Abstract
Background
CYP2D6 subfamily isoenzymes play an important role in the biotransformation of haloperidol, and their activity may influence the efficacy and safety of haloperidol. The use of haloperidol is often associated with the occurrence of adverse drug reactions (ADRs), such as dyskinesia, acute dystonia, and orthostatic hypotension. Previous studies have demonstrated the relationship between the CYP2D6*4 genetic polymorphism and CYP2D6 activity, as well as haloperidol efficacy and safety rates.
Purpose
To evaluate the association of CYP2D6*4 genetic polymorphism with the steady-state concentration of haloperidol in patients with acute alcohol-induced psychotic disorders (AIPDs).
Material and methods
The study involved 100 male patients with AIPD (average age 41.4 ± 14.4 years) who received haloperidol by injections in a dose of 5–10 mg/day. The efficacy profile was assessed using a validated psychometric PANSS scale (Positive and Negative Syndrome Scale). Therapy safety was assessed using the internationally validated UKU (Side-Effect Rating Scale) and SAS (Simpson-Angus Scale for Extrapyramidal Symptoms) scales. Genotyping was performed with the real-time polymerase chain reaction.
Results
We revealed the statistically significant results in terms of therapy safety evaluation (dynamics of the UKU scores: (GG) 8.00 [7.00; 10.00], (GA) 15.0 [9.25; 18.0], p < 0.001; dynamics of the SAS scores: (GG) 11.0 [9.0; 14.0], (GA) 14.50 [12.0; 18.0], p < 0.001. Pharmacokinetic study showed a statistically significant difference across the groups with different genotypes: (GG) 3.13 [2.32; 3.95], (GA) 3.89 [2.92; 5.26], p = 0.010.
Conclusion
It can be concluded that patients with the GA genotype have a higher risk of ADRs compared to patients who carry the GG genotype. It was shown that CYP2D6*4 genetic polymorphism has a statistically significant effect on the steady-state concentration of haloperidol.
Keywords: CYP2D6, pharmacogenetics, personalized medicine, alcohol-induced psychotic disorders, haloperidol
Introduction
Acute alcoholic hallucinoses, or alcohol-induced psychotic disorders (AIPDs) rank second in prevalence rates among alcohol-related psychotic disorders requiring emergency medical care.1 According to a study by Egorov, incidence rate ratio of delirium tremens to alcoholic hallucinosis is 2.27:1 for the group of patients with a family history of alcohol use disorders (AUDs) and 4.67:1 for the group with no history of AUDs.2 Available data indicate that the incidence of AIPD ranges from 5.6% to 22.8%.3,4 Prevalence rates of different AIPD variants vary: in a study by Nemkova, it was revealed that the most frequent AIPD variant is abortive (51%), followed by the typical AIPD (31%); AIPD with the elements of syndrome of the psychic automatism (5%); AIPD with predominant visual hallucinations (4%); AIPD with predominant delusional disorders (3%); and AIPD with predominant depression (1%).5
Treatment of patients with acute alcoholic hallucinosis requires obligatory prescription of neuroleptics with pronounced antipsychotic effect, for instance, haloperidol.6,7 Haloperidol belongs to the group of typical antipsychotic drugs and possesses an antidopaminergic effect through the pronounced actions in the dopamine D2 receptors. Haloperidol blocks the D2 receptors and therefore relieves positive psychopathological symptoms.8
It is suggested that antidopaminergic activity in the dorsolateral striatum may contribute to extrapyramidal adverse drug reactions (ADRs) associated with the action of typical antipsychotic drugs, including haloperidol.9 Extra-pyramidal ADRs such as acute dystonia, akathisia, malignant neuroleptic syndrome, parkinsonism, and tardive dyskinesia are common when using haloperidol. Haloperidol also shows noradrenergic, cholinergic, and histaminergic blocking actions which are associated with the occurrence of various ADRs.10
Haloperidol undergoes biotransformation in the liver with the participation of cytochrome P450 family enzymes.11,12 The main isoenzyme involved in haloperidol metabolism is CYP2D6 which is encoded by the gene of the same name.13 The CYP2D6 gene is located on chromosome 22 (22q13.1) and is translated into the CYP2D6 protein that is localized in the endoplasmic reticulum and expressed in the liver, brain, intestinal tissue and lymphoid cells.14 It is important to note that CYP2D6 accounts for only 2–4% of all cytochromes in the liver;15 it is the main drug-metabolizing enzyme involved in the metabolism of approximately 20% of commonly used medications.16
One of the most studied CYP2D6 genetic polymorphisms is CYP2D6*4 (rs3892097), which leads to CYP2D6 isoenzyme deactivation, resulting in the reduced metabolism of substrate drugs,17 including haloperidol.18
Categorization of CYP2D6 metabolizer status is based on the evaluation of the enzymatic activity, which allows to determine the type of metabolizer on the basis of genetic data.19 Thus, there are five categories of metabolizers: poor metabolizers (PM), intermediate metabolizers (IM), normal, or extensive metabolizers (EM), rapid metabolizers (RM), and ultrarapid metabolizers (UM).20 Current pharmacogenetic guidelines use these categories to provide the clinicians with recommendations for specific drugs to adjust doses or switch medications that are expected to cause ADRs in patients with a particular CYP2D6 metabolizer status.21
The results of the studies conducted to date show that the risk of ADRs during the administration of antipsychotic drugs, including haloperidol, depends on both clinical factors and individual characteristics of pharmacodynamics and pharmacokinetics of drugs.11
The study aimed to evaluate the association of CYP2D6*4 genetic polymorphism with the steady-state concentration of haloperidol in patients with AIPDs.
Material and Methods
Present study involved 100 male patients (average age—41.40 ± 14.40 years) who were hospitalized to Moscow Research and Practical Centre on Addictions due to the diagnosis of AIPD with predominant hallucinations (F10.52, according to ICD-10). Haloperidol in injections at a dose of 5–10 mg/day was prescribed to this cohort of patients for 5 days for the treatment of acute hallucinatory symptoms. Haloperidol was administered upon the admission of a patient to the emergency department. In addition to haloperidol, all patients received minimal standard therapy for 5 days, which included infusions and ion-containing solutions, as well as vitamins (see Table 1). Prescriptions were made in accordance with the national clinical guidelines for the therapy of AIPD.
Table 1. Minimal Standard Therapy.
| Medications | Average daily dose | |
| Sodium chloride solution 0.9% + potassium chloride 10% + magnesium sulfate 25% | 800 mL | |
| Thiamine hydrochloride solution 5% | 100 mg | |
| Pyridoxine hydrochloride solution 5% | 100 mg |
The inclusion criterion was the diagnosis of AIPD with predominant hallucinations (F10.52, according to ICD-10). Exclusion criteria were creatinine clearance values <50 mL/min, creatinine concentration in plasma >1.5 mg/dL (133 mmol/L), bodyweight less than 60 kg or greater than 100 kg, age of 75 years or more, presence of any other psychotropic medications in the treatment regimen, presence of chronic psychotic disorders, and presence of any contraindications for haloperidol use.
Each patient signed an informed consent to voluntarily participate in the study. The study was approved by the local ethical committee of the Russian Medical Academy of Continuing Professional Education of the Ministry of Health of Russia (Protocol No. 14 of October 27, 2020).
For genotyping, venous blood samples were collected into VACUETTE® (Greiner Bio-One, Austria) vacuum tubes on day 6 of haloperidol therapy. The single nucleotide polymorphism (SNP) rs3892097 (CYP2D6*4) was analyzed by real-time PCR using “Dtlite” DNA amplifiers (DNA Technology, Moscow, Russia) on a CFX96 Touch Real-Time System with CFX Manager software (Bio-Rad Laboratories Inc., Hercules, CA, USA) and the “SNP-screen” sets (Syntol, Moscow, Russia). In every set, two allele-specific hybridizations were used, which allowed simultaneous determination of both alleles of the respective SNP using two fluorescence channels.
To assess haloperidol efficacy, the Positive and Negative Syndrome Scale (PANSS) was used.22 The safety profile was evaluated using the UKU23 and SAS24 scales. Patients were examined on days 1 and 6 of haloperidol therapy.
Statistical analysis was performed in Statsoft Statistica v. 10.0 (Dell Statistica, Tulsa, OK, USA). The normality of sample distribution was evaluated using the Shapiro-Wilk test and was taken into account for selecting parametric or non-parametric tests. The differences were considered statistically significant at p < 0.05 (power above 80%). Two samples of continuous independent data were compared using the Mann-Whitney U-tests with further correction of the obtained p-value using the Benjamin-Hochberg test, due to the multiple comparison procedure. Research data are presented in the form of the median and interquartile range (Me [Q1; Q3]).
Results
The CYP2D6 genotyping performed on 100 subjects revealed the following results. In total, 70 out of 100 patients did not carry the CYP2D6*4 allele, whereas 30 patients were heterozygous for the respective variant.
Further study included a comparison of the therapy efficacy and safety rates in major allele carriers (main group) and minor allele carriers (comparison group).
The results of data analysis performed for psychometric assessments (PANSS) and side-effect rating scales (UKU and SAS) on days 1 and 6 in patients who received haloperidol are presented in Table 2.
Table 2. Data from the Psychometric Assessments and Side-Effect Rating Scales in Patients who Received Haloperidol, on Days 1 and 6 of the Study.
| Scale | GG (N = 70) | GA (N = 30) | P* | |||
| Day 1 | ||||||
| PANSS | 14.50 [13.00; 18.00] | 16.00 [15.00; 18.00] | 0.017 | |||
| SAS | 0 [0; 0] | 0 [0; 0] | > 0.999 | |||
| UKU | 0 [0; 0] | 0 [0; 0] | > 0.999 | |||
| Day 6 | ||||||
| PANSS | 1.00 [1.00; 2.00] | 2.00 [1.00; 2.75] | 0.006 | |||
| SAS | 11.00 [9.00; 14.00] | 14.50 [12.00; 18.00] | < 0.001 | |||
| UKU | 8.00 [7.00; 10.00] | 15.00 [9.25; 18.00] | < 0.001 |
Note: p* – p-value obtained in Benjamini–Hochberg multiple testing correction (based on the results of Mann–Whitney U test).
Then we compared the dynamics of changes in positive PANSS scale scores in patients with different genotypes (Figure 1). Statistical analysis of the clinical efficacy profile data obtained for the patients with different CYP2D6 genotypes revealed no statistically significant differences: (GG) −13.00 [−16.00; −11.00], (GA) −15.00 [−16.75; −13.00], p = 0.078.
Figure 1.
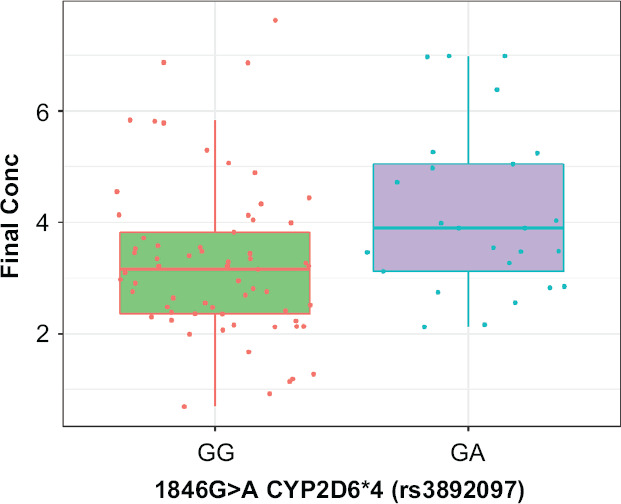
Effect of CYP2D6*4 Genetic Polymorphism on Haloperidol Steady-State Concentration Levels
Table 3 shows the dynamics of changes in SAS and UKU scale scores in patients carrying different genotypes. Statistical analysis of haloperidol safety profile data obtained using the SAS and UKU scale scores in patients with different genotypes showed statistically significant differences.
Table 3. Changes in SAS and UKU Scores From Day 1 to Day 6 in Patients with Different Genotypes.
| Scale | GG (N = 70) | GA (N = 30) | p | |||
| SAS | 11.00 [9.00; 14.00]. | 14.50 [12.00; 18.00]. | p < 0.001 | |||
| UKU | 8.00 [7.00; 10.00]. | 8.00 [7.00; 10.00] | p < 0.001 |
Table 4 shows the results of the pharmacokinetic study in patients with GG and GA genotypes.
Table 4. Haloperidol Steady-State Concentration Values in Patients with Different Genotypes.
| GG | GA | p | ||
| 3.13 [2.32; 3.95] | 3.89 [2.92; 5.26] | 0.010 |
The CYP2D6*4 genetic polymorphism has been shown to have a statistically significant effect on the equilibrium concentration of haloperidol when administered to patients with AIPD (Figure 1).
Therefore, the study revealed no statistically significant differences in the efficacy of haloperidol therapy in patients with acute AIPDs carrying different CYP2D6*4 genotypes. Meanwhile, a statistically significant difference in safety profile (as assessed by the UKU and SAS scales) was found. The dynamics were more obvious in the group of patients with the GA genotype compared with the carriers of the GG genotype. There was a statistically significant difference between the carriers of the GG and GA genotypes in haloperidol steady-state concentration levels, confirming the impact of the CYP2D6*4 genetic polymorphism on haloperidol concentrations in patients with AIPD.
The results of the study will enable clinicians to optimise the dosing regimen of haloperidol in patients with acute AIPD to reduce the risk of dose-dependent ADRs.
Discussion
Our findings are consistent with the results of our previous study that focused on pharmacogenetic aspects only.25 The results of the present study suggest that CYP2D6*4 genotype may be a potentially important predictor of haloperidol efficacy and safety in patients with acute AIPD.
Haloperidol starting dose should be decreased by 25% in carriers of the GA genotype, whereas homozygous GG carriers should be prescribed haloperidol at a standard therapeutic dose. Current DPWG guideline on haloperidol pharmacogenetics recommend a 50% reduction of the starting dose for mutant homozygotes.26 In our study, a worsening of the safety profile in heterozygous carriers was observed; therefore, an adjustment of the starting dose of haloperidol is needed. However, it is likely that dose correction should be not as pronounced as in the existing recommendations for homozygous patients.
This study has several important limitations: all patients were males; only one genetic polymorphism was included in the study; there were no homozygous carriers of the minor allele revealed.
A strength of the study is that patients had no mental or acute somatic comorbidities and received haloperidol monotherapy, which allows eliminating the effect of other medications on the efficacy of treatment.
Conclusion
Thus, in a study on a group of 100 patients with acute AIPD, an association between the CYP2D6*4 polymorphism and the safety profile of haloperidol was demonstrated. A statistically significant difference between the carriers of the GG and GA genotypes was revealed in haloperidol steady-state concentration levels, confirming the impact of the CYP2D6*4 genetic polymorphism on haloperidol concentrations in patients with AIPD.
Footnotes
Funding Information
This work was supported by the grant of the Russian Science Foundation (project No. 22-15-00190, https://rscf.ru/project/22-15-00190/).
Contributor Information
AA Parkhomenko, Parkhomenko, postgraduate student of addiction psychiatry department..
MS Zastrozhin, Zastrozhin, MD, PhD, associate professor of addiction psychiatry department, head of laboratory of genetics and fundamental studies, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation; Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia; University of California, San Francisco, San Francisco, CA, USA..
SA Pozdnyakov, Pozdnyakov, researcher of the laboratory of genetics and fundamental studies..
VV Noskov, Noskov, laboratory assistants, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
IA Zaytsev, Zaytsev, laboratory assistants, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
VA Ivanchenko, Ivanchenko, laboratory assistants, Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
NP Denisenko, Denisenko, PhD in Medicine, Head of the Department of Personalized Medicine..
KA Akmalova, Akmalova, Researcher in the Department of Molecular Medicine..
VYu Skryabin, Skryabin, MD, PhD, associate professor of addiction psychiatry department, head of clinical department..
EA Bryun, Bryun, MD, PhD, professor, head of addiction psychiatry department, president, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation; Moscow Research and Practical Centre on Addictions of the Moscow Department of Healthcare, Moscow, Russia..
DA Sychev, Sychev, corresponding member of the Academy of Sciences of Russia, MD, PhD, professor, rector, head of clinical pharmacology and therapy department, Russian Medical Academy of Continuous Professional Education of the Ministry of Health of the Russian Federation, Moscow, Russian Federation..
References
- 1.Masood B, Lepping P, Romanov D, Poole R. Treatment of Alcohol-Induced Psychotic Disorder (Alcoholic Hallucinosis)-A Systematic Review. Alcohol Alcohol . 2018;53(3):259–267. doi: 10.1093/alcalc/agx090. doi: [DOI] [PubMed] [Google Scholar]
- 2.Egorov AY, Aleksin DS, Petrova NN. Specific features of alcoholic psychosis in psychiatric practice. Medicine . 2012;1:29–40. Vestnik of Saint Petersburg University. [Google Scholar]
- 3.Kuzminov VN. Some aspects of the pathogenesis, clinic and treatment of delirium tremens. International Medical Journal . 2002;8(1–2):75–78. [Google Scholar]
- 4.Mostovoy SM. Dynamics of prevalence of alcoholism and alcohol-related psychoses in the Far East. Russian Journal of Psychiatry . 2002;4:45–48. [Google Scholar]
- 5.Nemkova TI, Gofman AG. Changes in the clinical picture of acute alcoholic hallucinosis over the past 50 years. Social and clinical psychiatry . 2018;28(2):25–29. [Google Scholar]
- 6.Gofman AG. Medical Information Agency . Moscow: 2019. Alcoholic hallucinosis; p. 464. [Google Scholar]
- 7.Sivolap YP, Savchenkov VA. Medicine . Moscow: 2001. Pharmacotherapy in narcology; p. 352. [Google Scholar]
- 8.Fan L, Tan L, Chen Z, Qi J, Nie F, Luo Z, Cheng J, Wang S. Haloperidol bound D2 dopamine receptor structure inspired the discovery of subtype selective ligands. Nat Commun . 2020;11(1):1074. doi: 10.1038/s41467-020-14884-y. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno Y, Kunisawa N, Shimizu S. Antipsychotic treatment of behavioral and psychological symptoms of dementia (BPSD): Management of extrapyramidal side effects. Front Pharmacol . 2019;10:1045. doi: 10.3389/fphar.2019.01045. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman S, Marwaha R. Treasure Island (FL): StatPearls Publishing; 2021. Haloperidol. StatPearls [Internet] [PubMed] [Google Scholar]
- 11.Nasyrova RF, Ivanov MV, Neznanov NG. St. Petersburg: Publishing center of the St. Petersburg V.M. Bekhterev Research Institute; 2015. Introduction to psychopharmacogenetics; p. 272. [Google Scholar]
- 12.Eum S, Lee AM, Bishop JR. Pharmacogenetic tests for antipsychotic medications: clinical implications and considerations. Dialog Clin Neurosci . 2016;18(3):323–337. doi: 10.31887/DCNS.2016.18.3/jbishop. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spina E, De Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm . 2015;122(1):5–28. doi: 10.1007/s00702-014-1300-5. doi: [DOI] [PubMed] [Google Scholar]
- 14.Gopisankar MG. CYP2D6 pharmacogenomics. Egypt J Med Hum Genet . 2017;18:309–313. doi: 10.1016/j.ejmhg.2017.03.001. doi: [DOI] [Google Scholar]
- 15.Williams IS, Gatchie L, Bharate SB, Chaudhuri B. Biotransformation, using recombinant CYP450-expressing baker’s yeast cells, identifies a novel cyp2d6.10 a122v variant which is a superior metabolizer of codeine to morphine than the wild-type enzyme. ACS Omega . 2018;3:8903–8912. doi: 10.1021/acsomega.8b00809. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis JP, Peter AP, Shaman JA. Consequences of CYP2D6 copy-number variation for pharmacogenomics in psychiatry. Front Psychiatry . 2019;10:1–14. doi: 10.3389/fpsyt.2019.00432. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanger U, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Arch Pharmacol . 2004;369(1):23–37. doi: 10.1007/s00210-003-0832-2. Naunyn Schmiedebergs. doi: [DOI] [PubMed] [Google Scholar]
- 18.Fursa OO, Kozlovsky VL. Pharmacogenetic features of cytochrome P450 system activity in metabolism of antipsychotic drugs. Journal of Neurology and Psychiatry . 2014;4:111–122. [Google Scholar]
- 19.Nofziger C, Turner AJ, Sangkuhl K, Whirl-Carrillo M, Agúndez JAG, Black JL, Dunnenberger HM, Ruano G, Kennedy MA, Phillips MS, Hachad H, Klein TE, Gaedigk A. PharmVar GeneFocus: CYP2D6. Clin Pharmacol Ther . 2020;107(1):154–170. doi: 10.1002/cpt.1643. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TE, Relling MV, Hoffman JM. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med . 2017;19(2):215–223. doi: 10.1038/gim.2016.87. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. A review of the important role of CYP2D6 in pharmacogenomics. Genes . 2020;11(11):1295. doi: 10.3390/genes11111295. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawanishi C, Furuno T, Kishida I, Matsumura T, Kosaka K. A patient with treatment-resistant schizophrenia and cytochrome P4502D6 gene duplication. Clin Genet . 2002;61(2):152–154. doi: 10.1034/j.1399-0004.2002.610211. doi: [DOI] [PubMed] [Google Scholar]
- 23.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. Acta Psychiatr Scand Suppl . 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. doi: [DOI] [PubMed] [Google Scholar]
- 24.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand . 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. doi: [DOI] [PubMed] [Google Scholar]
- 25.Parkhomenko AA, Zastrozhin MS, Skryabin V, Ivanchenko VA, Pozdniakov SA, Noskov VV, Zaytsev IA, Denisenko NP, Akmalova KA, Bryun EA, Sychev DA. Correlation of 1846G>A Polymorphism of CYP2D6 Gene with Haloperidol Efficacy and Safety in Patients with Alcoholic Hallucinoses. Psychopharmacol Bull . 2022 Jun 27;52(3):58–67. [PMC free article] [PubMed] [Google Scholar]
- 26.Annotation of DPWG Guideline for haloperidol and CYP2D6. Available at https://api.pharmgkb.org/v1/download/file/attachment/DPWG_May_2021.pdf. [Google Scholar]


