Abstract
Background and objective
Low birth weight (LBW) is a major risk factor of child mortality and morbidity during infancy (0–3 years) and early childhood (3–8 years) in low and lower-middle-income countries, including Bangladesh. LBW is a vital public health concern in Bangladesh. The objective of the research was to investigate the socioeconomic inequality in the prevalence of LBW among singleton births and identify the significantly associated determinants of singleton LBW in Bangladesh.
Materials and methods
The data utilized in this research was derived from the latest nationally representative Bangladesh Demographic and Health Survey, 2017–18, and included a total of 2327 respondents. The concentration index (C-index) and concentration curve were used to investigate the socioeconomic inequality in LBW among the singleton newborn babies. Additionally, an adjusted binary logistic regression model was utilized for calculating adjusted odds ratio and p-value (<0.05) to identify the significant determinants of LBW.
Results
The overall prevalence of LBW among singleton births in Bangladesh was 14.27%. We observed that LBW rates were inequitably distributed across the socioeconomic groups (C-index: -0.096, 95% confidence interval: [-0.175, -0.016], P = 0.029), with a higher concentration of LBW infants among mothers living in the lowest wealth quintile (poorest). Regression analysis revealed that maternal age, region, maternal education level, wealth index, height, age at 1st birth, and the child’s aliveness (alive or died) at the time of the survey were significantly associated determinants of LBW in Bangladesh.
Conclusion
In this study, socioeconomic disparity in the prevalence of singleton LBW was evident in Bangladesh. Incidence of LBW might be reduced by improving the socioeconomic status of poor families, paying special attention to mothers who have no education and live in low-income households in the eastern divisions (e.g., Sylhet, Chittagong). Governments, agencies, and non-governmental organizations should address the multifaceted issues and implement preventive programs and policies in Bangladesh to reduce LBW.
Introduction
Low birth weight (LBW) is a leading public health concern. It is a vital risk factor of perinatal survival, infant and child mortality, and morbidity in infancy and early childhood (3–8 years), and it contributes prominently to the overall burden of infant mortality [1, 2]. Infants with LBW may have digestive and breathing problems and complications in eating, gaining weight, and fighting off infections compared with normal birth weight infants [3]. As LBW infants grow into adulthood, they may have mental retardation, developmental disorders, physical disabilities, exhaustion/fatigue, depression, and other psychiatric conditions. They also have an increased risk of non-communicable diseases including hypertension, diabetes, chronic snoring (sleeping-disordered breathing), and cardiovascular disease [4–9]. Every year 15% to 20% of all live births have LBW across the globe [10, 11], of which 91% are from lower-middle-income countries (LMICs) [12] and around 50% occur in Bangladesh and India [12, 13]. Approximately 80% of annual newborn deaths are linked to LBW delivery globally [12, 14–17]. In Bangladesh, 38% of all newborn deaths are related to LBW [18]. The prevalence of LBW in Bangladesh was 17.7% in 2011 [19], 20% in 2014 [20], and 16% in 2017 [21]. Although the prevalence of LBW decreased in Bangladesh, it is still higher compared to most of the developed and developing countries [13]. To reduce the prevalence of LBW in LMICs, it is important to identify the most significant contributing factors. Socioeconomic factors such as wealth index, education, family income, occupation, and family size are prominent determinants of LBW [22, 23]. Pregnant women who live in the poor households (i.e., households with low socioeconomic status) may have less access to health care services, and greater food and nutritional insecurity compared to women living in wealthy households (i.e., households with high socioeconomic status), placing them at higher risk for LBW infants [24, 25]. Therefore, it is important to further investigate the role of socioeconomic inequality and its associated determinants in Bangladesh.
To the best of our knowledge, no existing study investigated the socioeconomic inequality in the prevalence of LBW using the nationally representative BDHS data. Thus, the objectives of the present study were to statistically investigate the socioeconomic inequality in the prevalence of LBW in Bangladesh and identify the significantly associated determinants of LBW, using data from the most recent BDHS (2017–2018).
Materials and methods
Data source
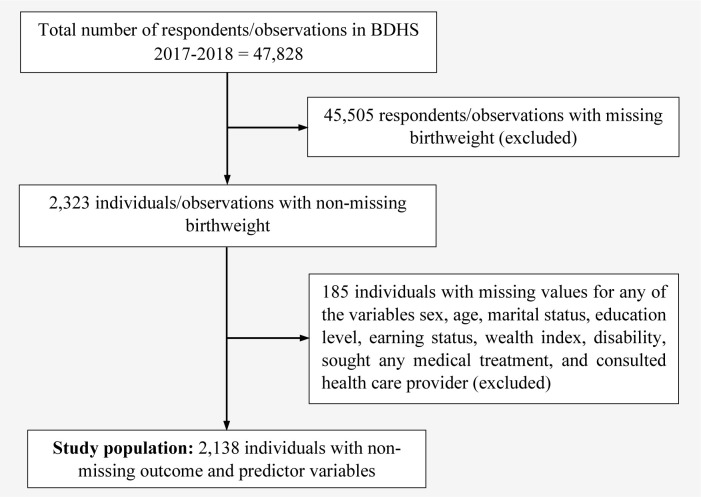
The dataset utilized in this study was taken from Bangladesh Demographic and Health Survey (BDHS), 2017–18 [21]. The BDHS data was collected through two-stage stratified cluster sampling. In the 1st stage, 675 enumeration areas (EAs) were selected via probability proportional sampling, wherein 250 were urban and 425 rural areas. In the 2nd stage, sorting the taken households and provided a complete sampling frame. A systematic random sample of 30 households was chosen from each EA in the 2nd stage to estimate the key demographic and health-related indicators. A total of 20,250 residential households were chosen to participate in face-to-face interviews with questionnaires. About 20,100 ever-married women with an age range of 15–49 were expected to complete the interviews [21]. Each respondent was asked to give the overall birth history for births during the survey period, and the birth weight was measured in grams. A total of 47,828 respondents provided their birth information in BDHS 2017–18. Implementing the sample weight variable, excluding unusual observations and missing values, 2,138 observations were selected for the final analysis. A brief description of the data extraction procedure is depicted in Fig 1.
Fig 1. Study flowchart of inclusion/exclusion of observations.
Ethical approval
The present study utilized current public domain survey datasets, which are freely accessible online; that is why it does not require any additional ethical approval. A detailed description of the ethical procedures followed by the DHS program (https://dhsprogram.com) can be found in the BDHS reports [21].
Dependent/Outcome variable
The outcome variable in this study was LBW, measured by grams based on the WHO cutoff (birth weight < 2500 g), and recorded a binary response variable with a membership class label: LBW and non-LBW [26]. The membership class label was coded as “1” for LBW and “0” for non-LBW.
Explanatory/Independent variables
The explanatory variables included in this study are based on the earlier research papers on literature [5, 27–34]. The explanatory variables included demographic characteristics: maternal age (≤20, 21–30, 31–40, ≥40), residence (urban, rural), region (Barisal, Chittagong, Dhaka, Khulna, Mymensingh, Rajshahi, Rangpur, Sylhet), religion (Muslim, non-Muslim), and sex of child (male, female); socioeconomic characteristics: mother’s education level (no education, primary, secondary, higher), husband/partner’s education level (no education, primary, secondary, higher), and wealth index (poorest, poorer, middle, richer, richest); physical and medical information: height, weight, maternal nutritional status (underweight, normal, overweight, obese), parity (≤3, >3), age at first birth (<15, 15–25, >25), marriage to 1st birth interval(≤30, >30), antenatal care (ANC) initiation at 1st trimester (yes, no), number of antenatal visits (<4, ≥4), during pregnancy iron tablet (yes, no), place of delivery (home, government sector, private sector, NGO and others), delivery by CS (yes, no), and child is alive (yes, no); environmental characteristic: toilet facility (hygienic, unhygienic); and exposure to mass media: newspaper (yes, no), and television (yes, no). Based on WHO criteria, nutritional status of mothers was measured by body mass index (BMI) with underweight (BMI<18.5 kg/m2), normal (18.5≤BMI≤24.9 kg/m2), overweight (25≤BMI≤29.9 kg/m2), and obese (≥ 30.0 kg/m2).
Statistical analysis
Data was prepared using the survey weights before the statistical analysis. In bivariate analysis, Pearson’s chi-squared test of independence [35–40] was implemented to examine the association between two categorical variables, and independent samples t-test was employed for determining the significant difference between the group means of the normally distributed data. Primarily, an unadjusted logistic regression (LR) model was performed to establish the strength of the associations between LBW and the explanatory variables and to calculate unadjusted/crude odds ratio (COR) along with 95% confidence interval (CI). Adjusted LR model with a stepwise forward selection method was employed to identify significantly associated risk factors for LBW [41, 42] and to calculate adjusted odds ratio (AOR) and it 95% CI. The explanatory variables with a p-value <0.05 from the bivariate analysis were included as independent variables in the LR models. The LR model was expressed by the following expression:
| (1) |
where, X = (X1: maternal age, X2: division, X3: mother’s education level, X4: wealth index, X5: height, X6: weight, X7: parity, X8: age at 1st birth, X9: antenatal care initiation at 1st trimester, X10: number of antenatal visits, X11: delivery by CS, X12: child is alive, X13: toilets; X14: newspaper) represent explanatory variables; β0 is intercept; β = (β1,β2,…,β14) represents the regression coefficients; and ε denotes random error term. Odds ratio (OR) with 95% confidence intervals (CIs) was calculated aimed at assessing the directions as well as the strength of the effect of the explanatory variables. The explanatory variable with a p-value <0.05 was considered statistically significant for the determinants of LBW. Data processing/preparation and all statistical analyses were carried out by SAS 9.4 software (SAS Institute, Inc., Cary, North Carolina).
Concentration index
The concentration index (C-index) was computed to quantify the degree of socioeconomic inequality of newly born babies with LBW among singleton births. C-index is a well-known and suitable measurement for measuring socioeconomic inequality in health-related variables [43]. The CI was calculated using the following formula:
| (2) |
where, yi: LBW, μ: mean of LBW, Ri: ith individual’s fractional rank in the socioeconomic distribution [44]. The range of C-index lies between − 1 to + 1. If the curve displays above the line of equality, the value of C-index is negative and indicates a disproportionally concentration of inequality among the poor [45, 46]. If the curve falls below the line of equality, the value of C-index is positive, demonstrating a disproportionally concentrated inequality among the rich. There is no socioeconomic inequality when the value of C-index is zero. The larger absolute value of C-index explores the higher inequities among socioeconomic households. Concentration curve (CC) with p-value was also portrayed for a clear illustration.
Results
Baseline characteristics of the participants
Table 1 shows the descriptive statistics of the study variables. In this study, the study population consisted of 2,138 respondents/mothers (age:15–49 years) with 22 LBW-related explanatory variables. The average age of the mothers was 24.79±5.505 years, with an average height of 151.47±5.73, average weight of 53.61±10.90, and average BMI of 23.32±4.32. In the study population, the majority of the mothers were younger (age≤30 years), among which the proportion of 21–30 years old mothers was 53.18% followed by the mothers of age ≤20 years (35.36%). The highest proportion of mothers had normal BMI (55.75%), while the overweighted mothers had the 2nd highest proportion (24.65%). More than half of the mothers were secondary educated (51.08%), whereas 2.67% of mothers had no education, 15.34% were primary literate, and 30.92% had higher degrees. About 52% of mothers initiated antenatal care during pregnancy at the 1st trimester, and most of mothers delivered their babies by cesarean section (62.72%). In addition, a larger proportion of mothers had ≥4 ANC visits (68.01%), 15–25 years age at first birth (89.52%), hygienic toilet facility (82.79%) and lived in the Dhaka division (17.59%).
Table 1. Distribution of the risk factors by LBW using BDHS, 2017–18.
| Overall, n (%) | LBW, n (%) | Non-LBW, n (%) | P-value | |
|---|---|---|---|---|
| Total | 2138 (100%) | 305 (14.27%) | 1,833 (85.73%) | |
| Maternal age (Years) | ||||
| < = 20 | 756 (35.36) | 118 (15.61) | 638 (84.39) | 0.023 |
| 21–30 | 1137 (53.18) | 151 (13.28) | 986 (86.72) | |
| 31–40 | 233 (10.90) | 31 (13.30) | 202 (86.70) | |
| >40 | 12 (0.56) | 5 (41.67) | 7 (58.33) | |
| Residence | ||||
| Urban | 931 (43.55) | 126 (13.53) | 805 (86.47) | 0.395 |
| Rural | 1207 (56.45) | 179 (14.83) | 1028 (85.17) | |
| Division | ||||
| Barisal | 188 (8.79) | 25 (13.30) | 163 (86.70) | 0.014 |
| Chittagong | 312 (14.59) | 60 (19.23) | 252 (80.77) | |
| Dhaka | 376 (17.59) | 51 (13.56) | 325 (86.44) | |
| Khulna | 300 (14.03) | 40 (13.33) | 260 (86.67) | |
| Mymensingh | 243 (11.37) | 23 (9.47) | 220 (90.53) | |
| Rajshahi | 240 (11.23) | 37 (15.42) | 203 (84.58) | |
| Rangpur | 292 (13.66) | 33 (11.30) | 259 (88.70) | |
| Sylhet | 187 (8.75) | 36 (19.25) | 151 (80.75) | |
| Religion | ||||
| Muslim | 1917 (89.66) | 271 (14.14) | 1646 (85.86) | 0.615 |
| Non-Muslim | 221 (10.34) | 34 (15.38) | 187 (84.62) | |
| Sex of child | ||||
| Male | 1159 (54.21) | 152 (13.11) | 1007 (86.89) | 0.098 |
| Female | 979 (45.79) | 153 (15.63) | 826 (84.37) | |
| Maternal education | ||||
| No education | 57 (2.67) | 14 (24.56) | 43 (75.44) | 0.002 |
| Primary | 328 (15.34) | 57 (17.38) | 271 (82.62) | |
| Secondary | 1092 (51.08) | 164 (15.02) | 928 (84.98) | |
| Higher | 661 (30.92) | 70 (10.59) | 591 (89.41) | |
| Husband education | ||||
| No education | 158 (7.39) | 25 (15.82) | 133 (84.18) | 0.001 |
| Primary | 513 (23.99) | 87 (16.96) | 426 (83.04) | |
| Secondary | 766 (35.83) | 123 (16.06) | 643 (83.94) | |
| Higher | 701 (32.79) | 70 (9.99) | 631 (90.01) | |
| Height, cm [mean (SD)] | 151.47 (5.73) | 149.68 (5.66) | 151.77 (5.69) | <0.001 |
| Weight, kg [mean (SD)] | 53.61 (10.90) | 51.49 (11.04) | 53.97 (10.84) | <0.001 |
| BMI | 23.32 (4.32) | 22.90 (4.33) | 23.40 (4.31) | 0.063 |
| Underweight | 265 (12.39) | 42 (15.85) | 223 (84.15) | 0.455 |
| Normal | 1192 (55.75) | 174 (14.60) | 1018 (85.40) | |
| Overweight | 527 (24.65) | 73 (13.85) | 454 (86.15) | |
| Obese | 154 (7.20) | 16 (10.39) | 138 (89.61) | |
| Parity | ||||
| ≤3 | 2013 (94.15) | 278 (13.81) | 1735 (86.19) | 0.016 |
| >3 | 125 (5.85) | 27 (21.60) | 98 (78.40) | |
| Age at 1st birth (years) | ||||
| <15 | 81 (3.79) | 18 (22.22) | 63 (77.78) | 0.005 |
| 15–25 | 1914 (89.52) | 277 (14.47) | 1637 (85.53) | |
| >25 | 143 (6.69) | 10 (6.99) | 133 (93.01) | |
| Marriage to 1st birth interval | ||||
| < = 30 | 1513 (70.77) | 226 (14.94) | 1287 (85.06) | 0.167 |
| >30 | 625 (29.23) | 79 (12.64) | 546 (87.36) | |
| ANC initiation at 1st trimester | ||||
| Yes | 1110 (51.92) | 137 (12.34) | 973 (87.66) | 0.008 |
| No | 1028 (48.08) | 168 (16.34) | 860 (83.66) | |
| Number of antenatal visits | ||||
| <4 | 684 (31.99) | 114 (16.67) | 570 (83.33) | 0.030 |
| > = 4 | 1454 (68.01) | 191 (13.14) | 1263 (86.86) | |
| During pregnancy iron tablet | ||||
| Yes | 1850 (86.53) | 254 (13.73) | 1596 (86.27) | 0.073 |
| No | 288 (13.47) | 51 (17.71) | 237 (82.29) | |
| Place of delivery | ||||
| Home | 198 (9.26) | 32 (16.16) | 166 (83.84) | 0.178 |
| Public sector | 528 (24.70) | 87 (16.48) | 441 (83.52) | |
| Private sector | 1235 (57.76) | 162 (13.12) | 1073 (86.88) | |
| NGO sector | 175 (8.19) | 23 (13.14) | 152 (86.86) | |
| Other | 2 (0.09) | 1 (50.00) | 1 (50.00) | |
| Delivery by CS | ||||
| Yes | 1341 (62.72) | 174 (12.98) | 1167 (87.02) | 0.027 |
| No | 797 (37.28) | 131 (16.44) | 666 (83.56) | |
| Child is alive | ||||
| Yes | 2095 (97.99) | 291 (13.89) | 1804 (86.11) | <0.001 |
| No | 43 (2.01) | 14 (32.56) | 29 (67.44) | |
| Toilet facility a | ||||
| Hygienic | 1770 (82.79) | 240 (13.56) | 1530 (86.44) | 0.041 |
| Unhygienic | 368 (17.21) | 65 (17.66) | 303 (82.34) | |
| Newspaper | ||||
| Yes | 383 (17.91) | 39 (10.18) | 344 (89.82) | 0.012 |
| No | 1755 (82.09) | 266 (15.16) | 1489 (84.84) | |
| Television | ||||
| Yes | 1621 (75.82) | 228 (14.07) | 1393 (85.93) | 0.639 |
| No | 517 (24.18) | 77 (14.89) | 440 (85.11) |
ANC: antenatal care, BMI: body mass index, CS: caesarean section, LBW: low birth weight, NGO: non-governmental organization, SD: standard deviation.
a Hygienic toilet facility includes flush toilet, flush to piped sewer system, flush to septic tank, flush to pit latrine, flush to somewhere else, flush to unknow place, pit toilet latrine, ventilated improved pit latrine (VIP), pit latrine with slab and composting toilet. Unhygienic toilet facility includes all other toilet facilities that are not included under hygienic toilet facility (pit latrine without slab/open pit, no facility, no facility/bush/field, bucket toilet, hanging toilet/latrine and other).
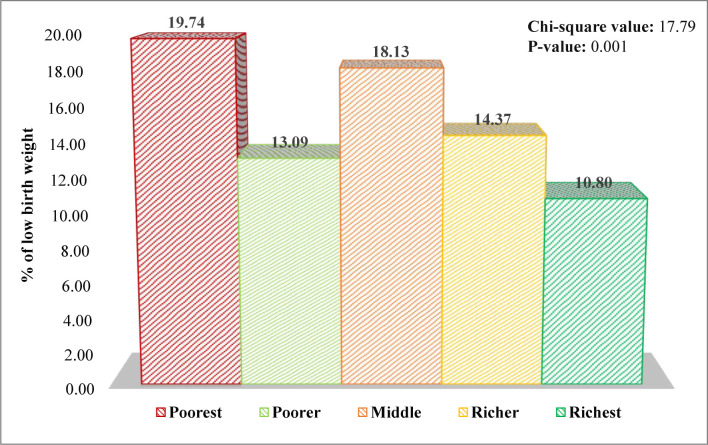
In our study population, the overall prevalence of LBW was 14.27% (Table 1). The prevalence of LBW among different groups of socioeconomic status has been displayed in Fig 2. It shows that the prevalence of LBW was the highest among the poorest mothers (19.74%) compared to those who had middle (18.13%) and richest (10.80%) socioeconomic status. The p-value of the chi-squared test was 0.001, which indicates a highly significant association between socioeconomic status and LBW status.
Fig 2. Prevalence of LBW among different groups of socioeconomic status.
The distribution of the explanatory variables by LBW status is presented in Table 1. The prevalence of LBW was higher among younger (age ≤20 years) and older (age > 40 years) mothers. The association between maternal age and LBW status was statistically significant (P = 0.023). The Sylhet division was found to have the highest prevalence rate of LBW (19.25%), while the Mymensingh division had the lowest LBW rate (9.47%). The division was significantly associated with LBW status. Non-educated mothers were found to have higher LBW babies (24.56%) compared to primary (17.38%), secondary (15.02%), and higher (10.59%) educated mothers. Parents’ education level and LBW were also statistically significant. Underweighted mothers exhibited the highest LBW rate (15.85%) followed by the mothers with normal BMI (14.60%). Mothers who gave birth to their first babies at age <15 years were found to have a higher prevalence of LBW (22.22%) than others (15–25 years: 14.47% and >25 years: 6.99%), exhibiting the statistically significant association between age at first birth and LBW status. Mothers who did not initiate ANC at the first trimester during their pregnancy period were found to have a significantly higher prevalence rate of LBW compared to their counterparts (16.34% versus 12.34%, P = 0.008). The prevalence of LBW was significantly higher among the mother who had < 4 ANC visits than those who had >4 ANC visits (16.67% versus 13.14%, P = 0.030). This indicates that number of ANC visits was significantly associated with LBW. Mothers who had normal delivery (i.e., spontaneous vaginal delivery) exhibited higher prevalence rate of LBW in a comparison with those who delivered by cesarean (16.44% versus 12.98%) and the association of LBW with model of delivery was statistically significant (P = 0.027). Families having no toilets facilities had a significantly higher prevalence of LBW than the families with hygienic toilets facilities (17.66% versus 13.56%, P = 0.041), which indicates a significant association between toile facility and LBW. In terms of exposure to mass media, not reading newspaper was significantly associated with LBW (read newspaper: 10.18% versus not read newspaper: 15.16%, P = 0.012). Area of residence, religion, sex of the child, mother’s BMI, marriage to 1st birth interval, wanted pregnancy, taking iron tablet during pregnancy, place of delivery, and watching television were not significantly associated with LBW.
S1 Table demonstrated that the predictors maternal age, residence, division, parental education, BMI, parity, age at first birth, Marriage to 1st birth interval, ANC initiation at 1st trimester, number of antenatal visits, taking iron tablet during pregnancy, place of delivery, mode of delivery, toilet facility, reading newspaper, watching television were significantly associated with the socio-economic status (P<0.005).
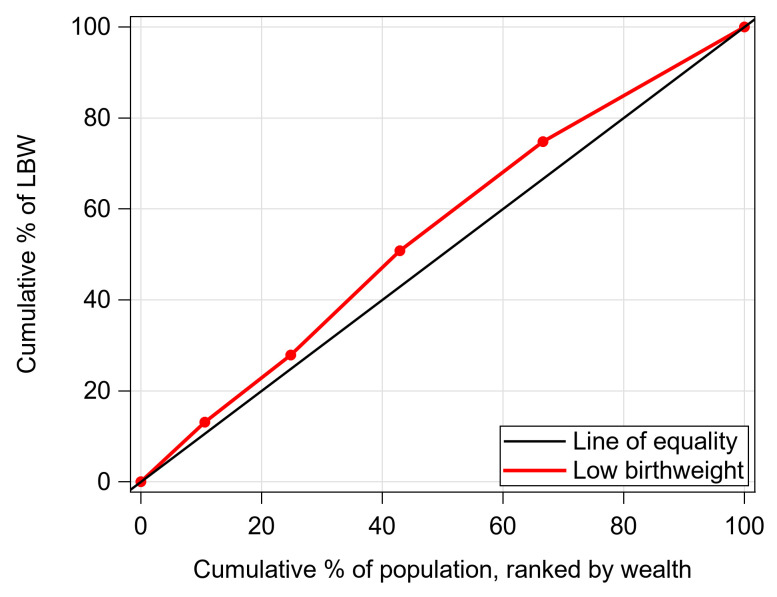
Fig 3 depicts the concentration curve for LBW rate ranked by wealth index. The CC showed that the line for LBW was above the line of equality, which indicated that the LBW babies were strongly concentrated in low socioeconomic groups (poorest). To clarify the result, the value of C-index was also presented in Table 2. The value of C-index: -0.096 (SE: 0.029; P = 0.029) demonstrated that there was a higher concentration of babies with LBW among mothers living in the lowest wealth quintile.
Fig 3. Concentration curve for LBW babies ranked by wealth in Bangladesh.
Table 2. Value of the concentration index.
| Concentration index | Standard Error | 95% CI | P-value |
|---|---|---|---|
| -0.096 | 0.029 | (-0.175, -0.016) | 0.029 |
Table 3 represents the prevalence and concentration index of LBW used by wealth quintile across the divisions in Bangladesh. The Sylhet division showed the highest C-index of -0.066, followed by the other divisions. LBW was more concentrated amongst the poorest socioeconomic households in the Sylhet division.
Table 3. Prevalence and concentration index of LBW used by wealth index across the divisions.
| Wealth index | Division | |||||||
|---|---|---|---|---|---|---|---|---|
| Barisal | Chittagong | Dhaka | Khulna | Mymensingh | Rajshahi | Rangpur | Sylhet | |
| Poorest | 13.04 | 35.29 | 30.77 | 4.55 | 14.29 | 28.57 | 17.50 | 33.33 |
| Poorer | 14.29 | 8.33 | 16.67 | 16.67 | 8.77 | 19.05 | 10.17 | 12.50 |
| Middle | 21.15 | 30.77 | 16.33 | 25.71 | 10.42 | 6.82 | 8.51 | 20.83 |
| Richer | 12.50 | 21.05 | 17.71 | 9.72 | 7.14 | 14.47 | 8.89 | 20.51 |
| Richest | 2.70 | 13.99 | 9.28 | 6.82 | 8.51 | 14.00 | 8.20 | 17.20 |
| Concentration Index | -0.166 | -0.136 | -0.174 | -0.1605 | -0.093 | -0.109 | -0.168 | -0.066 |
Unadjusted and adjusted logistic regression analysis
Table 4 represents the results of unadjusted and adjusted LR analyses to identify the potential risk factors for LBW. The unadjusted LR showed that mothers from the poorest (COR: 2.032, 95% CI: 1.362–1.3.031, P<0.001) and middle-class (COR: 1.830, 95% CI: 1.289–2.598, P<0.001) households were more likely to have LBW babies compared to the mothers who were from the richest households. After adjusting for other confounders in the MLR model, mothers from the poorest families (AOR: 1.653, 95% CI: 0.969–2.820, P = 0.044) and middle income households (AOR: 1.561, 95% CI: 1.043–2.334, P = 0.030) were more likely to deliver LBW babies than the richest mothers. In unadjusted LR, mothers aged >40 years exhibited almost 5-fold higher odds of having LBW babies compared to the 21–30 years old mothers (COR: 4.664, 95% CI: 1.462–14.884, P = 0.009); whereas in adjusted LR, they showed almost 4-fold higher odds of having LBW babies (AOR: 3.963, 95% CI: 1.098–14.305, P = 0.036). Simple LR analysis demonstrated 2.227 times, 2.28 times and 74% higher likelihood of having LBW babies among the mothers who were from Sylhet (COR: 2.280, 95% CI: 1.299–4.003, P = 0.004), Chittagong (COR: 2.277, 95% CI: 1.363–3.806, P = 0.002), and Rajshahi (COR: 1.743, 95% CI: 1.002–3.035, P = 0.049) division, respectively, compared to those who were from Mymensingh division. Likewise, adjusted LR revealed that mothers who lived in the Sylhet (AOR: 2.900, 95% CI: 1.598–5.264, P<0.001), Chittagong (AOR: 2.813, 95% CI: 1.632–4.848, P<0.001), Rajshahi (AOR: 2.048, 95% CI: 1.156–3.628, P = 0.014), and Dhaka (AOR: 1.842, 95% CI: 1.486–3.356, P = 0.019) division were more likely to have babies with LBW compared to mothers who lived in Mymensingh division. Both unadjusted and adjusted LR analyses showed that the mothers who had no education, primary education, and completed secondary education were more likely to give birth to LBW babies than the higher educated mothers. Maternal height was found to be a significant risk factor for LBW.
Table 4. Unadjusted and adjusted LR analysis of risk factors for LBW.
| Risk factors | Unadjusted LR | Adjusted LR | ||||||
|---|---|---|---|---|---|---|---|---|
| COR | 95% CI of COR | P-value | AOR | 95% CI of AOR | P-value | |||
| Lower | Upper | Lower | Upper | |||||
| Wealth index | ||||||||
| Richest® | 1.000 | 1.000 | ||||||
| Poorest | 2.032 | 1.362 | 3.031 | 0.0005 | 1.653 | 0.969 | 2.820 | 0.044 |
| Poorer | 1.244 | 0.824 | 1.877 | 0.2987 | 1.044 | 0.632 | 1.724 | 0.8679 |
| Middle | 1.830 | 1.289 | 2.598 | 0.0007 | 1.561 | 1.043 | 2.334 | 0.0303 |
| Richer | 1.386 | 0.984 | 1.953 | 0.0618 | 1.172 | 0.805 | 1.705 | 0.4073 |
| Maternal age (Years) | ||||||||
| 21–30 ® | 1.00 | 1.00 | ||||||
| < = 20 | 1.208 | 0.931 | 1.567 | 0.1558 | 1.119 | 0.842 | 1.486 | 0.4390 |
| 31–40 | 1.002 | 0.662 | 1.518 | 0.9921 | 1.033 | 0.647 | 1.647 | 0.8929 |
| >40 | 4.664 | 1.462 | 14.884 | 0.0093 | 3.963 | 1.098 | 14.305 | 0.0355 |
| Division | ||||||||
| Mymensingh® | 1.00 | 1.00 | ||||||
| Barisal | 1.467 | 0.804 | 2.677 | 0.2117 | 1.512 | 0.810 | 2.823 | 0.1939 |
| Chittagong | 2.277 | 1.363 | 3.806 | 0.0017 | 2.813 | 1.632 | 4.848 | 0.0002 |
| Dhaka | 1.501 | 0.891 | 2.528 | 0.1267 | 1.842 | 1.486 | 3.356 | 0.0193 |
| Khulna | 1.472 | 0.855 | 2.534 | 0.1635 | 1.751 | 0.995 | 3.079 | 0.0519 |
| Rajshahi | 1.743 | 1.002 | 3.035 | 0.0494 | 2.048 | 1.156 | 3.628 | 0.0140 |
| Rangpur | 1.219 | 0.695 | 2.138 | 0.4902 | 1.217 | 0.679 | 2.180 | 0.5094 |
| Sylhet | 2.280 | 1.299 | 4.003 | 0.0041 | 2.900 | 1.598 | 5.264 | 0.0005 |
| Maternal education | ||||||||
| Higher® | 1.00 | 1.00 | ||||||
| No education | 1.212 | 0.3327 | 9.2399 | 0.0024 | 1.358 | 0.620 | 2.973 | 0.043 |
| Primary | 1.143 | 0.1929 | 8.8619 | 0.0029 | 1.27 | 0.582 | 1.526 | 0.8080 |
| Secondary | 1.042 | 0.1522 | 6.9159 | 0.0085 | 1.08 | 0.689 | 1.416 | 0.9483 |
| Husband’s education | ||||||||
| Higher® | 1.00 | 1.00 | ||||||
| No education | 1.121 | 0.2518 | 4.387 | 0.0362 | 1.281 | 0.422 | 1.445 | 0.4315 |
| Primary | 1.183 | 0.1724 | 12.534 | 0.0004 | 1.186 | 0.710 | 1.662 | 0.7026 |
| Secondary | 1.020 | 0.1599 | 11.616 | 0.0007 | 1.074 | 0.813 | 1.695 | 0.3913 |
| Height | 0.994 | 0.991 | 0.996 | < .0001 | 0.994 | 0.992 | 0.997 | < .0001 |
| Weight | 0.998 | 0.997 | 0.999 | 0.0002 | 1.000 | 0.998 | 1.001 | 0.6931 |
| Parity | ||||||||
| ≤3 | 1.00 | 1.00 | ||||||
| >3 | 1.719 | 1.103 | 2.682 | 0.0168 | 1.175 | 0.675 | 2.044 | 0.5692 |
| Age at 1st birth (years) | ||||||||
| >25® | 1.00 | 1.00 | ||||||
| <15 | 3.800 | 1.658 | 8.706 | 0.0016 | 2.773 | 1.112 | 6.916 | 0.0287 |
| 15–25 | 2.250 | 1.169 | 4.333 | 0.0152 | 1.728 | 0.856 | 3.492 | 0.1272 |
| Antenatal care initiation at 1st trimester | ||||||||
| Yes® | 1.00 | 1.00 | ||||||
| No | 1.387 | 1.087 | 1.770 | 0.0084 | 1.160 | 0.881 | 1.526 | 0.2896 |
| Number of antenatal visits | ||||||||
| <4® | 1.00 | 1.00 | ||||||
| ≥4 | 1.323 | 1.028 | 1.702 | 0.0298 | 1.022 | 0.771 | 1.355 | 0.8783 |
| Delivery by CS | ||||||||
| Yes® | 1.00 | 1.00 | ||||||
| No | 1.319 | 1.032 | 1.687 | 0.0272 | 1.098 | 0.839 | 1.439 | 0.4957 |
| Child is alive | ||||||||
| Yes® | 1.00 | 1.00 | ||||||
| No | 2.993 | 1.563 | 5.732 | 0.0009 | 2.323 | 1.174 | 4.596 | 0.0155 |
| Toilet facility | ||||||||
| Hygienic® | 1.00 | 1.00 | ||||||
| Unhygienic | 1.368 | 1.013 | 1.847 | 0.0410 | 1.091 | 0.763 | 1.560 | 0.6328 |
| Newspaper | ||||||||
| Yes® | 1.00 | 1.00 | ||||||
| No | 1.576 | 1.104 | 2.249 | 0.0123 | 1.130 | 0.753 | 1.696 | 0.5564 |
COR: Crude odds ratio; OR: odds ratio; ®: Reference category.
Unadjusted LR showed that mothers who gave their first birth at age <15 years (COR: 3.800, 95% CI: 1.658–8.706, P = 0.002) or between age 15–25 years (COR: 2.250, 95% CI: 1.169–4.333, P = 0.015) had respectively 4-fold and 2-fold higher odds of giving birth to LBW babies than those who had their 1st babies at the age >25 years. Similarly, adjusted LR revealed that women who were <15 years old at 1st birth (AOR: 2.773, 95% CI: 1.112–6.916, P = 0.029) were more prevalent to give birth to babies with LBW than >25 years old mothers at 1st birth. Mothers who lost their previous child were found to have higher odds of LBW babies compared those whose previous child was alive. Similarly, parity, antenatal care initiation at 1st trimester, number of antenatal visits, delivery by CS, the child is alive, toilet facility, and newspaper reading habit were also statistically significant risk factors of LBW.
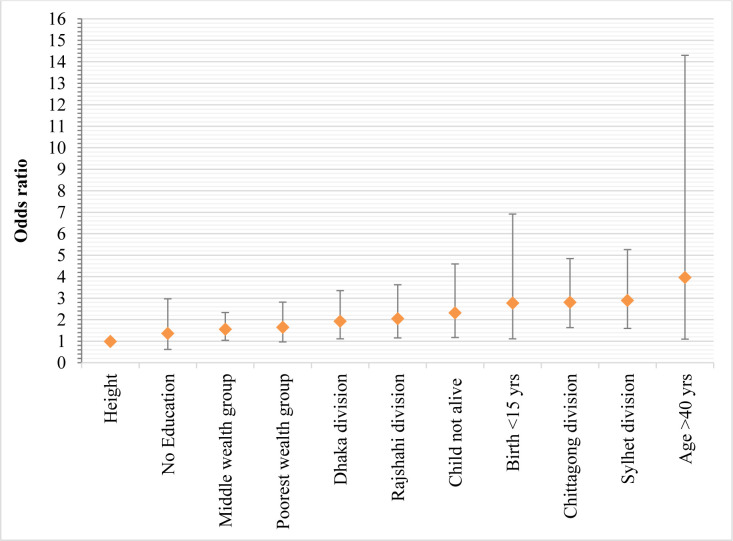
Fig 4 represents the results of the adjusted odds ratios of the significant groups of significant risk factors of LBW. Mothers with age >40 years exhibited the strongest association with LBW (AOR: 3.963, 95% CI: 1.098–14.305, P = 0.0355) followed by the mothers who lived in Sylhet (AOR: 2.900, 95% CI: 1.598–5.264, P = 0.0005) and Chittagong (AOR: 2.813, 95% CI: 1.632–4.848, P = 0.0002) division.
Fig 4. The odds ratio for significantly associated determinants of LBW.
Discussion
The present study aimed to investigate the socioeconomic inequality in the prevalence of LBW among singleton births in Bangladesh and to identify the significant determinants using the latest BDHS (2017–18) data. We found that the overall prevalence of LBW among singleton births in our study population was 14.27%, which is much higher than other countries, including Russia (6.0%), Germany (7.0%), and Japan (10.0%) [47]. Lorenz curve and the Gini coefficient are widely used statistical tools for measuring health inequality when the study population is ordered by the health variable being studied. However, their main disadvantage is that they overlook the socioeconomic dimension [48, 49]. When the study population is ordered by socioeconomic status, the CC and C-index are useful statistical techniques, which incorporate the social dimension. Thus, our study used the C-index and CC to quantify the degree of socioeconomic inequality in LBW in Bangladesh. Our analysis showed that the occurrence of LBW was inequitably distributed among the socioeconomic groups, with a higher concentration of LBW infants among mothers living in the poorest households. Similar findings were reported by Mallick [50] and Khan et al. [28]. To the best of our knowledge, no study had investigated LBW in relation to socioeconomic inequality in Bangladesh. However, similar studies in the United States, United Kingdom, Canada, and Australia revealed increased risk of LBW delivery among women from poor families [51]. Also, similar findings of socioeconomic inequality were documented for other outcomes, including child malnutrition, and mother’s underweight, overweight, and obesity in Bangladesh [43, 52–54] and other low and middle-income countries [55–57]. Additionally, the results of the C-index stratified by divisions revealed that the concentration of LBW infants varies from region to region. Among all divisions in Bangladesh, the Sylhet division exhibited the highest C-index, where the occurrence of LBW was highly concentrated amongst the mothers who belonged to the poorest households.
Unadjusted and adjusted binary logistic regression analysis found that the wealth index is a critical gradient because women from the poorest households were at a higher odds of having babies with LBW than those from the richest households. Earlier studies showed similar findings for LBW and other outcomes (noncommunicable diseases and underweight) in LMICs [58–61]. Women living in the lowest socioeconomic group, who are the most food insecure are more likely to be malnourished [62] and are less likely to receive proper care during pregnancy, conditions that increases the risk of having a LBW infant [63].
Specific to Bangladesh, our study indicates that women who live in eastern divisions (Sylhet and Chittagong divisions) are more likely to give birth to LBW infants. Similar findings were reported by Khan et al. [29]. In the hilly areas, the government should take the necessary steps to reduce the number of births of LBW babies. We also found that maternal education level is an important determinant of LBW. In our study, women with no education had a higher odds of LBW infants compared to educated women. This is consistent with previous studies that found uneducated mothers are more likely to have LBW infants [64, 65]. Educated women are more likely to have high income and therefore able to make healthier choices including attending ANC, better nutrition, etc. Moreover, educated women are more aware of the available healthcare facilities and have a better knowledge of nutritional practices compared to uneducated women [66, 67]. Mother’s age was also a significant determinant of LBW in our study. The likelihood of LBW was higher among young mothers, which is consistent with the findings of other existing studies [68–70]. Mothers with an age at first birth <15 years were more likely to have LBW infants than mothers whose age at first birth is ≥15 years.
Conclusion
The present study, based on BDHS 2017–18, revealed that singleton infants with LBW were more concentrated among mothers living in the poorest socioeconomic quintile in Bangladesh. Wealth index, maternal education level, maternal age, geographic region or administrative division, mother’s height, and maternal age at first birth were all significantly associated determinants of LBW. The highest risk of LBW was found among the infants born to uneducated women in the lowest socioeconomic quintile who lived in the eastern divisions (e.g., Sylhet and Chittagong) of Bangladesh. Intensive initiatives and efforts, by government and non-government organizations and agencies, to develop and implement policies and programs that addresses factors such as equitable access to health care, nutrition and education, focusing on communities at highest risk, are needed to reduce the prevalence of LBW infants in Bangladesh.
Supporting information
(DOCX)
Acknowledgments
Authors would like to thanks the PLOS ONE’s editor and reviewers for their valuable comments and suggestions to improve the quality of the manuscript.
Data Availability
The data underlying the results presented in the study are available from the Demographic and Health Surveys (DHS) Program (https://dhsprogram.com/).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Ballot DE, Chirwa T, Ramdin T, Chirwa L, Mare I, Davies VA, et al. Comparison of morbidity and mortality of very low birth weight infants in a Central Hospital in Johannesburg between 2006/2007 and 2013. BMC Pediatrics. 2015;15(1):20. doi: 10.1186/s12887-015-0337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manyeh AK, Kukula V, Odonkor G, Ekey RA, Adjei A, Narh-Bana S, et al. Socioeconomic and demographic determinants of birth weight in southern rural Ghana: evidence from Dodowa Health and Demographic Surveillance System. BMC Pregnancy and Childbirth. 2016;16(1):160. doi: 10.1186/s12884-016-0956-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devi WA. Low Birth Weight Baby. Journal for Research. 2016;2(4). [Google Scholar]
- 4.Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, et al. Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta‐Analysis of 7 646 267 Participants From 135 Studies. Journal of the American Heart Association. 2018;7(23):e008870. doi: 10.1161/JAHA.118.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal P, Garg S, Upadhyay HP. Prevalence of low birth weight babies and its association with socio-cultural and maternal risk factors among the institutional deliveries in Bharatpur, Nepal. Asian Journal of Medical Sciences. 2019;10(1):77–85. doi: 10.3126/ajms.v10i1.21665 [DOI] [Google Scholar]
- 6.Ediriweera DS, Dilina N, Perera U, Flores F, Samita S. Risk of low birth weight on adulthood hypertension—evidence from a tertiary care hospital in a South Asian country, Sri Lanka: a retrospective cohort study. BMC Public Health. 2017;17(1):358. doi: 10.1186/s12889-017-4268-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordman H, Jääskeläinen J, Voutilainen R. Birth Size as a Determinant of Cardiometabolic Risk Factors in Children. Hormone Research in Paediatrics. 2020;93(3):144–53. doi: 10.1159/000509932 [DOI] [PubMed] [Google Scholar]
- 8.Paavonen EJ, Strang-Karlsson S, Räikkönen K, Heinonen K, Pesonen A-K, Hovi P, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120(4):778–84. doi: 10.1542/peds.2007-0540 [DOI] [PubMed] [Google Scholar]
- 9.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in Young Adulthood for Very-Low-Birth-Weight Infants. New England Journal of Medicine. 2002;346(3):149–57. doi: 10.1056/NEJMoa010856 [DOI] [PubMed] [Google Scholar]
- 10.WHO. Global nutrition targets 2025: low birth weight policy brief (WHO/NMH/NHD/14.5). Geneva: World Health Organization. 2014. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5.
- 11.Eshete A, Alemu A, Zerfu TA. Magnitude and Risk of Dying among Low Birth Weight Neonates in Rural Ethiopia: A Community-Based Cross-Sectional Study. International Journal of Pediatrics. 2019;2019:9034952. doi: 10.1155/2019/9034952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. The Lancet Global Health. 2019;7(7):e849–e60. doi: 10.1016/S2214-109X(18)30565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Sixty-fifth world health assembly: Resolutions and Decisions Annexes. WHA65/2012/REC/1; 2012. p. 1–148. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA65-REC1/A65_REC1-en.pdf. [Google Scholar]
- 14.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 15.Katz J, Lee ACC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. The Lancet. 2013;382(9890):417–25. doi: 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn JE, Blencowe H, Oza S, You D, Lee ACC, Waiswa P, et al. Every Newborn: progress, priorities, and potential beyond survival. The Lancet. 2014;384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7 [DOI] [PubMed] [Google Scholar]
- 17.Doherty T, Kinney M. Low birthweight: will new estimates accelerate progress? The Lancet Global Health. 2019;7(7):e809–e10. 10.1016/S2214-109X(19)30041-5. Available from: https://www.sciencedirect.com/science/article/pii/S2214109X19300415. [DOI] [PubMed] [Google Scholar]
- 18.Yasmin S, Osrin D, Paul E, Costello A. Neonatal mortality of low-birth-weight infants in Bangladesh. Bull World Health Organ. 2001;79(7):608–14. Epub 2001/08/02. ; PubMed Central PMCID: PMC2566474. [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Population R, Training NB, Mitra, Associates/Bangladesh, International ICF. Bangladesh Demographic and Health Survey 2011. Dhaka, Bangladesh: NIPORT, Mitra and Associates, and ICF International, 2013. [Google Scholar]
- 20.National Institute of Population R, Training NB, Mitra, Associates, International ICF. Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh: NIPORT, Mitra and Associates, and ICF International, 2016. [Google Scholar]
- 21.National Institute of Population Research Training (NIPORT), and ICF. Bangladesh Demographic and Health Survey 2017–18. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT and ICF, 2020. [Google Scholar]
- 22.Asaduzzman M, Saydur MR. Maternal Socioeconomic Factors Affecting Birth Weight of New Born Babies Born in Combined Military Hospital Dhaka. Anwer Khan Modern Medical College Journal. 2019;10(2):138–42. doi: 10.3329/akmmcj.v10i2.44126 [DOI] [Google Scholar]
- 23.Mahmoodi Z, Karimlou M, Sajjadi H, Dejman M, Vameghi M, Dolatian M. Working conditions, socioeconomic factors and low birth weight: path analysis. Iran Red Crescent Med J. 2013;15(9):836–42. Epub 2013/09/05. doi: 10.5812/ircmj.11449 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhury N, Ahmed SM. Maternal care practices among the ultra poor households in rural Bangladesh: a qualitative exploratory study. BMC Pregnancy and Childbirth. 2011;11(1):15. doi: 10.1186/1471-2393-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali N, Sultana M, Sheikh N, Akram R, Mahumud RA, Asaduzzaman M, et al. Predictors of Optimal Antenatal Care Service Utilization Among Adolescents and Adult Women in Bangladesh. Health Services Research and Managerial Epidemiology. 2018;5:2333392818781729. doi: 10.1177/2333392818781729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes MM, Black RE, Katz J. 2500-g Low Birth Weight Cutoff: History and Implications for Future Research and Policy. Maternal and Child Health Journal. 2017;21(2):283–9. doi: 10.1007/s10995-016-2131-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kader M, Tripathi N. Determinants of low birth weight in rural Bangladesh. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 2013;2(2):130–4. doi: 10.5455/2320-1770.ijrcog20130604 [DOI] [Google Scholar]
- 28.Khan JR, Islam MM, Awan N, Muurlink O. Analysis of low birth weight and its co-variants in Bangladesh based on a sub-sample from nationally representative survey. BMC Pediatrics. 2018;18(1):100. doi: 10.1186/s12887-018-1068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MMA, Mustagir MG, Islam MR, Kaikobad MS, Khan HTA. Exploring the association between adverse maternal circumstances and low birth weight in neonates: a nationwide population-based study in Bangladesh. BMJ Open. 2020;10(10):e036162. doi: 10.1136/bmjopen-2019-036162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatun S, Rahman M. Socio-economic determinants of low birth weight in Bangladesh: a multivariate approach. Bangladesh Medical Research Council Bulletin. 2008;34(3):81–6. doi: 10.3329/bmrcb.v34i3.1857 [DOI] [PubMed] [Google Scholar]
- 31.Monawar Hosain GM, Chatterjee N, Begum A, Saha SC. Factors Associated with Low Birthweight in Rural Bangladesh. Journal of Tropical Pediatrics. 2006;52(2):87–91. doi: 10.1093/tropej/fmi066 [DOI] [PubMed] [Google Scholar]
- 32.Rahman MS, Howlader T, Masud MS, Rahman ML. Association of Low-Birth Weight with Malnutrition in Children under Five Years in Bangladesh: Do Mother’s Education, Socio-Economic Status, and Birth Interval Matter? PLoS One. 2016;11(6):e0157814. doi: 10.1371/journal.pone.0157814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen R, Roy M, Anny A, Shova NY, Hema T. Prevalence of Low Birth Weight in Urban Dhaka and its Association with Maternal Age and Socioeconomic Status. Risk. 2020;10:12. [Google Scholar]
- 34.Kader M, Perera NKPP. Socio-economic and nutritional determinants of low birth weight in India. N Am J Med Sci. 2014;6(7):302–8. doi: 10.4103/1947-2714.136902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plackett RL. Karl Pearson and the Chi-Squared Test. International Statistical Review / Revue Internationale de Statistique. 1983;51(1):59–72. doi: 10.2307/1402731 [DOI] [Google Scholar]
- 36.Ugoni A, Walker BF. The Chi square test: an introduction. COMSIG Rev. 1995;4(3):61–4. . [PMC free article] [PubMed] [Google Scholar]
- 37.Franke TM, Ho T, Christie CA. The Chi-Square Test: Often Used and More Often Misinterpreted. American Journal of Evaluation. 2011;33(3):448–58. doi: 10.1177/1098214011426594 [DOI] [Google Scholar]
- 38.Pandis N. The chi-square test. American journal of orthodontics and dentofacial orthopedics. 2016;150(5):898–9. doi: 10.1016/j.ajodo.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 39.Rana R, Singhal R. Chi-square test and its application in hypothesis testing. Journal of the Practice of Cardiovascular Sciences. 2015;1(1):69–71. doi: 10.4103/2395-5414.157577 [DOI] [Google Scholar]
- 40.Bewick V, Cheek L, Ball J. Statistics review 8: Qualitative data–tests of association. Critical Care. 2003;8(1):46. doi: 10.1186/cc2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islam MM, Ababneh F, Akter T, Khan HR. Prevalence and risk factors for low birth weight in Jordan and its association with under-five mortality: a population-based analysis. East Mediterr Health J. 2020;26:1273–84. doi: 10.26719/emhj.20.096 [DOI] [PubMed] [Google Scholar]
- 42.Maniruzzaman M, Suri HS, Kumar N, Abedin MM, Rahman MJ, El-Baz A, et al. Risk factors of neonatal mortality and child mortality in Bangladesh. Journal of global health. 2018;8(1). doi: 10.7189/jogh.08.010421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasan E, Khanam M, Shimul SN. Socio-economic inequalities in overweight and obesity among women of reproductive age in Bangladesh: a decomposition approach. Journal of global health. 2020;20(1):263. doi: 10.1186/s12905-020-01135-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerman RI, Yitzhaki S. Improving the accuracy of estimates of Gini coefficients. Journal of Econometrics. 1989;42(1):43–7. doi: 10.1016/0304-4076(89)90074-2 [DOI] [Google Scholar]
- 45.Kumar P, Sharma H, Sinha D. Socio-economic inequality in anaemia among men in India: a study based on cross-sectional data. BMC Public Health. 2021;21(1):1345. doi: 10.1186/s12889-021-11393-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shobhit S, Ratna P, Shekhar C, Pradeep K, Samriddhi SG, Dhananjay WB. Decomposing socio-economic inequality for routine medical check-ups among older adults in India. Research Square. 2020. doi: 10.21203/rs.3.rs-72308/v1 Available from: 10.21203/rs.3.rs-72308/v1. [DOI] [Google Scholar]
- 47.WHO. UNICEF-WHO low birthweight estimates: levels and trends 2000–2015. Geneva: World Health Organization. 2019. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-19.21. [Google Scholar]
- 48.Kunst AE, Mackenbach JP. Measuring socioeconomic inequalities in health. Copenhagen: World Health Organization; 1994. p. 115. [Google Scholar]
- 49.Schneider MC, Castillo-Salgado C, Bacallao J, Loyola E, Mujica OJ, Vidaurre M, et al. Methods for measuring health inequalities (Part III). Epidemiological Bulletin. 2005;26(2). Available from: https://www3.paho.org/english/dd/ais/be_v26n2-cover.htm. [PubMed] [Google Scholar]
- 50.Mallick A. Prevalence of low birth weight in India and its determinants: Insights from the National Family Health Survey (NFHS). Anthropol Anz. 2021;78(3):163–75. [DOI] [PubMed] [Google Scholar]
- 51.Martinson ML, Reichman NE. Socioeconomic Inequalities in Low Birth Weight in the United States, the United Kingdom, Canada, and Australia. American Journal of Public Health. 2016;106(4):748–54. doi: 10.2105/AJPH.2015.303007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pulok MH, Sabah MN-U, Enemark U. Socioeconomic inequalities of child malnutrition in Bangladesh. International Journal of Social Economics. 2016;43(12):1439–59. doi: 10.1108/IJSE-03-2015-0065 [DOI] [Google Scholar]
- 53.Nabeen A, Akanda M, Salam A. Association between economic inequality and under-five child malnutrition: Evidence from Bangladesh Demographic and Health Survey. Dhaka Univ J Sci. 2018;66(1):73–8. [Google Scholar]
- 54.Islam GMR. Inequality, chronic undernutrition, maternity, and diabetes mellitus as the determinant of anemia among ever-married women in Bangladesh. BMC Public Health. 2021;21(1):310. doi: 10.1186/s12889-021-10362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S, Srivastava S, Upadhyay AK. Socio-economic inequality in malnutrition among children in India: an analysis of 640 districts from National Family Health Survey (2015–16). International Journal for Equity in Health. 2019;18(1):203. doi: 10.1186/s12939-019-1093-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sengupta A, Angeli F, Syamala TS, Dagnelie PC, Schayck CPv. Overweight and obesity prevalence among Indian women by place of residence and socio-economic status: Contrasting patterns from ‘underweight states’ and ‘overweight states’ of India. Social Science & Medicine. 2015;138:161–9. doi: 10.1016/j.socscimed.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 57.Rai A, Gurung S, Thapa S, Saville NM. Correlates and inequality of underweight and overweight among women of reproductive age: Evidence from the 2016 Nepal Demographic Health Survey. PLOS ONE. 2019;14(5):e0216644. doi: 10.1371/journal.pone.0216644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaya S, Uthman OA, Ekholuenetale M, Bishwajit G. Socioeconomic Inequalities in the Risk Factors of Noncommunicable Diseases Among Women of Reproductive Age in Sub-saharan Africa: A Multi-Country Analysis of Survey Data. Frontiers in Public Health. 2018;6. doi: 10.3389/fpubh.2018.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdu J, Kahssay M, Gebremedhin M. Household Food Insecurity, Underweight Status, and Associated Characteristics among Women of Reproductive Age Group in Assayita District, Afar Regional State, Ethiopia. Journal of Environmental and Public Health. 2018;2018:7659204. doi: 10.1155/2018/7659204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasmeen S, Azim E. Status of low birth weight at a tertiary level hospital in Bangladesh for a Selected Period. South East Asia Journal of Public Health. 2013;1(1):24–7. doi: 10.3329/seajph.v1i1.13209 [DOI] [Google Scholar]
- 61.Dasgupta A, Basu R. Determinants of low birth weight in a Block of Hooghly, West Bengal: A multivariate analysis. Int J Biol Med Res. 2011;2(4):838–42. [Google Scholar]
- 62.Harris-Fry H, Azad K, Kuddus A, Shaha S, Nahar B, Hossen M, et al. Socio-economic determinants of household food security and women’s dietary diversity in rural Bangladesh: a cross-sectional study. Journal of Health, Population and Nutrition. 2015;33(1):2. doi: 10.1186/s41043-015-0022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhury M, Dibley MJ, Alam A, Huda TM, Raynes-Greenow C. Household Food Security and Birth Size of Infants: Analysis of the Bangladesh Demographic and Health Survey 2011. Current Developments in Nutrition. 2018;2(3):nzy003. doi: 10.1093/cdn/nzy003 Available from: 10.1093/cdn/nzy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muula A, Siziya S, Rudatsikira E. Parity and maternal education are associated with low birth weight in Malawi. African health sciences. 2011;11(1). [PMC free article] [PubMed] [Google Scholar]
- 65.C A K., Basel PL, Singh S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLOS ONE. 2020;15(6):e0234907. doi: 10.1371/journal.pone.0234907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammad KA, Zahura FT, Rahman MM. Importance of maternal education on antenatal care visits in Bangladesh. Bangladesh Journal of Scientific Research. 2018;30(1–2):23–33. doi: 10.3329/bjsr.v30i1-2.36117 [DOI] [Google Scholar]
- 67.Islam A, Islam N, Bharati P, Aik S, Hossain G. Socio-economic and demographic factors influencing nutritional status among early childbearing young mothers in Bangladesh. BMC Women’s Health. 2016;16(1):58. doi: 10.1186/s12905-016-0338-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terada M, Matsuda Y, Ogawa M, Matsui H, Satoh S. Effects of Maternal Factors on Birth Weight in Japan. Journal of Pregnancy. 2013;2013:172395. doi: 10.1155/2013/172395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karim MR, Mondal MNI, Rana MM, Karmaker H, Bharati P, Hossain MG. Maternal factors are important predictors of low birth weight: evidence from Bangladesh Demographic & Health Survey-2011. Malaysian Journal of Nutrition. 2016;22(2). [Google Scholar]
- 70.Aras R. Is maternal age risk factor for low birth weight? Archives of Medicine and Health Sciences. 2013;1(1):33–7. doi: 10.4103/2321-4848.113558 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from the Demographic and Health Surveys (DHS) Program (https://dhsprogram.com/).