Abstract
Lung neoplasm is the main cause of cancer-related mortality, and bone metastasis is among the most common secondary tumors. The vast majority of patients also present with multiple bone metastases, which makes systemic and adjuvant pain therapy preferable to surgery. The optimal approach for a resectable non-small-cell lung tumor that also presents a unique, resectable bone metastasis is not fully established. The number of papers addressing this subject is small, and most are case reports; nevertheless, survival rates seem to increase with radical surgery. The sequencing of local versus systemic treatment should always be discussed within the multidisciplinary team that will choose the best approach for each patient. As targeted systemic therapies become more accessible, radical surgery, together with existing reconstructive methods, will lead to an increase in life expectancy and a better quality of life.
Keywords: lung cancer, solitary bone metastasis, bone metastasis resection reconstruction, radical therapy, targeted therapy
1. Introduction
Lung cancer is the leading cause of cancer-related deaths, and bone metastasis is one of the most prevalent secondary malignancies. Because the vast majority of patients have numerous bone metastases, systemic and adjuvant pain treatment is preferred over surgery. With an average survival of less than a year, these patients have a poor prognosis [1].
Bone metastases are associated with increased morbidity, loss of function, and decreased quality of life [2,3]. The main locations of metastases secondary to non-small-cell lung cancers (NSCLC) are the spine in 50% of cases, the ribs in 27.1%, the iliac bone in 10%, the sacrum in 7.1%, and the femur in 5.7% of cases, followed by the humerus, scapula, and sternum at 2.9% [4]. We noted from this study that, in most cases, the curative treatment for a bone metastasis secondary to a lung neoplasm could not consist of a resection due to the anatomical location.
Bone metastases are often asymptomatic and represent the first symptom in only 2.5% of lung neoplasm cases [5]. During the spread of the disease, 80% of patients with lung neoplasm complain of bone pain [6].
A very small number, approximately 7%, of patients diagnosed with non-small-cell lung neoplasm present a single metastasis at the initial evaluation [7]. According to current published guidelines, a non-small-cell lung neoplasm with a single brain or adrenal metastasis can be removed by synchronous metastasectomy if the lung tumor is also resectable [8,9,10].
The best course of action for a resectable lung tumor that also has a solitary, resectable bone metastasis is still up for debate. Despite the fact that there are few articles on this topic and that the majority of them are case reports, after extensive surgery, patient survival rates appear to improve [11,12].
The present study analyzed a small category of patients with lung neoplasms that were associated with single bone metastasis. Synchronous surgical treatments to resect the non-small-cell lung neoplasms lead to significant increases in medium- and long-term survival. We reviewed 41 papers that presented several unique bone metastases that were secondary to pulmonary malignancies.
2. Pathophysiology of Bone Metastasis
Bone neoplastic development results from an alteration in the perfectly balanced bone-remodeling cycle between bone resorption by osteoclasts and bone tissue production mediated by osteoblasts [13]. Metastatic pathophysiology consists of excessive osteoclastic stimulation that leads to bone resorption. The bone matrix releases cytokines that stimulate tumor growth, thus creating a vicious circle of bone resorption and tumor stimulation. The main culprits in this mechanism are parathyroid-hormone-related peptides (PTHrP) and Interleukin 8 [14,15,16]. Disruption to the receptor activator of the nuclear factor kappa (RANK)–receptor activator of the nuclear factor kappa B ligand (RANKL)–osteoprotegerin (OPG) axis is the basis for the development of bone metastasis [17,18]. Those that are secondary to lung neoplasms are osteolytic, but osteoblastic forms have also been described.
An important aspect of the development of osteolytic bone metastases is the secondary hypercalcemia that can occur. Correct hydration, diuretics, and even glucocorticoids can be used in these cases. The intravenous administration of bisphosphonates, together with adequate hydration, can lead to the normalization of calcium levels [19].
3. Skeletal Metastasis Secondary to Lung Cancer
Lung neoplasms are among the most common primary malignancies that metastasize to bones. Patients with a bone tumor lesion are divided into two categories: those with and without a known history of lung neoplasm. In the face of a suspicious bone lesion, a primary bone neoplasm must always be excluded. Within a multidisciplinary team, all decisions regarding staging and preoperative diagnosis are made before the treatment begins. A bone biopsy is mandatory to establish the origin of the primary tumor formation.
A separate category of patients is represented by those who present at the emergency room with a pathological bone fracture. Their staging and preoperative diagnosis must follow the same steps, although the fracture itself may represent an emergency.
The preoperative imaging evaluation is very important because it must make sure that the bone lesion is unique; only when this has been confirmed can curative treatment be discussed. In this context, a full imaging evaluation is required (thoracic–abdominal and pelvic-computed tomography (CT) scan, brain MRI, and bone scintigraphy or whole-body PET). Mediastinoscopy is becoming mandatory to exclude other secondary determinations.
The incidence of single bone metastases is difficult to determine, given the rarity of cases. The vast majority of published works are case reports or limited case series; nevertheless, a resectable lung neoplasm with a resectable single bone metastasis must be treated aggressively so that long-term survival can be improved.
A 2016 study that analyzed five non-small-cell lung cancer (NSCLC) patients exhibiting solitary bone metastasis concluded that the progression-free survival time improved on those rare occasions when synchronous aggressive surgical management was performed [20].
Another study, published in 2005, analyzed the evolution of two cases of lung neoplasm with single bone metastasis and found a survival rate of over five years for patients treated with surgical resection [12]. Another case report presented a patient who survived for more than eight years from diagnosis after a metastasectomy [11]. This approach may seem to have been a solution in these cases, but it should be performed only when the primary tumor can be controlled and R0 resection is possible [21,22].
A correct and complete evaluation by an experienced multidisciplinary team should guide the diagnosis and therapeutic strategy for this category of patient.
4. Sequencing of Treatment
Oligometastatic NSCLC refers to a limited number of metastases in a limited number of organs. The biology of the disease in these patients may have a slower evolution. The standard number of metastases has not been established, but a patient with fewer than five is considered oligometastatic. Their presentation may occur at the same time as the diagnosis or at a distance from definitive treatment [23].
The sequencing of local versus systemic treatment is not yet established. It is preferable to start with systemic treatments to verify the mild nature of the disease, but many clinical situations––the presence of a cerebral or bone metastasis with the risk of fracture or pain––require local treatment first [24].
The choice of systemic treatment is made according to the molecular profile of the tumor. At the metastatic NSCLC stage, broad molecular profiling of the tumor is indicated to identify all biomarkers in a single analysis and to include biomarkers being evaluated for targetability. However, treatment availability depends on the reimbursement condition and accessible clinical trials.
Among the more important biomarkers we mentioned are PD-L1, EGFR mutations, ALK translocation, K-RAS, RAS1, BRAF, NTRK1/2/3, RET rearrangement, and METex14. Broad molecular profiling should be considered for lung adenocarcinoma and squamous cell carcinomas alike [25].
If the molecular tests are negative for all of the above, systemic treatment is selected according to PD-L1. If the PD-L1 values are negative or below 50%, the alternatives are combined immunotherapy and chemotherapy; doublet immunotherapy; or combined immunotherapy, chemotherapy, and antiangiogenic treatment. If the PD-L1 values are over 50%, immunotherapy alone will be chosen [26].
Table 1 shows the systemic treatment options when targetable molecular alterations are present [27].
Table 1.
Systemic treatment options depending on genetic alterations.
| Genetic Alteration | Possible Systemic Treatment |
|---|---|
| EGFR exon 19 deletion or L858R mutation | osimertinib |
| EGFR S768I, L861Q, G719X mutation | afatinib or osimertinib |
| EGFR insertion exon 20 | amivantamab or mobocertinib |
| KRAS G12C mutation | sotorasib |
| ALK rearrangement | alectinib or lorlatinib or brigatinib |
| ROS1 rearrangement | entrectinib or crizotinib |
| NTRK 1/2/3 gene fusion | larotrectinib or entrectinib |
| MET exon14 skipping mutation | capmatinib or tepotinib |
| RET rearrangement | serpercatinib |
5. Indications for Surgical Treatment
5.1. Surgical Treatment Indications and Techniques Used in Single Bone Metastases Associated with Lung Cancer
There is no generally valid management option for patients presenting with lung cancer and single bone metastasis in the extrathoracic area (M1b metastasis staging site according to the TNM 8 classification of lung cancer). Therapeutic intervention is usually individualized according to prognostic factors (prognosis after bone metastasis, the number of metastases, performance status) and pain level [28,29,30].
The aim of the primary approach to the bone mass is to establish with certainty its metastatic origin and alleviate symptoms. Metastases at the level of the thoracic cage, in addition to being painful, interfere with respiration [31]. This may exacerbate lung-cancer-related respiratory impairment; on the other hand, respiratory dysfunctions affecting tolerance to general anesthesia can also make surgery questionable [30].
Surgical treatment of lung cancer can be performed simultaneously with the resection of the bone tumor within oncological limits if it is located ipsilaterally or at a distance, possibly after several courses of conversion chemotherapy, depending on the tumor location and bronchial tree status (Figure 1).
Figure 1.
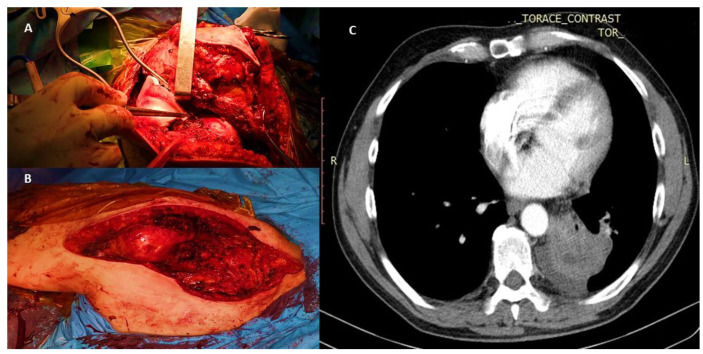
A 65-year-old man with a pulmonary neoplasm of the left-upper lobe confirmed by fibrobronchoscopy with right-scapular metastasis and invasion of adjacent soft tissues. Prior to radical pulmonary surgery, the patient underwent partial resection of the scapula (internal third), acromion, coracoid process, and lateral end of the clavicle (external third), together with the invaded soft tissue (subscapularis, suprascapularis, and infraspinatus muscles; humeral and clavicular insertion of the pectoralis major muscle; and insertion of the pectoralis minor and deltoid muscle). (A) Intra-operative view of scapular tumor mass (external third) invading the soft tissues and integument. (B) Post-excision tumoral aspect with soft tissue reconstruction that did not require prosthetic materials or skin graft. (C) CT scan of a left-upper lobe lung tumor diagnosed as lung cancer by fibrobronchoscopy.
Because the primary lung tumor may progress during the recovery period that would be required after a bone metastasectomy, the preferred initial approach to the primary tumor is by various types of anatomic and nonanatomic lung resection and radical mediastinal lymphadenectomy.
Depending on the location, size, and extension of the lung tumor and respiratory and general functional status, several types of classical/open or minimally invasive surgery can be performed, the latter being preferred due to reduced pain and a shorter hospital stay. The following allow a faster recovery so that the patient may continue the therapeutic algorithm (orthopedic surgery versus adjuvant chemotherapy): nonanatomic (wedge) resections and anatomic resections (segmentectomies, lobectomies, bilobectomies) and pneumonectomies. Of these, lobectomy has been shown to increase overall survival rates in patients with NSCLC and a single metastasis compared to other types of lung resection [32].
Furthermore, it has been established that surgical intervention for bone metastases should take into account expected survival times [33].
Biopsies of single bone metastases are mandatory for diagnosis in order to exclude primary synchronous bone tumors, which require a different therapeutic approach [34].
Radical lung resection is only possible when the lymph node component is N0–N2, with N3 requiring neoadjuvant chemotherapy.
The resection of bone metastases may precede lung resection in several situations, such as pathological bone fractures [35]
In the presence of a significant chronic algic syndrome secondary to bone metastasis, the sequence of interventions consists of a resection (within oncological limits) of the bone tumor followed by surgical treatment of the lung cancer with or without neoadjuvant chemotherapy [36].
In the case of neurological damage (paresis, paralysis), spinal decompression surgery is performed first (M1b spine vertebral decompression), followed by lung surgery [34,35].
In addition, for spinal metastases with a survival expectancy of more than six months, open surgery is recommended, usually followed by radiotherapy [37].
Regarding resection expansions, the survival rate in the metastatic bone stage of lung cancer is so poor that an extended perimetastatic resection is not justified [38].
M1b-rib requires a resection within the oncological limits of the affected rib segment with parietal reconstruction for the prevention of paradoxical breathing.
M1b-sternum requires a partial resection of the sternum (manubrium +/− resection of the clavicular head or internal third of the clavicle +/− adjacent costal cartilages, or body, depending on location) followed by solid and soft tissue reconstruction. The reconstruction can be performed either with synthetic materials (polypropylene mesh, methylmethacrylate) or different cutaneous or myocutaneous flaps [39].
In patients with M1b-clavicle, a partial or total claviculectomy may be performed and is followed, or not, by reconstruction to restore the scapular girdle function [40,41].
M1b-scapula requires a partial or total resection associated with the resection of the head/external third of the clavicle/humeral head according to the invasion type.
There are circumstances in which bone metastasis has invaded the adjacent soft tissue, requiring extensive resection (thoracic parietectomies) and scapular resection along with the adjacent invaded musculature.
5.2. Resection and Reconstruction of Solitary Bone Lesions in Lung Cancer
Following an interdisciplinary consensus, the correct therapeutic procedure is indicated. A single metastasis secondary to a lung carcinoma will be treated by a wide surgical resection within oncological limits, followed by a reconstruction with modular prostheses (Figure 2). The resection followed by reconstruction is the most effective technique for maintaining postoperative function and preserving quality of life, given that secondary interventions will be avoided, such as those that occur following the degradation of osteosynthesis materials.
Figure 2.
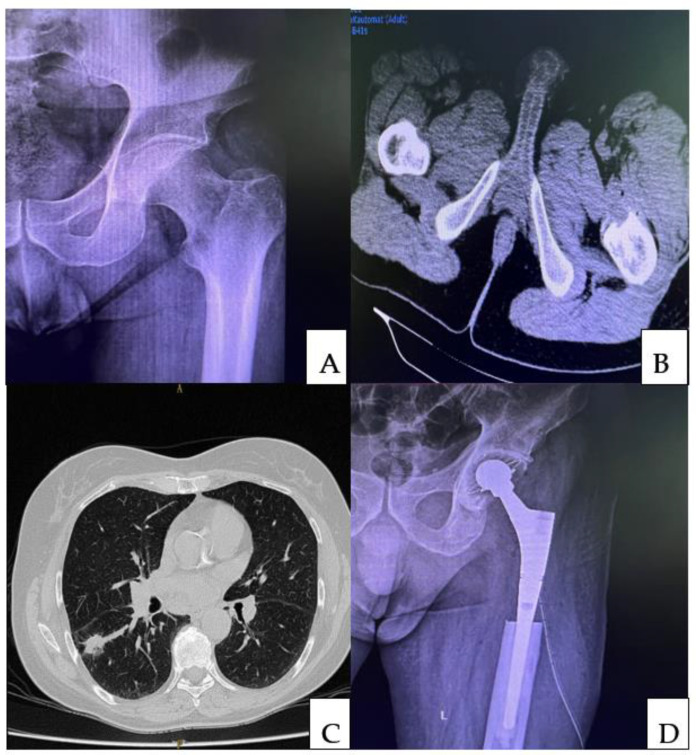
Pre-operative X-ray (A) of a 49-year-old male complaining of pain in the left hip that started three months earlier. An area of osteolysis at the level of the lesser trochanter was detected on the radiograph of the left hip. A computed tomographic (CT) scan of the pelvis (B) was performed, which revealed an osteolytic tumor at the level of the lesser trochanter. Following the biopsy, a diagnosis of bone metastasis secondary to a lung carcinoma was made. A chest CT scan revealed a spiculated, iodophilic nodule with retraction of the overlying pleura at the level of the apical segment of the right lower lobe (S6—Fowler) (C). The preoperative staging was a pulmonary tumor with single bone metastasis. Following multidisciplinary consensus, the patient was subjected to the radical resection of the pulmonary tumor followed by reconstruction of the left proximal femur (D).
Currently, the vast majority of joints can be reconstructed; moreover, an entire femur or humerus can be prosthetically reconstructed if the following series of requirements are respected: correct preoperative planning, precise resection margins, correct restoration of the length of a limb, optimal fixation to bear important loads, and the restoration of muscle insertion, so that muscle function is not altered.
In particular, metastases located at the level of the femoral neck can be treated by arthroplasty using long femoral stems. Cephalomedullary rods are not an option due to the high mechanical stress from the neck and the lesion that remains, which bcomes progressively larger.
5.3. Treatment of Complete or Impending Fractures in Unique Bone Metastasis following Lung Cancer—Resection Followed by Intramedullary Nails and Acrylic Bone Cement
When facing a fractured or prefractured bone metastasis, even if it is unique, clinicians must balance whether a resection followed by reconstruction with a tumoral prosthesis respects the desired goal and, more precisely, if the resection should be R0. In these cases, it is preferable to perform as wide a resection as possible and use centromedullary rods reinforced with acrylic cement as a reconstructive method, followed by careful imaging and monitoring (Figure 3). During the second phase of treatment, the osteosynthesis method is replaced by a modular prosthesis. It is a method that allows waiting for the distal femur and proximal tibia not only until the oncological prognosis is good but until a definitive reconstruction can be performed.
Figure 3.
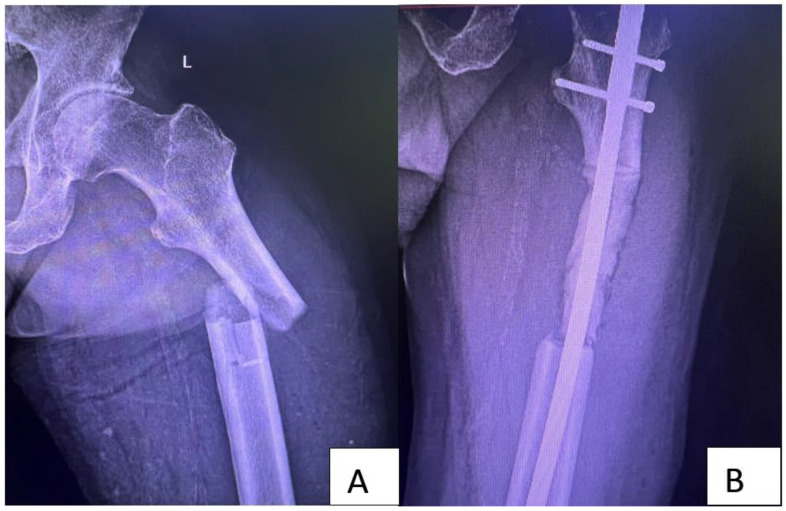
Preoperative X-ray (A) of a 56-year-old male with a history of recently diagnosed non-small-cell lung cancer (NSCLC) and a pathological bone fracture. After multidisciplinary evaluation, no other secondary tumor was found. Due to the fracture, the approach chosen was metastatic resection followed by intramedullary nail and acrylic bone cement (B). The conduct in the case of this patient was to carry out periodic imaging evaluations and to replace the nail if the local and general situation allowed a push-through modular component. Imaging evaluations performed at 12 months detected multiple metastases, so replacement with a modular component was abandoned.
6. Conclusions
Due to the relatively small number of patients with a lung neoplasm and single metastasis or oligometastasis, there are no large-scale randomized studies that investigate the therapeutic approach with certainty. Following the reporting of some case series and case presentations, it can be concluded that survival is considerably increased for these patients after radical therapy.
The sequencing of treatment will be conducted by discussing each clinical case within the multidisciplinary team. Depending on the location of the metastasis and its evolution, treatment can sometimes be urgent, such as a pathological bone fracture or pre fracture stage.
Correct and radical surgical treatment following a multidisciplinary consensus, together with the existing reconstructive methods, can lead both to an increase in life expectancy and an increase in the quality of life.
As targeted systemic therapies become more accessible, radical surgical treatment, along with local treatments, will increase the survival of these patients.
Acknowledgments
Not applicable.
Author Contributions
Conceptualization, C.-E.N., B.C. and C.S.; methodology, C.-E.N.; software, A.C. (Adrian Ciuche); validation, C.N., A.C. (Adrian Cursaru) and A.P.C.; formal analysis, C.N. and C.S.; investigation, B.C. and A.C. (Adrian Cursaru); resources, B.S.; data curation, A.P.C.; writing—original draft preparation, C.-E.N. and B.C.; writing—review and editing, C.-E.N., C.C. and C.N.; visualization, B.S.; supervision, C.-E.N., C.C. and C.N.; project administration, B.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University Emergency Hospital Bucharest (no. 40218/15 June 2022).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Further data concerning the study can be obtained by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coleman R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Berenson J.R., Rajdev L., Broder M. Managing bone complications of solid tumors. Cancer Biol. Ther. 2006;5:1086–1089. doi: 10.4161/cbt.5.9.3308. [DOI] [PubMed] [Google Scholar]
- 3.Mundy G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Tsuya A., Kurata T., Tamura K., Fukuoka M. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer. 2007;57:229–232. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Kagohashi K., Satoh H., Ishikawa H., Ohtsuka M., Sekizawa K. Bone metastasis as the first manifestation of lung cancer. Int. J. Clin. Pr. 2003;57:184–186. [PubMed] [Google Scholar]
- 6.Kosteva J., Langer C. Incidence and distribution of skeletal metastases in the era of PET. Lung Cancer. 2004;46:S45. [Google Scholar]
- 7.Ettinger D.S., Wood D.E., Akerley W., Bazhenova L.A., Borghaei H., Camidge D.R., Cheney R.T., Chirieac L.R., D’Amico T.A., Demmy T.L., et al. Non–Small Cell Lung Cancer, Version 6. J. Natl. Compr. Cancer Netw. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 8.Hu C., Chang E.L., Hassenbusch S.J., 3rd, Allen P.K., Woo S.Y., Mahajan A., Komaki R., Liao Z. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106:1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 9.Raviv G., Klein E., Yellin A., Schneebaum S., Ben-Ari G. Surgical treatment of solitary adrenal metastases from lung carcinoma. J. Surg. Oncol. 1990;43:123–124. doi: 10.1002/jso.2930430212. [DOI] [PubMed] [Google Scholar]
- 10.Reyes L., Parvez Z., Nemoto T., Regal A.-M., Takita H. Adrenalectomy for adrenal metastasis from lung carcinoma. J. Surg. Oncol. 1990;44:32–34. doi: 10.1002/jso.2930440108. [DOI] [PubMed] [Google Scholar]
- 11.Agarwala A.K., Hanna N.H. Long-Term Survival in a Patient with Stage IV Non–Small-Cell Lung Carcinoma After Bone Metastasectomy. Clin. Lung Cancer. 2005;6:367–368. doi: 10.3816/CLC.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 12.Hirano Y., Oda M., Tsunezuka Y., Ishikawa N., Watanabe G. Long-term survival cases of lung cancer presented as solitary bone metastasis. Ann. Thorac. Cardiovasc. Surg. 2005;11:401–404. [PubMed] [Google Scholar]
- 13.Diel I.J., Mundy G.R. Bisphosphonates in the adjuvant treatment of cancer: Experimental evidence and first clinical results. Br. J. Cancer. 2000;82:1381–1386. doi: 10.1054/bjoc.1999.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendre M.S., Margulies A.G., Walser B., Akel N.S., Bhattacharrya S., Skinner R.A., Swain F., Ramani V., Mohammad K.S., Wessner L.L., et al. Tumor-Derived Interleukin-8 Stimulates Osteolysis Independent of the Receptor Activator of Nuclear Factor-κB Ligand Pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 15.Guise T.A., Mohammad K.S., Clines G., Stebbins E.G., Wong D.H., Higgins L.S., Vessella R., Corey E., Padalecki S., Suva L., et al. Basic Mechanisms Responsible for Osteolytic and Osteoblastic Bone Metastases. Pt 2Clin. Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 16.Guise T.A., Yin J.J., Taylor S.D., Kumagai Y., Dallas M., Boyce B.F., Yoneda T., Mundy G.R. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones D.H., Nakashima T., Sanchez O.H., Kozieradzki I., Komarova S.V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C.V., et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 18.Berenson J.R., Rajdev L., Broder M. Pathophysiology of bone metastases. Cancer Biol. Ther. 2006;5:1078–1081. doi: 10.4161/cbt.5.9.3306. [DOI] [PubMed] [Google Scholar]
- 19.Lewis M.A., Hendrickson A.W., Moynihan T.J. Oncologic emergencies: Pathophysiology, presentation, diagnosis, and treatment. CA A Cancer J. Clin. 2011;61:287–314. doi: 10.3322/caac.20124. [DOI] [PubMed] [Google Scholar]
- 20.Zhao T., Gao Z., Wu W., He W., Yang Y. Effect of synchronous solitary bone metastasectomy and lung cancer resection on non-small cell lung cancer patients. Oncol. Lett. 2016;11:2266–2270. doi: 10.3892/ol.2016.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akram M., Zaheer S., Hussain A., Siddiqui S.A., Afrose R., Khalid S. Solitary fibular metastasis from nonsmall cell lung carcinoma. J. Cytol. 2017;34:113–115. doi: 10.4103/0970-9371.203577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai L., Dong S., Chen H. Olecranon metastases from primary lung cancer: Case report and review of the literature. J. Int. Med. Res. 2019;47:5312–5317. doi: 10.1177/0300060519875538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torok J.A., Gu L., Tandberg D.J., Wang X., Harpole D.H., Jr., Kelsey C.R., Salama J.K. Patterns of Distant Metastases After Surgical Management of Non–Small-Cell Lung Cancer. Clin. Lung Cancer. 2016;18:e57–e70. doi: 10.1016/j.cllc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Rusthoven K.E., Hammerman S.F., Kavanagh B.D., Birtwhistle M.J., Stares M., Camidge D.R. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48:578–583. doi: 10.1080/02841860802662722. [DOI] [PubMed] [Google Scholar]
- 25.Barlesi F., Mazieres J., Merlio J.-P., Debieuvre D., Mosser J., Lena H., Ouafik L.H., Besse B., Rouquette I., Westeel V., et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 26.Hanna N.H., Schneider B.J., Temin S., Jr., Baker S., Brahmer J., Ellis P.M., Gaspar L.E., Haddad R.Y., Hesketh P.J., Jain D., et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020;38:1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 27.Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors. J. Mol. Diagn. 2018;20:129–159. doi: 10.1016/j.jmoldx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Detterbeck F.C., Boffa D.J., Kim A.W., Tanoue L.T. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of Survival in Patients With Bone Metastasis of Lung Cancer. Clin. Orthop. Relat. Res. 2008;466:729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho Y.J., Cho Y.M., Kim S.H., Shin K.-H., Jung S.-T., Kim H.S. Clinical analysis of patients with skeletal metastasis of lung cancer. BMC Cancer. 2019;19:303. doi: 10.1186/s12885-019-5534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Felice F., Piccioli A., Musio D., Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8:25691–25699. doi: 10.18632/oncotarget.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Jiao F., Dong L., Li Q., Liu G., Hu X. Lobectomy Can Improve the Survival of Patients with Non-small Cell Lung Cancer with Lung Oligometastatic. Front. Surg. 2021;8:685186. doi: 10.3389/fsurg.2021.685186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willeumier J.J., van der Linden Y.M., van de Sande M.A., Dijkstra P.S. Treatment of pathological fractures of the long bones. EFORT Open Rev. 2016;1:136–145. doi: 10.1302/2058-5241.1.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukamoto S., Kido A., Tanaka Y., Facchini G., Peta G., Rossi G., Mavrogenis A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021;28:3347–3372. doi: 10.3390/curroncol28050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp B.J., Devarakonda S., Govindan R. Bone metastases in non-small cell lung cancer: A narrative review. J. Thorac. Dis. 2022;14:1696–1712. doi: 10.21037/jtd-21-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood T.J., Racano A., Yeung H., Farrokhyar F., Ghert M., Deheshi B.M. Surgical Management of Bone Metastases: Quality of Evidence and Systematic Review. Ann. Surg. Oncol. 2014;21:4081–4089. doi: 10.1245/s10434-014-4002-1. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh M.-K., Bowles D.R., Canseco J.A., Sherman M.B., Schroeder G.D., Vaccaro A.R. Is Open Surgery for Metastatic Spinal Cord Compression Secondary to Lung Cancer Really Beneficial? A Systematic Review. World Neurosurg. 2020;144:e253–e263. doi: 10.1016/j.wneu.2020.08.098. [DOI] [PubMed] [Google Scholar]
- 38.Bauer H.C.F. Controversies in the surgical management of skeletal metastases. J. Bone Jt. Surgery. Br. Vol. 2005;87:608–617. doi: 10.1302/0301-620X.87B5.16021. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad U., Yang H., Sima C., Buitrago D.H., Ripley R.T., Suzuki K., Bains M.S., Rizk N.P., Rusch V.W., Huang J., et al. Resection of Primary and Secondary Tumors of the Sternum: An Analysis of Prognostic Variables. Ann. Thorac. Surg. 2015;100:215–222. doi: 10.1016/j.athoracsur.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Huang X.-Y., Feng W.-Y., Luo X.-T., Wei C.-W., Liu J.-H., Lai Y.-J., Xiao Z.-M., Zhang X., Zhan X.-L. Analysis of the clinical efficacy of tumor resection methods used in 20 patients with clavicular tumor. World J. Surg. Oncol. 2019;17:106. doi: 10.1186/s12957-019-1642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capanna R., Campanacci D.A. The Treatment of Metastases in the Appendicular Skeleton. J. Bone Jt. Surgery. Br. Vol. 2001;83:471–481. doi: 10.1302/0301-620X.83B4.0830471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further data concerning the study can be obtained by contacting the corresponding author.