Abstract
Objective:
Leukocytes contribute to the development of abdominal aortic aneurysm (AAA). We evaluated whether associations of differential leukocyte counts (DLC) with AAA persist after accounting for traditional risk factors of AAA.
Approach and Results:
Among 11,217 adults from the Atherosclerosis Risk in Communities Study, we evaluated associations of DLC at baseline (1987–1989) with incident AAAs over a median follow-up of 22.5 years, using Cox proportional hazards regression. Each DLC was categorized into 5 groups—below normal, tertiles within the normal range, and above normal, with the first tertile serving as the referent. We identified 377 incident AAAs through 2011, using hospital discharge diagnoses, linked Medicare records, or death certificates. At baseline, higher neutrophil, monocyte and eosinophil counts were associated with higher risk of AAA, independent of smoking, other DLC, and other traditional risk factors. The association with incident AAA was strongest for above normal neutrophil count, with an adjusted hazard ratio (HR) (95% CI) of 2.17 (1.29, 3.64). Below normal neutrophil, lymphocyte, eosinophil and basophil counts were associated with higher risk of AAA with adjusted HR (95% CI) between 1.86 (1.04, 3.35) and 1.62 (1.10, 2.39).
Conclusions:
Higher neutrophil, monocyte, and eosinophil counts in midlife are associated with higher risk of AAA, even after accounting for traditional risk factors such as smoking, obesity and atherosclerosis. This suggests the need to identify non-traditional risk factors and treatment strategies to mitigate the residual risk of AAA conferred by midlife inflammation. Whether immunosuppression is associated with higher risk of AAA needs further investigation.
Keywords: Aneurysm, Epidemiology, Risk Factors, Inflammation, Primary Prevention
INTRODUCTION
Abdominal aortic aneurysm (AAA) is mostly asymptomatic during development, incidentally diagnosed, progressive, and has substantial morbidity and mortality associated with its rupture.1–3 The prevalence of AAA in the US ranges from 1.3% to 12.5% in males, and 0 to 5.2% in females.1 Traditional management of AAA includes risk factor modification and observation until the risk of rupture exceeds the risk of surgery, i.e. open and endovascular surgical repair, but surgery is associated with increased health care cost, perioperative mortality and other adverse outcomes.2, 4–6 Hence, there is a need to increase our understanding of the etiology and developmental mechanisms of AAA, to discover potential targets for early pharmacotherapeutic intervention and primary prevention.2–4
AAA development involves infiltration of the aortic wall by inflammatory and immune cells, progressive degradation and remodeling of extracellular matrix, proteolysis of elastin and collagen in the media and adventitia, and smooth muscle cell apoptosis with thinning of the media.3, 4, 7, 8 Previous studies in animal AAA models and human aneurysmal tissues have identified the presence of several immune cells, such as neutrophils, monocytes/macrophages, eosinophils and lymphocytes, in aortic aneurysmal wall tissue and have reported their association with the development of AAA.3, 4, 7 However, prospective epidemiologic evidence at the population level is sparse. 8–10 In a UK-based prospective cohort study of 775,231 individuals selected from electronic medical records, higher neutrophil and monocyte counts at baseline were associated with a higher risk of incident AAA independent of several cardiovascular risk factors (including smoking).9 This study had a very short follow-up period (median 3.8 years), which might lead to reverse causality.9 Hence, we investigated the associations of differential leucocyte count (DLC) measured in midlife with the future risk of incident AAA in the ARIC cohort over a median 22.5 years of follow-up. Additionally, we adjusted for a long list of established vascular risk factors for AAA measured in midlife, to account for their potential confounding effect on the association of midlife DLC, a biomarker of inflammation, with higher risk of incident AAA.
METHODS
Data Availability
In order to minimize the possibility of unintentionally sharing information that can be used to re-identify private information, a subset of the data generated for this study are available at the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) and can be accessed at [https://biolincc.nhlbi.nih.gov/studies/aric/].
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective community-dwelling cohort study that recruited 15,792 adults aged 45–64 years at baseline visit 1 in 1987–1989 from the northwest suburbs of Minneapolis, Minnesota; Forsyth County, North Carolina; Jackson, Mississippi (African Americans only); and Washington County, Maryland.11 Participants were followed by annual telephone contact and additional re-examinations in 1990–1992 (Visit 2), 1993–1995 (Visit 3), 1996–1998 (Visit 4) 2011–2013 (Visit 5), and beyond. Study protocols were approved by Institutional Review Boards at all study centers, and participants gave written informed consent.11 In this study, from the initial ARIC study sample of 15,792 individuals at baseline visit, the following participants were excluded [Figure 1]: participants not Black or White (n=48); those with prior AAA surgery (n=11); those with uncertain AAA status at follow-up (n=30); participants from Washington County because of missing differential counts (n=3992); those whose differential percentages summed to less than 95% (n=121) or greater than 105% (n=26); missing values for any of the differential counts (n=325); and Blacks in Minneapolis due to small numbers (n=22).
Figure 1.
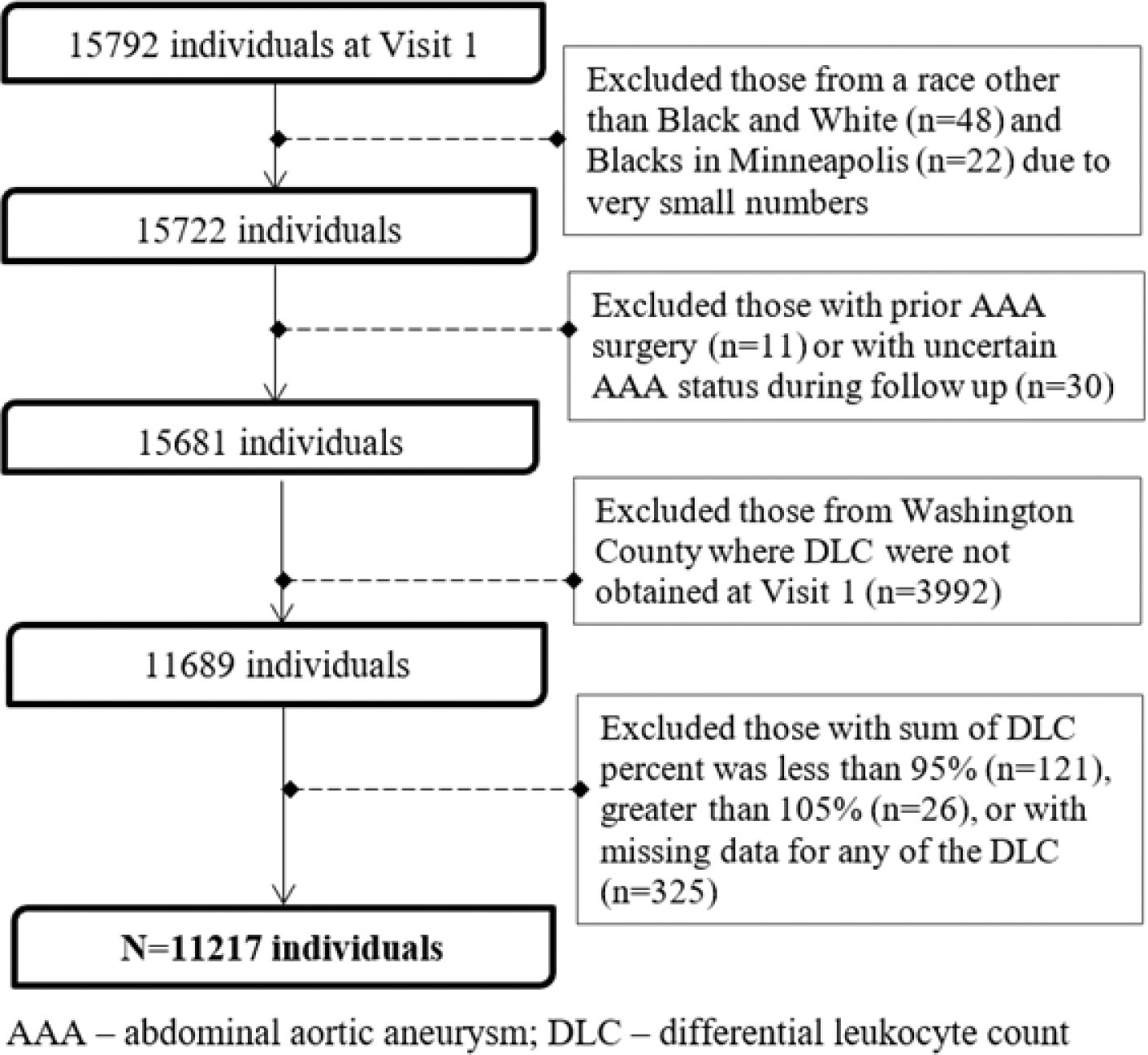
Flow Chart of Participants Excluded at Baseline, Atherosclerosis Risk in Communities Study, 1987 To 1989.
Outcome Ascertainment
An NIH funded ancillary study investigated AAAs in the ARIC cohort.; its design is briefly discussed here but has been described previously.8, 12 At the baseline visit (visit 1), participants were queried about any prior arterial surgery. We excluded participants reporting prior AAA surgery or aortic angioplasty. On follow-up, we prospectively ascertained incident, clinically diagnosed AAAs based on annual telephone surveys to identify hospitalizations, search of state death records and surveillance of local hospitals for admission or death. The ARIC study also linked participant identifiers with fee-for-service Medicare data from the Centers for Medicare and Medicaid Services for 1991 to 2011 to find any missing hospital or outpatient events for those ≥65 years of age. AAA in Medicare was defined as 1 inpatient or 2 outpatient claims at least 1 week apart. AAA was defined by International Classification of Diseases-9-CM codes of 441.3 (ruptured AAA) or 441.4 (AAA without mention of rupture), or procedure codes of 38.44 (AAA resection and replacement) or 39.71 (AAA endovascular repair) or a listed cause of death coded as International Classification of Diseases-9 441.3 or 441.4, or International Classification of Diseases-10 code I71.3 (ruptured AAA) or I71.4 (AAA without mention of rupture). AAAs based on procedure codes were required to be verified by diagnosis codes. Thoracic, thoracoabdominal, or unspecified aortic aneurysms were not included as AAA events.
Measurement of Exposure and Covariates
At the baseline visit, 8 hour fasting blood samples were drawn. Leukocytes were retrieved from whole anti-coagulated blood, and total leukocyte count (TLC) and DLC (percentages) were determined by automated particle Coulter Counters within 24 hours after venipuncture in local hospital hematology laboratories. The reliability coefficient for TLC measurements was greater than 0.96.13 Total neutrophil percent was calculated by adding segmented neutrophils and band neutrophils. Absolute differential counts were computed from TLC and differential percentages. TLC and differential percentages were obtained at 3 of 4 field centers at the baseline visit (Forsyth, Jackson, and Minneapolis).
At the baseline visit, technicians measured cardiovascular risk factors and conditions including anthropometrics (height in cm and weight in kilograms), behavior risk factors, history of physician-diagnosed cardiovascular conditions such as peripheral artery disease (PAD) and coronary heart disease (CHD). Questionnaires were used to assess self-reported information such as smoking status, smoking pack-years, drinking status, education level, and medication usage.8, 12 Pack-years were calculated as the average number of cigarettes per day divided by 20 cigarettes per pack and then multiplied by the number of years smoked. Seated blood pressure was measured after five minutes of rest using a random-zero sphygmomanometer, and systolic blood pressure was defined as the average of the last 2 of 3 consecutive measurements. Body mass index (BMI) was defined as weight in kilograms divided by height in meters squared. Plasma total cholesterol and triglycerides were measured by enzymatic methods.14,15 HDL cholesterol was measured after dextran-magnesium precipitation.16 Prevalent diabetes mellitus was characterized by a fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, a self-reported physician diagnosis, or self-reported use of anti-diabetic medications.8,12
Statistical analysis
Partial Pearson correlations among DLC were estimated adjusting for age, sex, and race. Initially, restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th and 95th percentiles were utilized to explore the shape of the dose-response association of each DLC with AAA risk, using Stata software version 12 (StataCorp LLC, College Station, TX). All subsequent analyses were done using SAS software version 9.4 (SAS Institute Inc., Cary, NC). All absolute DLC were divided into 5 categories--below the normal range; tertiles within the normal range; and above the normal range, with the first tertile in the normal range serving as the reference. We prepared direct adjusted survival curves for each category of each DLC. We built Cox proportional hazards models to estimate hazard ratios (HR) for time to incident AAA. We right censored death and loss to follow-up, and administratively censored those without events at December 31, 2011. We constructed 4 adjustment models in sequence. Model 1 adjusted for age, sex, race-center; model 2 further adjusted for smoking status and pack-years of smoking; model 3 further added other DLC as covariates; model 4 added other established AAA risk factors (such as height, total cholesterol and peripheral artery disease).12 In addition, model 5 replaced height by body mass index (BMI) and waist circumference and added baseline prevalent coronary heart disease. We computed p-values for trend across the 4 highest categories (i.e., omitting the lowest group, to address non-linearity as displayed in the spline graphs), assigning the lowest category as the reference group. The proportional hazards assumption was evaluated by testing for interaction between each differential count exposure and log survival time.
Sensitivity and Secondary Analyses
Since smoking and White race are important risk factors for AAA, and leukocyte counts are lower in Blacks than Whites, we conducted subgroup analyses using models 1–4 separately for Whites and ever smokers, to evaluate consistency of associations for the subgroups. We did not have adequate power to evaluate these associations for Blacks, due to low numbers. Since smoking is a strong risk factor for AAA and potentially a confounder for the association between DLC and AAA, we conducted another subgroup analysis in never smokers to evaluate the consistency in the patterns of associations as compared to the whole cohort, with awareness that statistical power would be low due to few AAA events (44 incident AAAs) in this subgroup analysis. To avoid model overfitting due to small number of events, we analyzed DLC in this subgroup analysis as continuous variables with the three primary adjustment models (similar to Models 1, 3, and 4). To address potential for reverse causality, we performed additional sensitivity analyses that excluded AAA cases who were diagnosed within the first 10 years of follow-up (n=76). These additional analyses were conducted only for DLC counts that showed significant and consistent associations in the primary analyses. Like the stratified analyses, only adjustment models 1–4 were evaluated. Additionally, to account for potential competing risks, we computed cause-specific HR for AAA by right censoring coronary heart disease, stroke, and heart failure. This technique is preferable if conducting etiologic/causal analyses as in our study, as opposed to modeling sub-distribution hazards which is preferable while constructing prognostic/risk-stratification models.17, 18 We conducted a secondary analysis to test the associations of DLC with severe AAA cases that required surgical intervention or had ruptured (n=111 events) at the time of detection. Since DLC measures can vary greatly over short periods of time, to assess the robustness of associations, we conducted another secondary analysis using measures of DLC from visit 2 (1990–1992) as an alternative baseline. This sensitivity analysis included 7927 participants after applying the same inclusion and exclusion criteria as in our primary analyses. At Visit 2, the study centers at both Washington County, MD and Jackson, MS (n=3343) did not measure DLC and therefore were excluded.
RESULTS
A total of 11,217 black and white participants from the 3 communities were followed, over a median 22.5 years [Interquartile interval: 17.5 years - 23.5 years]. A total of 377 participants developed AAA. Those who developed AAA were more often White, taller, non-diabetic males with greater propensity for smoking; had hypertension and peripheral artery disease; and had higher Low Density Lipoprotein (LDL) and total cholesterol and lower High Density Lipoprotein (HDL) cholesterol (Table 1). Neutrophils, lymphocytes, and monocytes were moderately correlated with each other, as were eosinophils and basophils (Supplemental Table I) after adjustment for age, race, and sex. Survival curves for incident AAA and severe AAA are presented in supplemental figure I and II, respectively.
Table 1.
Baseline Characteristics of Study Participants Stratified by Development of Abdominal Aortic Aneurysms: The Atherosclerosis Risk In Communities Study, 1987–2011.
| Baseline characteristics* | AAA (N=377) | No-AAA (N=10,840) | P-value |
|---|---|---|---|
|
| |||
| Age, years | 57±5 | 54±6 | <0.001 |
| Male, % | 275 (73) | 4664 (43) | <0.001 |
| African Americans, % | 71 (19) | 3796 (35) | <0.001 |
| BMI, kg/m2 | 27±4 | 28±5 | <0.001 |
| Height, cm | 173±9 | 169±9 | <0.001 |
| Smoking status | <0.001 | ||
| Current smoker, % | 196 (52) | 2927 (26) | |
| Former smoker, % | 135 (36) | 3469 (32) | |
| Never smoker, % | 46 (12) | 4444 (41) | |
| Pack-years smoking (including non- smokers) | 33±24 | 15±21 | <0.001 |
| Hypertension, % | 169 (45) | 3795 (35) | <0.001 |
| Peripheral Artery Disease, % | 22 (6) | 216 (2) | <0.001 |
| Diabetes, % | 26 (7) | 1306 (12) | 0.007 |
| Total cholesterol, mg/dL | 223±45 | 214±42 | <0.001 |
| LDL cholesterol, mg/dL | 148±41 | 136±40 | <0.001 |
| HDL cholesterol, mg/dL | 45±14 | 53±18 | <0.001 |
| Triglycerides, mg/dL | 148±92 | 127±91 | <0.001 |
| Use of cholesterol lowering medication, % | 18 (5) | 265 (3) | 0.005 |
| Use of aspirin, % | 191 (51) | 4909 (46) | 0.06 |
| WBC differentials †: | |||
| Neutrophil count/uL | 4,283±1,786 | 3,531±1,510 | <0.001 |
| Monocyte count/uL | 430±194 | 357±178 | <0.001 |
| Lymphocyte count/uL | 2,070±685 | 1,968±654 | 0.009 |
| Eosinophil count/uL | 165±157 | 144±135 | 0.04 |
| Basophil count/uL | 34±46 | 34±42 | 0.41 |
BMI-body mass index; LDL-low density lipoprotein; HDL-high density lipoprotein
Median length of follow-up for those who developed AAA over follow-up was 15.7 years, and for those who did not develop AAA was 22.5 years.
Continuous variables are presented as mean ± standard deviation (SD), categorical variables are presented as frequencies (%)
Values presented after excluding outliers beyond 6 SD from the mean
In our primary analyses of the whole cohort from visit 1 (Table 2), elevated neutrophil count, within and above the normal range, was monotonically associated with increased risk of AAA (p trend <0.0001). The trend was significant, even after accounting for traditional AAA risk factors and the other differentials (model 4: p trend = 0.001). The HR (95% CI) for the highest group compared with the reference group was 5.87 (95% CI 3.74, 9.21) after adjustment for age, sex and race, and 2.17 (1.29, 3.64) after additional adjustment for the other differentials and traditional risk factors (model 4). Neutropenia was also significantly and independently associated with greater AAA risk as compared to the reference group (HR 1.81, 95% CI 1.10, 2.97, model 4). Monocyte count in the third tertile of the normal range was associated with an increased risk of AAA compared to the referent group, independent of potential confounders (Table 2). After adjusting for potential confounders, lymphocyte group below the normal range had significantly greater risk of AAA than the reference (model 4: HR 1.86, 95%CI 1.04, 3.35; the HR from model 5 was similar). Eosinophils and basophils showed similar patterns of association with AAA. Specifically, low counts were significantly associated with increased risk of AAA, even after adjusting for other DLC and potential confounders (model 4: for eosinophils HR 1.62, 95% CI 1.10, 2.39, and for basophils HR 1.86, 95% CI 1.12, 3.07). For counts within and above the normal ranges, there was a positive and dose-response association with AAA risk (p for trend =0.038 for eosinophils, model 4), while the trend did not reach significance for basophils (p for trend=0.79). In model 4, HRs in the highest group vs the reference group were 2.01 (95% CI 1.08, 3.73) for eosinophil counts, and 1.94 (95% CI 1.04, 3.63) for basophil counts. The above associations were mostly consistent in the secondary fully adjustment model (i.e., model 5) except that the trend became stronger for basophils (p for trend=0.038 vs 0.79). Our primary findings, including positive associations of AAA with elevated neutrophil, monocyte, and eosinophil counts, were supported by the dose response curves constructed using restricted cubic splines (Figure 2).
Table 2.
Hazard Ratio (HR) and 95% Confidence Interval (CI) of Abdominal Aortic Aneurysm (AAA) by White Blood Cell Differential Count, Atherosclerosis Riskin Communities Study, 1987–1989 to 2011.
| Biomarkers (Visit 1) | Below normal range | Within Normal Range | Above normal range | p for trend* | ||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||||
|
| ||||||
| Neutrophils | ||||||
| Range | <2000 | 2000–3010 | 3011–4032 | 4033–7000 | >7000 | |
| N | 1391 | 3152 | 3176 | 3139 | 349 | |
| AAA events | 28 | 57 | 100 | 162 | 29 | |
| Hazard Ratio (95% CI) | ||||||
| Incidence Rate (per 100,000 person-years) | 98.4 | 88.4 | 157.7 | 275.7 | 500.2 | |
| Model 1 | 1.44(0.90–2.30) | 1 (Reference) | 1.55(1.12–2.16) | 2.75(2.03–3.74) | 5.87(3.74–9.21) | <0.0001 |
| Model 2 | 1.63(1.02–2.63) | 1 (Reference) | 1.32(0.94–1.84) | 1.70(1.24–2.35) | 2.53(1.57–4.07) | <0.0001 |
| Model 3 | 1.66(1.03–2.68) | 1 (Reference) | 1.26(0.9–1.77) | 1.57(1.13–2.19) | 2.14(1.30–3.52) | 0.0006 |
| Model 4 | 1.81(1.10–2.97) | 1 (Reference) | 1.22(0.86–1.73) | 1.52(1.08–2.15) | 2.17(1.29–3.64) | 0.0013 |
| Model 5 | 1.82(1.11–2.98) | 1 (Reference) | 1.28(0.9–1.82) | 1.60(1.13–2.27) | 2.33(1.40–3.87) | 0.0003 |
| Lymphocytes | ||||||
| Range | <1000 | 1000–1628 | 1629–2070 | 2071–3000 | >3000 | |
| N | 313 | 3359 | 3365 | 3359 | 814 | |
| AAA events | 14 | 90 | 102 | 135 | 36 | |
| Hazard Ratio (95% CI) | ||||||
| Incidence Rate (per 100,000 person-years) | 232.4 | 132.5 | 152.5 | 207.4 | 240.3 | |
| Model 1 | 1.74(0.99–3.07) | 1 (Reference) | 1.23(0.92–1.63) | 1.94(1.48–2.53) | 2.81(1.90–4.15) | <0.0001 |
| Model 2 | 1.83(1.04–3.21) | 1 (Reference) | 0.99(0.74–1.32) | 1.22(0.92–1.61) | 1.43(0.95–2.14) | 0.0411 |
| Model 3 | 1.80(1.02–3.17) | 1 (Reference) | 0.94(0.70–1.25) | 1.11(0.84–1.48) | 1.16(0.76–1.77) | 0.2858 |
| Model 4 | 1.86(1.04–3.35) | 1 (Reference) | 0.87(0.65–1.17) | 1.01(0.76–1.36) | 0.97(0.62–1.53) | 0.8203 |
| Model 5 | 1.90(1.05–3.41) | 1 (Reference) | 0.89(0.66–1.20) | 1.11(0.83–1.49) | 1.09(0.70–1.69) | 0.3486 |
| Monocytes | ||||||
| Range | <200 | 200–306 | 307–424 | 425–1000 | >1000 ** | |
| N | 1755 | 3158 | 3087 | 3134 | 75 | |
| AAA events | 36 | 76 | 87 | 175 | 3 | |
| Hazard Ratio (95% CI) | ||||||
| Incidence Rate (per 100,000 person-years) | 100.3 | 119.0 | 142.5 | 297.6 | 226.2 | |
| Model 1 | 0.89(0.60–1.32) | 1 (Reference) | 1.02(0.75–1.38) | 2.09(1.59–2.74) | N/A | <0.0001 |
| Model 2 | 1.28(1.11–1.48) | 1 (Reference) | 1.10(1.09–1.13) | 2.70(2.08–3.50) | N/A | 0.0007 |
| Model 3 | 0.86(0.57–1.29) | 1 (Reference) | 0.85(0.62–1.16) | 1.34(1.09–1.79) | N/A | 0.0320 |
| Model 4 | 0.85(0.56–1.30) | 1 (Reference) | 0.85(0.61–1.18) | 1.35(1.01–1.83) | N/A | 0.0420 |
| Model 5 | 0.86(0.56–1.31) | 1 (Reference) | 0.89(0.61–1.18) | 1.47(1.06–2.21) | N/A | 0.0051 |
| Eosinophils | ||||||
| Range | <20 | 20–110 | 111–195 | 196–500 | >500 | |
| N | 2369 | 2851 | 2874 | 2854 | 251 | |
| AAA events | 97 | 61 | 83 | 122 | 14 | |
| Hazard Ratio (95% CI) | ||||||
| Incidence Rate (per 100,000 person-years) | 204.4 | 106.2 | 146.5 | 223.6 | 307.3 | |
| Model 1 | 1.56(1.09–2.23) | 1 (Reference) | 1.21(0.87–1.69) | 1.72(1.26–2.35) | 2.38(1.33–4.25) | <0.0001 |
| Model 2 | 1.63(1.14–2.33) | 1 (Reference) | 1.16(0.83–1.63) | 1.40(1.02–1.92) | 1.86(1.03–3.35) | 0.0091 |
| Model 3 | 1.45(1.00–2.11) | 1 (Reference) | 1.14(0.81–1.61) | 1.32(0.95–1.82) | 1.79(0.99–3.24) | 0.0388 |
| Model 4 | 1.62(1.10–2.39) | 1 (Reference) | 1.20(0.84–1.71) | 1.35(0.96–1.89) | 2.01(1.08–3.73) | 0.0382 |
| Model 5 | 1.69(1.14–2.51) | 1 (Reference) | 1.26(0.89–1.8) | 1.42(1.01–1.99) | 2.18(1.18–4.03) | 0.0163 |
| Basophils | ||||||
| Range | <20 (all with value 0) | 20–51 | 52–65 | 66–100 | >100 | |
| N | 5734 | 1646 | 1501 | 1611 | 720 | |
| AAA events | 219 | 21 | 46 | 60 | 31 | |
| Hazard Ratio (95% CI) | ||||||
| Incidence Rate (per 100,000 person-years) | 194 | 62.2 | 152.7 | 196.3 | 227.7 | |
| Model 1 | 2.91(1.85–4.59) | 1 (Reference) | 2.22(1.32–3.72) | 2.89(1.76–4.76) | 4.05(2.31–7.08) | <0.0001 |
| Model 2 | 2.19(1.37–3.50) | 1 (Reference) | 1.76(1.03–3.00) | 1.78(1.06–2.97) | 2.50(1.41–4.43) | 0.0094 |
| Model 3 | 1.86(1.14–3.03) | 1 (Reference) | 1.61(0.94–2.77) | 1.48(0.86–2.53) | 1.91(1.04–3.51) | 0.5318 |
| Model 4 | 1.86(1.12–3.07) | 1 (Reference) | 1.53(0.88–2.66) | 1.49(0.86–2.57) | 1.94(1.04–3.63) | 0.7923 |
| Model 5 | 2.14(1.29–3.55) | 1 (Reference) | 1.67(0.95–2.94) | 1.79(1.04–3.09) | 2.56(1.38–4.75) | 0.0378 |
AAA- Abdominal Aortic Aneurysms
Model 1: adjusted for age, sex and race-center
Model 2: adjusted for Model 1 covariates plus smoking status and pack years of smoking
Model 3: adjusted for Model 2 covariates plus other differentials as a continuous variable
Model 4: adjusted for Model 3 covariates plus height, total cholesterol, HDL cholesterol, triglycerides, use of cholesterol lowering medication, hypertension, peripheral artery disease, and diabetes
Model 5: adjusted for Model 3 covariates plus BMI, waist circumference, total cholesterol, HDL cholesterol, triglycerides, use of cholesterol lowering medication, hypertension, peripheral artery disease, diabetes, and prevalent coronary heart disease at Visit 1
p-value for trend was calculated by excluding below the normal range group
This group had very few AAA events, hence for model stability we excluded this group from cox regression analyses
Figure 2.
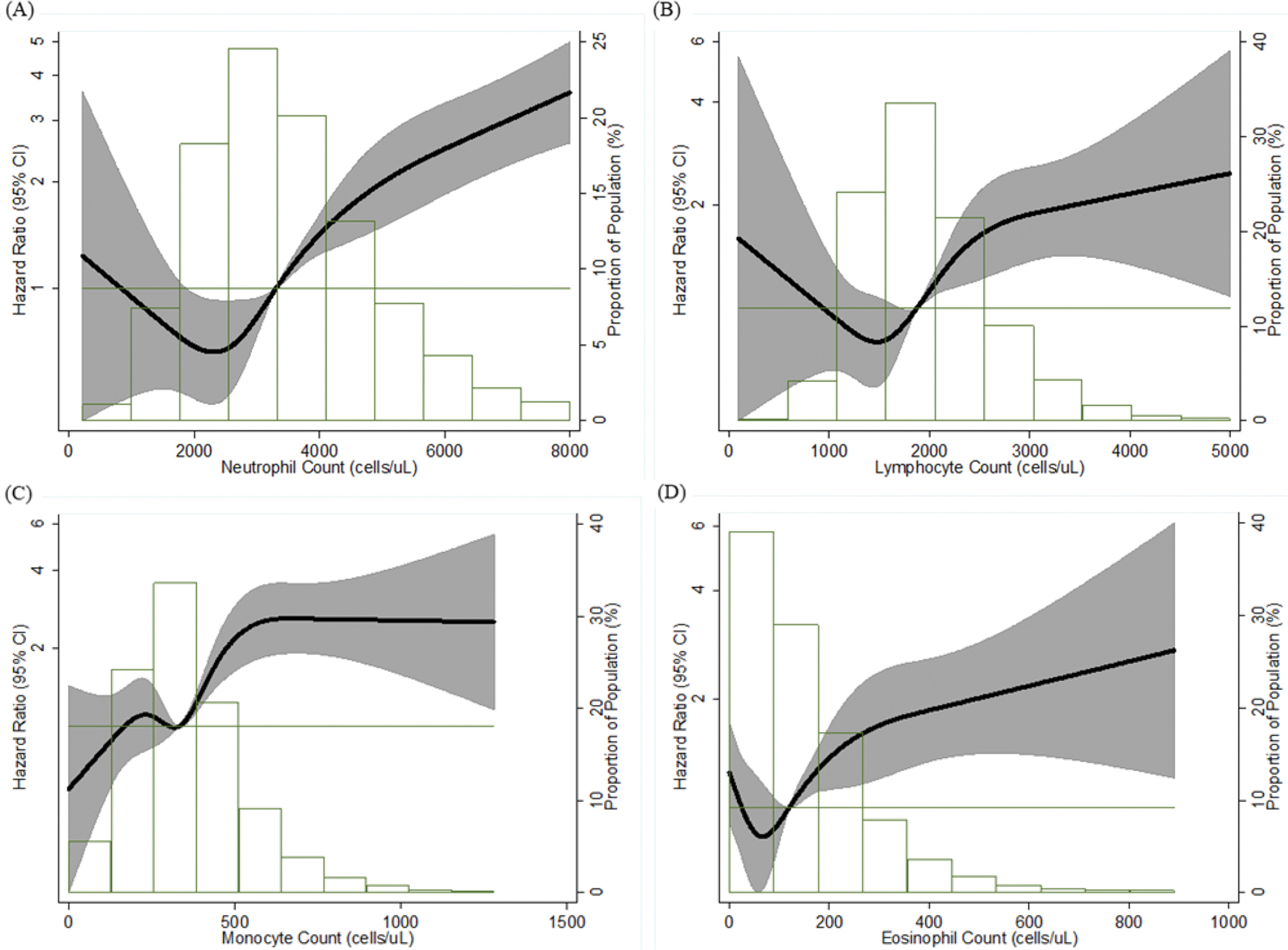
Association Between Each Differential Leukocyte Count and Incident AAA Presented as Hazard Ratio (Solid Line) and 95% Confidence Intervals (Shaded Area), Atherosclerosis Risk in Communities Study, 1987 – 2011.
(A) Neutrophil Count; (B) Lymphocyte Count; (C) Monocyte Count; (D) Eosinophil Count. Cox proportional hazards model using restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th and 95th percentiles and adjustment for age, race, and sex. The reference is the median value of each differential leukocyte count (hazard ratio = 1), and the histogram represents the frequency distribution of each differential leukocyte count in the study sample. AAA = abdominal aortic aneurysm
Out of 4533 participants who were never smokers, 44 participants developed AAA over the follow-up. The incidence rate among never smokers at baseline was 46.6 per 100,000 person years. Among never smokers (Table 3), neutrophil count was significantly associated with the risk of AAA after adjusting for demographic variables; however, this association was attenuated after adjustment for other potential confounders. Among never smokers, monocyte and eosinophil counts were significantly associated with the risk of developing AAA after adjustment for several potential confounders.
Table 3:
Hazard Ratio (95% Confidence Interval) of Abdominal Aortic Aneurysm per Standard Deviation Increment of White Blood Cell Differential Count Among Never Smokers, in the Atherosclerosis Risk in Communities Study, 1987–1989 to 2011.
| Standard Deviation, SD, cells/μl | HR* per SD increase (95% CI) |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
|
| ||||
| Neutrophils | 1252 | 1.33 (1.02–1.74) | 1.27 0.96–1.58) | 1.29 (1.00–1.80) |
| Monocytes | 157 | 1.36 (1.07–1.74) | 1.36 (1.03–1.81) | 1.35 (1.02–1.79) |
| Lymphocytes | 600 | 0.83 (0.59–1.17) | 0.76 (0.52–1.09) | 0.74 (0.51–1.09) |
| Eosinophils | 126 | 1.20 (1.00–1.54) | 1.31 (1.02–1.69) | 1.31 (1.01–1.69) |
| Basophils | 39 | 0.81 (0.57–1.15) | 0.76 (0.53–1.09) | 0.73 (0.51–1.05) |
HR (hazard ratio) per SD (standard deviation) increase in exposure.
Out of 4533 participants who were never smokers, 44 participants developed AAA over the follow-up. Incidence rate among never smokers at baseline was 46.6 per 100,000 person years.
Model 1: adjusted for age, sex and race-center
Model 2: adjusted for Model 1 covariates plus other differentials
Model 3: adjusted for Model 2 covariates plus height, total cholesterol, HDL cholesterol, triglycerides, use of cholesterol lowering medication, hypertension, diabetes, peripheral artery disease, and prevalent coronary heart disease at Visit 1
In our sensitivity analyses, excluding AAA cases who were diagnosed within the first 10 years of follow-up did not materially change the patterns of associations for neutrophils, monocytes, and eosinophils (Supplemental Table II). In stratum-specific analyses in ever-smokers (Supplemental Table III) and Whites only (Supplemental Table IV), patterns of associations for all of the differentials were similar to those of the whole cohort, although the associations for some categories did not reach statistical significance, likely due to reduced sample size. Even after accounting for potential competing risks by coronary heart disease, stroke and heart failure, associations were similar to the primary analysis which censored only death and loss to follow-up (Supplemental Table V). In our secondary analysis of DLC with severe AAA cases that required surgical intervention or had ruptured, the associations of DLC with severe AAA were similar in direction to the whole cohort but our analyses were likely underpowered due to the small number of cases (Supplemental Table VI). To account for the transient nature of DLC measures, we re-evaluated associations of DLC with the risk of incident AAA using DLC measures from visit 2 as an alternative baseline (Supplemental Table VII). Our findings remained similar suggesting the robustness of associations to short-term variability in DLC measures.
DISCUSSION
In our population-based cohort, over a median of 22.5 years of follow-up, we found that higher neutrophils, monocytes, and eosinophils were associated with increased risk of AAA, independent the other differentials and traditional AAA risk factors including smoking and plasma lipid levels. We also found that neutrophil, lymphocyte, eosinophil, and basophil count below the normal range were independently associated with increased risk of AAA compared with the first tertile of normal range (referent group). These associations were consistent in whites, ever smokers, after excluding AAA cases diagnosed within the first 10 years of follow-up, and additional accounting for potential competing risks by coronary heart disease, stroke, and heart failure. Furthermore, the patterns of associations between DLC and AAA with Visit 2 as an alternative baseline, in never smokers, or with restriction to severe AAA cases, were mostly similar to those observed in the primary analyses, although power was limited in those secondary analyses.
Leukocyte recruitment in inflammation is critical for host defense. However, excessive accumulation of these cells can compromise the structural integrity of vascular tissue. In the development of AAA, leukocytes are culpable through secretion of matrix metalloproteinases, cytokines such as interleukins (IL) and chemokines, which through several actions, contribute to loss of tensile strength of the aortic wall.7 Statin use is associated with lower leucocyte counts and other biomarkers of inflammation.19 Previous animal and human studies suggest that statins reduce collagen breakdown by stabilizing imbalances in matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases.20 Among patients with diagnosed AAA, statin therapy is associated with reduced AAA growth rate, rupture rate, and perioperative mortality following elective AAA repair.21–23 Future studies should also evaluate the potential effect of statins in primary prevention of AAA.
In the UK based CALIBER cohort, over a short follow-up (median 3.8 years), elevated neutrophil count was associated with increased risk of AAA.9 Our study extended this finding by showing robust linear associations over more than 20 years of follow-up. Canakinumab, a neutrophil count lowering agent, targets cytokine interleukin 1 beta (IL1b).24, 25 This has several downstream effects on various inflammation pathways including the interleukin 6 (IL6) pathway, independent of lipid pathways.25 The CANTOS trial reported protective effects of canakinumab against incident cardiovascular diseases (CVD) (not specifying whether AAA was included in CVD mortality).25 However, the trial also reported a significantly higher rate of fatal infection and sepsis in the treatment arm.25
In the CALIBER cohort, over a very short follow-up (median 3.8 years), AAA risk was higher in the top quintile vs middle quintile of monocyte count (HR 1.31, 95% CI 1.12, 1.52).9 Notably, the CALIBER cohort study data was collected from electronic medical records and death registries.9 In the ARIC cohort, we found a significant positive association between monocyte count and AAA risk. Monocytes and macrophages have been implicated in the development of AAA through secretion of several inflammatory mediators, including matrix metalloproteinases and IL6.3, 4, 5, 7 IL6 drives the pathophysiological feedback cycle of recruitment of monocytes, differentiation to macrophages and their infiltration into the aortic wall. Several longitudinal as well as genetic studies have provided additional robust evidence of causal effects of the IL-6 pathway in the development of AAA.26–29 Toclizumab, an IL-6 signal blocker, is associated with improved vascular function.30,31 Activated neutrophils are also a major source of circulating soluble IL6 receptor, that contributes to trans-signaling of immune cells.32, 33 It might be plausible to conduct well-designed clinical trials to evaluate the efficacy of IL6 receptor blockers in the management of small AAA and prevention of AAA in high risk populations. In our study, higher neutrophil and monocyte counts within their normal range were also significantly associated with higher risk of AAA independent of traditional risk factors such as smoking. These findings might be explained by chronic low-grade inflammation in midlife. The HUNT study reported that depression, a condition associated with chronic low-grade inflammation, is associated with higher risk of AAA.34 More population-based studies are required to evaluate whether conditions causing chronic low-grade inflammation, such as depression, may contribute to the risk of AAA in the community.
In our study, after adjusting for smoking and other potential confounders, neutrophils, lymphocytes, eosinophils, and basophils below the normal range were significantly associated with an increased risk of AAA as compared to those in the referent group. In the spline graphs, the confidence intervals for these associations appeared imprecise, but these graphs were not adjusted for smoking (which increases leukocyte counts35) due to inadequate power i.e. low number of non-smokers at baseline (Table 1). A few case reports/series from post-transplant patients on immunosuppressive therapy have hypothesized the potential role of immunosuppression in AAA pathophysiology.36,37 Future studies might consider testing this hypothesis in large population-based studies. The inverse associations of eosinophil and basophil counts below the normal range could be due to misspecification of exposure. Increases in more populous differentials, such as neutrophils (which are associated with increased AAA risk), may lead to lower measurements of the other sparse subpopulations of DLC such as eosinophils and basophils. However, simultaneous adjustment for the other differentials did not affect the observed associations. One study showed that in human and murine AAA lesions, eosinophil deficiency exacerbated AAA growth and discussed that eosinophils may play a protective role in AAA by favorably regulating macrophages and monocytes.38 In the CALIBER cohort, low lymphocyte and eosinophil counts were associated with an increased risk for CVD, but not AAA, during a median follow-up of 3.8 years.10 The very short follow-up time, limitations of using data from electronic medical records and differences in population characteristics might explain the different findings between CALIBER and our study.
In our study, there was a positive and dose-response association between eosinophil within and above normal ranges and AAA risk. In the Danish National Registry of Patients and Viborg Vascular screening trial, recent active asthma (which is marked by high eosinophil counts) was robustly associated with an increased risk of AAA.39 In the UK Biobank, higher eosinophil counts were associated with a higher risk of CVD over a median follow-up of 7 years.24 Previous case-control studies have reported a higher eosinophil count in those with AAA.38 To our knowledge, our study is the first large prospective study to provide evidence of an association of higher eosinophil counts in midlife with subsequent risk of AAA over a median follow-up of 22.5 years. Large population-based studies are warranted to investigate whether conditions associated with increased eosinophil count, such as allergies and certain infections, contribute to higher risk of AAA in the community.
The study findings should be interpreted considering its strengths and limitations. 1) The ARIC study has a relative advantage of being a large, community-dwelling cohort, with a long follow-up. 2) Misclassification of AAA due to underdiagnoses is plausible since AAA is often clinically silent and incidentally diagnosed, but such misclassification is likely non-differential with respect to leukocyte counts. This may lead to HRs being biased toward null. 3) DLC in a person may vary over time. Evaluating DLC measured only at the baseline visit may lead to misspecification of exposure and would typically bias HRs toward the null. 4) AAA is detected predominantly in the elderly, is usually asymptomatic and shares several risk factors, such as smoking, with other CVD. Hence, a threat to validity from competing events is particularly important for AAA research. We addressed this in our sensitivity analyses by modeling cause-specific hazards, i.e., right censoring competing CVD events.17, 18 There were no material differences in HRs after considering competing events. 5) Subpopulations of DLC might be correlated with each other through molecular signaling pathways (e.g. neutrophils, monocytes and macrophages are regulated by IL 6 pathways). However, in the ARIC study, DLC subpopulations were only weakly to moderately correlated with each other (Supplemental table I). Hence, we constructed models with mutual adjustment for DLC subpopulations. This may have led to over adjustment bias.40 Yet, in general, this adjustment had little impact on the associations. 6) Participants with history of AAA repair prior to the baseline evaluation were excluded but we may still have included some prevalent AAAs in the cohort at baseline due to lack of a baseline ultrasound screening. However, given that the baseline age of the cohort was 45–64 years, and the rarity of AAA in this age-group, the baseline prevalence of AAA would likely have been low1, 41 and our sensitivity analyses excluded initial AAA events occurring in the first 10 years of follow-up, which would have further reduced such bias. 7) Some subgroups had limited number of events, which may have led to inadequate power for those subgroup associations. 8) Finally, AAA growth or time to rupture from initial diagnosis was not measured in the ARIC cohort. Future studies might consider evaluating whether DLC may be associated with AAA growth or time to AAA rupture.
In summary, in this community-based longitudinal study with more than 20 years of follow-up, we found that higher neutrophil, monocyte, and eosinophil counts at baseline were positively associated with the risk of AAA, independent of traditional risk factors such as smoking, obesity and atherosclerosis. These associations were consistent after excluding AAA cases diagnosed within the first 10 years of follow-up, and additional accounting for potential competing risks by coronary heart disease, stroke, and heart failure. These findings suggest the need to identify non-traditional risk factors and treatment strategies to mitigate the residual risk of AAA conferred by midlife inflammation. Leukocyte lowering agents such as IL 6 receptor blockers may be a suitable candidate for early pharmacotherapeutic intervention and prevention of AAA. Below normal neutrophil, lymphocyte, eosinophil, and basophil counts were independently associated with a higher risk of AAA. These associations need to be replicated in larger, independent populations.
Supplementary Material
HIGHLIGHTS:
In a community-based longitudinal study, over a median follow-up of 22.5 years, we found that higher neutrophil and monocyte counts (both- above and within normal range) measured in midlife were significantly associated with higher risk of AAA, independent of other DLC and traditional risk factors such as smoking, obesity, and atherosclerosis.
Higher eosinophil count in midlife (baseline) was significantly associated with higher risk of AAA, independent of other DLC and traditional risk factors such as smoking.
These associations were consistent after excluding AAA cases diagnosed within the first 10 years of follow-up, and after additional accounting for potential competing risks by coronary heart disease, stroke, and heart failure.
Below normal neutrophil, lymphocyte, eosinophil, and basophil counts in midlife (baseline) were significantly associated with a higher risk of AAA, independent of other DLC and traditional risk factors.
ACKNOWLEDGEMENTS:
The authors thank the staff and participants of the ARIC study for their important contributions.
SOURCES OF FUNDING:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
In addition, this research is supported by the National Heart, Lung, and Blood Institute through grant R01 HL103695.
ABBREVIATIONS:
- AAA
Abdominal Aortic Aneurysm
- ARIC
Atherosclerosis Risk in Communities
- DLC
Differential Leukocyte Count
- TLC
Total Leukocyte Count
- CVD
Cardiovascular Diseases
- IL
Interleukin
Footnotes
DISCLOSURES:
None.
SUPPLEMENTAL MATERIALS
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019; 139: e56–528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014; 371: 2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 3.Raffort J, Lareyre F, Clément M, Hassen-Khodja R, Chinetti G, Mallat Z Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017; 14: 457. doi: 10.1038/nrcardio.2017.52. [DOI] [PubMed] [Google Scholar]
- 4.Chang TW, Gracon AS, Murphy MP, Wilkes DS. Exploring autoimmunity in the pathogenesis of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2015; 309: H719–727. doi: 10.1152/ajpheart.00273.2015. [DOI] [PubMed] [Google Scholar]
- 5.Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Review of Cardiovascular Therapy. 2015; 13(9): 975–987. doi: 10.1586/14779072.2015.1074861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dua A, Kuy S, Lee CJ, Upchurch GR, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010.J Vasc Surg. 2014; 59: 1512–1517. doi: 10.1016/j.jvs.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Quintana RA, Taylor WR. Cellular Mechanisms of Aortic Aneurysm Formation. Circ Res 2019; 124: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folsom AR, Yao L, Alonso A, et al. Circulating biomarkers and abdominal aortic aneurysm incidence: The atherosclerosis risk in communities (ARIC) study. Circulation. 2015; 132: 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AD, Denaxas S, Nicholas O, Hingorani AD and Hemingway H. Neutrophil Counts and Initial Presentation of 12 Cardiovascular Diseases: A CALIBER Cohort Study. J Am Coll Cardiol. 2017; 69: 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah AD, Denaxas S, Nicholas O, Hingorani AD and Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. 2016; 3: e000477. doi: 10.1136/openhrt-2016-000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The atherosclerosis risk in communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol. 1989; 129: 687–702. [PubMed] [Google Scholar]
- 12.Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, Lederle FA, Hunter DW, Bengtson LG, Guan W, Missov E, Folsom AR. Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the ARIC Study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol. 2016; 36: 2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto FJ, Szklo M, Folsom AR, Rock R, Mercuri M. Leukocyte count correlates in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 1992; 136: 525–537. [DOI] [PubMed] [Google Scholar]
- 14.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983; 29: 1075–1080. [PubMed] [Google Scholar]
- 15.Nägele U, Hägele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984. Feb;22(2):165–74. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Patsch JR, Sailer S, Kostner G, Sandhofer F, Holasek A, Braunsteiner H. Separation of the main lipoprotein density classes from human plasma by rate-zonal ultracentrifugation. J Lipid Res. 1974; 15: 356–366. [PubMed] [Google Scholar]
- 17.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016; 133(6): 601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noordzij M, Leffondré K, Van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrology Dialysis Transplantation. 2013; 28(11): 2670–2677. [DOI] [PubMed] [Google Scholar]
- 19.Yoon SS, Dillon CF, Carroll M et al. Effects of statins on serum inflammatory markers: The U.S. National Health and Nutrition Examination Survey. 1999–2004. J Atheroscler Thromb 2010;17:1176–1182. [DOI] [PubMed] [Google Scholar]
- 20.Evans J, Powell JT, Schwalbe E, Loftus IM, Thompson MM. Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur J Vasc Endovasc Surg. 2007; 34:302–303. [DOI] [PubMed] [Google Scholar]
- 21.Dunne JA, Bailey MA, Griffin KJ, Sohrabi S, Coughlin PA, Scott DJ. Statins: the holy grail of abdominal aortic aneurysm (AAA) growth attenuation? A systematic review of the literature. Curr Vasc Pharmacol. 2014; 12:168–172. [DOI] [PubMed] [Google Scholar]
- 22.Takagi H, Yamamoto H, Iwata K, Goto S, Umemoto T. Effects of statin therapy on abdominal aortic aneurysm growth: a meta-analysis and meta-regression of observational comparative studies. Eur J Vasc Endovasc Surg. 2012; 44:287–292. [DOI] [PubMed] [Google Scholar]
- 23.Salata K, Syed M, Hussain MA, et al. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018; 7(19): e008657. doi: 10.1161/JAHA.118.008657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welsh C, Welsh P, Mark PB, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler Thromb Vasc Biol 2018; 38: 1415–1423. 10.1161/ATVBAHA.118.310945 [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Everett BM, Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 26.Tang W, Yao L, Hoogeveen RC, et al. The Association of Biomarkers of Inflammation and Extracellular Matrix Degradation with the Risk of Abdominal Aortic Aneurysm: The ARIC Study. Angiology. 2019; 70(2):130–140. doi: 10.1177/0003319718785278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison SC, Smith AJ, Jones GT, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013; 34(48): 3707–3716. doi: 10.1093/eurheartj/ehs354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai T, Zhang Y, Ho YL, et al. Association of Interleukin 6 Receptor Variant With Cardiovascular Disease Effects of Interleukin 6 Receptor Blocking Therapy: A Phenome-Wide Association Study. JAMA Cardiol. 2018;3(9):849–857. doi: 10.1001/jamacardio.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paige E, Clément M, Lareyre F, et al. Interleukin-6 Receptor Signaling and Abdominal Aortic Aneurysm Growth Rates. Circ Genom Precis Med. 2019; 12(2): e002413. doi: 10.1161/CIRCGEN.118.002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011; 219(2): 734–736. doi: 10.1016/j.atherosclerosis.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Ikonomidis I, Pavlidis G, Katsimbri P et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol. 2019; 108: 1093–1101. doi: 10.1007/s00392-019-01443-9 [DOI] [PubMed] [Google Scholar]
- 32.Moots RJ, Sebba A, Rigby W, et al. Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology (Oxford). 2017; 56(4): 541–549. doi: 10.1093/rheumatology/kew370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012. Feb;32(2):281–90. doi: 10.1161/ATVBAHA.111.229435. Epub 2011 Nov 10. [DOI] [PubMed] [Google Scholar]
- 34.Nyrønning LÅ, Stenman M, Hultgren R, Albrektsen G, Videm V, Mattsson E. Symptoms of Depression and Risk of Abdominal Aortic Aneurysm: A HUNT Study. J Am Heart Assoc. 2019. Nov 5;8(21):e012535. doi: 10.1161/JAHA.119.012535. Epub 2019 Oct 23. Erratum in: J Am Heart Assoc. 2019 Dec 3;8(23):e014509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higuchi T, Omata F, Tsuchihashi K, Higashioka K, Koyamada R, Okada S. Current cigarette smoking is a reversible cause of elevated white blood cell count: Cross-sectional and longitudinal studies. Prev Med Rep. 2016;4:417–422. Published 2016 Aug 9. doi: 10.1016/j.pmedr.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Englesbe MJ, Wu AH, Clowes AW, Zierler RE. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplant patients. J Vasc Surg. 2003; 37: 27–31. [DOI] [PubMed] [Google Scholar]
- 37.Lindeman JHN, Rabelink TJ, van Bockel JH. Immunosuppression and the Abdominal Aortic Aneurysm: Doctor Jekyll or Mister Hyde? Circulation. 2011; 124(18): e463–465. [DOI] [PubMed] [Google Scholar]
- 38.Liu CL, Liu X, Zhang Y, Liu J, Yang C, Luo S, Liu T, Wang Y, Lindholt JS, Diederichsen ACP, Rasmussen LM, Dahl M, Sukhova GK, Lu G, Upchurch G Jr, Libby P, Guo J, Zhang JY, Shi GP. Eosinophils Protect Mice from Angiotensin-II Perfusion-Induced Abdominal Aortic Aneurysm. Circ Res. 2020. Nov 6. doi: 10.1161/CIRCRESAHA.120.318182. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu CL, Wemmelund H, Wang Y, et al. Asthma Associates With Human Abdominal Aortic Aneurysm and Rupture. Arterioscler Thromb Vasc Biol. 2016; 36(3): 570–578. doi: 10.1161/ATVBAHA.115.306497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009; 20(4): 488–495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao L, Folsom AR, Alonso A, et al. Association of carotid atherosclerosis and stiffness with abdominal aortic aneurysm: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis 2018; 270: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In order to minimize the possibility of unintentionally sharing information that can be used to re-identify private information, a subset of the data generated for this study are available at the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) and can be accessed at [https://biolincc.nhlbi.nih.gov/studies/aric/].


