Abstract
This review sets out the key evidence comparing subcutaneous ICDs (S-ICDs) and transvenous ICDs and uses it to empower clinical cardiologists and those who implant ICDs to make optimum patient selections for S-ICD use. The evidence demonstrates that clinical trials performed until recently have proven the performance of S-ICDs. However, the latest data now available from the ATLAS randomised controlled trial have added new insights to this body of evidence. ATLAS demonstrates the superiority of S-ICDs over transvenous ICDs regarding lead-related complications, findings that point to promising opportunities for patients who are at risk of sudden cardiac death.
Keywords: Subcutaneous, transvenous, complications, ICD, sudden cardiac arrest
Since 1980, when the first ICD was inserted in a patient, ICDs have evolved to become the mainstay therapy for both primary and secondary prevention of all-cause cardiac mortality in patients at risk of dying because of fatal cardiac arrhythmias. However, the risks of complications, in particular those related to lead failure and systemic infections, associated with traditional transvenous (TV-ICD) devices are an on-going concern.[1]
The subcutaneous ICD (S-ICD) was designed to overcome these intravascular complications by its entirely extrathoracic implantation. Over the past couple of decades, studies have focused on addressing the efficacy and safety of S-ICDs compared with TV-ICDs and have demonstrated the proven performance of S-ICDs.[2]
The PRAETORIAN trial was the first head-to-head randomised controlled trial (RCT) to compare S-ICDs with TV-ICDs.[3] In this trial, the authors reported the non-inferiority of the S-ICD for a primary composite endpoint of device-related complications or inappropriate shocks at 4 years. The study also showed a statistically significant difference in lead-related complications, with TV-ICD patients experiencing fourfold higher rates of lead-related complications than S-ICD patients (6.6% in the TV-ICD arm versus 1.4% in the S-ICD arm; p=0.001).[3]
Further clinical evidence has been obtained from non-randomised studies and a meta-analysis that demonstrated the non-inferiority of S-ICD in safety and efficacy, including a real-world S-ICD registry and patients with a left ventricular ejection fraction (LVEF) ≤35% in the UNTOUCHED trial.[2,4,5]
More recently, the first randomised superiority trial has been performed. The ATLAS trial was presented as a late-breaking clinical trial at the 2022 Heart Rhythm Society Cardiology Conference.[6] It demonstrated 92% fewer serious lead-related complications for S-ICDs compared with TV-ICDs at 6 months following implantation.[6] This evidence indicates that the S-ICD could be an appropriate choice of device for patients without the need for pacing, particularly those considered to be at a higher risk of lead-related complications.
Accordingly, the purpose of this review is to empower both clinical cardiologists and those who implant ICDs with an up-to-date review of the key clinical evidence comparing S-ICDs and TV-ICDs in order to help inform their patient selection.
Clinical Evidence
PRAETORIAN
PRAETORIAN was a RCT in which the primary endpoint was a composite of device-related complications and inappropriate shocks.[3] The trial randomised 849 eligible patients from clinical centres across the US and Europe between March 2011 and January 2017. Patient diagnoses at baseline are shown in Table 1.
Table 1: Summary of Study Characteristics.
| Study | Design | Patients (n) | Patient Baseline Characteristics | Primary Outcome(s) | Results | Key Findings |
|---|---|---|---|---|---|---|
| PRAETORIAN[3] (NCT01296022) | Non-inferiority RCT | 849 |
|
Composite of device-related complications and IAS | At median follow-up of 49.1 months, a primary endpoint event occurred in 68 patients in the S-ICD group and 68 patients in the TV-ICD group | S-ICD was non-inferior to the TV-ICD regarding device-related complications and IAS in patients with an indication for an ICD but no indication for pacing |
| UNTOUCHED[5] (NCT02433379) | Multi-national, prospective, non-randomised study | 1,111 |
|
IAS-free rate at 540 days (18 months) compared to a performance goal of 91.6% | 95.9% IAS-free rate at 18 months | S-ICD offers high efficacy and safety |
| EFFORTLESS[4] (NCT01085435) | An international, observational, non-randomised, standard of care registry | 994 |
|
|
|
The S-ICD maintains a high level of shock efficacy over time |
| Rordorf et al. 2021[2] (meta-analysis) | Meta-analysis of primary studies that directly compare clinical outcomes and complications between S-ICD and TV-ICD | 9,073 (13 studies) |
|
The composite of clinically relevant complications and IAS | No statistically significant difference in the risk of the primary outcome between S-ICD and TV-ICD | S-ICD is at least as effective and safe as TV-ICD for prevention of SCD in patients without the need for pacing |
| ATLAS[6] (NCT02881255) | Prospective RCT | 503 |
|
Reduction in the rate of major lead-related complications at 6 months post-implant | S-ICD reduces the rate of major, lead-related complications by 92% | S-ICD is superior to TV-ICD regarding serious lead-related complications |
IAS = inappropriate shock; LVEF = left ventricular ejection fraction; RCT = randomised controlled trial; S-ICD = subcutaneous ICD; SVT = supraventricular tachycardia; TV-ICD = transvenous ICD.
PRAETORIAN demonstrated that the S-ICD was not inferior to the TV-ICD regarding the composite endpoint (device-related complications and inappropriate shocks) at 4 years (Figure 1).
Figure 1: Time-to-first-event Curves for the Primary Endpoint and Its Components in the PRAETORIAN Study.
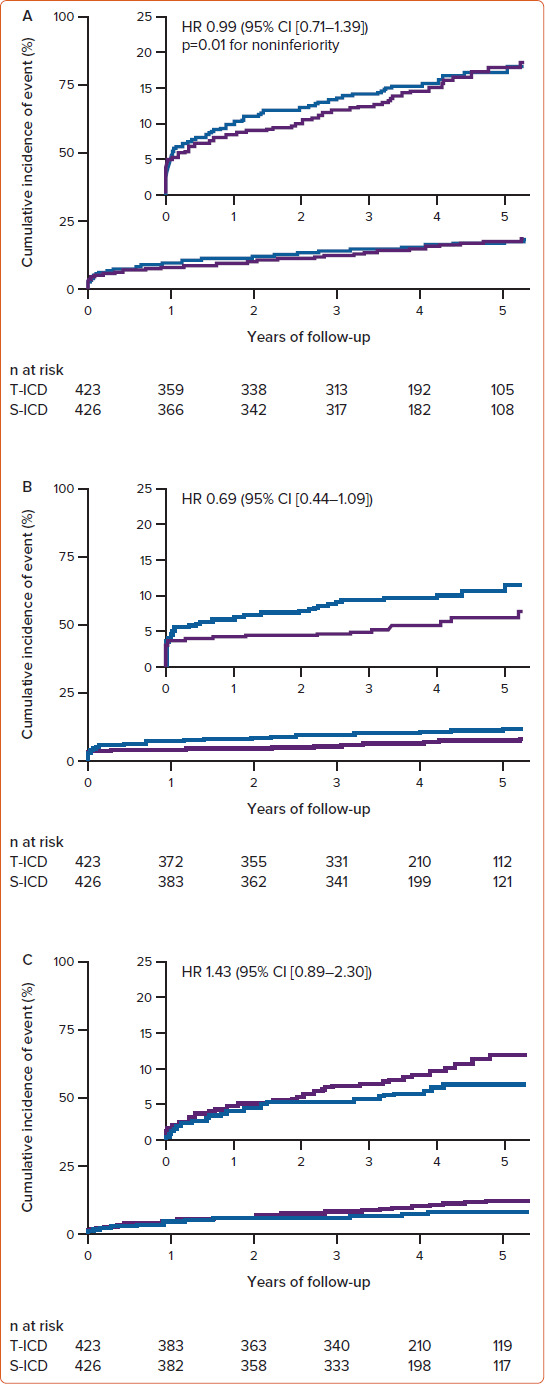
A: Primary composite endpoint (device-related complications or inappropriate shocks); B: Device-related complications; C: Inappropriate shocks. S-ICD = subcutaneous ICD; TV-ICD = transvenous ICD. Source: Knops et al. 2020.[3] Reproduced with permission from Massachusetts Medical Society.
In a secondary analysis of PRAETORIAN, appropriate therapy was evaluated along with assessment of whether anti-tachycardia pacing (ATP) reduces the number of appropriate shocks. It found no statistical difference in the number of patients treated with appropriate ICD therapy in the S-ICD and TV-ICD groups, and patients with an S-ICD were more likely to receive an appropriate shock. However, the overall number of appropriate shocks was comparable between the two groups, despite the inability of the S-ICD to deliver ATP.[7]
UNTOUCHED
UNTOUCHED was a multinational, prospective, non-randomised study designed to evaluate the rate of inappropriate shocks.[5] It included a more typical, contemporary ICD patient population (i.e. patients with LVEF ≤35%) implanted with an S-ICD compared with the patient populations of previous S-ICD studies. The primary endpoint was the inappropriate shock-free rate at 540 days (18 months) compared to a performance goal of 91.6%, derived from the results obtained with optimally programmed TV-ICD patients in the MADIT-RIT study.[8] The trial spanned almost 3 years and took place across 110 sites located in the US, Canada and Europe between June 2015 and February 2018. UNTOUCHED enrolled 1,111 patients with LVEF ≤35% (ischaemic or non-ischaemic heart disease) who were eligible for S-ICD therapy.
Overall, at 18 months the inappropriate shock-free rate was 95.9% with a lower confidence limit of 94.8%, meeting the performance goal of 91.6%. The inappropriate shock-free time course is presented in Figure 2.
Figure 2: Inappropriate Shock-free Rate in the UNTOUCHED Study.
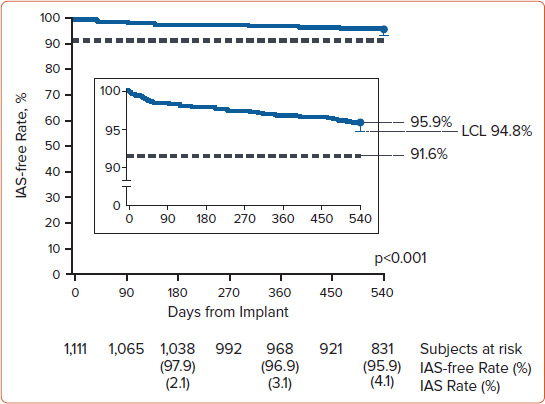
Kaplan–Meier curve illustrating the primary the endpoint result (IAS-free rate at 18 months) in the UNTOUCHED study.[5] IAS = inappropriate shock; LCL = lower confidence limit. Source: Gold et al. 2020.[5] Reproduced with permissions from Wolters Kluwer Health, Inc.
UNTOUCHED demonstrated that the inappropriate shock rates of S-ICDs, in a cohort of patients with more comorbidities and lower LVEFs than previous S-ICD studies, are comparable to those observed in previous studies with TV-ICDs. It should be noted that the 1-year inappropriate shock rates measured across S-ICD studies have decreased substantially over time because of the implementation of conditional zone programming and technology improvements to minimise T wave oversensing, including the SMART Pass filter (Boston Scientific) that was specially designed to reduce cardiac oversensing.
EFFORTLESS
The EFFORTLESS S-ICD registry was a non-randomised, standard of care, multicentre registry set up to collect long-term system-related, clinical, and patient reported outcome data from patients implanted with S-ICDs.[4] It is the largest study of long-term outcomes associated with the S-ICD. Study endpoints were the perioperative (30 days post implantation) S-ICD complication rate, 360-day S-ICD complication rate and the incidence of inappropriate shocks for AF or supraventricular tachycardia (SVT). The registry enrolled 994 patients with a diverse range of cardiac disease at 46 centres in 11 countries from February 2011 to November 2014. The 5-year outcomes were also reported, including spontaneous shock efficacy.
The results from EFFORTLESS showed an S-ICD complication-free rate of 99.9% at 30 days, 98.5% at 360 days and 94.5% after 5 years (Figure 3).
Figure 3: Complication-free Rates With Subcutaneous ICD Use in the EFFORTLESS Study.
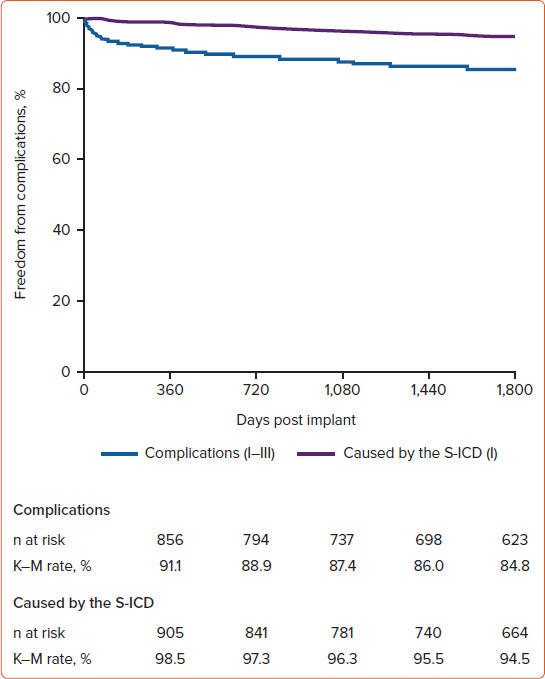
K–M curve of complication-free rates for overall complications (Types I–III) and complications caused by the S-ICD system (Type I) in the EFFORTLESS study.[4] K–M = Kaplan–Meier; S-ICD = subcutaneous ICD. Source: Lambiaise et al. 2022.[4] Reproduced with permissions from Oxford University Press.
In this long-term S-ICD registry, the majority of complications occurred in the first few months post implant, with the most common complication being infection requiring replacement. Erosion was the second most common complication, occurring more commonly in years 2–5. Moreover, EFFORTLESS provided valuable clinical evidence of the long-term efficacy of the S-ICD, demonstrating consistently high spontaneous shock efficacy (98%) over an average of 5 years of follow-up. This result is consistent with those of other TV-ICD studies.[7,9–12]
Meta-analysis
In a meta-analysis of the available clinical studies comparing S-ICD and TV-ICD, the primary outcome was the composite of all relevant complications and inappropriate shocks.[2] The analysis included 13 studies and a total of 9,073 patients. Patient baseline characteristics were comparable between those implanted with S-ICD (3,433 patients) and those with TV-ICD (5,640 patients). Mean LVEF was 40% ± 10%. Underlying cardiopathies were an ischaemic aetiology (46% of patients), non-ischemic cardiomyopathy (44% of patients) and channelopathy (9% of patients).
The risk of the composite of relevant complications and inappropriate shocks between S-ICD and TV-ICD patients was not statistically significant different. There was also no statistically significant difference between S-ICD and TV-ICD patients for the global risk of inappropriate shock (Figure 4). However, patients implanted with an S-ICD had a lower risk of lead complications than TV-ICD patients (Figure 5). Furthermore, patients implanted with an S-ICD had a lower risk of inappropriate shocks due to SVT but a higher risk of inappropriate shocks for cardiac oversensing.
Figure 4: Meta-analysis Findings for Inappropriate Shock.
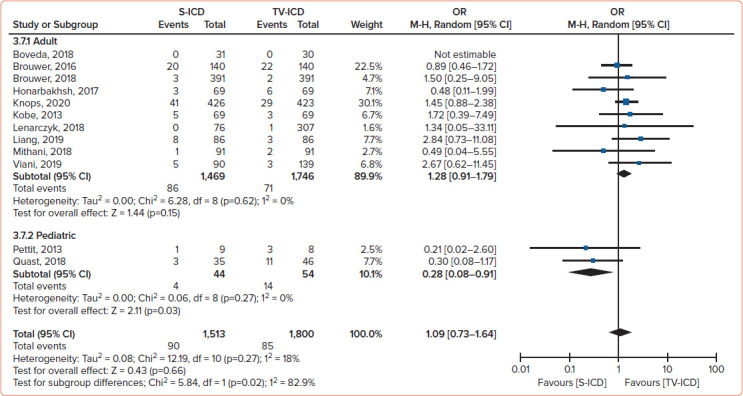
Meta-analysis findings comparing the risk of inappropriate shock in patients implanted with S-ICDs and TV-ICDs.[2] M-H = Mantel-Haenszel; S-ICD = subcutaneous ICD; TV-ICD = transvenous ICD. Source: Rordorf et al. 2021.[2] Reproduced with permission from Elsevier.
Figure 5: Meta-analysis Findings for Lead-related Complications.
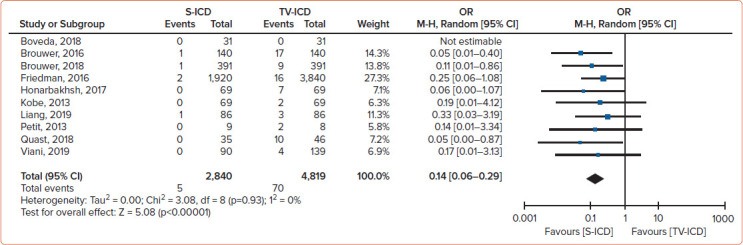
Meta-analysis findings comparing the risk of lead complications in patients implanted with S-ICDs and TV-ICDs.[2] M-H = Mantel-Haenszel; S-ICD = subcutaneous ICD; TV-ICD = transvenous ICD. Source: Rordorf et al. 2021.2 Reproduced with permission from Elsevier.
Finally, the risk of appropriate shocks was similar in S-ICDs versus TV-ICDs and major clinical endpoints, such as cardiovascular and non-cardiovascular death, were comparable among the two groups.
This meta-analysis – the largest of its kind – compared clinical outcomes and complications between patients implanted with S-ICDs versus TV-ICDs in those with an indication for an ICD without the need for pacing. It revealed that the overall risk of clinically relevant complications and inappropriate shocks was not different between two patient groups. The results also showed that S-ICDs and TV-ICDs are associated with different types of complications and different prevalent causes of inappropriate shocks. This should be taken into consideration by clinicians implanting the device when selecting the most appropriate choice of ICD for their patient.
ATLAS
ATLAS was a prospective, randomised controlled head-to-head trial.[6] The primary outcome was the rate of lead-related complications of S-ICD compared to TV-ICD measured at 6 months following implant. The study enrolled 503 patients with a primary or secondary standard indication for an ICD or with inherited cardiac arrhythmias or with cardiac conditions considered at increased risk for lead-related complications. It took place across 14 clinical centres in Canada. Patient diagnoses at baseline are shown in Table 1.
Serious lead-related complications occurred in 4.8% of patients with a TV-ICD versus 0.4% of those with an S-ICD at 6 months. This demonstrates that the S-ICD is superior to TV-ICD, achieving 92% fewer serious lead-related complications. Serious complications were defined as moderate-severe or severe tricuspid regurgitation, haemothorax/pneumothorax, cardiac perforation, tamponade, pericardial effusion or pericarditis, ipsilateral upper-extremity deep vein thrombosis and lead dislodgement or loss of sensing or pacing requiring revision.
ICD effectiveness, defined as the rate of failed first ICD shock, was similar between S-ICDs and TV-ICDs. The rate of inappropriate shocks showed a trend toward a higher risk with S-ICDs versus TV-ICDs (6.4 versus 2.8%), although the difference was not statistically significant.
Author Expertise
Dr Roberto Rordorf, cardiologist and electrophysiologist, is the Head of the Arrhythmias and Electrophysiology Unit at the Policlinico San Matteo Foundation in Pavia, Italy. The Arrhythmias Unit has had considerable experience over the past few decades in the field of device therapy for patients with heart failure and cardiac arrhythmias. Pavia is one of the few centres in Italy that participated in the design and conduct of one of the pivotal trials on cardiac resynchronisation therapy, the CARE-HF study.[13] It was also one of the first centres worldwide to test vagal stimulation in the treatment of chronic heart failure.[14] As a leading national centre in the treatment of patients with heart failure, cardiomyopathies and channelopathies, significant clinical and research activity on ICD therapy is conducted there. Furthermore, patients with complex atrial and ventricular arrhythmias are treated by means of catheter ablation on a regular basis. Beyond the percutaneous treatment of cardiac arrhythmias, Pavia is one of the few centres worldwide with recognised long-term experience in the neuromodulation of cardiac arrhythmias.[15,16]
Discussion
As ICD technology progresses and programming algorithms improve, ICD therapy has evolved dramatically and today it has become the cornerstone of sudden cardiac death. In addition, advancing age and increased comorbidities are forever putting forward more candidates for intervention. The safety and efficacy of the S-ICD have been demonstrated in several studies over the past couple of decades. While traditional TV-ICDs continue to bring risks of lead complications, the latest data from the ATLAS trial now further expand the findings from PRAETORIAN and other studies by demonstrating that the S-ICD is superior to the TV-ICD in preventing the serious complications associated with transvenous leads as early as 6 months after implant.[6,7]
Who Should Receive the Subcutaneous-ICD?
Evidence from clinical trials shows that S-ICDs are appropriate for a broad range of patient populations. A recent study on a large representative national cohort of older patients supported the use of S-ICD in patients aged >65 years who were at risk of sudden cardiac death.[17] The safety and efficacy of S-ICD in teenagers and young adults has also been demonstrated in a large, real-world cohort of S-ICD patients stratified by age at implantation.[18] The rates of inappropriate shocks and complications were not different in younger versus older patients. Historically, young patients have often represented the most suitable candidates for an entirely S-ICD system because they face a lifetime of device therapy and rarely have a pre-existing or concurrent pacing or cardiac resynchronisation therapy indication. It should also be taken into consideration that, even in in a patient implanted with an S-ICD who has a lead complication or a device infection, lead extraction is likely to be more straightforward and less risky with the S-ICD than with the TV-ICD.[19]
The data show that S-ICD use avoids many of the serious complications associated with invasive leads, including serious infection and lead-related complications. Further, the superiority of S-ICD occurs 6 months after implant in adults of all ages with the most common ICD indications.
Patients experiencing transvenous lead complications can also be very good candidates for S-ICD implantation, either where a transvenous lead abandonment strategy is adopted or following percutaneous lead extraction.20,21 This is also true for those cases treated with lead extraction and TV-ICD removal because of infection. Beyond patients who already have an infective complication with TV-ICD, other patients who are deemed at higher infective risk, such as those with end-stage renal insufficiency or diabetes, should be considered for an S-ICD as a first-line strategy. Furthermore, we have recently demonstrated positive patient acceptance of the S-ICD, even in groups at risk of psychological distress, such as females or those with a smaller body habitus, independent of the generator positioning.[22]
Patients with inherited cardiac arrhythmias and cardiomyopathies are usually younger and could be considered suitable for S-ICD therapy. Nevertheless, data from the literature in these scenarios are still scarce and debatable.
Regarding channelopathies, most available data are available for Brugada syndrome. Brugada patients are usually young and active with a long life expectancy, only rarely requiring pacing. Accordingly, although data with longer follow-up periods are needed, Brugada patients have been considered to be the ideal candidates for S-ICDs in many centres. Previous studies have demonstrated that the S-ICD is effective and associated with an acceptable rate of complications in patients with channelopathies.[23,24] Nevertheless, a recent study raised a potential eligibility limitation with the use of S-ICD in Brugada patients, with the authors demonstrating a screening pass rate of 85% at rest and only 70% when a type 1 Brugada ECG pattern was induced by means of the ajmaline test.[25]
Patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) and hypertrophic cardiomyopathy (HCM) are usually young and active and therefore also potentially good candidates for the S-ICD. S-ICDs have been proven to be highly effective in terminating both spontaneous and induced arrhythmias in ARVC.[26] Moreover, although the rate of ARVC patients that experienced inappropriate shocks was not negligible (14% at 1 year), it was in line with data from previous reports on TV-ICD (10–25%).[27–29]
In a large cohort of patients with HCM, the S-ICD was associated with a lower incidence of overall device therapies when compared to the TVICD. The difference was mainly driven by a significantly higher ATP therapy rate in the TV-ICD group, suggesting that ATP therapy is very likely to be unnecessary in HCM patients.[30]
Some aspects of the S-ICD system hold me back from proposing the S-ICD as the first choice in every patient in the need of an ICD. These include the need for conscious sedation or, in some cases, even the support of an anaesthesiologist during the implantation procedure, along with the relatively shorter battery life of 7–9 years and the larger size of the S-ICD compared with the TV-ICD. However, these relate to technical issues associated with any young technology and will undoubtedly be resolved with on-going developments. Indeed, the system has already evolved significantly from its original version, especially with the arrhythmia detection algorithms that have allowed a substantial reduction in inappropriate shocks, as demonstrated in the UNTOUCHED study.[5] Moreover, it must be recognised that, despite the initial concern about the size of the current S-ICD, patient acceptance is positive and its use is sometimes associated with better positive appraisal in comparison with the TV-ICD.[22]
Conclusion
Based on the current evidence presented so far, further strengthened by the results of the latest PRAETORIAN and ATLAS trials, I strongly believe that the use of the S-ICD for patients across all ages and with a wide range of indications who don’t require pacing should be significantly broadened in every day clinical practice. S-ICDs are particularly appropriate for young and active patients, and all those patients deemed at higher risk of infections and lead complications should be screened for the S-ICD as first-line therapy. With appropriate patient selection alongside modern device programming, S-ICDs can be chosen with confidence in order to reduce the rate of complications in our patients who are at risk of sudden cardiac death.
Footnotes
Publisher's Disclaimer: CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. 2022 Copyright © Boston Scientific Corporation or its affiliates. All rights reserved. CRM-1381404-AA
Publisher's Disclaimer: CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. 2022 Copyright © Boston Scientific Corporation or its affiliates. All rights reserved. CRM-1319702-AA CE 2797
References
- 1.Koneru JN, Jones PW, Hammill EF et al. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc. 2018;7:e007691. doi: 10.1161/JAHA.117.007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rordorf R, Casula M, Pezza L et al. Subcutaneous versus transvenous implantable defibrillator: an updated metaanalysis. Heart Rhythm. 2021;18:382–91. doi: 10.1016/j.hrthm.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Knops RE, Olde Nordkamp LRA, Delnoy PHM et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526–36. doi: 10.1056/NEJMoa1915932. [DOI] [PubMed] [Google Scholar]
- 4.Lambiase PD, Theuns DA, Murgatroyd F et al. Subcutaneous implantable cardioverter defibrillators: long-term results of the EFFORTLESS study. Eur Heart J. 2022;43:2037–50. doi: 10.1093/eurheartj/ehab921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold MR, Lambiase PD, El-Chami MF et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation. 2021;143:7–17. doi: 10.1161/CIRCULATIONAHA.120.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healey JS, Mondesert BA, Bashir J et al. LB-733-01: Subcutaneous versus transvenous defibrillators HRS late breaking clinical trials: the ATLAS trial. Heart Rhythm. 2022;19:1223–5. doi: 10.1016/j.hrthm.2022.04.018. [DOI] [Google Scholar]
- 7.Knops RE, van der Stuijt W, Delnoy PPHM et al. Efficacy and safety of appropriate shocks and antitachycardia pacing in transvenous and subcutaneous implantable defibrillators: analysis of all appropriate therapy in the PRAETORIAN trial. Circulation. 2022;145:321–9. doi: 10.1161/CIRCULATIONAHA.121.057816. [DOI] [PubMed] [Google Scholar]
- 8.Schuger C, Daubert JP, Brown MW et al. Multicenter automatic defibrillator implantation trial: reduce inappropriate therapy (MADIT-RIT): background, rationale, and clinical protocol. Ann Noninvasive Electrocardiol. 2012;17:176–85. doi: 10.1111/j.1542-474X.2012.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healey JS, Hohnloser SH, Glikson M et al. Cardioverter defibrillator implantation without induction of ventricular fibrillation: A single-blind, non-inferiority, randomised controlled trial (SIMPLE). Lancet. 2015;385:785–91. doi: 10.1016/S0140-6736(14)61903-6. [DOI] [PubMed] [Google Scholar]
- 10.Blatt JA, Poole JE, Johnson GW et al. No benefit from defibrillation threshold testing in the SCD-HeFT (sudden cardiac death in heart failure trial). J Am Coll Cardiol. 2008;52:551–6. doi: 10.1016/j.jacc.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Kutyifa V, Huth Ruwald AC, Aktas MK et al. Clinical impact, safety, and efficacy of single- versus dual-coil ICD leads in MADIT-CRT. J Cardiovasc Electrophysiol. 2013;24:1246–52. doi: 10.1111/jce.12219. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney MO, Wathen MS, Volosin K et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111:2898–905. doi: 10.1161/CIRCULATIONAHA.104.526673. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JGF, Daubert JC, Erdmann E et al. The CARE-HF study (CArdiac REsynchronisation in Heart Failure study): rationale, design and end-points. Eur J Heart Fail. 2001;3:481–9. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ, De Ferrari GM, Sanzo A et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–91. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Savastano S, Dusi V, Baldi E et al. Anatomical-based percutaneous left stellate ganglion block in patients with drug-refractory electrical storm and structural heart disease: a single-centre case series. Europace. 2021;23:581–6. doi: 10.1093/europace/euaa319. [DOI] [PubMed] [Google Scholar]
- 16.Dusi V, Pugliese L, De Ferrari GM et al. Left cardiac sympathetic denervation for long QT syndrome: 50 years' experience provides guidance for management. JACC Clin Electrophysiol. 2022;8:281–94. doi: 10.1016/j.jacep.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DJ, Qin L, Parzynski C et al. Longitudinal outcomes of subcutaneous or transvenous implantable cardioverter-defibrillators in older patients. J Am Coll Cardiol. 2022;79:1050–9. doi: 10.1016/j.jacc.2021.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Gulletta S, Gasperetti A, Schiavone M et al. Age-related differences and associated mid-term outcomes of subcutaneous implantable cardioverter defibrillators: a propensity-matched analysis from a multicenter European registry. Heart Rhythm. 2022;19:1109–15. doi: 10.1016/j.hrthm.2022.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Behar N, Galand V, Martins RP et al. Subcutaneous implantable cardioverter-defibrillator lead extraction. First multicenter French experience. JACC Clin Electrophysiol. 2020;6:863–70. doi: 10.1016/j.jacep.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Viani S, Migliore F, Tola G et al. Use and outcomes of subcutaneous ICD after transvenous ICD extraction: an analysis of current clinical practice and a comparison with transvenous ICD reimplantation. Heart Rhythm. 2019;16:564–71. doi: 10.1016/j.hrthm.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Russo V, Viani S, Migliore F et al. Lead abandonment and subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation in a cohort of patients with ICD lead malfunction. Front Cardiovasc Med. 2021;8:1–8. doi: 10.3389/fcvm.2021.692943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicentini A, Bisignani G, De Vivo SM et al. Patient acceptance of subcutaneous versus transvenous defibrillator systems: a multi-center experience. J Cardiovasc Electrophysiol. 2022;33:81–9. doi: 10.1111/jce.15297. [DOI] [PubMed] [Google Scholar]
- 23.Kuschyk J, Müller-Leisse J, Duncker D et al. Comparison of transvenous vs subcutaneous defibrillator therapy in patients with cardiac arrhythmia syndromes and genetic cardiomyopathies. Int J Cardiol. 2021;323:100–5. doi: 10.1016/j.ijcard.2020.08.089. [DOI] [PubMed] [Google Scholar]
- 24.Lambiase PD, Eckardt L, Theuns DA et al. Evaluation of subcutaneous implantable cardioverter-defibrillator performance in patients with ion channelopathies from the EFFORTLESS cohort and comparison with a metaanalysis of transvenous ICD outcomes. Heart Rhythm. 2020;1:326–35. doi: 10.1016/j.hroo.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conte G, Cattaneo F, de Asmundis C et al. Impact of SMART Pass filter in patients with ajmaline-induced Brugada syndrome and subcutaneous implantable cardioverterdefibrillator eligibility failure: results from a prospective multicentre study. Europace. 2022;24:845–54. doi: 10.1093/europace/euab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migliore F, Viani S, Bongiorni MG et al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: results from an Italian multicenter registry. Int J Cardiol. 2019;280:74–9. doi: 10.1016/j.ijcard.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Corrado DC, Wichter T, Link MS et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–53. doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olde Nordkamp LR, Wilde AA, Tijssen JG et al. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91–100. doi: 10.1161/CIRCEP.112.975268. [DOI] [PubMed] [Google Scholar]
- 29.Link MS, Wang PJ, Haugh CJ et al. Arrhythmogenic right ventricular dysplasia: clinical results with implantable cardioverter defibrillators. J Interv Card Electrophysiol. 1997;1:41–8. doi: 10.1023/A:1009714718034. [DOI] [PubMed] [Google Scholar]
- 30.Jankelson L, Garber L, Sherrid M et al. Subcutaneous versus transvenous implantable defibrillator in patients with hypertrophic cardiomyopathy. Heart Rhythm. 2022;19:759–67. doi: 10.1016/j.hrthm.2022.01.013. [DOI] [PubMed] [Google Scholar]


