DNA Fragments (COI, 16S RDNA, ITS2, 12S RDNA) for Species Identification of the Ixodida (Acari: Ixodida) Parasites Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheth B.P., Thaker V.S. DNA Barcoding and Traditional Taxonomy: An Integrated Approach for Biodiversity Conservation. Genome. 2017;60:618–628. doi: 10.1139/gen-2015-0167. [DOI] [PubMed] [Google Scholar]
- 39.Adjou Moumouni P.F., Aplogan G.L., Katahira H., Gao Y., Guo H., Efstratiou A., Jirapattharasate C., Wang G., Liu M., Ringo A.E., et al. Prevalence, Risk Factors, and Genetic Diversity of Veterinary Important Tick-Borne Pathogens in Cattle from Rhipicephalus microplus-Invaded and Non-Invaded Areas of Benin. Ticks Tick-borne Dis. 2018;9:450–464. doi: 10.1016/j.ttbdis.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Guo H., Adjou Moumouni P.F., Thekisoe O., Gao Y., Liu M., Li J., Galon E.M., Efstratiou A., Wang G., Jirapattharasate C., et al. Genetic Characterization of Tick-Borne Pathogens in Ticks Infesting Cattle and Sheep from Three South African Provinces. Ticks Tick Borne Dis. 2019;10:875–882. doi: 10.1016/j.ttbdis.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Labruna M.B., V S.M. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Revista MVZ Córdoba. 2011;16:2435–2457. doi: 10.21897/rmvz.282. [DOI] [Google Scholar]
- 42.Venzal J.M., Estrada Pena A., Castro O., De Souza C.G., Portillo A., Oteo J.A. Study on Seasonal Activity in Dogs and Ehrlichial Infection in Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) from Southern Uruguay. Parasitol. Latinoam. 2007;62:23–26. doi: 10.4067/S0717-77122007000100004. [DOI] [Google Scholar]
- 43.Arroyave E., Cornwell E.R., McBride J.W., Díaz C.A., Labruna M.B., Rodas J.D. Detection of Tick-Borne Rickettsial Pathogens in Naturally Infected Dogs and Dog-Associated Ticks in Medellin, Colombia. Rev. Bras. Parasitol. Vet. 2020;29 doi: 10.1590/s1984-29612020060. [DOI] [PubMed] [Google Scholar]
- 44.Papa A., Chaligiannis I., Xanthopoulou K., Papaioakim M., Papanastasiou S., Sotiraki S. Ticks Parasitizing Humans in Greece. Vector Borne Zoonotic Dis. 2011;11:539–542. doi: 10.1089/vbz.2010.0036. [DOI] [PubMed] [Google Scholar]
- 45.E.S.E Hospital César Uribe de Piedrahita. [(accessed on 25 May 2021)]; Available online: https://www.hcup.gov.co/quienes-somos/nuestro-hospital.
- 46.Atlas Interactivo - Climatológico - IDEAM. [(accessed on 26 May 2021)]; Available online: http://atlas.ideam.gov.co/visorAtlasClimatologico.html.
- 47.Sobotta K., Hillarius K., Jiménez P.H., Kerner K., Heydel C., Menge C. Interaction of Coxiella burnetii Strains of Different Sources and Genotypes with Bovine and Human Monocyte-Derived Macrophages. Front. Cell Infect. Microbiol. 2017;7:543. doi: 10.3389/fcimb.2017.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seong G., Han Y.-J., Chae J.-B., Chae J.-S., Yu D.-H., Lee Y.-S., Park J., Park B.-K., Yoo J.-G., Choi K.-S. Detection of Anaplasma sp. in Korean Native Goats (Capra Aegagrus Hircus) on Jeju Island, Korea. Korean J. Parasitol. 2015;53:765–769. doi: 10.3347/kjp.2015.53.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doyle C.K., Labruna M.B., Breitschwerdt E.B., Tang Y.-W., Corstvet R.E., Hegarty B.C., Bloch K.C., Li P., Walker D.H., McBride J.W. Detection of Medically Important Ehrlichia by Quantitative Multicolor TaqMan Real-Time Polymerase Chain Reaction of the Dsb Gene. J. Mol. Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madeddu G., Mancini F., Caddeo A., Ciervo A., Babudieri S., Maida I., Fiori M.L., Rezza G., Mura M.S. Rickettsia monacensis as Cause of Mediterranean Spotted Fever–like Illness, Italy. Emerg. Infect. Dis. 2012;18:702–704. doi: 10.3201/eid1804.111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howe G.B., Loveless B.M., Norwood D., Craw P., Waag D., England M., Lowe J.R., Courtney B.C., Pitt M.L., Kulesh D.A. Real-Time PCR for the Early Detection and Quantification of Coxiella burnetii as an Alternative to the Murine Bioassay. Mol. Cell Probes. 2009;23:127–131. doi: 10.1016/j.mcp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.H., Vigliotti V.S., Vigliotti J.S., Jones W., Pappu S. Increased Sensitivity and Specificity of Borrelia burgdorferi 16S Ribosomal DNA Detection. Am. J. Clin. Pathol. 2010;133:569–576. doi: 10.1309/AJCPI72YAXRHYHEE. [DOI] [PubMed] [Google Scholar]
- 53.Dergousoff S.J., Chilton N.B. Association of Different Genetic Types of Francisella-like Organisms with the Rocky Mountain Wood Tick (Dermacentor andersoni) and the American Dog Tick (Dermacentor variabilis) in Localities near Their Northern Distributional Limits. Appl. Environ. Microbiol. 2012;78:965–971. doi: 10.1128/AEM.05762-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biggs H.M. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis — United States. MMWR Recomm. Rep. 2016;65 doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 55.Mead P. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly Rep. 2019;68 doi: 10.15585/mmwr.mm6832a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Tularemia - United States, 2001–2010. MMWR Morb. Mortal. Wkly Rep. 2013;62:963–966. [PMC free article] [PubMed] [Google Scholar]
- 57.Manual de Campo Para la Vigilancia Entomológica. Ministerio de Salud, Dirección General de Salud Ambiental; Lima, Perú: 2002. [Google Scholar]
- 58.López V. Bioecología y Distribución de Garrapatas en Colombia. Instituto Colombiano Agropecuario; Medellín, Colombia: 1980. pp. 33–43. [Google Scholar]
- 59.Okello-Onen J., Hassan S.M., Essuman S. Taxonomy of African Ticks an Identification Manual. ICIPE; Nairobi, Kenya: 1999. [Google Scholar]
- 60.Rodríguez-Vivas R.I., Apanaskevich D.A., Ojeda-Chi M.M., Trinidad-Martínez I., Reyes-Novelo E., Esteve-Gassent M.D., Pérez de León A.A. Ticks Collected from Humans, Domestic Animals, and Wildlife in Yucatan, Mexico. Vet. Parasitol. 2016;215:106–113. doi: 10.1016/j.vetpar.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Manual de Vigilancia Entomológica de Dengue, Leishmaniasis, Chagas, Malaria y Fiebre Amarilla. Laboratorio Departamental de Salud Pública, Secretaría de Salud de Santander; Bucaramanga, Colombia: 2007. [Google Scholar]
- 62.Walker A.R., Bouattour A., Camicas J.-L., Estrada-Pena A., Horak I.G., Latif A.A., Pegram R.G., Preston P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. The University of Edinburgh; Edinburgh, UK: 2003. Edinburgh (Scotland) Bioscience Reports. [Google Scholar]
- 63.Barker S.C., Walker A.R. Ticks of Australia. The Species That Infest Domestic Animals and Humans. Zootaxa. 2014:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Nava S., Venzal J.M., González-Acuña D., Martins T.F., Guglielmone A.A., editors. Ticks of the Southern Cone of America. Academic Press; Cambridge, MA, USA: 2017. Front-Matter; pp. i–iii. [Google Scholar]
- 65.Ríos-Tobón S., Gutiérrez-Builes L.A., Ríos-Osorio L.A. Assessing Bovine Babesiosis in Rhipicephalus (Boophilus) Microplus Ticks and 3 to 9-Month-Old Cattle in the Middle Magdalena Region, Colombia. Pesq. Vet. Bras. 2014;34:313–319. doi: 10.1590/S0100-736X2014000400002. [DOI] [Google Scholar]
- 66.Binetruy F., Chevillon C., de Thoisy B., Garnier S., Duron O. Survey of Ticks in French Guiana. Ticks Tick-borne Dis. 2019;10:77–85. doi: 10.1016/j.ttbdis.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Rulison E.L., Kuczaj I., Pang G., Hickling G.J., Tsao J.I., Ginsberg H.S. Flagging versus Dragging as Sampling Methods for Nymphal Ixodes scapularis (Acari: Ixodidae) J. Vector Ecol. 2013;38:163–167. doi: 10.1111/j.1948-7134.2013.12022.x. [DOI] [PubMed] [Google Scholar]
- 68.Duron O., Jourdain E., McCoy K.D. Diversity and Global Distribution of the Coxiella Intracellular Bacterium in Seabird Ticks. Ticks Tick Borne Dis. 2014;5:557–563. doi: 10.1016/j.ttbdis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 70.Darriba D., Taboada G.L., Doallo R., Posada D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Letunic I., Bork P. Interactive Tree Of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available upon request from the corresponding author. C. burnetii sequences MN540438, MN540439, MN540440, MN540441, OM474972, and OM474973; CLB from Rhipicephalus sanguineus sequences OM475004, OM475005, OM475006, OM475007, OM475008, OM475009, OM475010, OM475011, and OM475012; Anaplasma platys sequences OM475363 and OM475364; Anaplasma marginale sequence OM475362; and uncultured Ehrlichia sp. sequence OM475361.