Abstract
Over the past two decades, scientific and technological advancements have revealed messenger ribonucleic acid (mRNA)-based vaccines as a well-tolerated and effective platform to combat infectious disease. The potential of mRNA-based vaccines was epitomized during the severe acute respiratory syndrome coronavirus 2 pandemic, wherein mRNA-based vaccines were rapidly developed and found highly efficacious with an acceptable safety profile. These properties together with the capability to quickly address pathogens of pandemic potential, pathogens with complex antigens, and multiple pathogens within a single vaccine have revitalized the field, and multiple mRNA-based vaccines have now entered clinical development. This review summarizes current mRNA-based vaccine technology, perspectives on ongoing clinical studies, and future prospects for the field.
Current Opinion in Immunology 2022, 77:102214
This review comes from a themed issue on Vaccines
Edited by Mariagrazia Pizza and Rino Rappuoli
For complete overview of the section, please refer to the article collection, “Vaccines (August 2022)”
Available online 4th June 2022
https://doi.org/10.1016/j.coi.2022.102214
0952-7915/© 2022 Elsevier Ltd. All rights reserved.
Introduction
Vaccination remains one of the most effective strategies to combat pathogens and avert public health crises worldwide. Over the last two centuries since the development and widespread use of the smallpox vaccine, vaccine technologies have remarkably progressed due to continued advancements in our understanding of vaccine science 1, 2. These new technologies may address existing hard-to-target pathogens as well as novel infectious diseases that pose additional threats to humanity.
One such technological advancement in the vaccine field is the use of messenger ribonucleic acid (mRNA), which has historically faced challenges, including instability, reactogenicity, inefficient delivery and translation, and poor immunogenicity. The recent development of two well-tolerated and highly efficacious mRNA-based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has highlighted the developmental progress of this technology and its potential to combat multiple infectious diseases. With more than two billion total doses of the two authorized SARS-CoV-2 mRNA vaccines administered worldwide (mRNA-1273 [Spikevax; Moderna, Inc., Cambridge, MA] or BNT162b2 [Comirnaty; BioNTech, Mainz, Germany; Pfizer Inc., New York, NY]) [3], the mRNA vaccine platform has demonstrated the ability for the expeditious and scalable development of well-tolerated and efficacious vaccines.
The global success of the mRNA-based SARS-CoV-2 vaccines has further reinvigorated interest in the mRNA platform and accelerated the development of mRNA vaccines against additional infectious-disease targets. The mRNA platform affords several advantages, including generation of difficult-to-manufacture multiprotein complexes, concomitant expression of multiple antigens (e.g. combination vaccines against multiple pathogens), and precise protein engineering enabling expression of structurally stabilized versions, virus-like particles, or multicopy antigen presentation. Further, the manufacture of mRNA-based vaccines follows well-defined and consistent processes, using similar reagents, regardless of the antigen encoded by the mRNA, which simplifies the production, scale-up, quality control, and overall development timelines. Finally, mRNA vaccines deliver a ‘digital code’ of the antigen without a need for protein purification or pathogen inactivation, which is particularly advantageous when a rapid response is essential to tackle outbreaks or pandemic situations [4]. Here, we summarize key features of mRNA-based vaccines against infectious disease, our current understanding of their mode of action, recent findings from ongoing clinical studies, and future prospects for the field.
Basic concepts of mRNA vaccine technology
Endogenously, mRNA acts as a blueprint for protein synthesis, first transcribed from genomic DNA in the nucleus and then transported to ribosomes in the cytoplasm for translation into proteins. In eukaryotes, mRNA follows a basic (5′–3′) structure, consisting of a 5′ cap, a 5′ untranslated region (UTR), an open reading frame (ORF) encoding the protein, a 3′ UTR, and a poly(A) tail. Each of these structural components are critical in regulating mRNA translation and stability, ultimately impacting protein expression and biological activity 5, 6, 7, 8, 9.
Owing to the length and structural characteristics of mRNA, its production and characterization are different than that of other RNA therapeutics, such as small-interfering RNA (siRNA) consisting of short double-stranded RNA (dsRNA). Production of mRNA is currently accomplished through in vitro transcription (IVT), which utilizes linearized DNA as template, an RNA polymerase, and nucleotide triphosphates in a buffered environment 10, 11. Purified IVT mRNA ultimately resembles fully processed, mature, endogenous mRNA molecules present in the cytoplasm of eukaryotic cells.
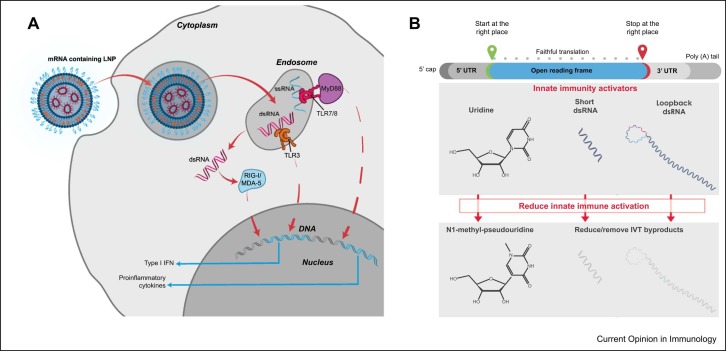
As mRNA and certain IVT by products are inherently immunostimulatory, different approaches to mRNA chemistry and manufacturing have been developed to modulate innate immune responses ( Figure 1) 12••, 13. Notably, mRNAs contained within mRNA-1273 and BNT162b2 replace the uridine nucleotide with a modified uridine (N1-methyl-pseudouridine [N1-methyl-Ψ]) to minimize innate immune activation through Toll-like receptor (TLR)7/8 signaling [12]. In contrast, other mRNA-based vaccine platforms have focused on unmodified, sequence-engineered mRNA. For example, CureVac uses unmodified mRNA for their SARS-CoV-2 vaccine with a codon-optimized ORF, containing enriched guanine and cytosine content, and sequence-engineered 5′ and 3′ UTRs 13, 14.
Figure 1.
Technological approaches to reduce innate immune activation by mRNA-based vaccines. (a) After entering the cell, IVT mRNA and certain IVT-related impurities (such as dsRNA and ssRNA fragments) can be recognized by a variety of innate immune receptors, including endosomal TLR3 or TLR7/8 and cytosolic sensors MDA5 or RIG-I, ultimately resulting in robust type-1 interferon signaling and proinflammatory cytokine induction. (b) Manufacture of IVT mRNA has been steadily advanced to limit innate immune activation, including by replacing uridine-5′-triphosphate with N1-methyl-pseudouridine-5′-triphosphate within the IVT mRNA, and removing or reducing the amount of short dsRNA and loopback dsRNA produced during the IVT process. MDA5, melanoma differentiation-associated protein 5; RIG-1, retinoic acid-inducible gene I; ssRNA, single-strand RNA.
Of note, mRNA does not integrate with the cellular genome and is quickly degraded by endogenous processes after injection, which decrease risks of metabolite-induced toxicity [15]. However, to function, mRNA must overcome certain physiological barriers for efficient delivery to cells and subsequent translation. In this regard, multiple delivery techniques have been developed to protect mRNA as well as to facilitate its cellular entry and delivery into the cytoplasm where translation occurs.
mRNA–lipid nanoparticle vaccine delivery and mechanism of action
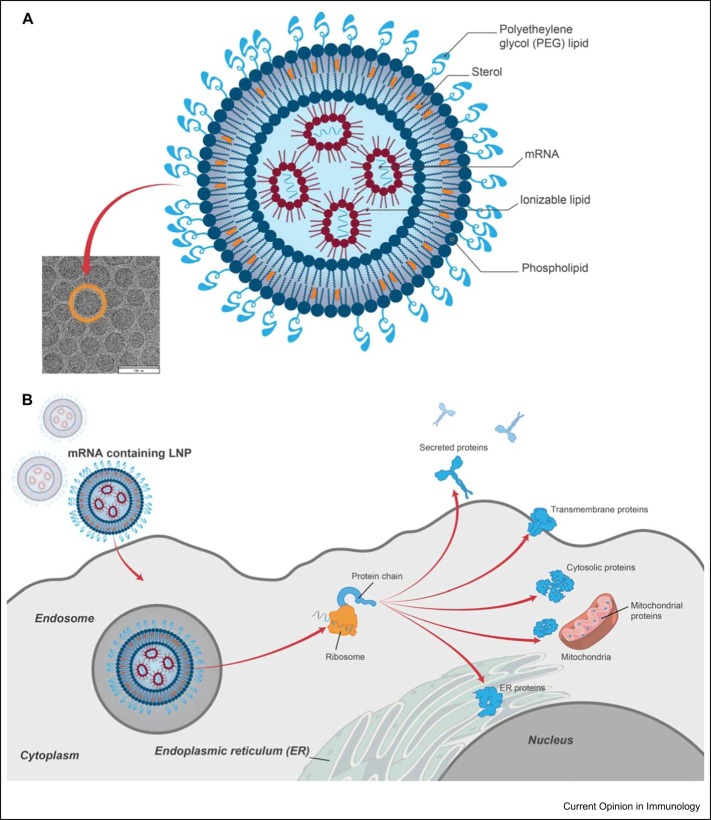
One of the leading delivery vehicles for mRNA-based vaccines is lipid nanoparticles (LNPs), approximately 100 nm in diameter and often composed of four components that together encapsulate and protect the mRNA: an ionizable lipid, a sterol, a lipid-linked polyethylene glycol (PEG), and a phospholipid [16] ( Figure 2). Integral to the function of LNPs is the ionizable amino lipid, which promotes mRNA encapsulation and endosomal escape for cytoplasmic release. While early work demonstrated the utility of positively-charged amino lipids in delivery systems for nucleic acids, tolerability challenges limited their application. Ionizable lipids that remain neutral at physiological pH were developed to overcome these initial hurdles, including Dlin-MC3-DMA (MC3) that is the ionizable component of the first FDA-approved RNA therapeutic formulated with LNP (Onpattro, an siRNA therapeutic); however, further development led to more biodegradable LNPs with improved efficiency and tolerability, including the ionizable lipids used in both mRNA-1273 (SM-102) and BNT162b2 (ALC-0315) 16, 17•, 18. Beyond the ionizable lipid, the remaining three lipid components also play key roles in LNP function: cholesterol is a structural component that aids in stabilizing the LNP, PEGylated lipids extend the half-life of the LNP, and distearoylphosphatidylcholine (DSPC), which modulates LNP bilayer fluidity [16].
Figure 2.
Current LNP technology in mRNA-based vaccines. (a) LNPs consist of an ionizable lipid, sterol, phospholipid, and a PEG lipid that together encapsulate and protect the mRNA core. Each lipid component has critical roles in LNP function. (b) The LNP-delivery system has been engineered to enable efficient cellular delivery of mRNA to cells and overcome certain physiological barriers, including protecting mRNA from degradation, uptake, and facilitating endosomal escape for subsequent translation of the encoded antigen(s).
After intramuscular administration, mRNA–LNPs are endocytosed by cells at the injection site and within the draining lymph nodes. Immune cells, specifically antigen-presenting cells (i.e. dendritic cells, monocytes, and subcapsular sinus macrophages), are the predominant populations that endocytose the mRNA-LNP, with the mRNA subsequently translated to produce the encoded protein antigen (Figure 2) [19]. Intracellular expression and processing of this antigen allows for efficient presentation on major histocompatibility complex class I and II proteins, inducing strong and persistent CD4+ and CD8+ T-cell responses, while expression of secreted or cell surface-anchored antigens engages B-cell receptors to activate B cells and antigen-specific antibody production. Follicular T-helper cell responses have also been demonstrated after mRNA vaccination, which are critical to the development of potent and durable neutralizing-antibody responses 20•, 21, 22, 23. Notably, some LNP-delivery vehicles have been shown to have a role in inflammation and immune stimulation in preclinical mouse models 24, 25. However, advancements in LNP technology have further optimized antigen expression, tolerability, and immunogenicity for mRNA vaccines, including those against SARS-CoV-2 16, 17•, 18.
Clinical and real-world studies of mRNA-1273 and BNT162b2 against SARS-CoV-2
Decades of research to optimize the mRNA platform and its potential to enable rapid vaccine development was put to the test in response to the emergence of SARS-CoV-2 and the ensuing COVID-19 pandemic. Two SARS-CoV-2 vaccines (mRNA-1273 and BNT162b2) were expeditiously developed and comprehensively characterized in preclinical and clinical evaluations. Initial studies in adults demonstrated the safety and tolerability profiles of these mRNA vaccines, the SARS-CoV-2 antigen-specific immune profile, and the high degree of efficacy against symptomatic and severe COVID-19 disease 26, 27••, 28, 29••. Results from these trials have led to vaccine licensure in the United States and multiple countries throughout the world. Multiple clinical trials are still ongoing in the United States and other global regions, including those to support use in different age populations and at-risk groups 30, 31. In addition, modified COVID-19 mRNA vaccines that match key variants of concern are under investigation as booster vaccinations in multiple nonclinical and clinical studies 32, 33, 34.
The post-authorization experience for these SARS-CoV-2 mRNA vaccines remains unprecedented, characterized by global, large-scale vaccination efforts enabling rapid and in-depth accumulation of real-world evidence on the immunity and effectiveness associated with mRNA vaccines 35, 36, 37, 38, 39, 40, 41, 42, 43, including in previously infected subjects (i.e. hybrid immunity) 44, 45, 46••, 47 or in heterologous booster regimens with adenoviral SARS-CoV-2 vaccines 48, 49, 50. Assessments of mRNA vaccine-elicited immunity have shown strong and persistent germinal center reactions post vaccination, potent neutralizing antibody titers that wane over time, Fc-mediated effector antibody functions, as well as generation of cross-reactive CD4+ and CD8+ memory T-cell and B-cell responses 20•, 51, 52, 53, 54, 55, 56. Further, the potential impact of newly emerged SARS-CoV-2 variants on immunogenicity and vaccine-mediated protection has also been continually characterized 39, 40, 57, 58, 59, 60, showing reduced and waning neutralizing antibody responses and vaccine effectiveness for certain variants, most notably the currently globally dominant omicron strain 61, 62, 63, 64.
Safety and immunogenicity in specific populations such as pregnant women have been assessed, with fetal antibody transfer and antibody Fc function demonstrated 65, 66, 67. Similar investigations have been performed in older adults, with some evidence of the impact of immunosenescence on immunogenicity [68], although vaccine effectiveness remains generally high 42, 47, 69. However, real-world evidence has also indicated that vaccine effectiveness is typically lower among immunocompromised populations, particularly solid organ or stem cell transplant recipients [70]. Additionally, although post-authorization safety signals have been identified, including mild cases of myocarditis/pericarditis in younger males 71, 72 and anaphylaxis [73], rates remain low and cases generally resolved. Overall, these studies have significantly contributed to the depth of our scientific knowledge on mRNA vaccines and have further advanced the field, as demonstrated by the multiple mRNA vaccines against other infectious pathogens now in clinical development.
Beyond SARS-CoV-2: clinical progress on mRNA vaccines against other pathogens
Before the clinical study of mRNA vaccines against SARS-CoV-2, mRNA-based vaccines against several other infectious disease targets had been tested in phase 1 and 2 clinical studies. The first mRNA based vaccine evaluated in a phase 1 trial was one formulated with protamine and encoding rabies virus glycoprotein (CV7201, CureVac) [74]. Though CV7201 was reasonably well-tolerated, immune responses were short-lived and induced only when the vaccine was administered with a needle-free device [74]. Two influenza vaccine candidates with a first-generation LNP formulation followed (expressing hemagglutinin from H10N8 or H7N9 strains, Moderna, Inc.) were among the first mRNA–LNP vaccine candidates to advance to clinical stage. Both vaccines were well-tolerated and raised protective immune responses [75], demonstrating that mRNA–LNP vaccines can be well-tolerated and immunogenic in humans. Subsequently, LNPs were utilized as a delivery vehicle for a second-generation rabies vaccine (CV7202, CureVac), which induced immune responses after two doses in a phase 1 trial, but showed high reactogenicity at the highest dose level (5 µg) [76].
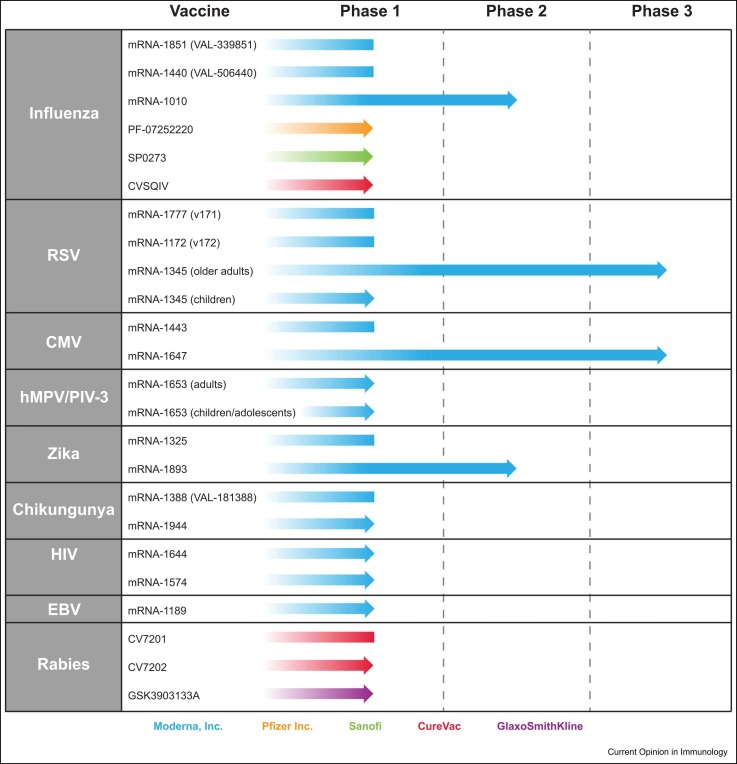
Since this initial work, numerous mRNA-based vaccines against infectious diseases have entered clinical development and a few have advanced to late-stage clinical trials ( Figure 3). Among those furthest progressed are mRNA-based vaccines against cytomegalovirus (CMV, mRNA-1647, Moderna, Inc.) and respiratory syncytial virus (RSV, mRNA-1345, Moderna, Inc.). Formulated with the proprietary LNP used for mRNA-1273, mRNA-1647 consists of six mRNAs encoding two CMV antigens (five mRNA sequences encoding for the five subunits of the pentameric complex and a single mRNA sequence encoding the full-length glycoprotein B). In a phase 1 trial, the vaccine was generally well-tolerated and three doses were immunogenic in CMV-seronegative participants, resulting in epithelial and fibroblast neutralizing antibody responses above the baseline CMV-seropositive levels, and strongly boosted immune responses among CMV-seropositive participants [77]. The mRNA-1647 vaccine, which has recently advanced to a phase 3 efficacy study, highlights the ability of the mRNA platform to target complex antigens and multiplex antigens in a single vaccine. A phase 3 clinical trial in adults aged ≥60 years was also recently initiated for the investigational mRNA-1345 vaccine against RSV, which encodes for the F glycoprotein stabilized in the prefusion state formulated with the same proprietary LNP of mRNA-1273. Phase 1 findings in older RSV-seropositive adults (aged 65–79 years) showed that a single dose of the vaccine was well-tolerated and boosted neutralizing responses 10-fold to 14-fold above baseline [78]; the vaccine is aimed to provide protection against RSV for both older adult and pediatric populations.
Figure 3.
mRNA vaccines against pathogens besides SARS-CoV-2 in clinical development. The progression and current clinical stage (phase 1, 2, or 3) of mRNA-based vaccines against infectious diseases beyond SARS-CoV-2 is shown, with blue arrows representing vaccines currently under active development and blue boxes representing vaccines not under active investigation. Two influenza vaccines by Sanofi/Translate Bio (MRT-5400 and MRT-5401) have also been under phase 1 clinical evaluation but are not included as the current status is unclear.
The ability to combine multiple mRNA sequences into a single vaccine also has potential to target multiple pathogens or viral strains. For example, a combination vaccine against human metapneumovirus (hMPV) and parainfluenza virus type 3 (PIV) has been developed (mRNA-1653, Moderna, Inc.), with a phase 1 study in healthy adults showing the vaccine was well-tolerated and elicited functional immune responses [79]; a phase 1b study in adults and hMPV/PIV3-seropositive children was the first clinical trial for a mRNA–LNP vaccine to be initiated in a pediatric population. Further, multiple mRNA-based vaccines against influenza are currently under early clinical evaluation (Figure 3), with this novel technology potentially allowing for rapid future tackling of influenza strains of pandemic potential, multiplexing against multiple antigens to broaden immunity, and reducing vaccine production time to enable more informed decision-making on the seasonal strains to be addressed.
Future prospects and overall conclusions
As additional mRNA-based vaccine programs enter late-stage clinical trials and further research is conducted on available SARS-CoV-2 vaccines, these findings can be anticipated to prompt further technological advancements and insight into the mode of action, safety, immunological properties, and protective efficacy of mRNA-based vaccines in humans. Key among those insights should be on the duration of mRNA-based vaccine-mediated protection against disease as well as breadth of protection, particularly for rapidly mutating pathogens such as SARS-CoV-2, influenza virus, and human immunodeficiency virus. Further knowledge on the immunological properties and efficacy of mRNA-based vaccines in certain populations is also warranted, including pregnant women, infants and children, the elderly, and the immunocompromised. Safety is also paramount and will be further informed by ongoing clinical studies and real-world monitoring of safety events following mRNA-based vaccination.
Improvements to certain mRNA vaccine characteristics are also warranted, including temperature stability to enable easier vaccine handling, storage, and access to the developing world. Efforts to increase the half-life of mRNA expression through optimization of coding sequences and UTRs are also predicted to increase potency of mRNA vaccines and could result in the lowering of effective doses. For example, while an initial unmodified SARS-CoV-2 vaccine developed by CureVac (CvnCoV) only achieved 48.2% efficacy against COVID-19 [80], continuous program developments led to a second-generation candidate (CV2CoV) with improvements to the noncoding regions and increased immunogenicity and protection in nonhuman primates [14].
Beyond the conventional mRNA-based vaccines, vaccines using self-amplifying RNA, which encodes virus-derived polymerase to amplify mRNA and thereby allow for high antigen expression at a low dose level, have now also entered clinical testing [81]. Further, although still at the early phase of development, vaccines based on circular RNA, which comprises highly stable RNA in a closed-ring structure without the need for certain features of mRNA, including a 5′ cap or 3′ poly-A tail, are being evaluated in preclinical studies [82]. Additional efforts to update or modify the LNP-delivery vehicle have the potential to enable mRNA vaccine formulations that target specific cells or tissues or may further improve the tolerability or potency of the mRNA vaccine platforms. Finally, alternative delivery routes of immunization (i.e. intranasal or intradermal delivery) could further expand the applications of this vaccine technology by providing optimal protection in mucosal tissues and directly at the site of infection.
Overall, the prospect for mRNA-based vaccine platforms to combat both pervasive and emerging infectious diseases remains optimistic, with the rapid development and deployment of well-tolerated and effective mRNA-based SARS-CoV-2 vaccines paving the path forward for this contemporary and transformative technology.
Funding
This work was funded by Moderna, Inc.
Author contributions
All authors contributed to the article conception and design, interpreting the relevant literature, drafting of the paper, and/or critically revising it for intellectual content.
Conflict of interest statement
All authors are employees of Moderna, Inc., and hold stock/stock options.
Acknowledgements
Medical writing and editorial assistance were provided by Emily Stackpole, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Plotkin S. History of vaccination. Proc Natl Acad Sci USA. 2014;111:12283–12287. doi: 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebre M.S., Brito L.A., Tostanoski L.H., Edwards D.K., Carfi A., Barouch D.H. Novel approaches for vaccine development. Cell. 2021;184:1589–1603. doi: 10.1016/j.cell.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie H, Mathieu E, Rodes-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M: Coronavirus Pandemic (COVID-19). Published online at OurWorldInData.org. Edited by; 2020. vol 2021.
- 4.Pizza M., Pecetta S., Rappuoli R. Vaccines 2020: the era of the digital vaccine is here. Sci Transl Med. 2021;13:eabm3249. doi: 10.1126/scitranslmed.abm3249. [DOI] [PubMed] [Google Scholar]
- 5.Leppek K., Das R., Barna M. Functional 5' UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat Rev Mol Cell Biol. 2018;19:158–174. doi: 10.1038/nrm.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauger D.M., Cabral B.J., Presnyak V., Su S.V., Reid D.W., Goodman B., Link K., Khatwani N., Reynders J., Moore M.J., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc Natl Acad Sci USA. 2019;116:24075–24083. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., Tureci O., Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 8.Guhaniyogi J., Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 9.Orlandini von Niessen A.G., Poleganov M.A., Rechner C., Plaschke A., Kranz L.M., Fesser S., Diken M., Lower M., Vallazza B., Beissert T., et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3' UTRs identified by cellular library screening. Mol Ther. 2019;27:824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics — developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 11.Pardi N., Muramatsu H., Weissman D., Kariko K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 12••.Nelson J., Sorensen E.W., Mintri S., Rabideau A.E., Zheng W., Besin G., Khatwani N., Su S.V., Miracco E.J., Issa W.J., et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci Adv. 2020;6:eaaz6893. doi: 10.1126/sciadv.aaz6893. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used multiple cellular and in vivo models to evaluate the role of mRNA and IVT byproducts on innate immune responses, highlighting that uridine modification of mRNA and reduction of certain byproducts can modify these responses.
- 13.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebre M.S., Rauch S., Roth N., Yu J., Chandrashekar A., Mercado N.B., He X., Liu J., McMahan K., Martinot A., et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature. 2022;601:410–414. doi: 10.1038/s41586-021-04231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a rational approach to identify amino lipid structural motifs that could improve lipid nanoparticle chemical stability, tissue clearance, and efficiency of mRNA delivery.
- 18.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang F., Lindgren G., Lin A., Thompson E.A., Ols S., Rohss J., John S., Hassett K., Yuzhakov O., Bahl K., et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in Rhesus Macaques. Mol Ther. 2017;25:2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Turner J.S., O'Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This small observational study evaluated the induction of germinal center reactions among healthy adults who received BNT162b2 vaccination, showing the vaccine-elicited persistent antigen-specific germinal center B cell responses.
- 21.Mudd P.A., Minervina A.A., Pogorelyy M.V., Turner J.S., Kim W., Kalaidina E., Petersen J., Schmitz A.J., Lei T., Haile A., et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185:603–613. doi: 10.1016/j.cell.2021.12.026. e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alameh M.G., Tombacz I., Bettini E., Lederer K., Sittplangkoon C., Wilmore J.R., Gaudette B.T., Soliman O.Y., Pine M., Hicks P., et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892. doi: 10.1016/j.immuni.2021.11.001. e2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyarto B.Z. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24 doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parhiz H., Brenner J.S., Patel P., Papp T.E., Shahnawaz H., Li Q., Shi R., Zamora M., Yadegari A., Marcos-Contreras O.A., et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE) J Control Release. 2021 doi: 10.1016/j.jconrel.2021.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]; The phase 3 study of BNT162b2 safety and efficacy against COVID-19 in adults.
- 28.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]; The phase 3 study on the safety and efficacy of mRNA-1273 against SARS-CoV-2 in adults.
- 30.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creech C.B., Anderson E., Berthaud V., Yildirim I., Atz A.M., Melendez Baez I., Finkelstein D., Pickrell P., Kirstein J., Yut C., et al. This phase 3 study showed the safety, immunogenicity, and efficacy of mRNA-1273 in children 6-11 years of age. New Eng J Medicine. 2022 [Google Scholar]
- 32.Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., Nunna N., Huang W., Oestreicher J., Colpitts T., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021 doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying B., Scheaffer S.M., Whitener B., Liang C.-Y., Dmytrenko O., Mackin S., Wu K., Lee D., Avena L.E., Chong Z., et al. Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice. bioRxiv. 2022 doi: 10.1016/j.cell.2022.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gagne M., Moliva J.I., Foulds K.E., Andrew S.F., Flynn B.J., Werner A.P., Wagner D.A., Teng I.-T., Lin B.C., Moore C., et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits comparable B cell expansion, neutralizing antibodies and protection against Omicron. bioRxiv. 2022 doi: 10.1016/j.cell.2022.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doria-Rose N., Suthar M.S., Makowski M., O'Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collier A.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montoya J.G., Adams A.E., Bonetti V., Deng S., Link N.A., Pertsch S., Olson K., Li M., Dillon E.C., Frosch D.L. Differences in IgG Antibody responses following BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.01162-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., Tian Y., Florea A., Aragones M., Tubert J.E., et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muniz M.J., Gagnon D.R., Gaziano J.M., Cho K., Casas J.P., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N Engl J Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., Krishnadasan A., Harland K.K., Smithline H.A., Hou P.C., et al. Effectiveness of mRNA Covid-19 vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385 doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arbel R., Hammerman A., Sergienko R., Friger M., Peretz A., Netzer D., Yaron S. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — Eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreano E., Paciello I., Piccini G., Manganaro N., Pileri P., Hyseni I., Leonardi M., Pantano E., Abbiento V., Benincasa L., et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600:530–535. doi: 10.1038/s41586-021-04117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammerman A., Sergienko R., Friger M., Beckenstein T., Peretz A., Netzer D., Yaron S., Arbel R. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med. 2022;386:1221–1229. doi: 10.1056/NEJMoa2119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Pape K.A., Dileepan T., Kabage A.J., Kozysa D., Batres R., Evert C., Matson M., Lopez S., Krueger P.D., Graiziger C., et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109823. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interim findings from a prospective cohort study on the effectiveness of the mRNA-1273 vaccine against COVID-19 infection and severe COVID-19, which showed high vaccine effectiveness among a large, diverse participant cohort in the United States.
- 47.Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., Tian Y., Florea A., Takhar H.S., Tubert J.E., Talarico C.A., Tseng H.F. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health – Am. 2022;6 doi: 10.1016/j.lana.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews N., Stowe J., Kirsebom F., Toffa S., Sachdeva R., Gower C., Ramsay M., Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;24:831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabricius D., Ludwig C., Scholz J., Rode I., Tsamadou C., Jacobsen E.M., Winkelmann M., Grempels A., Lotfi R., Janda A., et al. mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdOx1-primed subjects. Vaccines. 2021;9:918. doi: 10.3390/vaccines9080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaku C.I., Champney E.R., Normark J., Garcia M., Johnson C.E., Ahlm C., Christ W., Sakharkar M., Ackerman M.E., Klingstrom J., et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science. 2022;375:1041–1047. doi: 10.1126/science.abn2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roltgen K., Nielsen S.C.A., Silva O., Younes S.F., Zaslavsky M., Costales C., Yang F., Wirz O.F., Solis D., Hoh R.A., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040. doi: 10.1016/j.cell.2022.01.018. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ssemaganda A., Nguyen H.M., Nuhu F., Jahan N., Card C.M., Kiazyk S., Severini G., Keynan Y., Su R.-C., Ji H., et al. Expansion of tissue-resident CD8+ T cells and CD4+ Th17 cells in the nasal mucosa following mRNA COVID-19 vaccination. bioRxiv. 2021 doi: 10.1101/2021.05.07.442971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., Goodwin B., Rubiro P., Sutherland A., Wang E., et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., Nunna N., Huang W., Oestreicher J., Colpitts T., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mateus J., Dan J.M., Zhang Z., Rydyznski Moderbacher C., Lammers M., Goodwin B., Sette A., Crotty S., Weiskopf D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374 doi: 10.1126/science.abj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplonek P., Cizmeci D., Fischinger S., Collier A.R., Suscovich T., Linde C., Broge T., Mann C., Amanat F., Dayal D., et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abm2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi A., Koch M., Wu K., Dixon G., Oestreicher J., Legault H., Stewart-Jones G.B.E., Colpitts T., Pajon R., Bennett H., et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol. 2021 doi: 10.1128/JVI.01313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., Colpitts T., Bennett H., Boyoglu-Barnum S., Shi W., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine — preliminary report. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falsey A.R., Frenck R.W., Jr., Walsh E.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Bailey R., Swanson K.A., Xu X., et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muik A., Lui B.G., Wallisch A.K., Bacher M., Muhl J., Reinholz J., Ozhelvaci O., Beckmann N., Guimil Garcia R.C., Poran A., et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., Bruxvoort K.J., Tubert J.E., Florea A., Ku J.H., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O'Connell A.M., et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atyeo C., DeRiso E.A., Davis C., Bordt E.A., DeGuzman R.M., Shook L.L., Yonker L.M., Fasano A., Akinwunmi B., Lauffenburger D.A., et al. COVID-19 mRNA vaccines drive differential Fc-functional profiles in pregnant, lactating, and non-pregnant women. bioRxiv. 2021 doi: 10.1101/2021.04.04.438404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collier A.Y., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., Chandrashekar A., Patel S., Apraku Bondzie E., Sellers D., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitallé J., Pérez-Gómez A., Ostos F.J., Gasca-Capote C., Jiménez-León M.R., Bachiller S., Rivas-Jeremías I., del Mar Silva-Sánchez M., Ruiz-Mateos A., López-Cortes L.F., et al. Innate and adaptive immune defects associated with lower SARS-CoV-2 BNT162b2 mRNA vaccine response in elderly people. medRxiv. 2022 doi: 10.1101/2022.01.07.22268806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nanduri S., Pilishvili T., Derado G., Soe M.M., Dollard P., Wu H., Li Q., Bagchi S., Dubendris H., Link-Gelles R., et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., Dascomb K., Ong T.C., Klein N.P., Liao I.C., et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults — nine states, January–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., Olsha-Castell S., Arad D., Hasin T., Levi N., et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simone A., Herald J., Chen A., Gulati N., Shen A.Y., Lewin B., Lee M.S. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., Wickner P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 75.Feldman R.A., Fuhr R., Smolenov I., Mick Ribeiro A., Panther L., Watson M., Senn J.J., Smith M., Almarsson, Pujar H.S., et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 76.Aldrich C., Leroux-Roels I., Huang K.B., Bica M.A., Loeliger E., Schoenborn-Kellenberger O., Walz L., Leroux-Roels G., von Sonnenburg F., Oostvogels L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine. 2021;39:1310–1318. doi: 10.1016/j.vaccine.2020.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moderna Inc., Moderna Announces Positive Interim Results from Phase 1 Cytomegalovirus (CMV) Vaccine (mRNA-1647) Study and Progress Toward Phase 2 and Pivotal Trials. Edited by; 2019. vol March 4.
- 78.Moderna Inc., Moderna Announces First Participant Dosed in Phase 2/3 Study of its mRNA Respiratory Syncytial Virus (RSV) Vaccine. Edited by; 2021. vol 2022.
- 79.Shaw CL H., Knightly C., Kalidindi S., Zaks T., Smolenov I., Panther L. 2754. Phase 1 trial of an mRNA-based combination vaccine against hMPV and PIV3. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kremsner P.G., Ahuad Guerrero R.A., Arana-Arri E., Aroca Martinez G.J., Bonten M., Chandler R., Corral G., De Block E.J.L., Ecker L., Gabor J.J., et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22:329–340. doi: 10.1016/S1473-3099(21)00677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pollock K.M., Cheeseman H.M., Szubert A.J., Libri V., Boffito M., Owen D., Bern H., O'Hara J., McFarlane L.R., Lemm N.M., et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. EClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qu L., Yi Z., Shen Y., Lin L., Chen F., Xu Y., Wu Z., Tang H., Zhang X., Tian F., et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. bioRxiv. 2022;185:1728–1744. doi: 10.1101/2021.03.16.435594. https://www.sciencedirect.com/science/article/pii/S0092867422003944 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]