Abstract
Objective
The aim of this study was to investigate the impact of left atrial diverticula (LADs), left sided septal pouches (LSSPs) and middle right pulmonary veins (MRPVs) on recurrent atrial fibrillation (rAF) in patients undergoing laser pulmonary vein isolation procedure (PVI).
Material and methods
This retrospective study enrolled 139 patients with pre-procedural multiple detector computed tomography (MDCT) imaging and 12 months follow-up examination. LADs, LSSPs and MRPV were identified by two radiologists on a dedicated workstation using multiplanar reconstructions and volume rendering technique. Univariate and bivariate regression analyses with patient demographics and cardiovascular risk factors as covariates were performed to reveal independent factors associated with rAF.
Results
LADs were recorded in 41 patients (29%), LSSPs in 20 (14%) and MRPVs in 15 (11%). The right anterosuperior wall of the left atrium was the most prevalent location of LADs (68%). rAF occured in 20 patients, thereof, 15 exhibited an outpouching structure of the left atrium (LAD: 9, LSSP: 2 and MRPV: 3). Presence of an LAD (HR: 2.7, 95%CI: 1.0–8.4, p = 0.04) and permanent AF (HR: 4.8, 95%CI: 1.5–16.3, p = 0.01) were independently associated with rAF.
Conclusions
LAD, LSSP and MRPV were common findings on pre-procedural cardiac computed tomography. LADs were revealed as potential independent risk factor of rAF, which might be considered for treatment planning and post-treatment observation.
1. Introduction
Atrial fibrillation (AF) affects 1% to 2% of the general population, thus representing the most prevalent cardiac arrhythmia [1–3]. The lifetime risk of developing AF is >30% for both men and women [4]. The inefficient contraction of the left atrium creates a turbulent blood flow that increases the risk of developing cardiac thrombosis. AF is associated with a nearly 5-fold increased risk for ischemic stroke [5, 6]. The main approaches to the treatment of AF are rate control, rhythm control and stroke prevention [3, 7]. AF occurs due to complex electrical defects in the atrium including a rapidly firing focus, complex multiple reentrant circuit or rotors [8]. The development of these electrical defects is boosted by changes of three categories: electrical remodeling, structural remodeling and anatomic remodeling. As pulmonary vein sleeves are the most frequent anatomical source of ectopic cardiac pacemaker cells with proarrhythmogenic potential, catheter ablation is frequently used to uncouple these cells [9–11]. Ablational therapies not only include the PV but other ablation sites such as the left atrial roof, the posterior wall, the interatrial septum or the mitral isthmus line [12]. To improve the success rates and to avoid complications during ablation, knowledge on outpouchings of the left atrium and pulmonary veins is desirable for procedural planning, all of which can be reliably visualized by multiple detector computed tomography (MDCT), [13–15]. Middle right pulmonary veins (MRPV) are described as one of the most common variations of the PV, being present in almost 20% of patients and baring the risk to initiate AF [16, 17]. Left atrial diverticula (LAD) are defined as pouchy evaginations of the left atrial cavity with a broad body, wide neck and smooth contour [18]. LAD are a common finding in cardiac-CT, being present in up to 36% of patients undergoing AF ablation. They are reported to serve as an extra cardiac pacemaker focus, and comprise a risk factor for intracavitary thrombosis and cardiac perforation during ablation [19–23]. According to histopathological analyses, LADs contain trabeculated myocardium with the same wall structure as the surrounding myocardium and were firstly reported to show ectopic activity in a documented case report in 2009 [24, 25]. Left sided septal pouches (LSSP) are considered structures that occur when the patent foramen ovale (PFO) is absent but the septum primum and septum secundum are not completely fused [26]. Their clinical role is unclear however some data indicate that LSSP could be a trigger for atrial fibrillation and cryptogenic stroke [26–29].
The aim of this study is to investigate if there is a correlation between LAD, MRPV, LSSP and recurrence of atrial fibrillation (rAF) following pulmonary vein isolation.
2. Material and methods
The Ethics Commission of Cologne University’s Faculty of Medicine approved this retrospective, single-center study (reference number 19–1439). Due to the retrospective nature of the study, a written informed consent was waived.
2.1 Patient population
A structured database query of the picture archiving and communication system (PACS) revealed all patients who received a contrast enhanced electrocardiogram-gated multidetector computed tomography of the heart between January 2013 to September 2014 prior to pulmonary vein isolation. All ablations were performed by laser pulmonary vein ablation technique. This resulted in 150 patients screened for study inclusion. Patients with incomplete imaging (N = 6), incomplete ablation procedure with incomplete circumferential lines around the pulmonary veins during catheter ablation (N = 3) or incomplete follow-up visits (N = 5) were excluded from further analysis. In all three cases of incomplete ablation procedure, the anatomy of the esophagus was unfavorable and a complete ablation could not be performed to avoid heat damage.
2.2 CT techniques
Cardiac computed tomography was performed using a dedicated scan-protocol with visual triggering of the contrast medium bolus in the left atrium. All studies were carried out using a 64 row MDCT scanner (Discovery CT750 HD, GE Healthcare, Waukesha, WI). Scanning was performed in craniocaudal direction from above the aortic arch to the diaphragm using prospective ECG-gating. Using a power injector, 60 ml of an intravenous contrast agent (Imeron 400 mg/mL, Bracco, Milan, Italy) was administered at a flow rate of 5.0 ml/s through a cubital vein. The contrast bolus was followed by a saline chaser of 50ml at the same flow. No premedication was given. Further scan parameters comprised: 120kV, 200 mAs with tube current modulation. Images were reconstructed using the following parameters: CardIQ Xpress (ImageWorks™, GE Healthcare, Waukesha, WI), slice thickness 0.625 mm, section increment 0.1 mm.
2.3 CT data post processing and image analysis
All images were transferred to a dedicated cardiac work station (GE Medical, Advanced Workstation 4.6 and Cardiac iQ Express, GE Medical). Two radiologists with 5 and 15 years of experience in cardiac imaging (EC, TA) reviewed the images in consensus using multiplanar reconstructions and volume-rendering techniques. Radiologists were blinded to all clinical data except age and gender. Presence of LAD including the location, LSSP and MRPV were recorded (Fig 1). Left atrial diverticula were defined as pouchy evaginations of the left atrial cavity with a broad body, wide neck and smooth contour [18].
Fig 1.
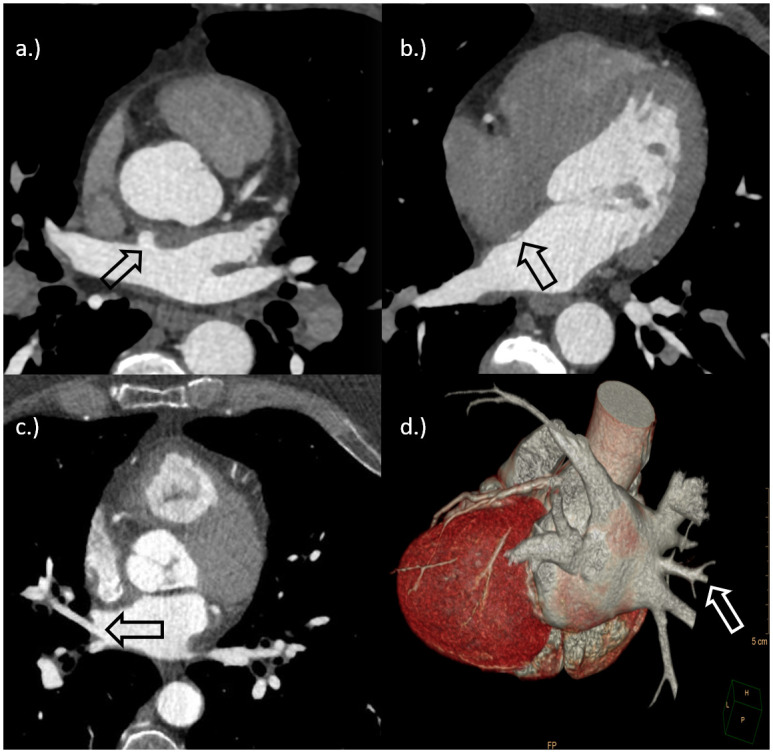
a.) Cardiac-CT of a 55 years old female patient with AF. The arrow shows a single left atrial diverticulum in the anterosuperior wall. b.) Cardiac-CT of a 54 years old female patient with AF. The arrow shows a left-sided septal pouch. c.) + d.) Cardiac-CT and three-dimensional volume rendering of the heart of a 44 years old male patient with AF. The arrows (white and black) show an accessory pulmonary vein merging into the left atrium from the right.
2.4 Ablation procedure
Pulmonary vein isolation was performed with the “CardioFocus HeartlightTM” system using laser energy (Heartlight™, CardioFocus, Marlborough, MA, US). It is a visually controlled laser ablation system consisting of a console, a 15-Charrière outer diameter steerable transseptal sheath, a reusable 2-Charrière endoscope, and the 12-Charrière catheter containing a size-adjustable balloon at the distal end. The console is used to deliver the laser energy, visualize the endoscopic image, and control the balloon size [30]. Balloon diameters can be adjusted between 9 mm and 35 mm. Ablation is induced by a 980 nm diode laser that generates a 30° arc of energy.
All procedures were carried out under anesthesiologic supervision in analgesia with propofol and remifentanil in a cardiac catheter laboratory with a biplane, swiveling X-ray fluoroscopy unit. During the procedure, the patients were heparinized in a controlled manner over the activated clotting time with a target value between 300 and 350 seconds to avoid thromboembolic complications. The measurements were made every 10 to 20 minutes. The left atrial appendage was checked for thrombus using transesophageal echocardiography. Access was via the femoral vein on both sides. A four-pole diagnostic catheter was placed in the right ventricle and a six-pole diagnostic catheter in the coronary sinus after puncturing the left femoral vein and using two 7 F-sheaths. Access to the left atrium was made after puncturing the right femoral vein and placing a long transseptal sheath (SL-0 ™, St. Jude Medical, St. Paul, USA) and subsequent fluoroscopic and transesophageal echocardiographically controlled single puncture of the atrial septum using a transeptal needle (BRK) XS 71cm ™, St. Jude Medical, St. Paul, USA). This was followed by a semi-selective angiographic display of all pulmonary veins in left anterior oblique 50° (LAO) and right anterior oblique 25° (RAO) with high-frequency stimulation of the right ventricle (rapid pacing), with a cycle length of between 240 ms and 300 ms. An esophageal temperature probe (SensiThermTM, St. Jude Medical, St. Paul, USA) was used to measure the esophageal temperature in all patients. To avoid phrenic lesions, the compound motor action potential method (CMAP) was used to isolate the right pulmonary veins. If present, MRPV were targeted by laser balloon. Accessory PVs were not specifically targeted, because they were not present. During the ablation of the right pulmonary veins, the phrenic nerve was stimulated with an electrophysiological diagnostic catheter (Response TM, St. Jude Medical, St. Paul, USA) in the superior vena cava and the resulting diaphragmatic electromyogram monitored with surface electrodes. After ablation is complete, isolation success is verified using a multipolar circular mapping catheter (LASSOTM, Johnson & Johnson, New Brunswick, USA).
2.5 Clinical information and patient follow-up
The following clinical parameters at time point of procedure were collected by a medical chart review of the hospital information system (ORBIS; Agfa HealthCare, Bonn, Germany): age, gender, presence of diabetes mellitus, periphery artery disease, atrial fibrillation, heart failure, hypertension and hyperlipidemia. Additionally, left atrial diameters were given in patients records after transthoracic echocardiogram. The antiarrhythmic drug regimen was as follows: If the patient presented with an existing antiarrhythmic medication (Amiodarone, Betablockers, single- or multi-channel blocker) prior to the intervention, it was continued for three additional months after PVI (blanking period). If the patient was not taking any antiarrhythmic medication, no antiarrhythmic medication was started after PVI. Clinical follow-up visits were scheduled 3, 6 and 12 months after ablation and included a holter monitoring for seven days. AF recurrence was defined as the presence of any episode of atrial tachycardia or AF lasting 30 s or more on 12-lead electrocardiogram. Patients with incomplete follow-up visits were excluded from further analysis (n = 5).
2.6 Statistical analysis
Categorical variables are presented as numbers with percentages and compared using the Chi-square or the Fisher exact test, when appropriate. Continuous variables are reported as mean ± standard deviation and compared using the two-sided Student’s t-test and the Mann-Whitney-U test, when appropriate. Cut-off values were evaluated with receiver-operating-characteristics curve analysis by determination of the Youden index. Factors with a p-value < 0.2 in the univariate analyses were entered into a binary logistic regression to identify independent factors associated with the respective outcome measure. Statistical significance was defined as p < 0.05. All analyses were performed using dedicated software (JMP v14, SAS Institute, Cary, NC, USA and SPSS Statistics v25, IBM, Armonk, NY, USA).
3. Results
The final study cohort consisted of 139 patients that received 139 cardiac ablations due to atrial fibrillation (median age 64.4 ± 12.7 years, 88 men). The most prevalent cardiovascular risk factors were coronary artery disease and arterial hypertension (63% each). The mean LAEDD was 4.4 ± 0.4 cm.
3.1 Prevalence of outpouching structures
A total of 41 (29%) LADs were identified (28 men; 12 women). Multiple LADs in one patient were not found. In 28 of these cases (68%), the LAD was located in the right anterosuperior wall of the left atrium, 7 (18%) in the left inferoposterior wall, and each 2 (5%) in the right inferoanterior, the left anterosuperior and the left inferoanterior wall, respectively (Fig 2).
Fig 2. Overview of the total amount of left atrial diverticula and their localisation.
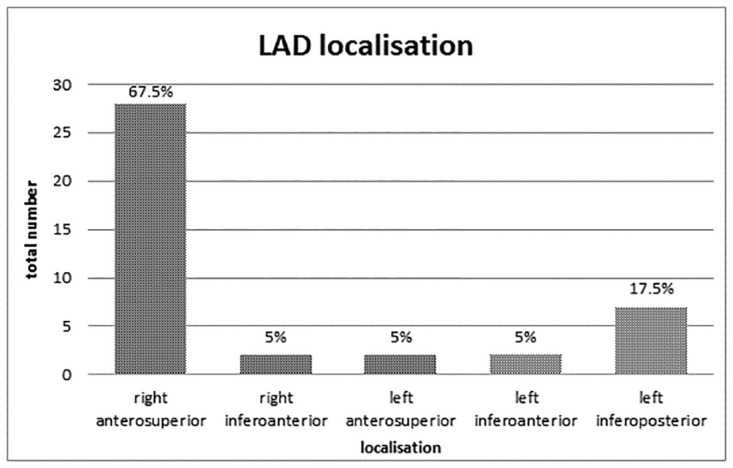
Most of the LAD were located close to the ostia of the pulmonary veins.
Accessory pulmonary veins were not found in the present study cohort. MRPVs were found in 15 (11%) cases and LSSPs in 20 (14%).
3.2 Correlation with recurrent atrial fibrillation
The overall rAF rate was 14% (20/139), including 8 PV reconnections and unclear cause in 12. Of these 20 patients, 13 (65%) had an outpouching of the left atrial anatomy: Nine (45%) had an LAD, 3 (11%) an MRPV and one (5%) an LSSP. In the univariate analysis, the presence of an LAD was by trend associated with rAF (HR = 2.2, 95%CI = 0.8–5.9; p = 0.1). The other two outpouching types were not significantly associated with rAF (Table 1). Among patient demographics and cardiovascular risk factors, persistent AF (HR = 2.8, 95%CI = 1.0–8.0; p = 0.043) and left atrial diameter > 4.6 cm (HR = 3.5, 95%CI = 1.2–10.2; p = 0.017) were further factors associated with rAF (Table 1). Covariates with a p-value < 0.2 in the univariate analysis were entered into the bivariate logistic regression model. Hereby, persistent AF and presence of a LAD were confirmed as independent risk factors for rAF (Table 2).
Table 1. Patient characteristics with and without AF recurrence.
| Parameter | AF recurrence (n = 20) | No recurrence (n = 119) | P-value |
|---|---|---|---|
| Age (years; mean ± SD) | 62.6 ± 14.1 | 64.7 ± 12.5 | 0.60 |
| Sex (male vs. female), n (%) | 12 (60%) | 76 (64%) | 0.80 |
| AF subtype (persistent vs. paroxysmal), n (%) | 7 (35%) | 19 (16%) | 0.04 * |
| LAD, n (%) | 9 (45%) | 32 (27%) | 0.10* |
| LSSP, n (%) | 1 (5%) | 19 (16%) | 0.20 |
| MRPV, n (%) | 3 (15%) | 12 (10%) | 0.52 |
| Coronary artery disease, n (%) | 11 (55%) | 77 (65%) | 0.41 |
| Hypertension, n (%) | 10 (50%) | 78 (88%) | 0.18 |
| Hyperlipidaemia, n (%) | 10 (50%) | 65 (66%) | 0.70 |
| Periphery artery disease, n (%) | 4 (20%) | 26 (22%) | 0.86 |
| Diabetes mellitus, n (%) | 2 (10%) | 21 (18%) | 0.40 |
| Heart failure, n (%) | 6 (30%) | 20 (17%) | 0.16 |
| LAEDD (cm; mean ± SD) | 4.66 ± 0.27 | 4.38 ± 0.38 | 0.001 * |
SD—standard deviation; AF–atrial fibrillation; LAD–left atrial diverticulum; LSSP–left-sided septal pouch; MRPV–middle right pulmonary vein; LAEDD, left atrial end diastolic diameter;
*—included in bivariate logistic regression analysis
Table 2. Binary logistic regression to determine independent predictors of AF recurrence.
| Risk factor | Standard error | P-value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Persistent AF subtype | 0.616 | 0.010 | 4.8 | 1.5–16.3 |
| LAD | 0.584 | 0.043 | 2.7 | 1.0–8.4 |
| Hypertension | 0.540 | 0.122 | 2.3 | 0.8–6.6 |
| Heart failure | 0.609 | 0.060 | 3.1 | 1.0–10.4 |
| LAEDD > 4,6 cm | 0.597 | 0.062 | 3.1 | 0.9–9.8 |
AF–atrial fibrillation; LAD–left atrial diverticulum; LAEDD–left atrial end diastolic diameter; CI–confidence interval.
4. Discussion
The aim of this study was to investigate the impact of left atrial diverticula, left sided septal pouches and middle right pulmonary veins on recurrent atrial fibrillation after pulmonary vein isolation. First, the results demonstrated that LAD, LSSP and MRPV were common findings in cardiac CT scans with prevalence rates ranging between 11% and 29%. Second, our data suggest that LADs are independently associated with rAF. Third, persistent AF was a further independent risk factor for rAF.
LADs represent a common finding in patients with AF with an imaging prevalence of 29% in the current studies, which is within the range of previous studies [19, 20]. LADs can be found in any location of the left atrium, yet, our data indicate a preference for the right anterosuperior wall (Fig 2), which is in line with other recently published studies [20, 22, 31]. In accordance with Abbara et al., we did not find other cardiac abnormalities in patients with LADs; furthermore, there were no patients with multiple LADs [22]. There are a few case reports describing that LADs and other accessory appendages are a potential source of thromboembolism and a potential cause of perforation risk during ablation [19, 20, 32]. Interestingly, histopathological analyses could demonstrate that LADs contain trabeculated myocardium which raises the hypothesis that LAD may be contractile and a source of ectopic activity, which was reported in a documented case report by Kileen et al. [24]. However, the role of LADs in the pathomechanism of AF remains unclear. In the present study, there were no procedural complications during catheter ablation in all patients with LAD. A recent study with comparable design by De Ponti et. al. could not establish a significant correlation between LAD and AF or rAF. However, a differentiation between “true” diverticula, aneurysms and accessory appendages has not been done and findings were all subsumed as LAD. This causes a blurring regarding the meaningfulness of the clinical impact of “true” LAD, which were targeted in our study. In contrast to their described study, we used ECG-gated CT protocols and breathing commands to eliminate disruptive factors of image quality such as respiratory movements and pulsation artifacts of the heart. Another recently published study by Demir et al. could not prove a correlation between the presence of a LAD and rAF. The main difference to our study is that two different types of ablation procedures were used (cryoballoon- and radiofrequency ablation) and the amount of rAF was significantly higher in the group that underwent radiofrequency ablation. Therefore we only included patients that underwent laser pulmonary vein isolation to avoid this possible bias [33]. However, none of these studies could prove LAD an independent risk factor for rAF.
Since it is known that muscular sleeves from the left atrium can extend into the wall of pulmonary veins, PVI has been successfully used to disconnect the electrical connection between PVs and the left atrium [34–37]. We detected a total number of 15 MRPVs in our study cohort. However, in this study MRPV showed no statistical correlation to rAF. This is in concordance with the results reported by Khoueiry et al. who did not find a difference in the incidence of AF recurrence in patients undergoing cryoballon ablation (17%) or radiofrequency ablation (14,1%). Neither a significant impact of MRPV on procedural success could be detected [38].
To our knowledge this is the first study to investigate a possible correlation between LSSP and rAF after PVI. LSSP are defined as “kangaroo pouch-like structures”, that occur when the PFO is absent but the septum primum and septum secundum are not completely fused. A recently published study by Holda et al. could demonstrate that there is an association between the presence of LSSP and cryptogenic stroke. LSSP are also described as a trigger for AF [26]. This matches to other research projects regarding LSSP and AF, since several studies before and around the turn of the millennium have shown a correlation between redundant LSSP and AF, in fetuses [39–41]. Whether there is a causality between LSSP in adult patients and AF has not been finally clarified, at least our quite small data showed no correlation with AF recurrence after PVI.
The drawbacks of our study are the retrospective study design and the small study population number. Furthermore, every patient included in our study had a history of AF. A control group of healthy normal rhythmic patients carrying LAD’s without AF would be helpful to better understand clinical implications from our findings. Furthermore, it is important to emphasize that all patients in this study received antiarrhythmic drugs for 12 months after ablation. This interval seems quite long, but does not distort the long-term results, since several studies could proof, that AADs can prevent early atrial arrhythmias within 2 months after catheter ablation, but do not prevent late arrhythmias [42]. Another limitation is that in addition to LAD, there are also other outpouchings in the left atrium that could have been falsely characterized as LADs. These outpouchings are mainly described as left atrial accessory appendages (LAAAs). However, LAAAs are typically characterized by an irregular contour [22]. To minimize this potential bias, we only include LADs with a smooth contour. Despite these limitations this study describes post-procedural data with a follow up to 1 year.
In conclusion LAD, LSSP and MRPV are common findings during cardiac computed tomography of the heart. The role of LAD in rAF is still controversial, however logistic regression analysis revealed that presence of a LAD and persistent AF as potential independent risk factors for rAF in our study cohort. At this point, further prospective multicenter studies are needed to fully clarify the clinical significance of these structures within the left atrium and recurrence of atrial fibrillation.
Supporting information
(XLSX)
Abbreviations
- AF
atrial fibrillation
- aPV
accessory pulmonary vein
- CI
confidence interval
- CMAP
compound motor action potential method
- kV
kilovolt
- LAD
left atrial diverticula
- LAEDD
left atrial end diastolic diameter
- LSSP
left sided septal pouch
- mAs
milliampere-seconds
- MDCT
multi detector computed tomography
- ms
milliseconds
- PACS
picture archiving and communication system
- PFO
patent foramen ovale
- PVI
pulmonary vein isolation procedure
- SD
standard deviation
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S (2014) The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 114: 1453–1468. doi: 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 2.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, et al. (2015) 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. The Lancet 386: 154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellman J, Sheikh F (2015) Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol 5: 649–665. doi: 10.1002/cphy.c140047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, et al. (2017) Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 136: 1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 6.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, et al. (2012) Antithrombotic Therapy for Atrial Fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: e531S–e575S. doi: 10.1378/chest.11-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, et al. (2017) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Interv Card Electrophysiol 50: 1–55. doi: 10.1007/s10840-017-0277-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nattel S, Dobrev D (2012) The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J 33: 1870–1877. doi: 10.1093/eurheartj/ehs079 [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P, Calkins H (2017) Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J 38: 20–26. doi: 10.1093/eurheartj/ehw260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, et al. (2014) Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 35: 3365–3376. doi: 10.1093/eurheartj/ehu374 [DOI] [PubMed] [Google Scholar]
- 11.Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, et al. (2014) The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J 35: 1466–1478. doi: 10.1093/eurheartj/ehu001 [DOI] [PubMed] [Google Scholar]
- 12.Holda MK, Koziej M, Holda J, Tyrak K, Piatek K, et al. (2017) Anatomic characteristics of the mitral isthmus region: The left atrial appendage isthmus as a possible ablation target. Ann Anat 210: 103–111. doi: 10.1016/j.aanat.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 13.Oda S, Honda K, Yoshimura A, Katahira K, Noda K, et al. (2016) 256-Slice coronary computed tomographic angiography in patients with atrial fibrillation: optimal reconstruction phase and image quality. Eur Radiol 26: 55–63. doi: 10.1007/s00330-015-3822-0 [DOI] [PubMed] [Google Scholar]
- 14.Kaseno K, Tada H, Koyama K, Jingu M, Hiramatsu S, et al. (2008) Prevalence and characterization of pulmonary vein variants in patients with atrial fibrillation determined using 3-dimensional computed tomography. Am J Cardiol 101: 1638–1642. doi: 10.1016/j.amjcard.2008.01.053 [DOI] [PubMed] [Google Scholar]
- 15.Thorning C, Hamady M, Liaw JV, Juli C, Lim PB, et al. (2011) CT evaluation of pulmonary venous anatomy variation in patients undergoing catheter ablation for atrial fibrillation. Clin Imaging 35: 1–9. doi: 10.1016/j.clinimag.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 16.Klimek-Piotrowska W, Hołda MK, Piątek K, Koziej M, Hołda J (2016) Normal distal pulmonary vein anatomy. PeerJ 4: e1579. doi: 10.7717/peerj.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsao HM, Wu MH, Yu WC, Tai CT, Lin YK, et al. (2001) Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 12: 1353–1357. doi: 10.1046/j.1540-8167.2001.01353.x [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yang ZG, Xu HY, Shi K, Long QH, et al. (2017) Assessments of pulmonary vein and left atrial anatomical variants in atrial fibrillation patients for catheter ablation with cardiac CT. Eur Radiol 27: 660–670. doi: 10.1007/s00330-016-4411-6 [DOI] [PubMed] [Google Scholar]
- 19.De Ponti R, Lumia D, Marazzi R, Mameli S, Doni LA, et al. (2013) Left atrial diverticula in patients undergoing atrial fibrillation ablation: morphologic analysis and clinical impact. J Cardiovasc Electrophysiol 24: 1232–1239. doi: 10.1111/jce.12213 [DOI] [PubMed] [Google Scholar]
- 20.Peng LQ, Yu JQ, Yang ZG, Wu D, Xu JJ, et al. (2012) Left atrial diverticula in patients referred for radiofrequency ablation of atrial fibrillation: assessment of prevalence and morphologic characteristics by dual-source computed tomography. Circ Arrhythm Electrophysiol 5: 345–350. doi: 10.1161/CIRCEP.111.965665 [DOI] [PubMed] [Google Scholar]
- 21.Killeen RP, Ryan R, MacErlane A, Martos R, Keane D, et al. (2010) Accessory left atrial diverticulae: contractile properties depicted with 64-slice cine-cardiac CT. Int J Cardiovasc Imaging 26: 241–248. doi: 10.1007/s10554-009-9511-9 [DOI] [PubMed] [Google Scholar]
- 22.Abbara S, Mundo-Sagardia JA, Hoffmann U, Cury RC (2009) Cardiac CT assessment of left atrial accessory appendages and diverticula. AJR Am J Roentgenol 193: 807–812. doi: 10.2214/AJR.08.2229 [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, He Z, Zhang L, Li B, Sun D, et al. (2009) The anatomical study of left atrium diverticulum by multi-detector row CT. Surg Radiol Anat 31: 191–198. doi: 10.1007/s00276-008-0427-1 [DOI] [PubMed] [Google Scholar]
- 24.Killeen RP, O’Connor SA, Keane D, Dodd JD (2009) Ectopic Focus in an Accessory Left Atrial Appendage. Circulation 120: e60–e62. [DOI] [PubMed] [Google Scholar]
- 25.Igawa O, Miake J, Adachi M (2008) The small diverticulum in the right anterior wall of the left atrium. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 10: 120. doi: 10.1093/europace/eum275 [DOI] [PubMed] [Google Scholar]
- 26.Holda MK, Koziej M (2018) Left-Sided Atrial Septal Pouch as a Risk Factor of Cryptogenic Stroke: A Systematic Review and Meta-Analysis. Cerebrovasc Dis 46: 223–229. doi: 10.1159/000495573 [DOI] [PubMed] [Google Scholar]
- 27.Holda MK, Koziej M, Wszolek K, Pawlik W, Krawczyk-Ozog A, et al. (2017) Left atrial accessory appendages, diverticula, and left-sided septal pouch in multi-slice computed tomography. Association with atrial fibrillation and cerebrovascular accidents. Int J Cardiol 244: 163–168. doi: 10.1016/j.ijcard.2017.06.042 [DOI] [PubMed] [Google Scholar]
- 28.Holda MK, Koziej M, Holda J, Piatek K, Tyrak K, et al. (2016) Atrial septal pouch—Morphological features and clinical considerations. Int J Cardiol 220: 337–342. doi: 10.1016/j.ijcard.2016.06.141 [DOI] [PubMed] [Google Scholar]
- 29.Wong JM, Lombardo DM, Barseghian A, Dhoot J, Hundal HS, et al. (2015) Left atrial septal pouch in cryptogenic stroke. Front Neurol 6: 57. doi: 10.3389/fneur.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzner A, Wissner E, Schoonderwoerd B, Burchard A, Tilz R, et al. (2012) The influence of varying energy settings on efficacy and safety of endoscopic pulmonary vein isolation. Heart Rhythm 9: 1380–1385. doi: 10.1016/j.hrthm.2012.03.059 [DOI] [PubMed] [Google Scholar]
- 31.Incedayi M, Ozturk E, Sonmez G, Saglam M, Sivrioglu AK, et al. (2012) The incidence of left atrial diverticula in coronary CT angiography. Diagn Interv Radiol 18: 542–546. doi: 10.4261/1305-3825.DIR.5388-11.1 [DOI] [PubMed] [Google Scholar]
- 32.Celik E, Pennig L, Laukamp KR, Hammes J, Maintz D, et al. (2020) Are left atrial diverticula and left-sided septal pouches relevant additional findings in cardiac CT? Correlation between left atrial outpouching structures and ischemic brain alterations. Int J Cardiol 317: 216–220. doi: 10.1016/j.ijcard.2020.05.038 [DOI] [PubMed] [Google Scholar]
- 33.Demir GG, Gunes HM, Seker M, Savur U, Guler GB, et al. (2019) Is the presence of left atrial diverticulum associated with recurrence in patients undergoing catheter ablation for atrial fibrillation? Arch Med Sci Atheroscler Dis 4: e25–e31. doi: 10.5114/amsad.2019.83508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chard M, Tabrizchi R (2009) The role of pulmonary veins in atrial fibrillation: a complex yet simple story. Pharmacol Ther 124: 207–218. doi: 10.1016/j.pharmthera.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Mahida S, Sacher F, Derval N, Berte B, Yamashita S, et al. (2015) Science Linking Pulmonary Veins and Atrial Fibrillation. Arrhythm Electrophysiol Rev 4: 40–43. doi: 10.15420/aer.2015.4.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH (1999) Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 10: 1525–1533. doi: 10.1111/j.1540-8167.1999.tb00211.x [DOI] [PubMed] [Google Scholar]
- 37.Lyen S, Wijesuriya S, Ngan-Soo E, Mathias H, Yeong M, et al. (2017) Anomalous pulmonary venous drainage: a pictorial essay with a CT focus. Journal of Congenital Cardiology 1: 7. [Google Scholar]
- 38.Khoueiry Z, Albenque JP, Providencia R, Combes S, Combes N, et al. (2016) Outcomes after cryoablation vs. radiofrequency in patients with paroxysmal atrial fibrillation: impact of pulmonary veins anatomy. Europace 18: 1343–1351. doi: 10.1093/europace/euv419 [DOI] [PubMed] [Google Scholar]
- 39.Necas M (2000) Redundant Septum Primum Flap in Fetus with Premature Atrial Contractions.
- 40.Stewart PA, Wladimiroff JW (1988) Fetal atrial arrhythmias associated with redundancy/aneurysm of the foramen ovale. J Clin Ultrasound 16: 643–650. doi: 10.1002/jcu.1870160905 [DOI] [PubMed] [Google Scholar]
- 41.Toro L, Weintraub RG, Shiota T, Sahn DJ, Sahn C, et al. (1994) Relation between persistent atrial arrhythmias and redundant septum primum flap (atrial septal aneurysm) in fetuses. Am J Cardiol 73: 711–713. doi: 10.1016/0002-9149(94)90942-3 [DOI] [PubMed] [Google Scholar]
- 42.Leong-Sit P, Roux JF, Zado E, Callans DJ, Garcia F, et al. (2011) Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 4: 11–14. doi: 10.1161/CIRCEP.110.955393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.


