Abstract
Mucosal immunization strategies are actively being pursued in the hopes of improving the efficacy of vaccines against the influenza virus. Our group investigated the oral immunization of mice via intragastric gavage with influenza hemagglutinin (HA) combined with mutant Escherichia coli heat-labile enterotoxins K63 (LT-K63) and R72 (LT-R72). These oral immunizations resulted in potent serum antibody and HA inhibition titers, in some cases stronger than those obtained with traditional intramuscular administration, in addition to HA-specific immunoglobulin A in the saliva and nasal secretions. This study demonstrates that it may be possible to develop effective oral influenza vaccines.
Influenza is a serious human disease exhibiting high mortality in vulnerable populations such as the very young and the very old, as well as causing significant morbidity in the general population (17). The social and economic costs associated with yearly influenza outbreaks are high (7). Formalin-inactivated whole-virus and split-virus vaccines administered intramuscularly (i.m.) are commercially available to control the spread and severity of influenza (15, 38). These prophylactic vaccines, although important agents in controlling influenza, suffer from a number of shortcomings that limit their efficacy and acceptability. Notably, inactivated whole-virus and split-virus vaccines are known to activate CD8+ cytotoxic T-lymphocyte responses only sporadically, have poor cross-reactivity to antigenic variants, and produce poor secretory immunoglobulin A (IgA) responses (4, 7, 17, 24, 34, 36). In addition, injection site reactogenicity and weak immune responses can be a problem in very young children (18, 19). Significant efforts are currently being pursued to improve the vaccines' efficacy and tolerability primarily through the development of mucosally active influenza vaccines (2, 7, 10, 33, 40). Oral immunization is considered by many to be a highly desirable form of vaccination, although numerous obstacles make oral immunization using subunit antigens a significant challenge (3, 6, 11). Many approaches have been investigated to develop viable orally active influenza vaccines (3, 21, 29, 30). Mucosal adjuvants, primarily Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT), are the most commonly employed vaccine enhancers (11, 12). Although potent mucosal adjuvants, LT and CT are toxic in humans at doses useful for adjuvanticity due to their ADP-ribosyltransferase activity (28). The nontoxic B subunit of CT (CTB) has also been investigated; however, studies have indicated that small amounts of the whole CT are required for sufficient adjuvant potency, inhibiting the potential of CTB in humans (44, 45, 46). Our group has investigated the mutant LT toxins LT-K63 and LT-R72, which demonstrate extremely low (LT-R72) to undetectable (LT-K63) levels of ADP-ribosyltransferase activity yet maintain potent mucosal adjuvant activity, demonstrating that ADP-ribosyltransferase activity may not be linked to the adjuvant activity (2, 13, 16). In this study, the influenza hemagglutinin (HA) antigens A/Beijing8-9/93 HA and A/Johannesburg/97 HA were administered orally in mice with LT-K63 and LT-R72 and the results were compared to those obtained with i.m. immunization for induction of serum antibody and mucosal IgA responses as well as serum HA inhibition titers. Dosing studies were conducted to determine the optimum dose levels of both antigen and adjuvant.
Vaccines used.
Purified monovalent A/Beijing8-9/93 (H3N2) and A/Johannesburg/97 (H1N1) split-virus influenza antigens were provided by Chiron Vaccines, Siena, Italy. Dosing was based on HA content as assayed by single radial immunodiffusion as described previously (25). LT-K63 and LT-R72 were prepared as described previously (35). Wild-type LT (wtLT) was obtained from Sigma (Escherichia coli heat-labile enterotoxin, lyophilized powder; Sigma-Aldrich, St. Louis, Mo.). All immunogen preparations were formulated in phosphate-buffered saline. Immunogens prepared for intragastric gavage (i.g.) administration included 1.5% (wt/vol) sodium bicarbonate.
Immunization and sample collection.
Groups of 10 female BALB/c mice (Charles River Labs, Wilmington, Mass.), 6 to 10 weeks old, were i.m. or i.g. immunized at days 0, 21, and 35 using immunogen preparations as described below. Mice were fasted 12 h prior to each immunization to minimize the possibility of lectins (or other agents) in the feed from inhibiting uptake of the orally delivered immunogens (9). Immunizations were made either by i.m. injection (50 μl) into the posterior thigh muscle or by direct i.g. (200 μl) into the stomach using a 20-gauge stainless steel feeding needle attached to a 1-ml syringe. Animals were not anesthetized during immunizations. Serum, saliva wash (SW), and nasal wash (NW) samples were collected from individual animals 2 weeks after the final immunization (day 49) using methods described previously (47).
Antibody ELISA.
Serum samples from individual animals were assayed for total anti-HA Ig (IgG plus IgA plus IgM) titers by a 3,3′,5,5′-tetramethylbenzidine-based colorimetric enzyme-linked immunosorbent assay (ELISA) as previously described, with A/Beijing8-9/93 or A/Johannesburg/97 as appropriate as coating antigen (20). A490 was measured using a standard ELISA reader. The titers represent reciprocal serum dilutions giving an A490 of 0.5 and were normalized to a serum standard assayed in parallel. SW and NW samples from individual animals were assayed for HA-specific IgA titers using a bioluminescence immunosorbent assay as previously described, with A/Beijing8-9/93 or A/Johannesburg/97 as appropriate as coating antigen (47). The goat anti-mouse IgA biotin conjugate (EY Labs, San Mateo, Calif.) used was presaturated with purified mouse IgG (Sigma Chemical Company, St. Louis, Mo.) to reduce cross-reactivity. Quantitation was based on the number of relative light units representing total luminescence integrated over 3 s (arbitrary units). Titers represent log dilution values linearly extrapolated from the log of the relative light units to a cutoff value at least 2 standard deviations above mean background.
HI assay.
Serum samples pooled by group were assayed for hemagglutination inhibition (HI) titer by the Viral and Rickettsial Disease Laboratory (Department of Health Services, Berkeley, Calif.). The HI assay is based on the ability of sample sera to inhibit the agglutination of goat erythrocytes in the presence of HA antigen. The resulting titers are expressed as the reciprocal dilution required for complete inhibition (22, 23).
Statistics.
Log anti-A/Beijing8-9/93 and anti-A/Johannesburg/97 HA serum Ig, saliva IgA, and nasal IgA titers from individual animals were analyzed for differences between test groups using a Fisher least-significant-difference procedure using a statistical significance of >5% (P ≤ 0.05) as the cutoff interval (1). Additionally, the resulting data were graphically represented as mean titers ± standard errors (SE) in the usual manner.
Effects of enterotoxin types and doses on antibody responses after i.g. immunization.
A dose-ranging study was conducted to determine the dose-response relationship for LT-K63 and LT-R72 for i.g. immunization with A/Beijing8-9/93 HA. Groups of 10 mice were immunized by the i.g. route with 20 μg of A/Beijing8-9/93 HA in combination with three dose levels of wtLT (1, 10, and 25 μg), LT-K63 (1, 10, and 100 μg), and LT-R72 (1, 10, and 100 μg). Significant mortality in the mice subjected to high doses of wtLT limited the upper dose of wtLT to 25 μg. An unadjuvanted A/Beijing8-9/93 HA control group (HA only) was immunized by the i.g. route at the highest dose level (20 μg) for comparison purposes.
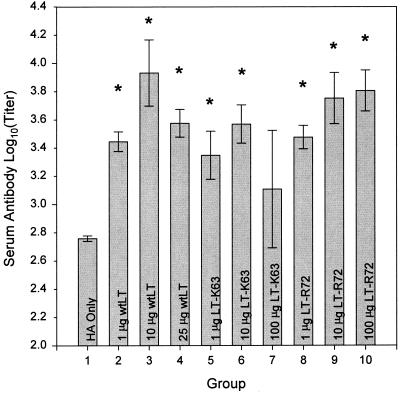
Serum antibody responses (Fig. 1) were significantly higher in most cases in animals that received A/Beijing8-9/93 HA in combination with either of the enterotoxins tested than in animals that received unadjuvanted A/Beijing8-9/93 HA. A dose response was not clearly demonstrated, although, in general, groups that received A/Beijing8-9/93 HA in combination with LT-R72 showed serum antibody responses comparable to those of groups receiving A/Beijing8-9/93 HA in combination with wtLT.
FIG. 1.
Comparison of the effects of enterotoxin doses on antigen-specific serum antibody responses after i.g. administration. Shown are means ± SE of anti-A/Beijing8-9/93 HA antibody titers in the sera of mice immunized with 20-μg doses of A/Beijing8-9/93 HA antigen either alone (HA only) or in combination with wtLT, LT-K63, and LT-R72 as indicated. Asterisks indicate groups whose values are significantly greater than that of the HA only group (P ≤ 0.05).
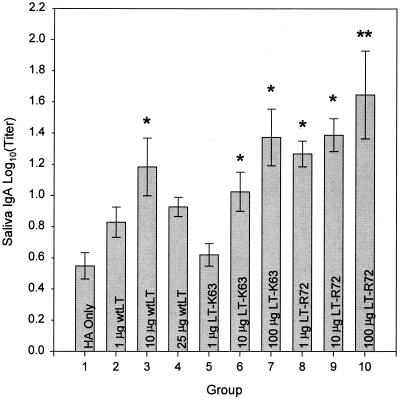
A clearer adjuvant dose response was found in the antigen-specific saliva IgA responses (Fig. 2). Significantly stronger saliva IgA responses were demonstrated for all but one of the LT-K63- and LT-R72-adjuvanted groups than for animals that received A/Beijing8-9/93 HA alone. Additionally, animals dosed i.g. with 20 μg of A/Beijing8-9/93 HA in combination with 100 μg of LT-R72 were found to have an antigen-specific saliva IgA response significantly higher (P ≤ 0.05) than that of animals dosed i.g. with either 10 or 25 μg of wtLT.
FIG. 2.
Comparison of the effects of enterotoxin doses on antigen-specific SW IgA responses after i.g. administration. Shown are means ± SE of anti-A/Beijing8-9/93 HA SW IgA antibody titers of groups of mice immunized with 20-μg doses of A/Beijing8-9/93 HA antigen either alone (HA only) or in combination with enterotoxins as indicated. Asterisks indicate groups whose values are significantly greater than that of the HA only group (P ≤ 0.05). Double asterisks indicate a value that is significantly greater than that of the groups immunized with 10 and 25 μg of wtLT (groups 3 and 4), in addition to that of the HA only group (P ≤ 0.05).
Effects of A/Beijing8-9/93 HA dose on antibody responses at two dose levels of LT-R72.
A second dose-ranging study was conducted to determine the optimum dose of A/Beijing8-9/93 HA for i.g. immunization when adjuvanted with LT-R72. Groups of 10 mice were immunized by the i.g. route with three dose levels of A/Beijing8-9/93 HA (1, 5, and 20 μg) in combination with either 10 or 100 μg of LT-R72. An unadjuvanted A/Beijing8-9/93 HA control group (HA only) was immunized at the highest dose level (20 μg) for comparison purposes.
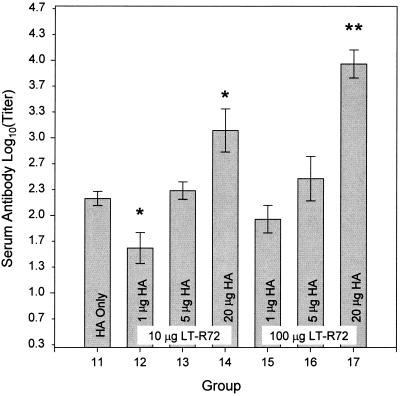
The antigen-specific serum antibody responses (Fig. 3) demonstrated a dose-response trend with respect to the dose level of A/Beijing8-9/93 and the dose level of LT-R72 with which the animals were immunized. Serum antibody responses were significantly higher (P ≤ 0.05) in animals immunized i.g. with 20-μg doses of A/Beijing8-9/93 HA in combination with either 10 or 100 μg of LT-R72 than in the unadjuvanted HA control group. The group that received the highest dose level tested (20 μg of A/Beijing8-9/93 in combination with 100 μg of LT-R72) had a significantly higher (P ≤ 0.05) antigen-specific serum antibody response than those of all other groups tested.
FIG. 3.
Comparison of the effects of HA doses on antigen-specific serum antibody responses after i.g. administration. Shown are means ± SE of anti-A/Beijing8-9/93 HA antibody titers in the sera of mice immunized either with 20 μg of HA administered alone (HA only) or with 1-, 5-, or 20-μg doses of A/Beijing8-9/93 HA in combination with either 10 or 100 μg of LT-R72 as indicated. Asterisks indicate groups whose values are significantly different from that of the HA only group (P ≤ 0.05). Double asterisks indicate a value that is significantly greater than that of all other groups shown (P ≤ 0.05).
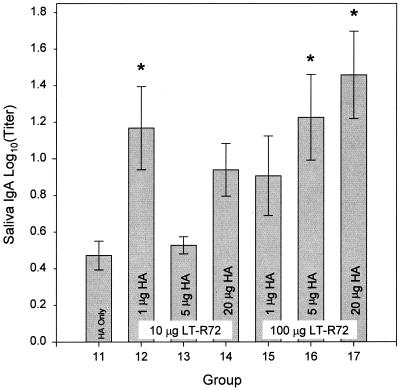
The antigen-specific saliva IgA responses (Fig. 4) matched the trend seen with the serum antibody responses with the exception of the group that received 1 μg of A/Beijing8-9/93 in combination with 10 μg of LT-R72 (group 12). Animals that received either 5 or 20 μg of A/Beijing8-9/93 HA in combination with 100 μg of LT-R72 demonstrated a significantly higher (P ≤ 0.05) antigen-specific saliva IgA response than that of animals that received unadjuvanted A/Beijing8-9/93 HA.
FIG. 4.
Comparison of the effects of HA doses on antigen-specific SW IgA responses after i.g. administration. Shown are means ± SE of anti-A/Beijing8-9/93 HA SW IgA antibody titers of groups of mice immunized either with 20 μg of HA administered alone (HA only) or with 1-, 5-, or 20-μg doses of A/Beijing8-9/93 HA in combination with either 10 or 100 μg of LT-R72 as indicated. Asterisks indicate groups whose values are significantly greater than that of the HA only group (P ≤ 0.05).
Comparison of i.g. and i.m. immunizations.
The serum antibody responses of mice i.g. immunized with A/Johannesburg/97 HA either alone or in combination with an LT were compared to those of mice immunized with A/Johannesburg/97 HA by the i.m. route. Groups of 10 mice were immunized by the i.g. route with 20 μg of A/Johannesburg/97 HA either alone or in combination with two dose levels of wtLT (1 and 10 μg), LT-K63 (10 and 100 μg), or LT-R72 (10 and 100 μg). A group receiving 1 μg of A/Johannesburg/97 HA by the i.m. route was included. The 1-μg HA i.m. dose level was chosen such that, based on data from previous experiments, a strong, protective, immunogenic response in mice would result (data not shown), and it was used here in order to make comparisons with the i.g. responses.
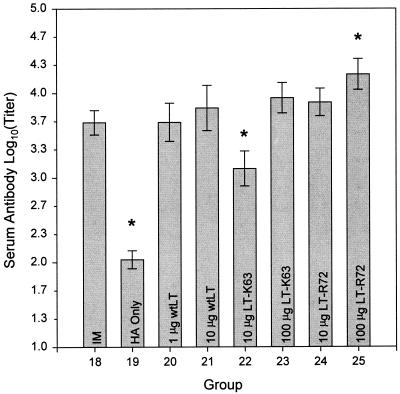
Serum antigen-specific antibody responses (Fig. 5) for mice immunized i.g. with 20 μg of A/Johannesburg/97 HA adjuvanted with an LT were equivalent to or higher than those for i.m. immunized mice. Mice i.g. immunized with 20 μg of A/Johannesburg/97 HA either unadjuvanted (HA only) or in combination with 10 μg of LT-K63 showed serum antigen-specific antibody responses significantly lower (P ≤ 0.05) than those of mice immunized by the i.m. route; nevertheless, i.g. immunization in the presence of 10 μg of LT-K63 resulted in antibody responses 1 log higher than those obtained with i.g. immunization with unadjuvanted A/Johannesburg/97 HA. Mice i.g. immunized with 20 μg of A/Johannesburg/97 HA in combination with 100 μg of LT-R72 showed serum antigen-specific antibody responses that were significantly (P ≤ 0.05) higher than those found with i.m. immunization.
FIG. 5.
Comparison of the effects of i.m. and i.g. administrations of A/Johannesburg/97 HA on antigen-specific serum antibody responses. Shown are means ± SE of anti-A/Johannesburg/97 HA antibody titers in the sera of mice immunized i.m. with 1 μg of A/Johannesburg/97 HA unadjuvanted (IM) or immunized i.g. with 20-μg doses of A/Johannesburg/97 HA alone (HA Only) and in combination with either 1 or 10 μg of wtLT, 10 or 100 μg of LT-K63, or 10 or 100 μg of LT-R72 as indicated. Asterisks indicate groups whose values are significantly different from that of the i.m. immunized group (P ≤ 0.05).
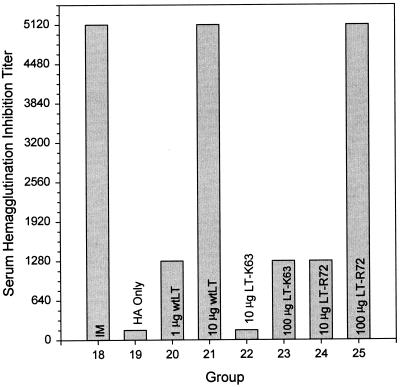
Serum HI titers (Fig. 6) for mice i.g. immunized with 20 μg of A/Johannesburg/97 HA in combination with either 10 μg of wtLT or 100 μg of LT-R72 were comparable in potency to those for i.m. immunized mice. Mice that were i.g. immunized with 20 μg of A/Johannesburg/97 HA in combination with either 1 μg of wtLT, 10 μg of LT-R72, or 100 μg of LT-K63 showed modest HI titer levels. Significant HI titers were not demonstrated for mice i.g. immunized with 20 μg of A/Johannesburg/97 HA either alone or in combination with 10 μg of LT-K63.
FIG. 6.
Comparison of the effects of i.m. and i.g. administrations of A/Johannesburg/97 HA on serum HI titers. The data shown are for pooled sera from groups of mice immunized i.m. with 1 μg of A/Johannesburg/97 HA unadjuvanted (IM), or immunized i.g. with 20-μg doses of A/Johannesburg/97 HA alone (HA Only), and in combination with either 1 or 10 μg of wtLT, 10 or 100 μg of LT-K63, or 10 or 100 μg of LT-R72 as indicated.
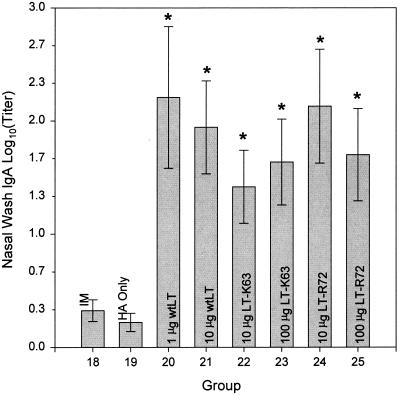
Antigen-specific NW IgA responses (Fig. 7) were found to be significant only in those mice immunized i.g. with 20 μg of A/Johannesburg/97 HA in combination with either wtLT, LT-K63, or LT-R72. Mice receiving i.m. immunization or immunized i.g. with 20 μg of A/Johannesburg/97 HA alone did not show significant antigen-specific NW IgA responses. No significant differences were seen in the antigen-specific IgA responses as a consequence of enterotoxin dose level or type.
FIG. 7.
Comparison of the effects of i.m. and i.g. administrations of A/Johannesburg/97 HA on antigen-specific NW IgA antibody responses. Shown are means ± SE of anti-A/Johannesburg/97 HA NW IgA antibody titers of mice immunized i.m. with 1 μg of A/Johannesburg/97 HA unadjuvanted (IM) or immunized i.g. with 20-μg doses of A/Johannesburg/97 HA alone (HA Only) and in combination with either 1 or 10 μg of wtLT, 10 or 100 μg of LT-K63, or 10 or 100 μg of LT-R72 as indicated. Asterisks indicate groups whose values are significantly greater than that of the i.m. immunized group (P ≤ 0.05).
Current commercial influenza vaccines are shown to induce in healthy adult humans serum antibody responses that are protective against viral challenge, but this protective immunity tends to be variable in potency and is relatively short lived, particularly in the elderly and infant populations (7, 15, 24, 34, 38). Mucosal immunization strategies have been extensively investigated as a means to improve the efficacy and duration of influenza vaccination by providing a broader immune response than that afforded by i.m. immunization (14, 31, 32, 33, 42). Like intranasal immunization, oral immunization has been shown to induce strong secretory IgA responses, improve protective cellular immune responses, and result in significant serum antibody responses as well (5, 14, 26, 29, 31, 32, 43). The secretory IgA responses for oral immunization have been shown for human subjects to be strongest in the urogenital and rectal tracts, and when compared to intranasal immunization, oral immunization has resulted in somewhat muted upper respiratory, nasopharyngeal, and salivary secretory IgA responses (39). These relatively weak upper respiratory IgA responses would seem to be a problem with respect to achieving effective protection against viral challenge against viruses whose primary mode of entry is via the upper respiratory tract (such as influenza). Other studies, however, have shown that there are sufficient local secretory IgA responses, and more importantly, there is evidence of antigen-primed B- and T-cell migration to the upper respiratory sites to induce potent protective immunity (26, 43). Furthermore, oral immunization has been shown to promote memory B-cell maintenance in the bone marrow, a factor that may be important in the development of the persistence of immunity against viral challenge (5). However, to obtain strong immune responses from many antigens, a potent mucosal adjuvant, usually an enterotoxin, must be coadministered (9).
Studies have shown that immune responses to orally immunized antigens were significantly stronger if the antigen by itself had mucosal binding properties or could be made to have mucosal binding properties by chemically coupling to agents with mucoadhesive, lectin, or receptor-binding properties (8, 9, 21, 30). CTB has been used for these purposes with some success (8, 9). Influenza HA binds neuraminic acid-rich glycoproteins, while LT-R72 and LT-K63 bind GM1 ganglioside, as well as galactose containing glycoproteins and lipopolysaccharides, all of which ligands are found ubiquitously in the gut (27, 37, 41). Our group has found that antigens that do not have any mucoadhesive or gut-associated binding properties have minimal immunogenicity when delivered orally in mice, other than at very high dose levels, either in the absence of LTs or as mixtures of soluble antigen with soluble LT (unpublished data). With influenza antigens, however, our group and others have shown that modest immune responses occur when reasonable dose levels are delivered orally but substantial and broad immune responses result when the antigens are adjuvanted with LT or CT (26).
Our group has demonstrated here that potent antigen-specific serum antibody titers that are comparable to or stronger than i.m. immunization, as well as modest salivary and nasal IgA responses, can be induced in mice with influenza HA antigens using i.g. immunization by adjuvanting with mutant LTs that demonstrate significantly reduced (LT-R72) and unmeasurable (LT-K63) levels of ADP-ribosyltransferase activity (16). The dose level of influenza HA antigen (20 μg) and mutant LTs (10 to 100 μg) found necessary for the strongest immune responses may be relatively high. Further formulation efforts are under way in our group to improve the efficiency of oral immunization and, if possible, lower the dose requirements.
Acknowledgments
We acknowledge the contributions made by Rino Rappuoli and Mariagrazia Pizza for advice and technical support for LT-K63 and LT-R72, Mildred Ugozzoli for developing the luminometer-based ELISA, Russell Reeve for help with statistical analyses, and Diana Atchley for her skills in animal husbandry.
REFERENCES
- 1.Andrews H P, Snee R D, Sarner M H. Graphical display of means. Am Statistician. 1980;34:195–199. [Google Scholar]
- 2.Barackman J D, Ott G, O'Hagan D T. Intranasal immunization of mice with influenza vaccine in combination with the adjuvant LT-R72 induces potent mucosal and serum immunity which is stronger than that with traditional intramuscular immunization. Infect Immun. 1999;67:4276–4279. doi: 10.1128/iai.67.8.4276-4279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barackman J D, Singh M, Ugozzoli M, Ott G S, O'Hagan D T. Oral immunization with poly(lactide-co-glycolide) microparticles containing an entrapped recombinant glycoprotein (gD2) from herpes simplex type 2 virus. S T P Pharma Sci. 1998;8:41–46. [Google Scholar]
- 4.Bender B S, Johnson M P, Small P A. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514–519. [PMC free article] [PubMed] [Google Scholar]
- 5.Benedetti R, Lev P, Massoch E, Fló J. Long-term antibodies after an oral immunization with cholera toxin are synthesized in the bone marrow and may play a role in the regulation of memory B-cell maintenance at systemic and mucosal sites. Res Immunol. 1998;149:107–118. doi: 10.1016/s0923-2494(98)80294-0. [DOI] [PubMed] [Google Scholar]
- 6.Challacombe S J, Rahman D, Jeffery H, Davis S S, O'Hagan D T. Enhanced secretory IgA and systemic IgG antibody responses after oral immunization with biodegradable microparticles containing antigen. Immunology. 1992;76:164–168. [PMC free article] [PubMed] [Google Scholar]
- 7.Clements M L, Stephens I. New and improved vaccines against influenza. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 545–570. [Google Scholar]
- 8.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Aizpurua H J, Russell-Jones G J. Oral vaccination: identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988;167:440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Haan A, Geerligs H J, Huchshorn J P, Van Scharrenburg G J M, Palache A M, Wilschut J. Mucosal immunoadjuvant activity of liposomes: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with an influenza subunit vaccine and coadministered liposomes. Vaccine. 1995;13:155–162. doi: 10.1016/0264-410x(95)93129-w. [DOI] [PubMed] [Google Scholar]
- 11.Dickingson, B. L., and J. D. Clements. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant, p. 73–87. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, New York, N.Y.
- 12.Elson C O. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 59–72. [Google Scholar]
- 13.Freytag L C, Clements J D. Bacterial toxins as mucosal adjuvants. Curr Top Microbiol Immunol. 1999;236:215–236. doi: 10.1007/978-3-642-59951-4_11. [DOI] [PubMed] [Google Scholar]
- 14.Gallichan W S, Rosenthal K L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghendon Y. The immune response of humans to live and inactivated influenza vaccines. Adv Exp Med Biol. 1989;257:37–45. doi: 10.1007/978-1-4684-5712-4_6. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knock-out of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glezen P W. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 18.Groothuis J R, Levin M V, Levin M J. Safety and immunogenicity of a purified haemagglutinin antigen in very young high-risk children. Vaccine. 1994;12:139–141. doi: 10.1016/0264-410x(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 19.Groothuis J R, Levin M J, Rabalais G P, Meiklejohn G, Lauer B A. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–828. [PubMed] [Google Scholar]
- 20.Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 553–612. [Google Scholar]
- 21.Harokopakis E, Hajishengallis G, Michalek S M. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hierholzer J C, Suggs M T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969;18:816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hierholzer J C, Suggs M T, Hall E C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969;18:824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoskins T W. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979;i:33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 25.Johannsen R, Moser H, Hinz J, Freisen H J, Gruschkau H. Quantitation of haemagglutinin of influenza tween-ether split vaccines by immunodiffusion. Vaccine. 1985;3:235–240. doi: 10.1016/0264-410x(85)90114-8. [DOI] [PubMed] [Google Scholar]
- 26.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 27.Kuziemko G M, Stroh M, Stevens R C. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 28.Lyche N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 29.Meitin C A, Bender B S, Small P A., Jr Enteric immunization of mice against influenza with recombinant vaccinia. Proc Natl Acad Sci USA. 1994;91:11187–11191. doi: 10.1073/pnas.91.23.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neutra M R, Kraekenbuhl J. Antigen uptake by M cells for effective mucosal vaccines. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 41–55. [Google Scholar]
- 31.Novak M, Yamamoto M, Fujihashi K, Moldoveanu Z, Kiyono H, McGhee J R, Mesechy J. Ig-secreting and interferon-γ-producing cells in mice mucosally immunized with influenza virus. Adv Exp Med Biol. 1995;371B:1587–1590. [PubMed] [Google Scholar]
- 32.Ogra P L. Mucosal immunoprophylaxis: an introductory overview. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 3–14. [Google Scholar]
- 33.Oh Y, Ohta K, Kuno-Sakai H, Kim R, Kimura M. Local and systemic influenza haemagglutinin-specific antibody responses following aerosol and subcutaneous administration of inactivated split influenza vaccine. Vaccine. 1998;10:506–511. doi: 10.1016/0264-410x(92)90348-n. [DOI] [PubMed] [Google Scholar]
- 34.Patriarca P A, Weber J A, Parker R A, Hall W N, Kendal A P, Bregman M S, Schonberger L B. Efficacy of influenza vaccine in nursing homes: reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985;253:1136–1139. [PubMed] [Google Scholar]
- 35.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 36.Powers D C. Influenza A virus-specific cytotoxic T-lymphocyte activity declines with advancing age. J Am Geriatr Soc. 1993;41:1–5. doi: 10.1111/j.1532-5415.1993.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 37.Pritchett T J, Brossmer R, Rose U, Paulson J C. Recognition of monovalent sialosides by influenza virus H3 hemagglutinin. Virology. 1987;160:502–506. doi: 10.1016/0042-6822(87)90026-2. [DOI] [PubMed] [Google Scholar]
- 38.Riddiough M A, Sisk J E, Bell J C. Influenza vaccination: cost-effectiveness and public policy. JAMA. 1983;249:3189–3195. [PubMed] [Google Scholar]
- 39.Rudin A, Johansson E, Bergquist C, Holmgren J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago N, Haas S, Baughman R A. Vehicles for oral immunization. In: Powell F M, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 413–438. [DOI] [PubMed] [Google Scholar]
- 41.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staats H F, McGhee J R. Application of basic principles of mucosal immunity to vaccine development. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 17–39. [Google Scholar]
- 43.Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine. 1996;14:1651–1656. doi: 10.1016/s0264-410x(96)00128-4. [DOI] [PubMed] [Google Scholar]
- 44.Tamura S, Funato H, Hiroabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 45.Tamura S, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 46.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T, Aizawa C, Oya A, Kurata T. Escherichia coli heat-labile enterotoxin B subunit supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:1083–1089. doi: 10.1016/0264-410x(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 47.Ugozzoli M, O'Hagan D T, Ott G S. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. 1998;93:563–571. doi: 10.1046/j.1365-2567.1998.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]