Abstract
Pathogens and mycotoxins are serious public health risks for humans and food safety in milk. This study concentrated on detecting Staphylococcus aureus and Ochratoxin A (OTA) in 210 pasteurized milk from ten urban Beijing districts to suggest the co-occurrence of S. aureus with toxin-producing genes and OTA in milk and the possible risk. S. aureus was identified by physiological and biochemical experiments and molecular biology experiments, and enterotoxin genes were identified by PCR. OTA was detected by LC-MS/MS. The study found 29 isolates of S. aureus, of which 17.24% had the sea gene encoding enterotoxin A. OTA was detected in 31 out of 120 samples and the maximum amount of detection was 18.8 μg/kg. The results of this study indicate that when failing to guarantee the cold chain, the presence of S. aureus with enterotoxin genes in milk will present a risk to food safety. Furthermore, the high detection rates and levels of OTA in milk suggest that OTA is a hidden risk. The co-occurrence of S. aureus and OTA in milk is a food safety concern and there is a need to control the occurrence of these two biohazards in milk.
Keywords: Ochratoxin A, Staphylococcus aureus, pasteurized milk, Beijing, AFM1
1. Introduction
Milk is a highly nutritious food containing many macronutrients and micronutrients including proteins, different types of fatty acids and lactose, minerals, antioxidants, and vitamins, that are essential for the growth and maintenance of human health, especially for infants, children, and older adults [1,2]. Therefore, increasing consumption of milk has been observed owing to its high nutritional role in human health throughout the world [3,4]. According to the 2021 China Dairy Quality Report, in 2020, China produced 27.804 million tons of dairy products, and low-temperature milk including pasteurized milk and low-temperature yogurt reached 2.308 million tons, accounting for 8.3%. However, the nutritional richness of milk also makes it susceptible to contamination by microorganisms and toxins [5]. Staphylococcus aureus and Ochratoxin A (OTA) are biohazards, which commonly occur in milk and milk products [6]. Milk is an important food source of foodborne illness due to contamination with S. aureus [7]. Furthermore, mycotoxin contamination in milk is an emerging concern around the globe [8]. Therefore, it makes sense to test for microorganisms and biotoxins in milk.
Staphylococcal food poisoning (SFP) is one of the most common foodborne diseases worldwide. It is mainly caused by staphylococcal enterotoxins (SEs) secreted by S. aureus [9]. SEs that have been found include SEA, SEB, SEC, SED, SEE, etc., which are the main cause of SFP, accounting for more than 90% of global SFP cases. SEA is the most common cause of food poisoning in the United States, accounting for 77.8% of all SFP cases [10]. In China, microbial food poisoning accounted for 53.70% of food poisoning emergencies in 2015. Furthermore, S. aureus was an important pathogenic factor in these cases [11]. According to the outbreak reports from 15 European countries, milk and dairy products represented 1–9% of all the incriminated foods in staphylococcal outbreaks [12]. Milk is an important source of SFP. There are several foodborne outbreaks of S. aureus intoxications associated with the consumption of contaminated milk [7]. In 1985, there was an outbreak of food poisoning caused by enterotoxin-contaminated milk in a school in Kentucky, and more than 1000 children were affected [13]. In 2000, 13,420 people suffered from food poisoning due to drinking low-fat milk in Osaka, Japan. Eventually, enterotoxin was detected in the milk [14]. In 2007, 166 people were exposed to food poisoning from milk, cacao milk, and vanilla milk, contaminated with staphylococcal enterotoxin in Elementary school, in Austria [15].
SEs are resistant to many environmental conditions, such as high temperatures, low pH [9], freezing, and drying. For instance, crude enterotoxin A remains active at 100 °C for 2 h in broth and at 121 °C for 28 min in mushrooms. SEs are not completely destroyed during pasteurization (15 s at 72 °C) and are considered to be a potential biological hazard. They are also resistant to human proteolytic enzymes and retain their activity in the digestive tract after ingestion [9]. Children will suffer SFP by ingesting as little as 100 ng of SEs, and vulnerable populations may develop staphylococcal food poisoning with a few micrograms of toxin [16]. Therefore, differentiation between virulent and non-virulent strains is significant for evaluating the potential implications of the presence of this microorganism for food safety and public health [17]. The detection of enterotoxin genes has been used to assess the risk of milk and other foods [12].
Ochratoxin A (OTA) is one of the most important and deleterious mycotoxins [18], which is a secondary metabolite produced by various Aspergillus and Penicillium species [19]. A great deal of animal or cell experiments have reported that exposure to OTA can result in various toxicological effects, including teratogenicity, carcinogenicity [20], mutagenicity, hepatotoxicity [21], and especially nephrotoxicity [22]. Different species have different LD50. The tests have shown that dogs and pigs are very susceptible, with oral LD50 of 0.2 and 1 mg/kg b.w. [23]. Apart from the toxicity of OTA, it is not easy to remove, and can only be destroyed when heated above 250 °C for several minutes [24]. OTA has been reported to extensively occur in feed and food, such as beans, coffee beans, cereals, milk, meat, etc. [5]. Despite efforts to control fungal contamination, extensive mycotoxin contamination has been reported in both developing and developed countries in animal feed [25]. When the animals consume contaminated feeds, one part of the toxin is degraded by bovine rumen microorganisms and the other part remains in the body, resulting in contaminated animal products like egg, milk, liver products etc. [6,26]. Furthermore when the concentration of OTA in the feed is high, there is a high risk of residual OTA in the milk [27]. Few studies have been carried out for monitoring mycotoxins other than AFM1 in milk [28]. OTA has been previously reported in milk and its products [18]. Additionally, a sample contaminated at 2.730 μg/L has been found in Sudan, which indicates public health hazards [29]. For several reasons, it is critical to detect OTA in milk. The first is that OTA is extremely toxic and difficult to remove, the second is that OTA can access cow’s milk through feed, etc. and pose a hidden risk, and the third is that studies to detect OTA in milk are rare and have not received more attention, despite reports of high levels of OTA contamination in milk. Thus, OTA in milk could present an hidden risk. Therefore, testing for OTA in milk is necessary.
Another prominent toxin after aflatoxin is OTA, and S. aureus is a common pathogenic bacterium in milk. Pasteurized milk from ten urban districts in Beijing was sampled over the span of a year to evaluate the enterotoxin genes and the fungal toxin OTA as well as the relationship between enterotoxin genes and enterotoxin. As a result, this study focuses on biological risk factors in milk, including S. aureus and OTA, with a focus on toxins, to suggest the co-occurrence of S. aureus with enterotoxin genes and OTA in milk and the potential risk.
2. Results
2.1. Identification of S. aureus and Detection of Enterotoxin Genes
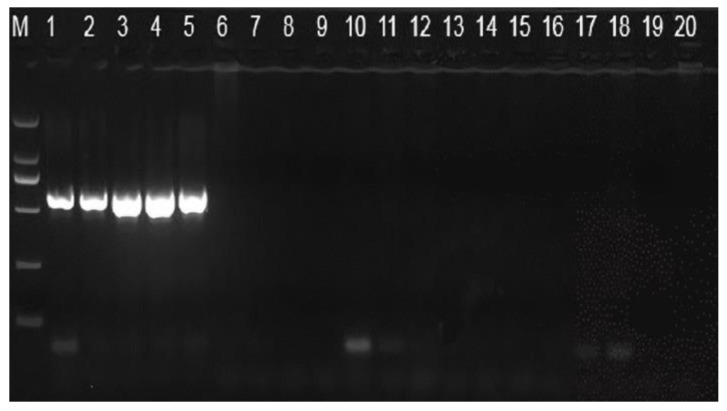
Forty-seven isolates of presumed S. aureus were isolated in 210 pasteurized milk. Twenty-nine out of 47 isolates were confirmed as S. aureus after coagulase, thermonuclear, biochemical tests and Polymerase Chain Reaction technology. As shown in Figure 1, in lanes 1–5, there is a bright band at 592 bp, which is the nuc gene. S. aureus isolates were further analyzed by PCR for the presence of the sea, seb, sec, sed, and see genes. The most frequently detected gene was seb (7; 24.14%) followed by sec (6; 20.69%), sea (5; 17.24%), sed (4; 13.79%), see (3; 10.34%) (Table 1).
Figure 1.
PCR amplification specificity detection of the nuc gene. M: D2000 marker; 1: Staphylococcus aureus ATCC25923; 2: S. aureus ATCC6538; 3: S. aureus CGMCC 1.89; 4: S. aureus CICC10786; 5: S. aureus MW2; 6: Salmonella; 7: Pseudomonas aeruginosa; 8: Bacillus cereus; 9: B. amyloliquefaciens; 10: Lactobacillus rhamnosus; 11: Lactobacillus; 12: L. Casei; 13: S. lentus; 14: S. haemolyticus; 15: S. Arlette; 16: S. epidermidis; 17: S. chromogenes; 18: S. cohnii; 19: S. sciuri; 20: S. saprophyticus.
Table 1.
Detection of enterotoxin genes of S. aureus.
| Enterotoxin Gene | sea | seb | sec | sed | see |
|---|---|---|---|---|---|
| Number | 5 | 7 | 6 | 4 | 3 |
| Proportion (%) | 17.24 | 24.14 | 20.69 | 13.79 | 10.34 |
2.2. Occurrence of OTA in Pasteurized Milk
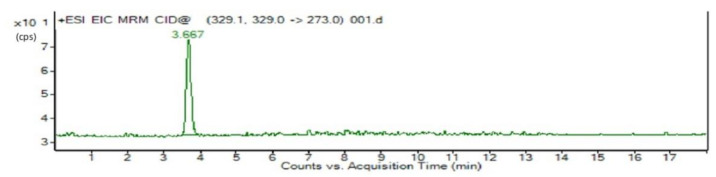
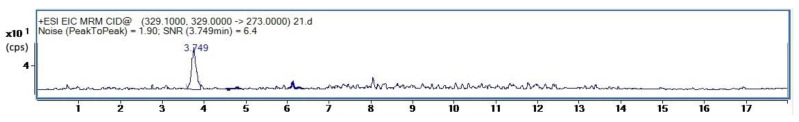
Figure 2 and Figure 3 respectively show the LC-MS/MRM chromatograms for the standard OTA and milk samples. In both figures, there is a peak that matches retention times of 3. 667 and 3.749 min, respectively.
Figure 2.
Chromatogram of OTA standard detection.
Figure 3.
Chromatogram of OTA detection in pasteurized milk.
The contamination levels of OTA in 120 pasteurized milk were evaluated in this work. The limit of detection (LOD) is 0.015 μg/L and the limit of quantification (LOQ) is 0.049 μg/L. OTA was found in 31 pasteurized milk samples (range 0.11–18.8 μg/L). 25.83% (31/120) of pasteurized milk was contaminated with OTA. 16.13% (5/31) of the samples had a contamination level of more than 10 μg/L (Table 2). Table S3 has provided detailed detect results for the entire year.
Table 2.
Contamination level of OTA in pasteurized milk.
| Contamination Level of OTA (μg/L) | Total | ||||
|---|---|---|---|---|---|
| >0.049 and <1.0 | 1.0–5.0 | 5.0–10.0 | >10.0 | ||
| Number | 21 | 2 | 3 | 5 | 31 |
| Proportion (%) | 17.50 | 1.67 | 2.5 | 4.16 | 25.83 |
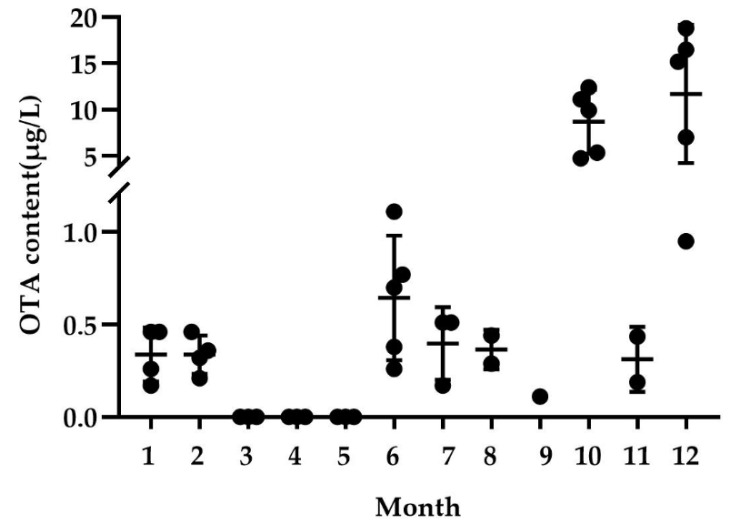
In this study, OTA was monitored throughout the year. The results show that the content of OTA detected in winter (October and December) was higher than that in summer (July–September). OTA was not detected from March to May. The content of OTA detected in December reached 18.80 μg/L (Figure 4).
Figure 4.
OTA-detection level in each month in pasteurized milk. Data from December 2014 to September 2015.
3. Discussion
Our results show the highest detection rate of seb. Some studies have also examined the classical enterotoxin genes in milk, with the highest detection rate of sed in Bianchi’s study at 25% (120/481) [12] and the highest detection rate of see in Grispoldi’s study at 47.06% (8/17) [17]. The most common enterotoxins produced by S. aureus isolated from dairy products of bovine or sheep origin were found in the literature to be SEC and SED [30]; the most common enterotoxin produced by S. aureus involved in food poisoning outbreaks was SEA [31]. This is probably related to the differences in the ecological reservoir of S. aureus in different countries and regions of the world [17]. The presence of enterotoxin-producing isolates of S. aureus in pasteurized milk means that failing to guarantee the cold chain could present a food safety risk, particularly if all enterotoxigenic isolates could potentially produce SEA in milk [17]. Research shows that more than half of S. aureus isolates contain at least one gene coding enterotoxin, indicating that milk contaminated with S. aureus is likely to cause food poisoning [32]. Our study results show that 17.24% of S. aureus in pasteurized milk contained sea gene. Therefore, pasteurized milk in these ten urban districts of Beijing may have potential food safety risks.
It is generally accepted that SE production constitutes a risk when S. aureus bacteria exceed a threshold of 105 S. aureus CFU/mL of milk during manufacture [33,34]. For example, the production of enterotoxins SEA and SEB are detected in milk when the count of S. aureus exceeds the critical level of 105 CFU/mL [35]. Many studies indicate that temperature and pH might also influence the expression of genes that code for the production of enterotoxins [36,37]. A study shows that the production of SEA can usually be detected at 10–45 °C and the yield of SEA increases with the increasing temperature [33,38]. A study indicated that the conditions for SEA production were pH of > 5.0 and aw of > 0.86 at temperatures of > 15 °C [36]. Undissociated lactic acid (1.6 mM compared to 0.2 mM) was reported to be able to increase SEA production of strain cocktails grown in BHI broth [39]. Another study observed sorbic acid stress (0.15%, pH 5) reduced SEA levels using S. aureus Sa17 [40]. In addition, different growth substrates lead to different growth behaviors of bacteria, which can also affect the production of enterotoxins [41]. Therefore, there are a variety of factors that influence the production of toxins.
There are few studies on the detection of OTA in milk because of dietary changes (high concentrate ratios and high feeding levels) that reduce protozoa’s capacity for OTA degradation, rumen microbial communities shift, increasing the likelihood that OTA may contaminate milk despite the fact that the rumen microbiota of cattle can degrade OTA [5]. Table 3 shows the detection of OTA in milk in China and abroad. Compared with our results, all are below our maximum detection. Although there are no regulations in other countries of the European Union for OTA in milk, Slovakia sets a limit of 5 µg/kg for milk [42]. Thus, 25.81% of the samples exceeded the Slovak limit for OTA in milk. Mycotoxin-producing strains in feed can multiply and produce toxins during the growth, harvest, and storage of crops. When cows consume contaminated feed, OTA is left in the milk through metabolism [43]. In addition, differences in climate and animal farming systems in different geographical regions may also lead to differences in OTA levels in milk [2]. In addition, different types of milk, such as organic and conventional milk, may also lead to differences in OTA levels due to differences in processing methods and the nutrients contained [44,45]. Some studies have also shown that pasteurized milk is more contaminated in the cold season than in the warm season. A study conducted by Ansari et al. in 2019 on pasteurized cow milk showed that during the cold seasons of the year compared to the warm seasons pasteurized milk samples were more contaminated [46]. Similar results were found in the study of Mokhtari [45]. In the cold season, due to the high humidity in the forage storage area, the possibility of growth of various fungi, including A. flavus and A. ochraceus in the forage and forage, will increase, so the contamination rate of OTA will increase. However, in the warmer season, starting around March, dairy farms have access to fresh feed, reducing the OTA content in milk [2]. At the same time, we noticed that the results for November, January, and February were anomalous compared to the results for October and December. The most important reason for this is that the samples from these three months were produced by cows late to feed of better quality.
Table 3.
Occurrence of OTA in milk at home and abroad.
| Country | Sample | Method of Analysis | LOD μg/kg | LOQ μg/kg |
Prevalence (%) | Range (μg/L or μg/kg |
Reference |
|---|---|---|---|---|---|---|---|
| China | raw cow milk | UHPLC-MS/MS | 0.004 | 0.012 | - | 0.0567–0.0841 | [47] |
| liquid cow milk | 0.003 | 0.009 | 0.0268–0.0579 | [47] | |||
| Italy | organic | LC-FD | - | 0.05 | 3/63 (4.8%) | 0.07–0.11 | [48] |
| Sudan | raw cow milk | HPLC-UV | - | - | 1/5 (20%) | 0.000–2.730 | [29] |
| France | raw cow milk | LC-FLD | - | - | 3/264 (1.1%) | 0.005–0.0066 | [49] |
| Sweden | raw cow milk | HPLC-FD | - | - | 5/36 (14%) | 0.010–0.040 | [50] |
| Norway | organic | LC-FLD | - | - | 5/47 (11%) | 0.015–0.028 | [51] |
| conventional | 6/40 (15%) | 0.011–0.058 | [51] |
“-”: Not detected.
Although aflatoxins, especially AFM1 are most commonly found in milk and dairy products in many other countries [1], our study found that AFM1 in milk from the Beijing area was well-controlled in the sample. AFM1 was not detected in 120 pasteurized milk. AFM1 was detected in two samples of 360 UHT milk, and the detected amounts were 0.27 μg/kg and 0.10 μg/kg, respectively. In contrast, OTA was detected in 22.22% (80/360) of UHT milk (Table S4.). In 2019, it was reported that the mean value of AFM1 and OTA in pasteurized milk was 0.01286 μg/kg and 0.135 μg/kg, respectively [44], which is consistent with our results. OTA is classified as a Group IIB carcinogen to humans by the International Agency for Research on Cancer [52]. A review of studies on OTA over the past 50 years suggests that the carcinogenicity of OTA may also occur in humans [18]. Although no direct evidence of carcinogenicity to the human body has been found at present, the contamination range of OTA is very wide, the contamination level is very high, and its harm is very great. Therefore, research on the real toxicity of OTA should be paid more attention to by more scholars.
Our research results show that OTA is a hidden risk in milk and it serves as a warning and calls attention to the detection of OTA in milk. Several studies in the last two years have examined the prevalence of OTA in different types of milk. A study conducted in 2016 reported OTA levels ranging from 0.34 to 13 μg/L. The detection rate was 80% (32/40) [5]. The detection levels of OTA in this reference were similar to our results. However, the detection rate was even higher than ours. The OTA detection values of several other papers were relatively small. As a result, OTA in milk is not now garnering more attention than before, and high levels of OTA are still detected in milk samples. Consequently, our work still has warning implications.
4. Conclusions
This study monitored S. aureus and OTA in pasteurized milk samples in Beijing throughout the year for the first time. The results of this study indicate that when failing to guarantee the cold chain, the presence of S. aureus with enterotoxin genes in milk will present a risk to food safety. Furthermore, the high detection rates and levels of OTA in milk suggest that OTA is a hidden risk. As a result, the findings of this study have some bearing and can be used as a reference point for biological risk factors in milk.
5. Materials and Methods
5.1. Sampling
From October 2014 to September 2015, a total of 210 pasteurized milk (including 90 copies of Brand A and 120 copies of Brand B) were bought from supermarkets located in 10 urban districts in Beijing, China. (Figure 5). All samples were delivered at 4 °C and analyzed within 24–48 h. In total, 210 samples of pasteurized milk were detected for S. aureus, and only 120 samples of Brand B were detected for OTA.
Figure 5.
Sampling location map. The red dot represents the actual sampling location.
5.2. Isolation and Detection of S. aureus
We carried out the culture and identification of S. aureus according to the methods described by GB 4789.10-2016. The samples were cultured at 37 °C and 200 rpm for 12 h for 18 h, then crossed on the selective Baird-Parker plate and cultured at 37 °C for 48 h. S. aureus colonies on B-P plates were round, 2–3 mm in diameter, gray or black in color, and surrounded by a turbid zone. The suspected colonies were selected for Gram staining and plasma coagulase test. Gram staining microscopic examination showed that S. aureus was Gram-positive cocci. The experiment on plasma coagulase is as follows. A single suspicious colony was picked from a Baird-Parker plate, inoculated into 5 mLBHI broth, and incubated at 37 °C for 18 h. In the ultra clean table, 0.5 mL of saline was added to the lyophilized rabbit plasma, shaken to dissolve it, then 0.2–0.3 mL of BHI culture was added, shaken well, placed in 37 °C incubator, and observed every half hour for 6 h. The positive result was determined if the volume of clotting or clotting was greater than half of the original volume. The broth culture of the positive plasma coagulase test was also used as the control.
Then the suspected colonies to increase the bacteria and extract the genome were picked out. DNA was extracted with the TIANamp Bacteria DNA Kit (OSR-M502, Tiangen, China). The nuc gene acts as a marker and also the presence of heat resistant nuclease gene (nuc) is strongly associated with the production of enterotoxin and it can be considered an indicator of infection with enterotoxin producer S. aureus [14]. Therefore, the nuc gene was amplified by PCR to identify the S. aureus. PCR was also used to detect the presence of the classic enterotoxin genes sea–see. The PCR reaction was conducted on a C1000 Toucah Thermal Cycler (Bio-Rad, Johannesburg, South Africa). The cycling conditions were initial denaturation at 94 °C for 5 min; followed by 30 cycles of 94 °C for 30 s, 64 °C for 30 s, 72 °C for 60 s, and a final elongation step of 72 °C for 10 min. The PCR products were stored at 4 °C and later separated by 1% agarose gel electrophoresis. D2000 DNA Marker was used. The PCR primers were designed with NCBI according to the nuclease gene sequence, as shown in Table 4. (GenBank: V01281.1, http://www.ncbi.nlm.nih.gov/nuccore/V01281.1, accessed on 2 March 2015).
Table 4.
Primers used in the detection of S. aureus and enterotoxin genes.
| Gene | Primer | Sequence (5’-3’) | Size (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|---|
| nuc | NUC-F | AGGGCAATACGCAAAGAGGTT | 592 | 62 | This work |
| NUC-R | TGAATCAGCGTTGTCTTCGC | ||||
| sea | SEA-F | TTGGAAACGGTTAAAACGAA | 120 | 50 | [53] |
| SEA-R | GAACCTTCCCATCAAAAACA | ||||
| seb | SEB-F | TCGCATCAAACTGACAAACG | 478 | 50 | [53] |
| SEB-R | GCAGGTACTCTATAAGTGCC | ||||
| sec | SEC-F | GACATAAAAGCTAGGAATTT | 257 | 50 | [53] |
| SEC-R | AAATCGGATTAACATTATCC | ||||
| sed | SED-F | CTAGTTTGGTAATATCTCCT | 317 | 50 | [53] |
| SED-R | TAATGCTATATCTTATAGGG | ||||
| see | SEE-F | TAGATAAAGTTAAAACAAGC | 170 | 50 | [53] |
| SEE-R | TAACTTACCGTGGACCCTTC |
“5’” and “3’” stand for the DNA sequence’s 3’ and 5’ ends, respectively.
5.3. Detection of OTA
5.3.1. OTA Extraction
We extracted OTA from samples according to the methods described by the GB 5009.96-2016. Pipette 5 mL of fresh milk into 50 mL centrifuge tube, add 20 mL of acetonitrile (84%), add 2 g of anhydrous magnesium sulfate and 1 g of sodium chloride, vortex 2 min, ultrasonic 20 min, and centrifuge at 1650 g for 5 min, 10 mL of the supernatant was evaporated dry in a rotary evaporator at 60 °C. Dissolve with 1mL of methanol, then add 1mL of water, mix well, and pass through a 0.22 μm filter membrane, to be analyzed. Each sample was analyzed in triplicate. OTA standard was purchased from J&K Scientific Ltd. Beijing, China.
5.3.2. LC-MS/MS Analysis
The LC-MS/MS analysis was performed as previously reported [47]. The sample extracts were analyzed in an isocratic elution with an Agilent Pro shell 120 EC C18 column (50 mm × 2.1 mm, 3.5 μm; Agilent Technologies, Little Fall, DE, USA) by using Agilent 1260 Infinity Quaternary LC system. The mobile phase consisted of (A) 0.1% formic acid aqueous solution and (B) Acetonitrile. The following linear gradient program was used: 20% B in 0–1 min; 20–65% B in 1–5 min; 65–85% B in 5–8 min; 85% B in 8–10 min; 85–20% B in 10–10.1 min; 20% B in 10.1–16 min. The flow rate was 0.3 mL/min while the injection volume was 10 μL.
Following separation, the column effluent was connected to a triple-quadrupole mass spectrometer (Agilent Technologies 6460, CA, U.S.A.) equipped with an ESI source. OTA was detected in positive mode using MRM. Data acquisition and mass spectrometric evaluation were carried out on a Mass Hunter Workstation (Agilent Technologies, Santa Clara, CA, USA). Operating conditions were: Spray Voltage–5500 V, Curtain Gas–30 psi, Temperature–500 °C, Gas 1–30 psi, Gas 2–50 psi, Entrance Potential (EP)–10 V, Collision Energy (CE)–21 eV. De-clustering Potential (DP)–61 V, Exit Potential (CXP)–20 V. The precursor ion was monitored and collision induced dissociation was used to generate product ions. The precursor ion was m/z 404, the product ion was m/z 358 and m/z 238.8, and the ion ratio was 1:1. To evaluate the linearity, five-point calibration curves were constructed to calculate the determination coefficients (R2). The signal-to-noise (S/N) approach was used to estimate the LOD and the LOQ. The gradient OTA standard solution was injected on the liquid chromatograph. The LOD and LOQ were defined based on signal (S)-to-noise (N) ratios of S/N > 3 and S/N > 10, respectively.
LC-MS/MS analysis was done at the Supervision, Inspection & Testing Center for Agricultural Products Quality.
5.3.3. Statistical Analysis
The data were analyzed by SPSS. The significance level was set at p < 0.05, and all experiments were replicated at least three times.
Acknowledgments
We gratefully acknowledge all the sampling members and the driver. We also thank Shengli Li from the State Key Laboratory of Animal Nutrition of CAU for supplying the raw milk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins14100718/s1, Table S1: Detection of Staphylococcus aureus in pasteurized milk. Data from December 2014 to September 2015. Unit is CFU/mL; Table S2: Detection rate of bacteria in pasteurized milk; Table S3: OTA detection level of each district in each month in pasteurized milk. Data from December 2014 to September 2015. “-”: not detected; Table S4: Detection of OTA in UHT milk and Pasteurized milk.
Author Contributions
Experiment, Y.S. and L.M.; data curation, Z.Z.; writing, original draft preparation, Y.S.; writing, review and editing, Z.Z.; supervision, Z.L.; project administration, Z.L.; funding acquisition, Z.L. and K.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
A year-round monitoring of the co-occurrence of S. aureus and OTA in pasteurized milk in ten urban districts of Beijing was carried out in this study. S. aureus and its classical enterotoxin genes were detected. The detection of OTA showed that the contamination rate and level were high, which is a hidden risk that is easy to be overlooked and needs attention.
Funding Statement
This work was supported by the Beijing Food Quality and Safety Laboratory, the National Natural Science Foundation of China, and the Beijing Advanced Innovation Center for China Food Nutrition and Human Health.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iqbal S.Z., Jinap S., Pirouz A.A., Ahmad Faizal A.R. Aflatoxin M1 in Milk and Dairy Products, Occurrence and Recent Challenges: A Review. Trends Food Sci. Technol. 2015;46:110–119. doi: 10.1016/j.tifs.2015.08.005. [DOI] [Google Scholar]
- 2.Mohammedi-Ameur S., Dahmane M., Brera C., Kardjadj M., Ben-Mahdi M.H. Occurrence and Seasonal Variation of Aflatoxin M1 in Raw Cow Milk Collected from Different Regions of Algeria. Vet. World. 2020;13:433–439. doi: 10.14202/vetworld.2020.433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahbazi Y., Ahmadi F., Fakhari F. Voltammetric Determination of Pb, Cd, Zn, Cu and Se in Milk and Dairy Products Collected from Iran: An Emphasis on Permissible Limits and Risk Assessment of Exposure to Heavy Metals. Food Chem. 2016;192:1060–1067. doi: 10.1016/j.foodchem.2015.07.123. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami R., Shahbazi Y., Nikousefat Z. Aflatoxin M1 in Milk and Traditional Dairy Products from West Part of Iran: Occurrence and Seasonal Variation with an Emphasis on Risk Assessment of Human Exposure. Food Control. 2016;62:250–256. doi: 10.1016/j.foodcont.2015.10.039. [DOI] [Google Scholar]
- 5.Younis G., Ibrahim D., Awad A., El Bardisy M.M. Determination of Aflatoxin M1 and Ochratoxin A in Milk and Dairy Products in Supermarkets Located in Mansoura City, Egypt. Adv. Anim. Vet. Sci. 2016;4:114–121. doi: 10.14737/journal.aavs/2016/4.2.114.121. [DOI] [Google Scholar]
- 6.Becheva Z.R., Ivanov Y.L., Godjevargova T.I., Tchorbanov A.I. Simultaneous Determination of Ochratoxin A and Enterotoxin A in Milk by Magnetic Nanoparticles Based Fluorescent Immunoassay. Food Addit. Contam. Part A. 2021;38:1218–1236. doi: 10.1080/19440049.2021.1914866. [DOI] [PubMed] [Google Scholar]
- 7.Dai J., Wu S., Huang J., Wu Q., Zhang F., Zhang J., Wang J., Ding Y., Zhang S., Yang X., et al. Prevalence and Characterization of Staphylococcus Aureus Isolated from Pasteurized Milk in China. Front. Microbiol. 2019;10:641. doi: 10.3389/fmicb.2019.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta R., Shetty S.A., Young M.F., Ryan P.B., Rangiah K. Quantification of Aflatoxin and Ochratoxin Contamination in Animal Milk Using UHPLC-MS/SRM Method: A Small-Scale Study. J. Food Sci. Technol. 2021;58:3453–3464. doi: 10.1007/s13197-021-04986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennekinne J.-A., De Buyser M.-L., Dragacci S. Staphylococcus aureus and Its Food Poisoning Toxins: Characterization and Outbreak Investigation. FEMS Microbiol. Rev. 2012;36:815–836. doi: 10.1111/j.1574-6976.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 10.Bania J., Dabrowska A., Bystron J., Korzekwa K., Chrzanowska J., Molenda J. Distribution of Newly Described Enterotoxin-like Genes in Staphylococcus Aureus from Food. Int. J. Food Microbiol. 2006;108:36–41. doi: 10.1016/j.ijfoodmicro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Wu S., Huang J., Wu Q., Zhang F., Zhang J., Lei T., Chen M., Ding Y., Xue L. Prevalence and Characterization of Staphylococcus Aureus Isolated from Retail Vegetables in China. Front. Microbiol. 2018;9:1263. doi: 10.3389/fmicb.2018.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi D.M., Gallina S., Bellio A., Chiesa F., Civera T., Decastelli L. Enterotoxin Gene Profiles of Staphylococcus aureus Isolated from Milk and Dairy Products in Italy. Lett. Appl. Microbiol. 2014;58:190–196. doi: 10.1111/lam.12182. [DOI] [PubMed] [Google Scholar]
- 13.Evenson M.L., Ward Hinds M., Bernstein R.S., Bergdoll M.S. Estimation of Human Dose of Staphylococcal Enterotoxin A from a Large Outbreak of Staphylococcal Food Poisoning Involving Chocolate Milk. Int. J. Food Microbiol. 1988;7:311–316. doi: 10.1016/0168-1605(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 14.Asao T., Kumeda Y., Kawai T., Shibata T., Oda H., Haruki K., Nakazawa H., Kozaki S. An Extensive Outbreak of Staphylococcal Food Poisoning Due to Low-Fat Milk in Japan: Estimation of Enterotoxin A in the Incriminated Milk and Powdered Skim Milk. Epidemiol. Infect. 2003;130:33–40. doi: 10.1017/S0950268802007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid D., Fretz R., Winter P., Mann M., Höger G., Stöger A., Ruppitsch W., Ladstätter J., Mayer N., de Martin A., et al. Outbreak of Staphylococcal Food Intoxication after Consumption of Pasteurized Milk Products, June 2007, Austria. Wien. Klin. Wochenschr. 2009;121:125–131. doi: 10.1007/s00508-008-1132-0. [DOI] [PubMed] [Google Scholar]
- 16.Wu S., Duan N., Gu H., Hao L., Ye H., Gong W., Wang Z. A Review of the Methods for Detection of Staphylococcus Aureus Enterotoxins. Toxins. 2016;8:176. doi: 10.3390/toxins8070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grispoldi L., Massetti L., Sechi P., Iulietto M.F., Ceccarelli M., Karama M., Popescu P.A., Pandolfi F., Cenci-Goga B.T. Short Communication: Characterization of Enterotoxin-Producing Staphylococcus Aureus Isolated from Mastitic Cows. J. Dairy Sci. 2019;102:1059–1065. doi: 10.3168/jds.2018-15373. [DOI] [PubMed] [Google Scholar]
- 18.Malir F., Ostry V., Pfohl-Leszkowicz A., Malir J., Toman J. Ochratoxin A: 50 Years of Research. Toxins. 2016;8:191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisvad J.C., Hubka V., Ezekiel C.N., Hong S.-B., Nováková A., Chen A.J., Arzanlou M., Larsen T.O., Sklenář F., Mahakarnchanakul W., et al. Taxonomy of Aspergillus Section Flavi and Their Production of Aflatoxins, Ochratoxins and Other Mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfohl-Leszkowicz A., Manderville R.A. An Update on Direct Genotoxicity as a Molecular Mechanism of Ochratoxin a Carcinogenicity. Chem. Res. Toxicol. 2012;25:252–262. doi: 10.1021/tx200430f. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J., Zhang Y., Xu W., Luo Y., Hao J., Shen X.L., Yang X., Li X., Huang K. Zinc Protects HepG2 Cells against the Oxidative Damage and DNA Damage Induced by Ochratoxin, A. Toxicol. Appl. Pharmacol. 2013;268:123–131. doi: 10.1016/j.taap.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T., Shen X.L., Chen W., Liao X., Yang J., Wang Y., Zou Y., Fang C. Advances in Research of Nephrotoxicity and Toxic Antagonism of Ochratoxin, A. Toxin Rev. 2017;36:39–44. doi: 10.1080/15569543.2016.1243560. [DOI] [Google Scholar]
- 23.Samuel M.S., Jeyaram K., Datta S., Chandrasekar N., Balaji R., Selvarajan E. Detection, Contamination, Toxicity, and Prevention Methods of Ochratoxins: An Update Review. J. Agric. Food Chem. 2021;69:13974–13989. doi: 10.1021/acs.jafc.1c05994. [DOI] [PubMed] [Google Scholar]
- 24.Boudra H., Le Bars P., Le Bars J. Thermostability of Ochratoxin A in Wheat under Two Moisture Conditions. Appl. Env. Microbiol. 1995;61:1156–1158. doi: 10.1128/aem.61.3.1156-1158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinotti L., Ottoboni M., Giromini C., Dell’Orto V., Cheli F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins. 2016;8:45. doi: 10.3390/toxins8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar P., Mahato D.K., Sharma B., Borah R., Haque S., Mahmud M.M.C., Shah A.K., Rawal D., Bora H., Bui S. Ochratoxins in Food and Feed: Occurrence and Its Impact on Human Health and Management Strategies. Toxicon. 2020;187:151–162. doi: 10.1016/j.toxicon.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Tale H., Joshaghani H., Rahimzadeh H., Niknejad F., Kiaie M. Ochratoxin a in Cow’s Milk Collected from Cattle Farms of Golestan Province. Med. Lab. J. 2016;10:13–16. [Google Scholar]
- 28.Flores-Flores M.E., Lizarraga E., López de Cerain A., González-Peñas E. Presence of Mycotoxins in Animal Milk: A Review. Food Control. 2015;53:163–176. doi: 10.1016/j.foodcont.2015.01.020. [DOI] [Google Scholar]
- 29.Elzupir A.O., Makawi S., Elhussein A.M. Determination of Aflatoxins and Ochratoxin a in Dairy Cattle Feed and Milk in Wad Medani, Sudan. J. Anim. Vet. Adv. 2009;12:2508–2511. [Google Scholar]
- 30.Vitale M., Gaglio S., Galluzzo P., Cascone G., Piraino C., Di Marco Lo Presti V., Alduina R. Antibiotic Resistance Profiling, Analysis of Virulence Aspects and Molecular Genotyping of Staphylococcus aureus Isolated in Sicily, Italy. Foodborne Pathog. Dis. 2018;15:177–185. doi: 10.1089/fpd.2017.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastos C.P., Bassani M.T., Mata M.M., Lopes G.V., da Silva W.P. Prevalence and Expression of Staphylococcal Enterotoxin Genes in Staphylococcus Aureus Isolated from Food Poisoning Outbreaks. Can. J. Microbiol. 2017;63:834–840. doi: 10.1139/cjm-2017-0316. [DOI] [PubMed] [Google Scholar]
- 32.Seyoum E.T., Mekonene T.K., Woldetsadik D.A., Zewudie B.M., Gebreyes W.A. Enterotoxin Gene Profile of Staphylococcus Aureus Isolates Recovered from Bovine Milk Produced in Central Ethiopia. J. Infect. Dev. Ctries. 2016;10:138–142. doi: 10.3855/jidc.6797. [DOI] [PubMed] [Google Scholar]
- 33.Babić M., Pajić M., Radinović M., Boboš S., Bulajić S., Nikolić A., Velebit B. Effects of Temperature Abuse on the Growth and Staphylococcal Enterotoxin A Gene (Sea) Expression of Staphylococcus aureus in Milk. Foodborne Pathog. Dis. 2019;16:282–289. doi: 10.1089/fpd.2018.2544. [DOI] [PubMed] [Google Scholar]
- 34.Duquenne M., Fleurot I., Aigle M., Darrigo C., Borezée-Durant E., Derzelle S., Bouix M., Deperrois-Lafarge V., Delacroix-Buchet A. Tool for Quantification of Staphylococcal Enterotoxin Gene Expression in Cheese. Appl. Environ. Microbiol. 2010;76:1367–1374. doi: 10.1128/AEM.01736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Necidová L., Janštová B., Karpíšková R. Dynamics of Staphylococcal Enterotoxin Production in Model Experiments Simulating the Fresh Cheese Environment. Acta Vet. Brno. 2012;81:391–396. doi: 10.2754/avb201281040391. [DOI] [Google Scholar]
- 36.Ding T., Yu Y.-Y., Hwang C.-A., Dong Q.-L., Chen S.-G., Ye X.-Q., Liu D.-H. Modeling the Effect of Water Activity, PH, and Temperature on the Probability of Enterotoxin A Production by Staphylococcus Aureus. J. Food Prot. 2016;79:148–152. doi: 10.4315/0362-028X.JFP-15-161. [DOI] [PubMed] [Google Scholar]
- 37.Schelin J., Susilo Y.B., Johler S. Expression of Staphylococcal Enterotoxins under Stress Encountered during Food Production and Preservation. Toxins. 2017;9:401. doi: 10.3390/toxins9120401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Homsombat T., Boonyayatra S., Awaiwanont N., Pichpol D. Effect of Temperature on the Expression of Classical Enterotoxin Genes among Staphylococci Associated with Bovine Mastitis. Pathogens. 2021;10:975. doi: 10.3390/pathogens10080975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosengren Å., Lindblad M., Lindqvist R. The Effect of Undissociated Lactic Acid on Staphylococcus Aureus Growth and Enterotoxin A Production. Int. J. Food Microbiol. 2013;162:159–166. doi: 10.1016/j.ijfoodmicro.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Zeaki N., Rådström P., Schelin J. Evaluation of Potential Effects of NaCl and Sorbic Acid on Staphylococcal Enterotoxin A Formation. Microorganisms. 2015;3:551–566. doi: 10.3390/microorganisms3030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker-York-Moore L., Moore S., Fox E. Characterization of Enterotoxigenic Bacillus Cereus Sensu Lato and Staphylococcus Aureus Isolates and Associated Enterotoxin Production Dynamics in Milk or Meat-Based Broth. Toxins. 2017;9:225. doi: 10.3390/toxins9070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altafini A., Roncada P., Guerrini A., Minkoumba Sonfack G., Fedrizzi G., Caprai E. Occurrence of Ochratoxin A in Different Types of Cheese Offered for Sale in Italy. Toxins. 2021;13:540. doi: 10.3390/toxins13080540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadi E. Potential Public Health Risk Due to Consumption of Contaminated Bovine Milk with Aflatoxin M1 and Coxiella burnetii in the West of Iran. Int. J. Dairy Technol. 2020;73:479–485. doi: 10.1111/1471-0307.12687. [DOI] [Google Scholar]
- 44.Turkoglu C., Keyvan E. Determination of Aflatoxin M1 and Ochratoxin A in Raw, Pasteurized and UHT Milk in Turkey. Acta Sci. Vet. 2019:47. doi: 10.22456/1679-9216.89667. [DOI] [Google Scholar]
- 45.Mokhtari S.A., Nemati A., Fazlzadeh M., Moradi-Asl E., Ardabili V.T., Seddigh A. Aflatoxin M1 in Distributed Milks in Northwestern Iran: Occurrence, Seasonal Variation, and Risk Assessment. Environ. Sci. Pollut. Res. 2022;29:41429–41438. doi: 10.1007/s11356-021-18212-9. [DOI] [PubMed] [Google Scholar]
- 46.Ansari F., Pourjafar H., Christensen L. A Study on the Aflatoxin M1 Rate and Seasonal Variation in Pasteurized Cow Milk from Northwestern Iran. Env. Monit. Assess. 2018;191:6. doi: 10.1007/s10661-018-7141-1. [DOI] [PubMed] [Google Scholar]
- 47.Huang L.C., Zheng N., Zheng B.Q., Wen F., Cheng J.B., Han R.W., Xu X.M., Li S.L., Wang J.Q. Simultaneous Determination of Aflatoxin M1, Ochratoxin A, Zearalenone and α-Zearalenol in Milk by UHPLC–MS/MS. Food Chem. 2014;146:242–249. doi: 10.1016/j.foodchem.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 48.Pattono D., Gallo P.F., Civera T. Detection and Quantification of Ochratoxin A in Milk Produced in Organic Farms. Food Chem. 2011;127:374–377. doi: 10.1016/j.foodchem.2010.12.051. [DOI] [Google Scholar]
- 49.Boudra H., Barnouin J., Dragacci S., Morgavi D.P. Aflatoxin M1 and Ochratoxin A in Raw Bulk Milk from French Dairy Herds. J. Dairy Sci. 2007;90:3197–3201. doi: 10.3168/jds.2006-565. [DOI] [PubMed] [Google Scholar]
- 50.Anna B.E., Monica O., Agneta O., Ira P., Karl H. Ochratoxin A in Cow’s Milk and in Human Milk with Corresponding Human Blood Samples. J. AOAC Int. 1993;76:842–846. doi: 10.1016/0165-2370(93)85018-T. [DOI] [PubMed] [Google Scholar]
- 51.Skaug M.A. Analysis of Norwegian Milk and Infant Formulas for Ochratoxin, A. Food Addit. Contam. 1999;16:75–78. doi: 10.1080/026520399284235. [DOI] [PubMed] [Google Scholar]
- 52.Vainio H., Heseltine E., Wilbourn J. Report on an IARC Working Group Meeting on Some Naturally Occurring Substances. Int. J. Cancer. 1993;53:535–537. doi: 10.1002/ijc.2910530402. [DOI] [PubMed] [Google Scholar]
- 53.Mehrotra M., Wang G., Johnson W.M. Multiplex PCR for Detection of Genes for Staphylococcus Aureus Enterotoxins, Exfoliative Toxins, Toxic Shock Syndrome Toxin 1, and Methicillin Resistance. J. Clin. Microbiol. 2000;38:1032–1035. doi: 10.1128/JCM.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.