Abstract
BACKGROUND:
Outcomes and safety of budesonide maintenance therapy in microscopic colitis (MC) are not well known.
METHODS:
Adult residents of Olmsted County, Minnesota diagnosed with MC (2002–2019) and treated with budesonide were identified using the Rochester Epidemiology Project. Response was assessed at 12 +/− 4 weeks after initiation of therapy and defined as complete (resolution of diarrhea), partial (≥50% improvement in number of bowel movements), nonresponse (<50% improvement), and intolerance (discontinued due to side-effects). For safety outcomes, cases (budesonide maintenance) and MC controls (no budesonide therapy) were matched by gender and age at diagnosis (+/−2 years).
RESULTS:
A total of 450 patients were identified, of which 162 (36.0%) were treated with budesonide for induction of clinical remission [median age 67 (23–91) years and 126 (77.8%) female]. Clinical outcomes for induction were as follows: 130 (80.2%) complete response, 22 (13.6%) partial response, 8 (4.9%) no response, and 2 (1.2%) intolerance. After induction, 96 (63.2%) had recurrence after discontinuation; 27 (28.1%) required further budesonide induction treatment without maintenance, 56 (58.3%) required long-term budesonide maintenance, and 13 (13.5%) were treated with other therapies. Of those receiving budesonide maintenance, all responded [55 (98.2%) complete, 1 (1.8%) partial]. No patient stopped maintenance from adverse events. The median duration of follow-up was 5.6 years (0.3–18.9). There was no significant difference between cases and controls in the incidence of osteopenia/ osteoporosis, diabetes mellitus, hypertension, glaucoma, or cataracts.
CONCLUSION:
The long-term use of budesonide in MC appears to be effective and generally well tolerated with limited adverse effects.
Keywords: microscopic colitis, budesonide, maintenance, collagenous colitis, lymphocytic colitis
Introduction
Microscopic colitis (MC) is a common cause of chronic diarrhea and is comprised of two subtypes, lymphocytic colitis (LC) and collagenous colitis (CC) distinguished by their histological findings.1 The American Gastroenterological Association recommends budesonide as first-line therapy in those with moderate-severe symptoms.2 However, after discontinuation of budesonide, relapse is common, ranging from 40–81%, requiring many patients to remain on maintenance therapy.3–4. The lowest possible dose that maintains remission is typically used in patients requiring long-term budesonide therapy.
Potential adverse effects associated with systemic corticosteroids include metabolic bone disease, hypertension, hyperglycemia, as well as ophthalmologic disorders such as glaucoma and cataracts. Budesonide is preferred to systemic corticosteroids, such as prednisone, due to a lower risk of side effects due to its high first-pass metabolism. However, there are limited data evaluating the tolerability and long-term safety of budesonide in MC. We performed a population-based study on the use of budesonide in MC for maintenance to assess treatment outcomes and adverse events.
Methods
The Rochester Epidemiology Project (REP) is a medical records linkage system containing longitudinal medical data on all medical encounters for residents of Olmsted County, Minnesota.5–9 Mayo Clinic and Olmsted County Medical Center are the two main health systems for Olmsted County. Residents age ≥18 years diagnosed with MC between January 1, 2002 through December 31, 2019 were identified based on pathology reports using the REP and confirmed by chart review. Patients with at least 4 months of follow up were included, and those with inflammatory bowel disease before or after MC diagnosis were excluded.
Data was collected on MC subtype (LC or CC), age, body mass index, smoking status (never, former, current), presence of other medical conditions (hypertension, diabetes mellitus, osteopenia/osteoporosis, cataracts, glaucoma) at diagnosis, treatment with budesonide (induction and maintenance), treatment with concomitant medications for MC (loperamide, bismuth subsalicylate, bile acid sequestrant, mesalamine), budesonide treatment response (complete, partial, nonresponse, intolerance), concurrent use of CYP3A4-inhibitors (strong, moderate) with budesonide,10–11 and safety outcomes (new diagnosis or worsening of hypertension, diabetes mellitus, osteopenia/osteoporosis, cataracts, glaucoma) during and within 12 months of completing budesonide maintenance therapy.
Cases were MC patients that received budesonide maintenance therapy while controls were MC patients not treated with budesonide or other corticosteroids. Cases and controls were matched 1:1 by gender and age at MC diagnosis (+/−2 years) to evaluate safety outcomes, as above, during and within 12 months of completing budesonide maintenance therapy or within 12 months of MC diagnosis, respectively.
Treatment response for those on budesonide was assessed at 12 +/− 4 weeks after initiation of therapy and defined a priori as complete (resolution of diarrhea), partial (≥50% improvement in number of bowel movements), nonresponse (<50% improvement), and intolerance (budesonide discontinued due to side-effects). Recurrence was defined as new diarrhea (≥3 bowel movements per day) after initial symptom improvement and lack of other causes of diarrhea. Duration of follow-up was determined from date of MC diagnosis to date of last clinic visit.
Continuous variables were reported as means with standard deviation (SD) and categorical variables as median (range) or frequency (percentages). Data analysis was performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.6.2. This study was approved by Mayo Clinic and Olmsted Medical Center institutional review boards.
Results
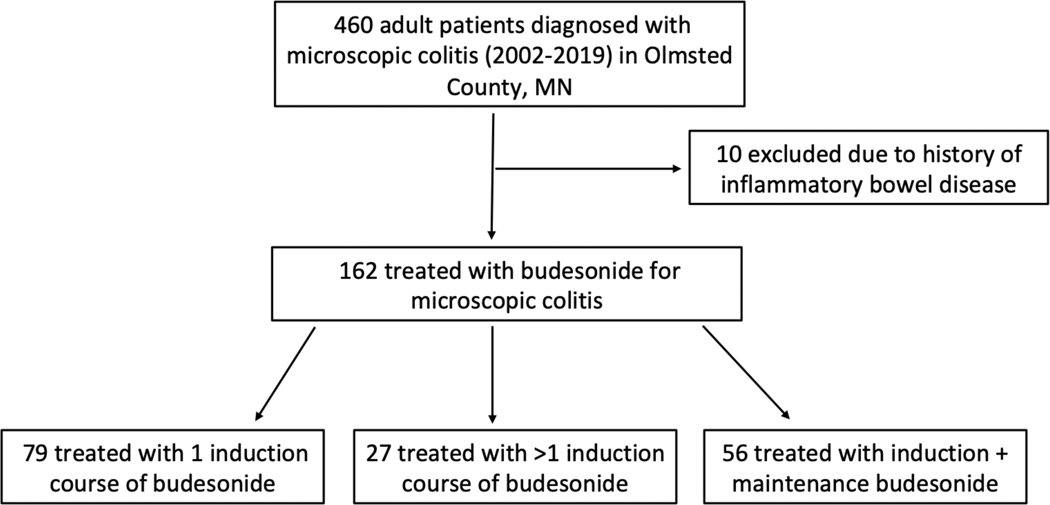
There were 450 patients diagnosed with MC between 2002–2019 in Olmsted County, of which 162 (36.0%) were treated with budesonide [median age 67 years (23–91); 126 (77.8%) female] (Figure 1). Clinical characteristics are displayed in Table 1 with no significant differences between MC subtypes. Budesonide was initial therapy in 55/162 (33.9%) patients while 107/162 (66.1%) had failed another medication or combination of medications prior to budesonide initiation, including loperamide 88/107 (82.2%), bismuth subsalicylate 46/107 (43.0%), bile acid sequestrant 9/107 (8.4%), and mesalamine 5/107 (4.7%).
Figure 1.
Study flow diagram.
Table 1.
Baseline characteristics of microscopic colitis patients.
| Median (range) or n (%) | |||||
|---|---|---|---|---|---|
| Characteristics | All patients (n=162) | Lymphocytic colitis (n=78) | Collagenous colitis (n=84) | p-value | |
| Age | 67 (23–91) | 67 (28–91) | 67 (23–88) | 0.52 | |
| Sex, female | 126 (77.8%) | 61 (78.2%) | 65 (77.4%) | 0.89 | |
| Race, white | 161 (99.4%) | 78 (100%) | 83 (98.8%) | 0.33 | |
| Body mass index (kg/m2) | 26.7 (15.8–58.4) | 27.5 (15.8–58.4) | 26.1 (18.2–48.2) | 0.06 | |
| Medical Conditions | Hypertension | 100 (61.7%) | 44 (56.4%) | 56 (66.7%) | 0.18 |
| Diabetes mellitus | 17 (10.5%) | 5 (6.4%) | 12 (14.3%) | 0.10 | |
| Osteopenia | 60 (37.0%) | 27 (34.6%) | 33 (39.3%) | 0.54 | |
| Osteoporosis | 25 (15.4%) | 9 (11.5%) | 16 (19.0%) | 0.19 | |
| Glaucoma | 14 (8.6%) | 7 (9.0%) | 7 (8.3%) | 0.88 | |
| Cataracts | 54 (33.3%) | 27 (34.6%) | 27 (32.1%) | 0.74 | |
Pre-existing medical conditions prior to budesonide treatment
All 162 patients received budesonide for induction of clinical remission, of which 130 (80.2%) had complete response, 22 (13.6%) partial response, 8 (4.9%) no response, and 2 (1.2%) intolerance. A total of 39/162 (24.1%) patients were treated concomitantly with budesonide and another medication for induction, including loperamide 32/39 (82.1%), bismuth subsalicylate 4/39 (10.2%), and bile acid sequestrant 3/39 (7.7%). In addition, 12/162 (7.4%) patients were treated with a concurrent CYP3A4-inhibitor during induction with budesonide [strong inhibitor 7/12 (58.3%), moderate inhibitor 5/12 (41.7%)].
After induction with budesonide, 96/152 (63.2%) had recurrence after discontinuation; 27/96 (28.1%) required further budesonide induction treatment without maintenance, 56/96 (58.3%) required long-term budesonide maintenance, and 13/96 (13.5%) were treated with other therapies (bile acid sequestrant or bismuth subsalicylate). The median time to recurrence after induction was 98 (7–3,221) days. The initial and lowest effective maintenance dose for those receiving long-term therapy is shown in Table 2. A total of 14/56 (25.0%) patients were treated concomitantly with budesonide maintenance and another medication, including loperamide 12/14 (85.7%), bismuth subsalicylate 1/14 (7.1%), and bile acid sequestrant 1/14 (7.1%). Additionally, 4/56 (7.1%) patients were treated with a concurrent CYP3A4-inhibitor during budesonide maintenance [strong inhibitor 2/4 (50%), moderate inhibitor 2/4 (50%)]. Of those receiving budesonide maintenance, all responded [55 (98.2%) complete, 1 (1.8%) partial] and none recurred while on maintenance therapy. After discontinuation of maintenance, 26/56 (46.4%) had recurrence, and budesonide was restarted in most [23/26 (88.5%)] for maintenance. The median time to recurrence after discontinuation of the first maintenance was 336 (7–2,885) days. The median duration of the first maintenance treatment was 43 (8–323) weeks and cumulative duration of all maintenance treatments was 62 (16–469) weeks. The median duration of follow-up was 5.6 years (0.3–18.9) and no patient stopped maintenance therapy due to adverse events.
Table 2.
First maintenance treatment with budesonide.
| Median (range) or n (%) | ||||
|---|---|---|---|---|
| First maintenance | All patients (n=56) | Lymphocytic colitis (n=19) | Collagenous colitis (n=37) | |
| Bowel movements, number | 5 (3–15) | 5 (3–15) | 6 (3–15) | |
| Smoking history | 39 (69.6%) | 10 (52.6%) | 29 (78.4%) | |
| Maintenance duration, days | 302 (56–2,258) | 113 (56–1,387) | 379 (84–2,258) | |
| Maintenance initial dose | 6 mg daily | 43 (76.8%) | 12 (63.2%) | 31 (83.8%) |
| 3 mg daily | 13 (23.2%) | 7 (36.8%) | 6 (16.2%) | |
| Maintenance lowest dose | 6 mg daily | 10 (17.9%) | 5 (26.3%) | 5 (13.5%) |
| 3 mg daily | 40 (71.4%) | 13 (68.4%) | 27 (72.9%) | |
| 3 mg every other day | 6 (10.7%) | 1 (5.3%) | 5 (13.5%) | |
| Maintenance outcome | Complete | 55 (98.2%) | 19 (100%) | 36 (97.3%) |
| Partial | 1 (1.8%) | 0 (0%) | 1 (2.7%) | |
| No response | 0 (0%) | 0 (0%) | 0 (0%) | |
| Intolerance | 0 (0%) | 0 (0%) | 0 (0%) | |
| Recurrence | 26 (46.4%) | 4 (21.1%) | 22 (59.5%) | |
Patients developed the following conditions during budesonide maintenance treatment or within 12 months of discontinuation: osteopenia 6/56 (10.7%), osteoporosis 3/56 (5.4%), diabetes mellitus 1/56 (1.8%), hypertension 0/56 (0%), glaucoma 4/56 (7.1%), cataracts 6/56 (10.7%), and mood changes 6/56 (10.7%) (Table 3). Two (50%) patients on a concurrent CYP3A4-inhibitor during budesonide maintenance treatment developed one of the conditions above. One (1.8%) patient with pre-existing diabetes and 10 (17.9%) with pre-existing hypertension required initiation of a new medication or increased dose of existing medication. Thirty (53.6%) patients received calcium or vitamin D supplementation during maintenance. MC patients not treated with budesonide developed the following conditions within 12 months of diagnosis: osteopenia 6/56 (10.7%), osteoporosis 6/56 (10.7%), diabetes mellitus 0/56 (0%), hypertension 1/56 (1.8%), glaucoma 1/56 (1.8%), and cataracts 7/56 (12.5%). There were no significant differences in individual adverse events between cases and controls [osteopenia (p = 0.47), osteoporosis (p = 0.49), diabetes mellitus (p = 1.0), hypertension (p = 1.0), glaucoma (p = 0.36), cataracts (p = 0.74)] and overall safety outcomes (p = 0.53).
Table 3.
Safety outcomes within 12 months after completing first maintenance treatment.
| Median ± Range or n (%) | ||||
|---|---|---|---|---|
| First Maintenance | All patients (n=56) | Lymphocytic colitis (n=19) | Collagenous colitis (n=37) | |
| Osteopenia* | 6 (10.7%) | 3 (15.7%) | 3 (8.1%) | |
| Osteoporosis* | 3 (5.4%) | 0 (0%) | 3 (8.1%) | |
| Glaucoma* | 4 (7.1%) | 1 (5.3%) | 3 (8.1%) | |
| Cataracts* | 6 (10.7%) | 2 (10.5%) | 4 (10.8%) | |
| Mood changes | 6 (10.7%) | 2 (10.5%) | 4 (10.8%) | |
| Hypertension | New diagnosis | 0 (0%) | 0 (0%) | 0 (0%) |
| New or increased dose of current medication | 10 (17.9%) | 1 (5.3%) | 9 (24.3%) | |
| Diabetes mellitus | New diagnosis | 1 (1.8%) | 0 (0%) | 1 (2.7%) |
| New or increased dose of current medication | 1 (1.8%) | 0 (0%) | 1 (2.7%) | |
n=39 (69.6%) and n=5 (8.9%) did not have DEXA or ophthalmology exam during or within 12 months after maintenance, respectively
Discussion
In this study, we describe the clinical outcomes and safety profile of budesonide maintenance in MC. While budesonide is the recommended first-line treatment for moderate-severe MC, only 162 (36.0%) were treated with budesonide during the study period, reflecting that patients with mild symptoms were managed with bismuth subsalicylate and antidiarrheals. Furthermore, fewer patients were treated with budesonide during the first half of the study period while it became a more commonly used treatment in the second half of the study period.
Consistent with prior studies, we found a high rate of response and a high rate of recurrence after discontinuation of budesonide.12–17 In these patients, low-dose budesonide is often used for maintenance therapy. In our cohort, most patients maintained symptomatic response with budesonide 3 mg per day or every other day. Table 4 summarizes previous randomized placebo-controlled trials evaluating both budesonide induction and maintenance treatment for MC, including the clinical outcomes and adverse events.
Table 4A.
Randomized control trials of budesonide for induction in microscopic colitis.
| Studies | Efficacy | Safety |
|---|---|---|
| Miehlke et al. (2002) 12 | 86.9% clinical remission in budesonide group vs. 13.6% placebo group (p < 0.001) | 7.7% discontinued budesonide due to side effects vs. 4.0% placebo group |
| Baert et al. (2002) 13 | 8/14 patients in budesonide group had clinical remission vs. 3/14 in placebo (p = 0.05) | No serious adverse events reported in either group |
| Bonderup et al. (2003) 14 | 10/10 patients in budesonide group had clinical remission vs. 2/10 in placebo (p < 0.001) | No side effects were reported in either group |
| Miehlke et al. (2009) 15 | 86% clinical remission in budesonide group vs. 48% placebo group (p = 0.10) | 10% had side effects in budesonide group vs. 14% in placebo group |
| Miehlke et al. (2014) 16 | 80% clinical remission in budesonide group vs. 37.8% placebo group (p = 0.0006) | The rate of adverse events did not differ between groups |
| Miehlke et al. (2018) 17 | 79% clinical remission in budesonide group vs. 42% placebo group (p = 0.01) | 47.4% adverse events in budesonide group vs. 42.1% placebo group |
As reported previously18–21, long-term budesonide appears to be generally well tolerated; no patients on maintenance therapy discontinued budesonide due to side effects in our cohort. Additionally, the overall incidence of possible budesonide-related adverse effects (metabolic bone disease, hypertension, diabetes mellitus, and ophthalmologic conditions) was relatively low. We found a similar incidence of adverse events in patients with MC from the REP, matched by age and gender, who were not treated with budesonide. MC occurs frequently in older patients with a female predominance, both known risk factors for metabolic bone disease. It is possible that the incidence of osteopenia/osteoporosis seen in our patients may be in part attributed to these factors, rather than budesonide use alone, although studies have demonstrated the risk of osteoporosis in MC is not increased.22–25 Similarly, the incidence of ophthalmologic diseases, such as cataracts, seen in our population may also be in part attributed to increasing age.26–27
Although budesonide is preferred to other corticosteroids, such as prednisone, due to its high first-pass hepatic metabolism with fewer systemic adverse effects, its metabolism is subject to drug interactions. Medications that inhibit CYP3A4 may have the potential to accentuate the adverse effects of budesonide. About 50% of the patients on a concomitant CP3A4-inhibitor during budesonide maintenance treatment developed a side effect, suggesting this may be an important consideration when selecting maintenance therapy for patients. Although our study is limited by the relatively small sample size, its strengths include the population-based cohort and longitudinal follow-up.
In summary, the long-term use of budesonide in MC appears to be effective and generally well tolerated with limited adverse effects not dissimilar from those with MC not treated with budesonide. Larger, prospective studies are needed to determine the efficacy and safety of long-term budesonide for maintenance in MC.28
Table 4B.
Randomized control trials of budesonide for maintenance in microscopic colitis.
| Studies | Efficacy | Safety |
|---|---|---|
| Miehlke et al. (2008) 18 | 78% clinical remission in budesonide group vs. 39% placebo group (p = 0.007) | No serious adverse events reported in either group |
| Bonderup et al. (2009) 19 | 76.5% clinical remission in budesonide group vs. 12% placebo group (p <0.001) | Side effects did not differ between groups (1/17 patients in budesonide vs. 1/17 placebo) |
| Munch et al. (2016) 20 | 61.4% clinical remission in budesonide group vs. 16.7% placebo group (p < 0.001) | No serious adverse events reported in either group |
Funding:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: DSP has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta, Seres, Abbvie, Immunic, and Otsuka. The other authors have no conflicts of interest to report.
References:
- 1.Pardi DS. Diagnosis and Management of Microscopic Colitis. Am J Gastroenterol. 2017; 112(1):78–85. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen GC, Smalley WE, Vege SS, et al. Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Medical Management of Microscopic Colitis. Gastroenterology. 2016; 150(1):242–6. [DOI] [PubMed] [Google Scholar]
- 3.Loreau J, Duricova D, Gower-Rousseau C, et al. Long-Term Natural History of Microscopic Colitis: A Population-Based Cohort. Clin Transl Gastroenterol. 2019; 10(9):e00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentile NM, Abdalla AA, Khanna S, et al. Outcomes of Patients with Microscopic Colitis Treated with Corticosteroids: a Population-Based Study. Am J Gastroenterol. 2013; 108(2):256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocca WA, Grossardt BR, Brue SM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project Medical Records-Linkage System (E-REP). Int J Epidemiol. 2018; 1;47(2):368–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc. 2012; 87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: the Rochester Epidemiology Project. Am J Epidemiol. 2011; 173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of Epidemiological Findings and Public Health Decisions: an Illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tome J, Sehgal K, Kamboj AK, et al. The Epidemiology of Microscopic Colitis in Olmsted County, Minnesota: Population-Based Study From 2011 to 2019. Clin Gastroenterol Hepatol. 2021:S1542–3565(21)00691–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagir A, Schmitt M, Dilger K, et al. Inhibition of Cytochrome P450 3A: Relevant Drug Interactions in Gastroenterology. Digestion. 2003;68(1):41–8. [DOI] [PubMed] [Google Scholar]
- 11.Manikandan P, Nagini S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr Drug Targets. 2018;19(1):38–54. [DOI] [PubMed] [Google Scholar]
- 12.Miehlke S, Heymer P, Bethke B, et al. Budesonide Treatment for Collagenous Colitis: a Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Gastroenterology 2002;123:978–984. [DOI] [PubMed] [Google Scholar]
- 13.Baert F, Schmit A, D’Haens G, et al. Budesonide in Collagenous Colitis: a Double-Blind Placebo-Controlled Trial with Histologic Follow-Up. Gastroenterology 2002;122:20–25. [DOI] [PubMed] [Google Scholar]
- 14.Bonderup OK, Hansen JB, Birket-Smith L, et al. Budesonide Treatment of Collagenous Colitis: a Randomised, Double Blind, Placebo Controlled Trial with Morphometric Analysis. Gut 2003; 52:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miehlke S, Madisch A, Karimi D, et al. Budesonide is Effective in Treating Lymphocytic Colitis: a Randomized Double-Blind Placebo-Controlled Study. Gastroenterology. 2009; 136(7):2092–100. [DOI] [PubMed] [Google Scholar]
- 16.Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is More Effective than Mesalamine or Placebo in Short-Term Treatment of Collagenous Colitis. Gastroenterology. 2014;146(5): 1222–30. [DOI] [PubMed] [Google Scholar]
- 17.Miehlke S, Aust D, Mihaly E, et al. Efficacy and Safety of Budesonide, vs. Mesalazine or Placebo, as Induction Therapy for Lymphocytic Colitis. Gastroenterology. 2018;55(6):1795–1804. [DOI] [PubMed] [Google Scholar]
- 18.Miehlke S, Madisch A, Bethke B, et al. Oral Budesonide for Maintenance Treatment of Collagenous Colitis: a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2008; 135:1510–1516. [DOI] [PubMed] [Google Scholar]
- 19.Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long-term Budesonide Treatment of Collagenous Colitis: a Randomized, Double-Blind, Placebo-Controlled Trial. Gut. 2009; 58:68–72. [DOI] [PubMed] [Google Scholar]
- 20.Münch A, Bohr J, Miehlke S, et al. Low-dose budesonide for Maintenance of Clinical Remission in Collagenous Colitis: a Randomised, Placebo-Controlled, 12-Month Trial. Gut. 2016;65(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian S, Wilhelm A, Jessica L, et al. Budesonide Treatment for Microscopic Colitis: Systematic Review and Meta-Analysis. Eur J Gastroenterol Hepatol. 2019; 31(8):919–927. [DOI] [PubMed] [Google Scholar]
- 22.Miehlke S, Acosta MB, Bouma G, et al. Oral Budesonide in Gastrointestinal and Liver Disease: A Practical Guide for the Clinician. J Gastroenterol Hepatol. 2018; 33:1574–1581. [DOI] [PubMed] [Google Scholar]
- 23.Wildt S, Munck LK, Becker S, et al. Risk of Osteoporosis in Microscopic Colitis. Postgrad Med. 2018; 130(3):348–354. [DOI] [PubMed] [Google Scholar]
- 24.Reilev M, Hallas J, Thomsen Ernst M, et al. Long-term Oral Budesonide Treatment and Risk of Osteoporotic Fractures in Patients with Microscopic Colitis. Aliment Pharmacol Ther. 2020; 51(6):644–651. [DOI] [PubMed] [Google Scholar]
- 25.Amin S, Achenbach SJ, Atkinson EJ, et al. Trends in Fracture Incidence: a Population-Based Study Over 20 Years. J Bone Miner Res. 2014; 29(3):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoff EO, Hattenhauer MG, Ing HH, et al. Estimated Incidence of Open-Angle Glaucoma in Olmsted County, Minnesota. Ophthalmology. 2001; 108(5):882–6. [DOI] [PubMed] [Google Scholar]
- 27.Gollogly HE, Hodge DO, St Sauver JL, et al. Increasing Incidence of Cataract Surgery: Population-Based Study. J Cataract Refract Surg. 2013; 39(9):1383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shor J, Churrango G, Hosseini N, et al. Management of Microscopic Colitis: Challenges and Solutions. Clin Exp Gastroenterol. 2019; 12:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]