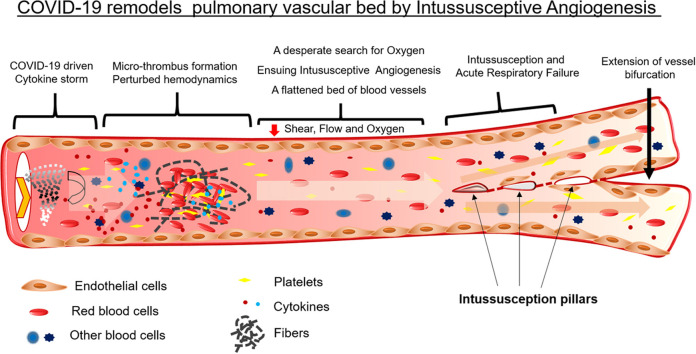
Abstract
Coronavirus disease-2019 (COVID-19), the disease caused by severe acute respiratory syndrome-coronavirus-2, has claimed more than 4.4 million lives worldwide (as of 20 August 2021). Severe cases of the disease often result in respiratory distress due to cytokine storm, and mechanical ventilation is required. Although, the lungs are the primary organs affected by the disease, more evidence on damage to the heart, kidney, and liver is emerging. A common link in these connections is the cardiovascular network. Inner lining of the blood vessels, called endothelium, is formed by a single layer of endothelial cells. Several clinical manifestations involving the endothelium have been reported, such as its activation via immunomodulation, endotheliitis, thrombosis, vasoconstriction, and distinct intussusceptive angiogenesis (IA), a unique and rapid process of blood-vessel formation by splitting a vessel into two lumens. In fact, the virus directly infects the endothelium via TMPRSS2 spike glycoprotein priming to facilitate ACE-2-mediated viral entry. Recent studies have indicated a significant increase in remodeling of the pulmonary vascular bed via intussusception in patients with COVID-19. However, the lack of circulatory biomarkers for IA limits its detection in COVID-19 pathogenesis. In this review, we describe the implications of angiogenesis in COVID-19, unique features of the pulmonary vascular bed and its remodeling, and a rapid and non-invasive assessment of IA to overcome the technical limitations in patients with COVID-19.
Keywords: COVID-19, Endothelium, Vascular remodeling, Intussusceptive angiogenesis, Functional respiratory imaging
Graphical Abstract
1. Coronavirus disease-2019 (COVID-19) and the associated clinical challenges
COVID-19, the disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has infected more than 210 million people and claimed more than 4.4 million lives worldwide over a period of nearly two years [1] (as of 20 August 2021). The major symptoms of COVID-19 include adult respiratory distress syndrome (ARDS), which originates from post-infection-cytokine storm [2]. Despite the active research on appropriate therapeutic strategies, a definitive and specific treatment is not available for the disease. Remdesivir [3] and convalescent plasma [4], [5] have shown positive effects, whereas hydroxychloroquine, an anti-malarial drug, has yielded contradictory results [6]. Although the respiratory system is the primary site affected by the disease [7], increasing clinical evidence suggests the involvement of multiple-organs [8]. Therefore, our treatment approaches should have a broad perspective. Unlike other SARS diseases, COVID-19 exhibits atypical symptoms such as cardiovascular complications, blood clotting, encephalitis, kidney damage, skin rashes, and inflammation of the heart, which led the experts to think that all these symptoms share a common feature, i.e., the "vasculature" [9], [10], [11].
The vascular network acts as a transport system to carry blood throughout the body, delivering nutrients and oxygen to the tissues and eliminating the tissue -waste products. SARS-CoV-2, like a strategic war enemy, attacks the transport system of the body, and hence causes multiorgan dysfunctions. Accumulating evidence suggests the development of dysfunctional vasculature and endothelial inflammation in the lungs as well as in the other organs [12]. This finding is further strengthened by the fact that the endothelium expresses high levels of ACE2 receptor [13] and furin, the primary binding molecules for SARS-CoV-2 spike protein, and protease to help viral entry [14]. Therefore, COVID-19 is much more of a vascular disease than estimated.
2. COVID-19, cytokine storm, and endothelitis
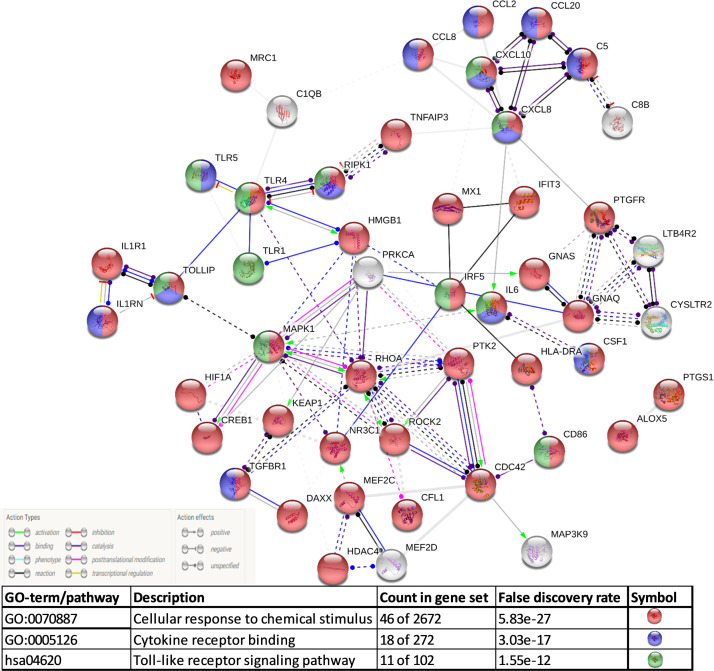
Most of the mechanisms of SARS-CoV-2 infection are postulated based on its 79.5% genome-sequence similarities with SARS-CoV owing to the lack of research on its infection [15]. The COVID-19 pathogenesis has been classified into four phases that range from infection to multiorgan failure. Phase I involves the entry of the viral particles into the goblet and ciliated cells in the upper respiratory tract as a result of binding to ACE2 and via TMPRSS2-assisted endocytosis [16]. This process leads to rapid viral replication and development of pyrogenic (>80% patients) and pneumonia-like(>20% patients) symptoms [9]. The viral invasion also activates the host immune response via pattern recognition receptors (PRRs), toll-like receptor (TLR), and NOD-like receptor (NLR), which is followed by the release of interferons (IFNs) [17]. Moreover, the viral nucleoprotein (N) helps the virus to breach the host immune system [18]. These events lead to phase 2 of the infection in which the damaged host cells release damage-associated molecular patterns (DAMPs) and undergo pyroptosis. The release of DAMPs triggers the generation of cytokines and chemokines in endothelial, epithelial, and alveolar macrophages followed by the attraction of mobile immune cells, macrophages, monocytes, neutrophils, and T-cells [19]. The accumulation of elevated pro-inflammatory molecules and activated immune cells results in a cytokine storm, which in turn makes the pulmonary endothelium leaky, hypoxic, and dysfunctional and results into endothelitis [20]. These events lead to phase 4 of ARDS during which low blood oxygenation and breathing difficulties culminate in multiorgan failure. However, unlike SARS-CoV, SARS-CoV-2 has an additional furin-like cleavage site in the spike protein, which makes making the entire endothelium more susceptible to infection [21]. Of all these events, cytokine storm is the most critical phase because it acts as a "wildfire" through the endothelium, the single lining of the whole circulatory system across the body. Endothelitis disrupts the protective function of the endothelium and paves the way for an imbalance in the vascular homeostasis. The disturbed vascular homeostasis in turn leads to the failure of multiple organs. Therefore, patients with pre-existing conditions in which the endothelium is already compromised, such as hypertension, diabetes, coronary heart disease, and old age, are more susceptible to fatal COVID-19 [12]. Furthermore, in correlation to metabolic activity, the lungs have the largest capillary network and, therefore, the largest endothelial surface area. The endothelium is a critical regulator of vascular homeostasis. Similar to other endothelia, pulmonary endothelial cells also prevent thrombosis by binding to tissue factor pathway inhibitors (TFPIs) and by blocking the action of the factor-VIIa–tissue-factor complex [22]. A quiescent or healthy endothelium inhibits platelet activation and aggregation as well as the adhesion of platelets and leukocytes to the vessel wall [23]. However, pulmonary inflammation or lung injury could cause uncontrolled activation of the coagulation cascade, leading to vascular thrombosis or lethal fibrotic-lung symptoms, the ARDS. In fact, the comparative transcriptome profile of the lungs obtained during autopsy from patients who died from COVID-19 or influenza A(H1N1) showed 79 differentially regulated inflammation-related genes [24]. The pathways involving these differentially regulated genes were identified and enriched, and their protein-protein interaction (PPI) networks were predicted. As expected, the major enriched pathways were ‘cellular response to chemical stimulus’, ‘cytokine receptor binding’ and ‘toll-like receptor signaling pathway’. The central proteins with the maximum PPI were MAPK1, RHOA, PTK2, and CDC42 ( Fig. 1). MAPKs signaling was found to be at the cross-road for the development of respiratory disorders [25], [26], [27]. In addition, inhibition of p38 MAPK improved lung permeability and attenuated systemic inflammation [28], whereas RhoA inhibition aided acute lung injury [29]. Furthermore, Rho-kinase inhibitors have been proposed in the therapeutic strategies against SARS-CoV-2 induced acute respiratory distress syndrome [30].
Fig. 1.
Predicted protein-protein interaction network based on differentially regulated inflammation-associated genes in COVID-19 compared to influenza (A/H1N1) lungs
(data from Ackermann et al., [24]).
3. Pulmonary vasculature and its remodeling
The pulmonary vasculature is a highly specialized circulatory network connecting two functionally interdependent organs, namely, the heart and the lungs. The pulmonary vasculature is morphologically adapted to: 1) deliver the entire cardiac output under low pressure from the right ventricle to the pulmonary microvessels for gaseous exchange; 2) act as a source of production, release, and processing of humoral mediators; and 3) serve as a barrier for the exchange of fluid and solutes and thus maintain the lung fluid balance. The vessel size in the pulmonary vasculature ranges from approximately 6 µm to 3 cm in diameter, with a surface area of approximately 70 m2. The lung vasculature responds to changes in blood pressure, shear stress, fluid viscosity, and vascular resistance, which, when present in excess, lead to vascular remodeling. In the lungs, vascular remodeling is a predominant phenomenon during development; however, it also occurs in many pathological conditions such as pulmonary hypertension (PH) and chronic obstructive pulmonary disease (COPD). In PH and COPD, vascular remodeling is characterized by thickening of the arterial wall and, reduced vessel lumen, which ultimately lead to increased pulmonary vascular resistance. Hypoxia drives vascular wall alterations in PH and COPD and further controls the hypoxia-dependent factors such as erythropoietin, glucose transporters, vascular endothelial growth factor (VEGF), endothelin-1, and nitric oxide (NO) production. At the cellular level, the major events involving vascular remodeling are proliferation, migration, and hypertrophy of the smooth muscle cells (SMCs) and the epithelial-mesenchymal transition of the endothelial cells to SMCs. Although pulmonary vascular remodeling occurs in PH and COPD, the degree of remodeling reported in the lungs of patients with COVID-19 is much higher. These patients display show a rapid increase in angiogenesis, along with increased thrombosis, endothelitis, and vascular inflammation.
4. COVID-19 and angiogenesis
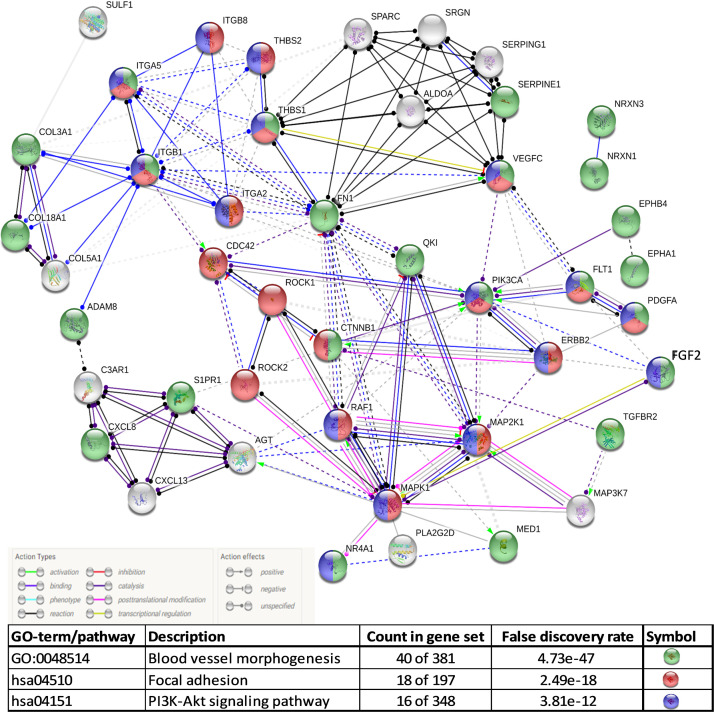
Angiogenesis refers to the formation of new blood vessels from pre-existing ones. Although there have been studies relating COVID-19 to endothelitis. Ackermann et al. reported for the first time the association between COVID-19 and angiogenesis. The researchers found 2.7 times higher (p < 0.001) angiogenesis in the lungs of patients with COVID-19 than in patients with influenza [24]. Comparative transcriptome profile of the lungs obtained during autopsy from patients who died of COVID-19 or influenza A(H1N1) showed 69 differentially regulated angiogenesis-related genes [24]. The enriched pathways involving these differentially regulated genes and their predicted protein-protein interaction (PPI) networks were identified. The major enriched pathways included ‘blood vessel morphogenesis, ‘focal adhesion’ and ‘PI3K-Akt signaling pathway’ ( Fig. 2). Although angiopoietin-2 (angpt-2) in the plasma is reported to be a marker of endothelial activation for intensive care unit admission in patients with COVID-19 [31], it was not differentially regulated at mRNA level in the lungs in patients with COVID-19 when compared to those with influenza A(H1N1) [24].
Fig. 2.
Predicted protein-protein interaction network based on differentially regulated angiogenesis-associated genes in COVID-19 compared to influenza (A/H1N1) lungs.
(data from Ackermann et al., [24]).
Based on the biophysical features, angiogenesis is classified as sprouting or intussusceptive (splitting). Historically, sprouting angiogenesis (SA) has been studied extensively since its discovery over two centuries ago, whereas Burri et al. were the first investigators to describe IA in the late 1990 s [32]. Both types of angiogenesis occur in virtually all the organs during embryonic development as well as in adults [33]. In SA, a single cell called “tip-cell” leads the growth of the vessel toward the stimulus; generally, the surrounding tissues that are deprived of oxygen and nutrients secrete proangiogenic factors, such as VEGFA and HIF1. The process includes the basic steps of enzymatic degradation of the capillary basement membrane, endothelial cell (EC) proliferation, directed migration of the ECs, tubulogenesis, vessel fusion, vessel pruning, and pericyte stabilization [33]. In a competitive manner, an endothelial cell with high VEGFR2 activity becomes a tip cell and suppresses the neighboring cells, making them remain stalked cells by activating DLL4-NOTCH1-VEGFR2 signaling [34]. Furthermore, NO regulates the direction of tip cell migration via the eNOS-NO-cGMP pathway [35].
In contrast to SA, IA is recently discovered and underexplored. Subsequent to Ackermann el al.’s report on its role in COVID-19 pathology [24], IA has become the focus of research and a key target to develop strategic treatment against COVID-19 during the ongoing pandemic. In fact, Burri discovered the intussuscepted mode of vascular growth in pulmonary capillaries in rats [32].
5. IA and its regulation
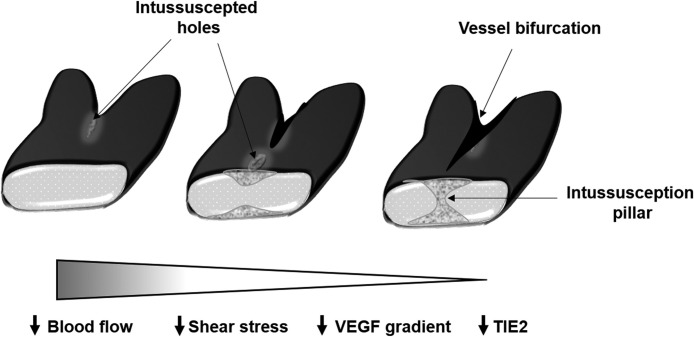
The word intussusception originates from a Latin word meaning ‘growth within itself’, which also describes the morphological features of transluminal pillars in the vessels. These pillars are formed when endothelial cells of opposite luminal walls protrude and join together in the lumen, followed by the invasion of pericytes and fibroblasts ( Fig. 3) [36]. IA is a rapid process of expansion and remodeling of the vascular bed in the tissue and is highly sensitive to the shear stress concentrations of growth factors [37], [38]. In several models, it has been shown that sprouting is the initial mode of angiogenesis, whereas IA occurs later on during the maturation and expansion of the vascular bed [39], [40], [41]. Although no biomarker has been identified for IA, several factors have been described for its regulation, such as hemodynamic forces [36], growth factors, and angiogenic markers. Among the hemodynamics forces, reduced blood flow has been shown to enhance IA in a chick chorioallantoic membrane (CAM) model [42]. Contradictory findings have been reported regarding the effect of growth factors on IA. For example, reduction in VEGF has been shown to induce vascular remodeling by IA [43]; however, in other studies, the VEGF exposer has been documented to induce IA in the CAM model [44], [45]. Although further investigations are required, researchers speculate that the differential effects of VEGF on IA could be due to the quantity of VEGF, expression of VEGFRs, and the qualitative distribution of VEGF in the tissue microenvironment [46]. In general, tip-cell-markers demonstrate similar effects on both IA and SA. For example, Tie-2 induces tip-cell formation in SA, whereas its deletion results in a reduction of intraluminal pillar formations, along with other cardiovascular malfunctions [47]. Similarly, overexpression of Ang-1 causes vessel enlargement without affecting SA [48]. Other than Tie-2 and Ang-1, Ephirin B2 [49] and recombinant erythropoietin [50] also affect IA, as a inferred from different models.
Fig. 3.
Schematic representation of intussusception pillar formation in blood vessel and factors involved
(adapted from [51]).
6. Aggressive remodeling of the pulmonary vasculature in COVID-19
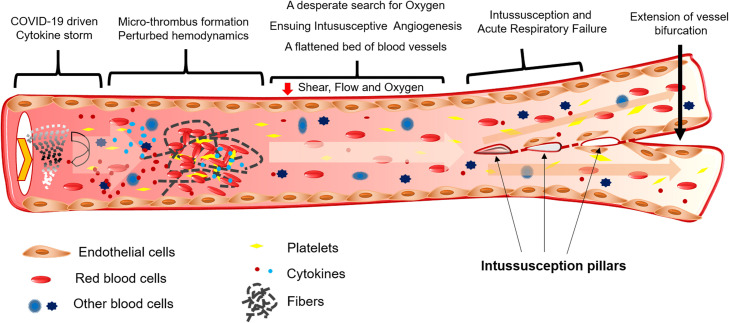
The lungs of patients with COVID-19 exhibit damaged endothelium, distorted vascularity, and intussusceptive pillars, which are not observed in the lungs of patients with influenza. IA features are more prominent in the lungs of patients with COVID-19 than in those with influenza [24]. Comparative gene expression studies in the lungs of patients with influenza and COVID-19 revealed the upregulation of angiogenic markers such as HIF1α, RBPJ, FGF2, PDGFA, NOS3, VEGFA, CTNNB1, and WNT5A [24]. Hypoxia is one of the main pathophysiological drivers of vascular remodeling in both health and disease [52]. Upregulation of HIF1α in the lungs of patients with COVID-19 could be a result of decreased blood flow because of thrombus formation in the blood vessels ( Fig. 4).
Fig. 4.
Schematic representation of cytokine storm-driven events in a blood vessel of the lungs of patients with COVID-19.
In an experimental model, the HIF-1-VEGFA-VEGFR2 signaling axis mediated pathological vascular remodeling via intussusception [53]. Additionally, RBPJ is a transcriptional repressor of Notch signaling [54] and is upregulated in the lungs of patients with COVID-19 [24]. Although Notch inhibition promotes SA, its inhibition in pre-existing vascular beds induces IA [55]. Therefore, upregulation of RBPJ in the lungs of patients with COVID-19 could also induce IA by suppressing Notch signaling. A recent meta-analysis as well as deep sequencing data from our laboratory suggested the upregulation of IA-like marker genes, such as HMOX1, SMA, CTNNB1, SULF2, NG2, ENG, TEM8, PECAM1, F8, CD34, ANGPT, CXR4, and CALD1, in a tumor cell-induced IA milieu [56]. Among them, HMOX1 and CTNNB1 were upregulated in the lungs of patients with COVID-19 [24].
Another characteristic feature of COVID-19 is the cytokine storm. “Inflammation” links for the set of genes were individually checked in the deep sequencing data to examine their implications in dysregulated IA in COVID-19-affected lungs. An interplay between Glu-Leu-Arg tripeptide-containing CXC chemokines and CXCL helps the mobilization and recruitment of inflamed cells under inflammatory conditions [57], [58]. As previously described, endothelitis is a major event recorded in COVID-19 that is correlated with microvasculopathy in the lungs. The circulating level of angpt-2 is associated with increased pulmonary edema and mortality in patients with ARDS [59]. In this connection, a clinical trial has been enrolled in clinicaltrials.gov for exploring the effect of targeting angpt-2 in patients with COVID-19 [60]. A study by Kümpers et al. demonstrated a direct link between the blood levels of angpt-2, and mortality in 43 critically ill patients with sepsis [61]. Furthermore, in connection with IA-associated genes and inflammation, PECAM-1, an endothelial marker, is known for its pro-inflammatory role in leukocyte diapedesis [62] and also facilitates chemokine-mediated directional migration of leukocytes to the inflammatory sites [63]. TEM-8 is not an inflammatory signaling molecule; however, a recent study has signified that a TEM-8-specific chimeric antigen receptor treatment to mice is effective against pulmonary inflammation in the lungs [64]. Soluble endoglin is a glycoprotein produced by the cleavage of the extracellular domain of the membrane-bound endoglin [65], which binds to several ligands such as TGF-β1, BMP-9, and BMP-10 [66] and acts as a cytokine modulator. Increased level of soluble endoglin in the plasma is associated with cardiac conditions [67]. Sulf2 is upregulated in inflammation [68]. A previous work has shown that the inflammatory cytokine tumor necrosis factor-α (TNF-α) induces Sulf1 expression in human lung fibroblasts [69], and the Sulf1 has also been reported to be upregulated in the lungs of patients with COVID-19 [24]. Li et al. demonstrated that MALAT1-driven inhibition of Wnt signal impedes the proliferation and inflammation in CTNNB1 promoter methylation in rheumatoid Fibroblast-Like Synoviocytes. MALAT1 suppresses the transcription and expression of CTNNB1, and the silenced MALAT1 stimulates the nucleation of β-catenin and the secretion of inflammatory cytokines, including interleukin-6, interleukin-10, and TNF-α [70].
7. Degree of vascular intussusceptions and COVID-19 severity
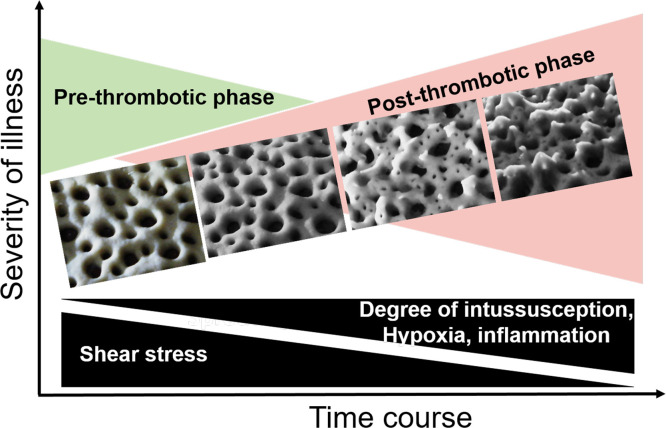
Progressive respiratory failure occurs in patients with COVID-19. IA and an increase in the vascular bed are also observed in the lungs of the patients. As stated previously, intussusception is a rapid mode of vascular remodeling and could be directly correlated with the increase in the vascular bed in the lungs. Therefore, it is obvious to look for the correlation between the severity of COVID-19 and the degree of vascular intussusception in the lungs of the patients. Here, we present a hypothetical model postulating the progressive IA with four different phases of COVID-19 severity ( Fig. 5 ).
Fig. 5.
Hypothetical model of progressive intussusceptive angiogenesis in the lungs of patients with COVID-19: the images represent flour- dough construction of an artistic impression based on the observations by Ackermann et al. [24].
8. Vascular remodeling-driven chronic hypoxia in patients with COVID-19
The work of Taylor et al. suggested that IA is a tissue-level vascular adaptation to chronic systemic hypoxia in the adult mouse retina [71]. Silent hypoxia is emerging as a hallmark of COVID-19. In general, thrombofibrosis and endothelial–mesenchymal transition in patients with COVID-19 is believed to be promoted by hypoxia-induced activation of endothelial cells, IA, and the activation of mesenchymal cells and immune cells. Apart from the findings of IA in COVID-19 post-mortem lung tissue, the work of Ackermann et al. [72] demonstrated intussusception of the vessels in the heart, liver, kidney, brain, and lymphoreticular organs. Therefore, hypoxia is a common denominator for the systemic damages caused by silent hypoxia in patients with COVID-19 [72], [73], [74], [75]. Silent hypoxia induces alarmingly low oxygen saturation levels (~ 50–80% saturation) without any breathing trouble in patients with COVID-19 [76], which is a matter of concern since the patient feels no discomfort. One of the manifestations of COVID-19 is the lack of hypoxic vasoconstriction in the lungs, which promotes hyperfusion within a lung that has already undergone respiratory damage [76], [77].
9. Challenges pertaining to IA assessment in patients with COVID-19: could functional respiratory imaging (FRI) be helpful?
As stated previously, IA is a rapid process, and the lack of distinguished biomarkers makes it difficult to diagnose the condition. However, a pulmonary scan based on the changes in vascular hemodynamics could overcome these challenges.
FRI is based on multidetector CT (MDCT) and computational fluid dynamics (CFD); the method combines the structural and functional assessment of the airways and their vasculature [78]. A low-dose CT scan of the lungs, obtained at functional residual capacity and total lung capacity levels, allows a patient-specific reconstruction of the airways [79]. FRI has been successfully used previously in examining microvascular pruning and CT-derived markers of pulmonary blood loss in the smallest caliber vessels during pulmonary vascular remodeling in smokers [80]. Recently, the technique has also been used in pulmonary scanning to examine the vascular hemodynamics and the size of blood vessels in patients with COVID-19 [81]. However, there is no correlation between IA, the size of blood vessels, and blood volume in the lungs of patients affected by COVID-19, if any.
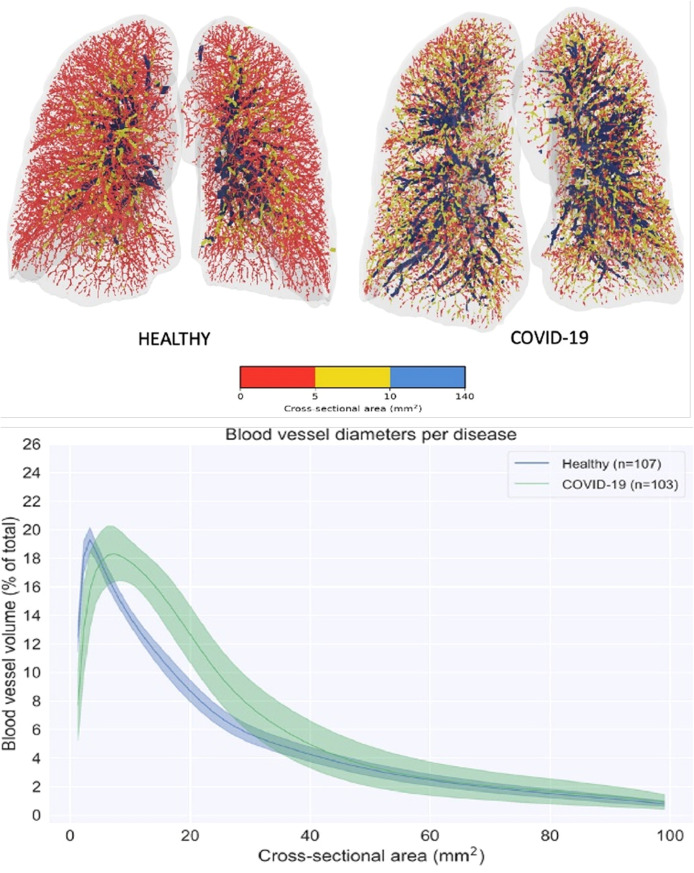
In this study, the authors included healthy volunteers (n = 107) and patients with COVID-19 (n = 103). They scanned the pulmonary vasculatures, analyzed the size of the blood vessels, and classified the blood vessels into three types based on their cross-sectional area: small vessels (0- -<5 mm2), mid-sized vessels (5- -<10 mm2), and large vessels (10- -<140 mm2). The volume of the vessels with more than 40 mm2 did not change between the lungs of healthy volunteers and those affected by COVID-19. The vessels between 5 and 30 mm2 were increased in the lungs of the patients with COVID-19; however, the volume of small vessels (1- -<5 mm2) was reduced ( Fig. 6 ).
Fig. 6.
Visual representation of the blood vessels colored according to their size. Red denotes the small vessels, yellow the mid-size vessels, and blue the larger vessels (top). Spectrum plot describing the blood volume as a function of the blood vessel cross-sectional area for the large spectrum reflecting most blood vessels visible in the HRCT (bottom). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The figure is adapted from [81] with prior permission (License number: 5162351383355).
Hence, it is apparent that the small vessels are merged to form mid-sized vessels in the lungs of patients with COVID-19. The same phenomenon occurs in IA, and multiple studies have shown that the smaller vessels join together and form larger vessels or perhaps bag-like structures. Here, it is unlikely that the larger vessels split into mid-sized vessels because as their volume remained unchanged in the lungs of patients affected by COVID-19.
Another interesting point is that there was no difference in the blood volume between the lungs of healthy volunteers and those affected by COVID-19, which signifies the redistribution of blood among the blood vessels of different sizes [81].
10. Summary
The present review has summarized our understanding of vascular patterning and angiogenesis in the pulmonary vascular bed and has hinted the possible research directions for deciphering the concept of IA in the lungs of patients affected by COVID-19. The patients exhibit severe pulmonary complications in different phases of the disease. COVID-19 induces pneumonia, ARDS, and sepsis. Pulmonary edema fills the air sacs with fluid, limiting their access to oxygen and causing shortness of breath, cough, and other symptoms. Since the lung is the most difficult organ to manage clinically in patients with COVID-19, there is a desperate attempt to comprehend the remodeling process of the lung vascular bed. Especially, in the scenario of long-term complications related to COVID-19, it is critical to investigate the pulmonary vascular bed and the interplaying microenvironment of angiocentric T-cell inflammation, thrombotic microangiopathy, and flow-regulated IA. In the past year, several investigators asserted the pro-intussusceptive milieu of the pulmonary vascular bed. The work by Ackermann et al. has convincingly presented IA in the lungs of patients with COVID-19 who succumbed to the disease. Subsequently, the concept of lung IA has been fortified by the contributions of other groups, including Ackermann et al. [24], [75], [82], [83]. However, an authentic and high-resolution analysis of pulmonary bed vascular patterning and perfusion in the lungs of patients affected by COVID-19 is required. An emerging technology, IA-supported FRI, could be beneficial in elucidating the functional aspects of pulmonary vascular perfusion in COVID-19 pathology.
CRediT authorship contribution statement
P.K.J. and S.C. conceptualized, wrote, and revised the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
S.C. acknowledges a grant from University Grants Commission – Faculty Recharge Programme, (UGC-FRP) (F.4-5(25)/2013(BSR)), Government of India. We are grateful to Gayatri Chattopadhay for preparing an artistic flour-dough model presented in Fig. 5.
References
Prof. Suvro Chatterjee is a senior scientist at the Department of biotechnology, University of Burdwan, India. He leads a group focused on vascular biology research through cell biology platforms. His core strength in using various cell based models has been revealed through a large number of publications –http://bio.au-kbc.org/faculty/suvro/liver-mainpage.html. Chatterjee group’s major contribution is in studying the mechanism underlying nitric oxide implications in vascular biology. Specifically, he has contributed significantly to understanding the molecular mechanism of controlling endothelial nitric oxide synthase (eNOS) and thereby nitric oxide production.
- 1.Coronavirus COVID-19 (2019-nCoV) Available online: 〈https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6〉 (accessed on May 31, 2020).
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA - J. Am. Med. Assoc. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. 0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Jiang S., Xia S., Ying T., Lu L. A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome. Cell. Mol. Immunol. 2020;17:554. doi: 10.1038/s41423-020-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y., Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for C. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 2020;382(382):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández Nieto D., Rodríguez-Villa Lario A., Navarro Fernández I., Ruiz-Villaverde R., Falkenhain-López D., Llamas Velasco M., García-Gavín J., Baniandrés O., González-Cruz C., Morillas-Lahuerta V., Cubiró X., Figueras Nart I., Selda-Enriquez G., Romaní J., Fustà-Novell X., Melian-Olivera A., Roncero Riesco M., Burgos-Blasco P., Sola Ortigosa J., Feito Rodriguez M., García-Doval I. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183:19163–19177. doi: 10.1111/bjd.19163. (bjd) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 14.Abassi Z.A., Skorecki K., Heyman S.N., Kinaneh S., Armaly Z. Covid-19 infection and mortality: a physiologist’s perspective enlightening clinical features and plausible interventional strategies. Am. J. Physiol. Cell. Mol. Physiol. 2020;318:1020. doi: 10.1152/ajplung.00097.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K., Sen, Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- an update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, M.; Kleine-Weber, H.; Krueger, N.; Mueller, M.A.; Drosten, C.; Poehlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020, 2020.01.31.929042.
- 17.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X., Pan J., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42:37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:1–3. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 23.Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015;15:15. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renda T., Baraldo S., Pelaia G., Bazzan E., Turato G., Papi A., Maestrelli P., Maselli R., Vatrella A., Fabbri L.M., Zuin R., Marsico S.A., Saetta M. Increased activation of p38 MAPK in COPD. Eur. Respir. J. 2008;31:62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 26.Bei Y., Hua-Huy T., Nicco C., Duong-Quy S., Le-Dong N.N., Tiev K.P., Chéreau C., Batteux F., Dinh-Xuan A.T. RhoA/Rho-kinase activation promotes lung fibrosis in an animal model of systemic sclerosis. Exp. Lung Res. 2016;42:44–55. doi: 10.3109/01902148.2016.1141263. [DOI] [PubMed] [Google Scholar]
- 27.Wheaton A.K., Agarwal M., Jia S., Kim K.K. Lung epithelial cell focal adhesion kinase signaling inhibits lung injury and fibrosis. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2017;312:L722–L730. doi: 10.1152/ajplung.00478.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang W., Cai S.X., Wang C.L., Sun X.X., Li K., Yan X.W., Sun Y.B., Sun X.Z., Gu C.K., Dai M.Y., Wang H.M., Zhou Z. Modulation of mitogen-activated protein kinase attenuates sepsis-induced acute lung injury in acute respiratory distress syndrome rats. Mol. Med. Rep. 2017;16:9652–9658. doi: 10.3892/mmr.2017.7811. [DOI] [PubMed] [Google Scholar]
- 29.Abedi F., Hayes A.W., Reiter R., Karimi G. Acute lung injury: the therapeutic role of Rho kinase inhibitors. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104736. [DOI] [PubMed] [Google Scholar]
- 30.Calò L.A., Bertoldi G., Davis P.A. Rho kinase inhibitors for SARS-CoV-2 induced acute respiratory distress syndrome: support from Bartter’s and Gitelman’s syndrome patients. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smadja D.M., Guerin C.L., Chocron R., Yatim N., Boussier J., Gendron N., Khider L., Hadjadj J., Goudot G., Debuc B., Juvin P., Hauw-Berlemont C., Augy J.L., Peron N., Messas E., Planquette B., Sanchez O., Charbit B., Gaussem P., Duffy D., Terrier B., Mirault T., Diehl J.L. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;1:3–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burri P.H., Tarek M.R. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat. Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- 33.Adair, T.H.; Montani, J.-P. Overview of Angiogenesis. 2010. [PubMed]
- 34.Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A., Schulte-Merker S., Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 35.Priya M.K., Sahu G., Soto-Pantoja D.R., Goldy N., Sundaresan A.M., Jadhav V., Barathkumar T.R., Saran U., Jaffar Ali B.M., Roberts D.D., Bera A.K., Chatterjee S. Tipping off endothelial tubes: nitric oxide drives tip cells. Angiogenesis. 2015;18:175–189. doi: 10.1007/s10456-014-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djonov V.G., Kurz H., Burri P.H. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev. Dyn. 2002;224:391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- 37.Eldridge L., Wagner E.M. Angiogenesis in the lung. J. Physiol. 2019;597:1023–1032. doi: 10.1113/JP275860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsia C.C.W. Comparative analysis of the mechanical signals in lung development and compensatory growth. Cell Tissue Res. 2017;367:687–705. doi: 10.1007/s00441-016-2558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makanya A.N., Hlushchuk R., Baum O., Velinov N., Ochs M., Djonov V. Microvascular endowment in the developing chicken embryo lung. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2007;292 doi: 10.1152/ajplung.00371.2006. 292-46. [DOI] [PubMed] [Google Scholar]
- 40.Schlatter P., König M.F., Karlsson L.M., Burri P.H. Quantitative study of intussusceptive capillary growth in the chorioallantoic membrane (CAM) of the chicken embryo. Microvasc. Res. 1997;54:65–73. doi: 10.1006/mvre.1997.2022. [DOI] [PubMed] [Google Scholar]
- 41.V D., AC A., A Z. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 2001:52. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Djonov V., Schmid M., Tschanz S.A., Burri P.H. Intussusceptive angiogenesis. Its role in embryonic vascular network formation. Circ. Res. 2000;86:286–292. doi: 10.1161/01.RES.86.3.286. [DOI] [PubMed] [Google Scholar]
- 43.Hlushchuk R., Ehrbar M., Reichmuth P., Heinimann N., Styp-Rekowska B., Escher R., Baum O., Lienemann P., Makanya A., Keshet E., Djonov V. Decrease in VEGF expression induces intussusceptive vascular pruning. Arterioscler. Thromb. Vasc. Biol. 2011;31:2836–2844. doi: 10.1161/ATVBAHA.111.231811. [DOI] [PubMed] [Google Scholar]
- 44.Baum O., Suter F., Gerber B., Tschanz S.A., Buergy R., Blank F., Hlushchuk R., Djonov V. VEGF-A promotes intussusceptive angiogenesis in the developing chicken chorioallantoic membrane. Microcirculation. 2010;17:447–457. doi: 10.1111/j.1549-8719.2010.00043.x. [DOI] [PubMed] [Google Scholar]
- 45.Hagedorn M., Balke M., Schmidt A., Bloch W., Kurz H., Javerzat S., Rousseau B., Wilting J., Bikfalvi A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev. Dyn. 2004;230:23–33. doi: 10.1002/dvdy.20020. [DOI] [PubMed] [Google Scholar]
- 46.Chapter 6. Vasculogenesis and Angiogenesis | Elsevier Enhanced Reader Available online: 〈https://reader.elsevier.com/reader/sd/pii/B9780128023853000061?token=30C399D7E9732106B89AEF11AC0C75D916E35DD7DD8DB21FB13A87D9912D95AF0668C504C7D737DDACF6427BDAC09A25〉 (accessed on Jun 7, 2020).
- 47.Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- 48.Thurston G., Wang Q., Baffert F., Rudge J., Papadopoulos N., Jean-Guillaume D., Wiegand S., Yancopoulos G.D., McDonald D.M. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 49.Shin D., Garcia-Cardena G., Hayashi S.I., Gerety S., Asahara T., Stavrakis G., Isner J., Folkman J., Gimbrone M.A., Anderson D.J. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 50.Crivellato E., Nico B., Vacca A., Djonov V., Presta M., Ribatti D. Recombinant human erythropoietin induces intussusceptive microvascular growth in vivo. Leukemia. 2004;18:331–336. doi: 10.1038/sj.leu.2403246. [DOI] [PubMed] [Google Scholar]
- 51.Kurz H., Burri P.H., Djonov V.G. Angiogenesis and vascular remodeling by intussusception: from form to function. News Physiol. Sci. 2003;18:65–70. doi: 10.1152/nips.01417.2002. [DOI] [PubMed] [Google Scholar]
- 52.Stenmark K.R., Fagan K.A., Frid M.G. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ. Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 53.Ali Z., Mukwaya A., Biesemeier A., Ntzouni M., Ramsköld D., Giatrellis S., Mammadzada P., Cao R., Lennikov A., Marass M., Gerri C., Hildesjö C., Taylor M., Deng Q., Peebo B., Del Peso L., Kvanta A., Sandberg R., Schraermeyer U., Andre H., Steffensen J.F., Lagali N., Cao Y., Kele J., Jensen L.D. Intussusceptive vascular remodeling precedes pathological neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019;39:1402–1418. doi: 10.1161/ATVBAHA.118.312190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulic I., Robertson G., Chang L., Baker J.H., Lockwood W.W., Mok W., Fuller M., Fournier M., Wong N., Chou V., Robinson M.D., Chun H.J., Gilks B., Kempkes B., Thomson T.A., Hirst M., Minchinton A.I., Lam W.L., Jones S., Marra M., Karsan A. Loss of the Notch effector RBPJ promotes tumorigenesis. J. Exp. Med. 2015;212:37–52. doi: 10.1084/jem.20121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dimova I., Hlushchuk R., Makanya A., Styp-Rekowska B., Ceausu A., Flueckiger S., Lang S., Semela D., Le Noble F., Chatterjee S., Djonov V. Inhibition of notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16:921–937. doi: 10.1007/s10456-013-9366-5. [DOI] [PubMed] [Google Scholar]
- 56.Vimalraj S., Bhuvaneswari S., Lakshmikirupa S., Jyothsna G., Chatterjee S. Nitric oxide signaling regulates tumor-induced intussusceptive-like angiogenesis. Microvasc. Res. 2018;119:47–59. doi: 10.1016/j.mvr.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Burdon P.C.E., Martin C., Rankin S.M. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br. J. Haematol. 2008;142:100–108. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- 58.De Filippo K., Dudeck A., Hasenberg M., Nye E., Van Rooijen N., Hartmann K., Gunzer M., Roers A., Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 59.Terpstra M.L., Aman J., Van Nieuw Amerongen G.P., Groeneveld A.B.J. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit. Care Med. 2014;42:691–700. doi: 10.1097/01.ccm.0000435669.60811.24. [DOI] [PubMed] [Google Scholar]
- 60.A Study of LY3127804 in Participants With COVID-19 - Full Text View - ClinicalTrials.gov Available online: 〈https://clinicaltrials.gov/ct2/show/NCT04342897〉 (Accessed on Jun 6, 2020).
- 61.Kümpers P., Lukasz A., David S., Horn R., Hafer C., Faulhaber-Walter R., Fliser D., Haller H., Kielstein J.T. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit. Care. 2008;12:12. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newman P.J., Berndt M.C., Gorski J., White G.C., Lyman S., Paddock C., Muller W.A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 63.Wu Y., Stabach P., Michaud M., Madri J.A. Neutrophils lacking platelet-endothelial cell adhesion molecule-1 exhibit loss of directionality and motility in CXCR2-mediated chemotaxis. J. Immunol. 2005;175:3484–3491. doi: 10.4049/jimmunol.175.6.3484. [DOI] [PubMed] [Google Scholar]
- 64.Petrovic K., Robinson J., Whitworth K., Jinks E., Shaaban A., Leeid S.P. TEM8/ANTXR1-specific CAR T cells mediate toxicity in vivo. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y.M., Bdolah Y., Lim K.H., Yuan H.T., Libermann T.A., Stillman I.E., Roberts D., D'Amore P.A., Epstein F.H., Sellke F.W., Romero R., Sukhatme V.P., Letarte M., Karumanchi S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 66.Castonguay R., Werner E.D., Matthews R.G., Presman E., Mulivor A.W., Solban N., Sako D., Pearsall R.S., Underwood K.W., Seehra J., Kumar R., Grinberg A.V. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapur N.K., Heffernan K.S., Yunis A.A., Parpos P., Kiernan M.S., Sahasrabudhe N.A., Kimmelstiel C.D., Kass D.A., Karas R.H., Mendelsohn M.E. Usefulness of soluble endoglin as a noninvasive measure of left ventricular filling pressure in heart failure. Am. J. Cardiol. 2010;106:1770–1776. doi: 10.1016/j.amjcard.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Te Velde A.A., Pronk I., De Kort F., Stokkers P.C.F. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur. J. Gastroenterol. Hepatol. 2008;20:555–560. doi: 10.1097/MEG.0b013e3282f45751. [DOI] [PubMed] [Google Scholar]
- 69.Sikora A.S., Hellec C., Carpentier M., Martinez P., Delos M., Denys A., Allain F. Tumour-necrosis factor-α induces heparan sulfate 6-O-endosulfatase 1 (Sulf-1) expression in fibroblasts. Int. J. Biochem. Cell Biol. 2016;80:57–65. doi: 10.1016/j.biocel.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 70.Li G.Q., Fang Y.X., Liu Y., Meng F.R., Wu X., Zhang C.W., Zhang Y., Liu D., Gao B. MALAT1-driven inhibition of Wnt signal impedes proliferation and inflammation in fibroblast-like synoviocytes through CTNNB1 promoter methylation in rheumatoid arthritis. Hum. Gene Ther. 2019;30:1008–1022. doi: 10.1089/hum.2018.212. [DOI] [PubMed] [Google Scholar]
- 71.Taylor A.C., Seltz L.M., Yates P.A., Peirce S.M. Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina. Microvasc. Res. 2010;79:93–101. doi: 10.1016/J.MVR.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ackermann M., Tsuda A., Secomb T.W., Mentzer S.J., Konerding M.A. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvasc. Res. 2013;87:75–82. doi: 10.1016/J.MVR.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackermann M., Stark H., Neubert L., Schubert S., Borchert P., Linz F., Wagner W.L., Stiller W., Wielpütz M., Hoefer A., Haverich A., Mentzer S.J., Shah H.R., Welte T., Kuehnel M., Jonigk D. Morphomolecular motifs of pulmonary neoangiogenesis in interstitial lung diseases. Eur. Respir. J. 2020;55:55. doi: 10.1183/13993003.00933-2019. [DOI] [PubMed] [Google Scholar]
- 74.Yanagihara T., Jones K.D. Demystifying morphomolecular alterations of vasculature in interstitial lung diseases. Eur. Respir. J. 2020;55:55. doi: 10.1183/13993003.02446-2019. [DOI] [PubMed] [Google Scholar]
- 75.Ackermann M., Mentzer S.J., Kolb M., Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur. Respir. J. 2020;56:56. doi: 10.1183/13993003.03147-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.“Silent hypoxia” may be killing some COVID-19 patients. But there’s hope. | Live Science Available online: 〈https://www.livescience.com/silent-hypoxia-killing-covid-19-coronavirus-patients.html〉 (Accessed on Aug 18, 2021).
- 77.RM L. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad. Emerg. Med. 2020;27:785–786. doi: 10.1111/ACEM.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern F., Wilson R.V., Coleman H.W., Paterson E.G. Comprehensive approach to verification and validation of CFD simulations—Part 1: methodology and procedures. J. Fluids Eng. 2001;123:793–802. doi: 10.1115/1.1412235. [DOI] [Google Scholar]
- 79.Vanhaverbeke K., Slaats M., Al-Nejar M., Everaars N., Snoeckx A., Spinhoven M., El Addouli H., Lauwers E., Van Eyck A., De Winter B.Y., Van Hoorenbeeck K., De Dooy J., Mahieu L., Mignot B., De Backer J., Mulder A., Verhulst S. Functional respiratory imaging provides novel insights into the long-term respiratory sequelae of bronchopulmonary dysplasia. Eur. Respir. J. 2021;57:57. doi: 10.1183/13993003.02110-2020. [DOI] [PubMed] [Google Scholar]
- 80.Rahaghi F.N., Argemí G., Nardelli P., Domínguez-Fandos D., Arguis P., Peinado V.I., Ross J.C., Ash S.Y., De La Bruere I., Come C.E., Diaz A.A., Sánchez M., Washko G.R., Barberà J.A., San José Estépar R. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.00370-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lins M., Vandevenne J., Thillai M., Lavon B.R., Lanclus M., Bonte S., Godon R., Kendall I., De Backer J., De Backer W. Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad. Radiol. 2020;27:1449–1455. doi: 10.1016/j.acra.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ackermann M., Werlein C., Länger F., Kühnel M.P., Jonigk D.D. COVID-19: effects on the lungs and heart. Pathologe. 2021;42:164–171. doi: 10.1007/S00292-021-00918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bavishi A.A., Mylvaganam R.J., Agarwal R., Avery R.J., Cuttica M.J. Timing of intubation in coronavirus disease 2019: a study of ventilator mechanics, imaging, findings, and outcomes. Crit. Care Explor. 2021;3 doi: 10.1097/CCE.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]