Abstract
Objective:
The aim of the study was to assess the relationship between the expression of elastin, collagen type I, II,III and the degeneration of the facet joint capsule and the ligamentum flavum.
Methods:
10 patients (4 male, 6 female) (mean age 61 ± 14,9) undergoing surgery for degenerative lumbar spine syndrome and 5 cadavers (3 male, 2 female) (age of death 87 ± 8,6 years) were included in this study. One set of tissue samples was taken from each patient in the patient group intraoperatively and two sets of samples were taken from each cadaver in the cadaver group posthumosly from the ligamentum flavum (medial and lateral) and from the facet joint capsules (superior and inferior articular process) at the L4/5 segment.Western blot analysis was performed for collagen types I, II, III and for elastin. Disc degeneration was scored according to the Pfirmann Classification, facet joint arthrosis was scored according to the Fujiwara Classification and their relationship with protein expression was investigated.
Results:
There was a strong expression of Collagen type I in the patient group (PG) compared to the body donor group (BDG) in the facet joint capsule (FJC) and in the lateral samples of the ligamentum flavum. Samples of the FJC showed lower expression of elastin in the PG compared with the BDG, but without statistical significance. An increased expression of collagen type I compared to elastin in the PG could be shown. In contrast, elastin predominated in the samples of the BDG group compared to collagen type I (collagen type I/ elastin PG: PAsup 2,78; PAinf 2,61; LFmed 2,23; 225 LFlat 1,83; BDG: PAsup 0,15; PAinf 0,2; LFmed 0,2; LFlat 0,27). Rank correlation coefficient according to Spearman showed low to moderate correlations for collagen type I, III and elastin for the degree of disc degeneration according to Pfirrmann and the degree of facet joint osteoarthritis according to Fujiwara, all of them without statistical significance.
Conclusion:
This study has shown us that in the context of degenerative changes of the lumbar spine, there is an increased expression of collagen type I and a dominance over elastin.
Level of Evidence:
Level III, Diagnostic Study
Keywords: Lumbar spine, Ligamentum flavum, Facet joint capsule, Collagen, Elastin
HIGHLIGHTS
The difference in composition of collagen I, II, III or elastin in patients with and without degenerative lumbar spinal disease has not been investigated.The aim of this study was to evaluate the possible differences in collagen and elastin production of the facet joint capsules and ligamentum flavum between patients who underwent surgery due to degenerative lumbar spine disease and human body donors.
Significant collagen type I expression in facet joint capsule and ligamentum flavum of patients group was observed, although the difference was not significant for collagen type III and elastin.
A change in the proteins of the extracellular matrix of the FJC and the LF with an increase in collagen type I and a decrease in elastin seems to play a central role in degeneration. Moreover, the results of this study provide further evidence that degenerative changes occur also in the dorsal structures of the lower lumbar spine.
Introduction
Lumbar degenerative disease is a wide clinical picture and results in frequent physician consultations with low back pain, leg pain, or neurogenic claudication.
Degeneration of a lumbar segment begins with changes in the intervertebral disc with loss of water content and bulging of the annulus. In the second stage, the collapse of the disc space, there is a decrease in the height of the intervertebral space and the formation of osteophytes. Shortening of the ventral column results in a higher axial load on the facet joints. This leads to arthritic changes in the facet joints with joint hypertrophy and the formation of osteophytes. Furthermore, hypertrophy of the ligamentum flavum (LF) takes place.1
These degenerative changes may result in lumbar spinal stenosis (LSS) of the spinal canal or the lateral recesses.
Considering the LF and its role in the formation of this clinical picture, different researches have been done so far on the origin of LF hypertrophy.
The LF is a posterior structure of the vertebral column and connects the laminae of the adjacent vertebrae to each other. It contains collagen fibers and elastic fibers.2 Degenerative changes with disc bulking and hyperplasia of the facet joints leads to LF in-folding, hypertrophy, and fibrosis.
The LF is made of elastin and collagen fibers in a 2 : 1 ratio. Fetus LF consists of 75% of elastic fibers. The elastin fibers provide the elasticity, while the collagen fibers provide the stiffness and stability.
The LF can be divided into 2 layers: the superficial layer as a light-yellow structure on the outer side and the thinner deep layer with a dark yellow color adjacent to the dura that contains much more elastic fibers.3 In non-hypertrophied LF, the elastic fibers have a strict parallel order.4
Regardless of LF hypertrophy, the collagen types I, III, VI, and VIII are highly expressed at mRNA level in LF. In addition, a moderate correlation between the cross-sectional area of the LF and the mRNA expression level of collagen types I, III, and VI could be found.5 As life progresses, the ratio of elastin to collagen decreases in the dorsal side of the LF, resulting in reduced elasticity.6 Higher mechanical load at the dorsal side of LF is considered to be the cause of the loss of elastic fibers over time. These age-related changes of the LF lead to a reduction of elastic fibers, a loosening of their angular orientation of the fibers, and a regional substitution with collagen fibers.
Little is known about the molecular changes of the facet joints during the degeneration process. The facet joints are surrounded by a capsular ligament that is divided into 2 layers. The outer layer of the capsule contains parallel arranged fibers in a strong connective tissue and is classified as facet joint capsule ligament (FCL). The inner layer of the facet joint capsule (FJC) consists of soft connective tissue with yellow fibers. A clear separation of this inner layer from the LF of the spinal canal is difficult.7 The facet joint complex allows an articulation with 6 degrees of freedom during motion.8 To resist the strong tensile and shear loads acting on the facet joint, the FCL is composed of collagen fiber bundles, especially collagen type I and elastin.9,10 In immunohistochemical analysis of the dorsal FJC, collagen I, II, III, and VI could be found in patients who underwent spine surgery at the segment L4/5.11
To date, it has not been investigated if patients with degenerative lumbar spine disease requiring surgery show changes in the composition of collagen I, II, or III or elastin in the area of the FJC and LF compared to people without known spinal disease.
The aim of this study was to evaluate the possible differences in collagen and elastin production of the FJC and the LF between patients who underwent surgery due to degenerative lumbar spine disease and human body donors.
Materials and Methods
To investigate the research question, 2 study groups consisting of a patient group (PG) and a control group with body donors (BDG) were formed. The study was approved by the local ethics committee (AZ 443-15-21122015) and was performed in accordance with the Declaration of Helsinki.
Patient population
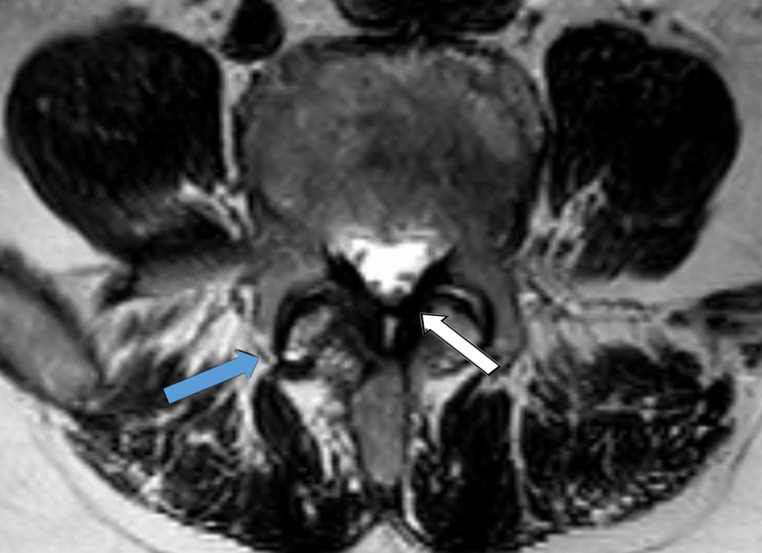
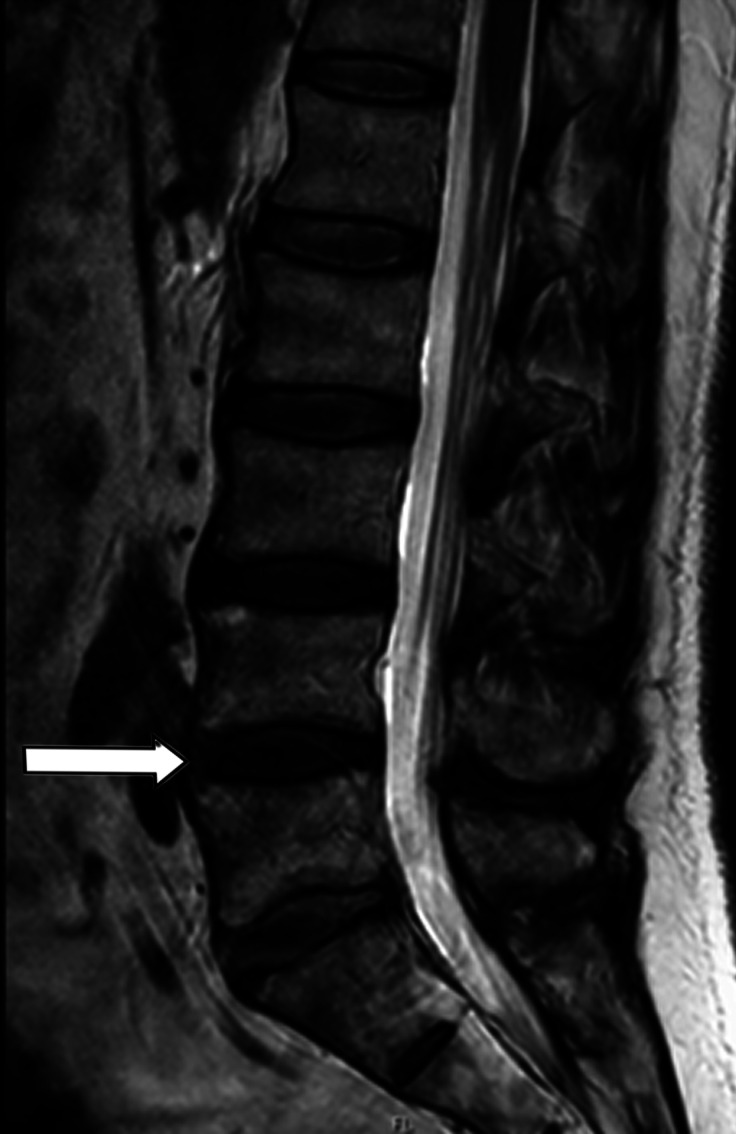
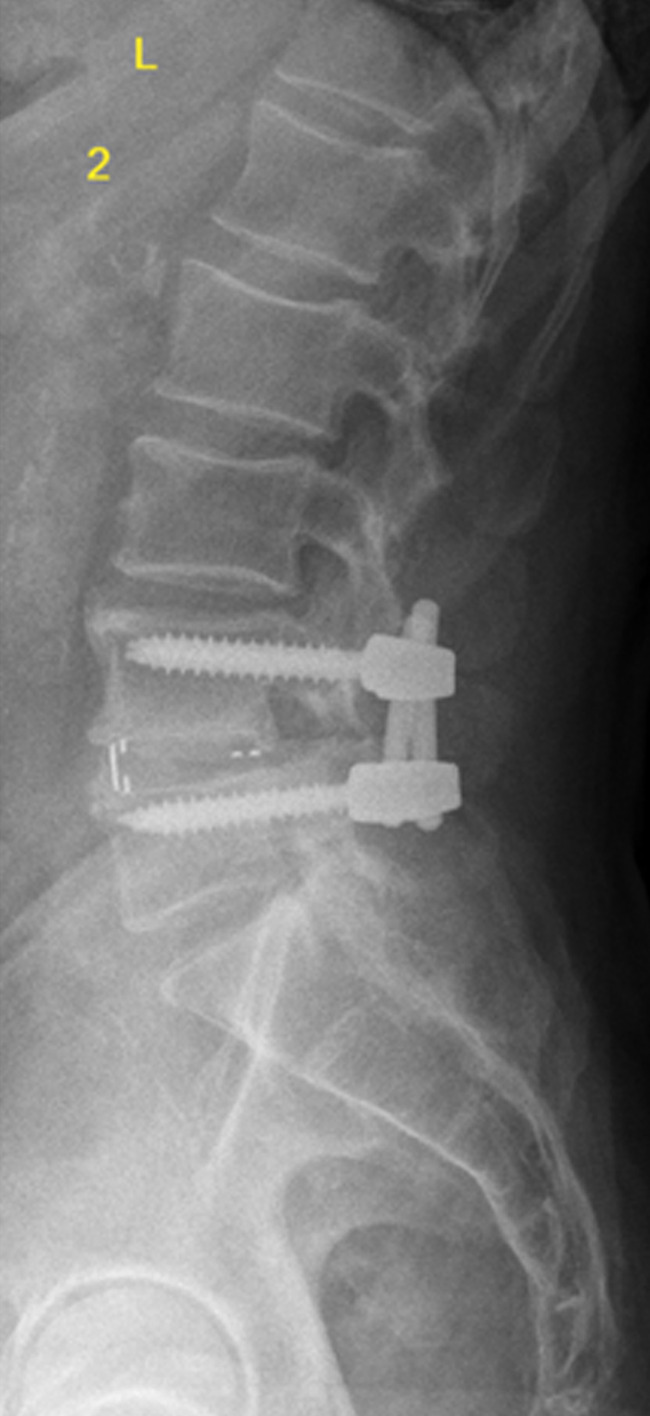
A total of 10 patients who underwent lumbar spine surgery (4 males, 6 females) could be included in this study. All patients underwent spine surgery because of degenerative lumbar spine syndrome (e.g., spinal canal stenosis (LSS), osteochondrosis, neuroforaminal stenosis, degenerative spondylolisthesis). We classified the degree of degenerative lumbar spine disease based on preoperative performed magnetic resonance imaging (MRI) of the lumbar spine to Pfirrmann and Fujiwara classification (Figures 1 and 2). Human probes of around 2 g were taken intraoperatively during a single-segment transforaminal interbody fusion (TLIF) of the segment L4/5 of the lumbar spine (Figure 3). One sample of the FJC of the superior and inferior articular process and one medial and one lateral sample of the LF were obtained for each examinee.
Figure 1.
Magnetic resonance imaging (T2 sequence) with axial view of the segment L4/5. Blue arrow shows the hypertrophy of the facet joint with type 3 classification according to Fujiwara. White arrow shows the ligamentum flavum with hypertrophy.
Figure 2.
Magnetic resonance imaging (T2 sequence) with sagittal view of the lumbar spine. White arrow points to the intervertebral disc of the segment L4/5. Degenerative changes are classified as type 3 according to Pfirrmann.
Figure 3.
Lateral plane x-ray postoperative. The posterior stabilization of L4/5 and the interbody fusion in TLIF technique is seen. TLIF, transforaminal interbody fusion.
Consent for sample collection and use was obtained using an informed consent form in accordance with the ethics vote of the Leipzig University Medical Faculty (AZ 443-15-21122015). Exclusion criteria were age < 18 years, pregnancy, previous lumbar spine surgery, and inflammatory-infectious, rheumatic, or neoplastic disease. There was no selection for age or sex.
Human body donor group
The human BDG was used as a control group in this study. In total, 5 human body donors could be included (3 males, 2 females). Two sets of samples were obtained from the segment L4/L5, 1 on the right and 1 on left side (n = 10).
No one had relevant previous lumbar disease. All body donors gave their signed consent for exploring their cadavers for research and educational purposes, before passing away. Institutional approval was obtained in accordance with the Saxonian Death and Funeral Act of 1994. Signed body donor consents are available on request. Exclusion criteria were age < 18 years, previous lumbar spine surgery, destroying lumbar spine disease as well as unattended postmortem state not to exceed 3 days.
Tissue samples obtained were standardized according to the sampling of the operative TLIF technique from the articular capsules of the facet joints and the medial and lateral LF from all included body donors. Additionally, the tissue samples could be obtained from the direct surrounding tissue of the spinal nerve at the neuroforamen.
The body donor probes were proceeded as done for the patient probes and described in detail in the following section.
Tissue sample processing of the patients group and the human body donor group
Liquid nitrogen was added to the tissue samples. Then, the probes were prepared in a fragmented state and suspended in 200-400 μL of single concentrated sample buffer. Between all steps, the samples were placed on ice. This was followed by 3 homogenization steps each for 10 seconds. The samples were then heated to 70°C for 20 minutes and centrifuged at 4°C for 15 minutes at 13 000 rpm. The supernatants were pipetted off and stored at −80°C. For collagen type I and type III, a protein amount of 20 µg and for collagen type II and elastin, a protein amount of 10 µg showed the best results of detection in pretests.
Protein determination
The Pierce BCA Protein Assay Kit (Thermo Scientific™ Pierce™ BCA Protein Assay Kit, Schwerte, Germany) was used for protein determination. Blank and bovine serum albumin (BSA) standard series were plotted as duplicate samples and each tissue sample was plotted as a single sample, resulting in 25 measurements for the patient group and 50 measurements for the BDG. Per well, 25 μL of standard or sample to be analyzed and 200 μL of BCA reagent (BCA reagent A and B 50 : 1 ratio) were applied. Sample buffer was used as blank. The BSA standard series was prepared using the enclosed BSA standard ampoules with a concentration of 2 mg/mL. The sample suspensions were used diluted 1 : 5 with sample buffer.
After addition of the BCA reagent, incubation followed at 37°C for 30 minutes. Protein concentrations were then measured using Biochrom's EZ Read 400 Microplate Reader (Biochrom™) at a wavelength of 562 nm.
Western blotting
Proteins were separated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred to polyvinylidene difluoride membrane (Trans-Blot®Turbo™V1.02 Transfer System, Bio-Rad Laboratories GmbH; Feldkirchen, Germany). The antibodies used in this study were listed here: anti-collagen I (ab34710, Abcam, Cambridge, UK), anti-collagen II (ab85266, Abcam), anti-collagen III (ab7778, Abcam), and anti-elastin (ab21610, Abcam). Membranes were then washed with phosphate-buffered saline and incubated with secondary antibodies peroxidase-conjugated goat anti-rabbit IgG (PI-1000, Abcam).
Total protein normalization was performed using Ponceau S staining for all samples tested. Immediately following protein transfer to the nitrocellulose membrane, the membrane was stained with Ponceau S and photographed. All measured target proteins were then related to the measured total protein amount of the corresponding Ponceau S image.
Magnetic resonance imaging analysis
Preoperative MRI scans of the operated patients were evaluated according to 2 criteria. Disc degeneration in the L4/L5 segment was scored according to the Pfirrmann classification. The degree of facet joint arthrosis in the L4/L5 segment was classified according to Fujiwara. The analysis was performed by a senior spine surgeon and a junior resident in orthopedics. The agreement was 100%.
Statistical analysis
Graphs and analyses were generated using Graph Pad Prism software 7 (GraphPad Software, La Jolla, Calif, USA) and Excel (Microsoft Excel). Data are presented as mean ± standard deviation. The significance of the average of both groups was tested using the unpaired t-test for normal distribution, and the Mann-Whitney U test was performed in case of non-normal distribution of the values. The significance level was set at P < 0.05. To augment the significance test, the effect size Cohen's d was determined for all mean differences (d > I0.8I: strong effect, d between I0.5I and I0.8I: medium effect). To clarify the relationship between collagen type I and elastin, collagen type I/elastin ratios were calculated for each sample location of both study groups.
Spearman's rank correlation coefficient was performed for correlation analysis of the degree of degeneration according to Pfirrmann and Fujiwara classification in the preoperative MRI image with the corrected protein amount of the target proteins in the tissue probes. The interpretation of Spearman`s correlation coefficients was performed according to Chan.12
Results
Consecutively, a total of 10 patients who underwent lumbar spine surgery (4 males, 6 females) could be included in this study (n = 10). The mean age was 61 ± 14.9 years (range 37, 44-81 years).
In total, 5 human body donors could be included (3 males, 2 females). Two sets of samples were obtained from the segment L4/L5, 1 on the right and 1 on the left side (n = 10). The mean age of the body donors at the time of death was 87 ± 8.6 years (range 22, 74-96 years). Descriptive data are shown in Table 1.
Table 1.
Patient-related descriptive data of all investigated probes
| Patient Group | Body Donor Group | |
|---|---|---|
| No. of patients | 10 | 5 |
| Gender (ratio male : female) | 4 : 6 | 3 : 2 |
| Age (years, mean ± standard deviation) | 61 ± 14.9 | 87 ± 8.6 |
To evaluate the degree of degeneration of the operated L4/5 segments in the patient group, the sagittal and transverse MRI images of the region were classified according to Pfirrmann and Fujiwara. Pfirrmann classification in the PG was distributed as follows: type 3, n = 1; type 4, n = 7; type 5, n = 2. The Fujiwara Classification was graduated as follows: type 2, n = 1; type 3, n = 7; type 4, n = 2 (Table 2) (Figures 1 and 2).
Table 2.
Graduation of degenerative changes based on magnetic resonance imaging of patient group according to Pfirrmann and Fujiwara classification
| Pfirrmann classification (n) | Fujiwara classification (n) | |
|---|---|---|
| Type 1 | 0 | 0 |
| Type 2 | 0 | 1 |
| Type 3 | 1 | 7 |
| Type 4 | 7 | 2 |
| Type 5 | 2 | - |
Using Western blot analysis, all samples were analyzed for 4 target proteins: collagen type I, collagen type II, collagen type III, and elastin. Collagen type I and type III as well as elastin could be detected in all samples investigated. Detection of collagen type II was only successful in 23 of 93 samples.
Collagen type I
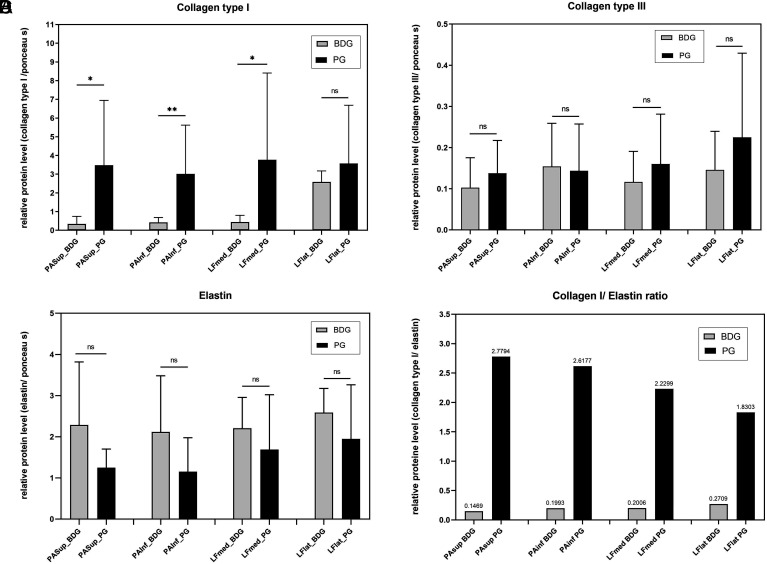
Collagen type I showed a strong expression in the PG compared with the samples from the BDG group. In the samples of the FJC of the superior and inferior articular processes (PAsup and PAinf), significantly more collagen type I was detected in the PG than in the BDG. For both groups, there was a strong effect size of d > 0.8 (PAsup: d = 1.4; PAinf: d = 1.7). The LF samples also showed a higher expression of collagen type I in the PG compared with the BDG, but it was only significant in the probes of the LFlat. For both sample localizations, large effect sizes could be calculated with d > 0.8 (LFmed: d = 1.0; LFlat: d = 1.3) for the mean differences of collagen type I expression (Figure 4A) (Table 3).
Figure 4. A-D.
Western blot analyses with relative protein levels normalized to Ponceau S are shown for (A) collagen type I, (B) collagen type III, (C) elastin, (D) ratio of collagen type I to elastin for the patient group (PG) and the body donor group (BDG).
Table 3.
Effect size (Cohen’s d) to measure the strength of relationship of the mean results between both sample groups (PG and BDG)
| PAsup ( d) | PAinf ( d) | LFmed ( d) | LFlat ( d) | |
|---|---|---|---|---|
| Collagen type I | 1.4 | 1.7 | 1.0 | 1.3 |
| Collagen type III | 0.5 | −0.1 | 0.5 | 0.5 |
| Elastin | −0.9 | −0.9 | −0.5 | −0.7 |
Collagen type II
In total, the expression of collagen type II was very low in all examined samples regardless of the group. Overall, only 8 of 50 samples in the BDG and 14 of 50 samples in the PG showed an expression of collagen II. A further statistical analysis of the detected protein levels was not performed.
Collagen type III
The expression of collagen type III was low in all samples. In the joint capsules of the vertebral joints (PAsup, PAinf), no relevant differences in collagen type III expression between the study groups could be shown. Collagen type III distribution in the samples of LF (LFmed, LFlat) showed the tendency to decrease in samples of BDG. The study was not significant and showed only medium effect sizes. The effect sizes of d = 0.5 for samples of PAsup, LFmed, and LFlat indicate an intermediate effect and effect sizes of d = −0.1 for samples of PAinf indicate a low effect when comparing the samples of both groups from the facet joints (Figure 4B) (Table 3).
Elastin
Figure 4C shows elastin expression by sample location and study groups. Furthermore, the corresponding effect size measurements of the mean differences of both groups are shown. Overall, elastin was more detectable than collagen type II and collagen type III in both groups. Samples of the FJC showed lower expression of elastin in the PG compared with the BDG, but without significance. However, effect sizes of d > 0.8 (d PAsup: −0.9, d PAinf: −0.9) indicate strong effects when comparing the samples of both groups from the facet joints. Samples from the LF showed only an intermediate effect size (d LFmed: −0.5; d LFlat: −0.7) (Table 3).
Collagen type I/elastin ratio
In order to specify the relationship between collagen type I and elastin in the samples of both study groups and to simplify their comparison, corresponding quotients were formed. Consideration of the quotients of both study groups showed that they differed by a power of 10. An increased expression of collagen type I compared to elastin in the PG could be shown. In contrast, elastin predominated in the samples of the BDG group compared to collagen type I. The detailed values of the collagen type I/elastin quotients in both groups are as follows: PG: PAsup 2.78; PAinf 2.61; LFmed 2.23; LFlat 1.83; BDG: PAsup 0.15; PAinf 0.2; LFmed 0.2; LFlat 0.27 (Figure 4D).
Correlation of degree of disc degeneration/facet joint degeneration to collagen type I, II, III, and elastin
The degree of disc degeneration according to Pfirrmann as well as the degree of facet joint arthrosis according to Fujiwara were tested for relevant correlations in the PG against the measured protein levels of all 4 target proteins. For this calculation, the rank correlation coefficient according to Spearman was used. The values from the tissue probes of the FJC (PAsup + PAinf) and the LF (LFmed+LFlat) were considered as a unity. Results are shown in Table 4.
Table 4.
Spearman rank correlation coefficient according to degree of disc degeneration/facet joint degeneration and expression of collagen type I, III, and elastin
| Facet joint capsules (PAsup + PAinf) | Ligament flavum (LFmed + LFlat) | |||||
|---|---|---|---|---|---|---|
| Collagen I | Collagen III | Elastin | Collagen I | Collagen III | Elastin | |
| Degree of disc degeneration (Pfirrmann) |
rs = 0.37 P = 0.33 |
rs = 0.5 P = 0.14 |
rs = 0.03 P = 0.94 |
rs = 0.46 P = 0.22 |
rs = 0.56 P = 0.09 |
rs = −0.55 P = 0.1 |
| Degree of facet joint degeneration (Fujiwara) |
rs = −0.46 P = 0.22 |
rs = 0.63 P = 0.051 |
rs = 0.31 P = 0.39 |
rs = −0.55 P = 0.13 |
rs = 0.11 P = 0.77 |
rs = −0.29 P = 0.43 |
The degree of disc degeneration showed a fair correlation to collagen type I (r = 0.37) and a moderate correlation of collagen type III (r = 0.5). The correlation of elastin and disc degeneration is poor (r = 0.03). The LF samples showed fair correlations of both collagen types I (r = 0.46) and III (r = 0.56) compared to the severity of disc degeneration. Elastin expression in LF samples was also fair (r = −0.56) correlated with disc degeneration. None of these results reached the significance level.
The degree of facet joint arthrosis showed a fair correlation to the expression of collagen type I (r = 0.46), a moderate correlation to collagen type III (r = 0.63), and a fair correlation for elastin (r = 0.31) in the FJC. All results were not significant. Elastin expression was fair correlated with the degree of facet joint degeneration. None of these results reached the significance level.
Furthermore, LF samples showed moderate correlation to collagen type I (r = 0.55) and poor correlation to collagen type III (r = 0.11) compared to the severity of facet joint degeneration. The elastin expression of the LF is fair (r = 0.29) correlated to the degree of facet joint degeneration.
Discussion
The purpose of the study was to investigate if there is a difference in protein expression of collagen I, II, and III as well as elastin in the FJC and the LF of patients with degenerative diseases who underwent surgery in the L4/5 segment compared to body donors as a control group.
A segment of the spine is described as a “3-joint complex” consisting of the intervertebral disc and the facet joints. Degenerative changes in one structure of the complex lead to changes in spinal mobility, thereby affecting the other components of the segment. It is known that the process of disc degeneration leads to biomechanical changes of the facet joints and may lead to subluxation or osteoarthritis.13,14 Biomechanical studies showed greater cartilage damage in the superior pole of the superior articular process and in the inferior pole of the inferior articular process due to the forces acting during extension and flexion.15,16 For this reason, separate samples of the FJC (a superior and inferior portion) were collected and examined in our study.
The FJC can be divided into a dorsal and a ventral part. Previous studies have shown that the ventral part of the capsule is like the structure of the LF, yellow and rich in elastin fibers.14,17 Yoshida et al18 showed that pathological changes and hypertrophy of the LF were more pronounced in the parts close to the joint capsule. Therefore, the decision to examine samples from the medial and lateral portions of the LF separately in our study was made.
Type I collagen is highly expressed in tendons and ligaments, among others. It gives a tissue tensile strength, making it resistant to tensile forces.19 Significantly, more collagen I was expressed in PG than in BDG in all 4 samples of each group. We found no difference in the expression of collagen type I between the PAsup and the PAinf in both groups. This could be explained by the fact that pathological changes affect the joint capsule as a unit and result in changes in the extracellular matrix of all capsular components.
Fujiwara et al20 demonstrated that the range of motion decreased with increasing subchondral sclerosis of the facet joints. The authors hypothesized that this finding indirectly indicated increased tightness of the surrounding soft tissues, which limited the range of motion in the segment. Our finding supports this hypothesis. More collagen type I expression could indicate that more solid surrounding tissue is formed by the degenerative changes in the segment.
Different studies could show that collagen type I is higher expressed in LF hypertrophy. Collagen type I was significantly more expressed in the PG samples of the lateral LF than in the BDG. Study data are inconsistent regarding different regions of LF hypertrophy in degenerative diseases. Yoshida et al18 described pathological changes in the lateral parts of the ligaments, which were located in the direct neighborhood of the facet joints. In contrast, an MRI study by Munns et al21 measured more thickened LF medially than laterally in almost all lumbar segments.
In addition to mechanical induction of LF hypertrophy, a cellular mechanism caused by disc herniation is thought to be due to inflammatory cytokines, including IL-a, IL-6, TNF-a, PGE2, and NO, diffusing through the spinal canal into the LF and stimulating cell proliferation. This condition leads to upregulation of the expression of collagen types I, V, and XI and osteocalcin mRNA, which are known markers of fibrogenesis and osteogenesis.22 Our results showed significantly more collagen type I in the LF samples of the PG. It could be hypothesized that the degenerative changes in the entire segment L4/5 including the intervertebral disc lead to a release of cytokines, resulting in an increased expression of type I collagen in the PG in addition to direct mechanical stress.
Elastic fibers were present in all FJC and LF samples of both groups. This supports the results of previous studies indicating that there is an anatomical smooth transition from the LF to the FJC.7,14,17 However, we could not find any significant difference in elastin expression between the two subgroups.
To clarify the relationship between collagen type I and elastin in the LF of our study groups, collagen type I/elastin ratios were calculated. In the BDG, quotients of 0.2 and 0.3 were obtained for the medial and lateral portions of the LF, respectively. This ratio between collagenous and elastic fibers in nondegenerative altered LF is consistent with the results of other studies.3,23 For the LF of the PG group, collagen type I/elastin quotients of 2.4 and 3.5 could be calculated. Compared to the current literature, we could confirm a significant change of the extracellular matrix into collagen fibers in the LF of patients with degenerative diseases in the segment L4/5.
Type III collagen was detectable in all tissue samples in both groups, but without significance. Collagen type III could be detected in degenerative changed LF as well as in LF of the control groups in various studies.24,25 Nakatani et al26 demonstrated the inducibility of collagen type III mRNA by recombinant TGF-β.26 On the other site, Park et al22 showed no remarkable expression of collagen type III mRNA by inflammatory cytokines in degenerative lumbar spines. The LF of the investigated BDG may have age-related changes in collagen type III expression that could lead to the resulting data. However, it could be possible that collagen type III plays a minor role in pathological changes of the LF and FJC.
Spearmann’s rank correlation showed no significant correlations between the degrees of disc degeneration and facet joint osteoarthritis on MRI images from PG. One explanation would be that due to the different subdivisions within the respective classification (type III to V for Pfirrmann classification; type II to IV for Furiwara classification) and the small study group, no effect is detectable.
We see the protein relations measured here as an expression of function and of malfunction. Small probes were necessary for our measurements. Research in this field may allow to measure protein changes in smaller probes, for example, by collecting tissue probes intraoperatively by decompression, for further diagnosis, as for example, Takeda’s group recently assumed.5
Nevertheless, the current study has some limitations. First, we investigated a small group of operated patients with degenerative lumbar spine disease. The BDG as control group was also limited. In addition, there was a significant age difference between the PG (61 ± 14.9 years) and the BDG (87 ± 8.6 years). The high average age in the body donor group is due to the fact that life expectancy in Germany is very high. There are very few body donors who die of natural causes and donate their bodies to science. For this reason, scientists have to accept the restriction of the high age in this control group. Due to the age difference in both groups, it cannot be excluded whether the results are a cause of the normal aging process. Because samples from patients were compared to samples from body donors, any postmortem tissue alterations could also lead to a change in the protein composition of the tissue in the body donors, which would not be the case in the samples from patients taken during operation.
Conclusively, the pathophysiological genesis of degenerative changes in the lumbar spine is currently not sufficiently clarified. A change in the proteins of the extracellular matrix of the FJC and the LF with an increase in collagen type I and a decrease in elastin seems to play a central role. The results of this study provide further evidence that degenerative changes occur also in the dorsal structures of the lower lumbar spine. This point is important in the adequate therapy decision before planned surgical therapy because different surgical methods address different clinical aspects and should be considered.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of University Leipzig (approval No: AZ 443-15-21122015).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Author Contributions: Concept – H.S., C.E.; Design – F.S., A.V., H.S., C.H.; Supervision – C.H.; Data Collection and/or Processing – F.S.; Analysis and/or Interpretation – F.S., H.S., A.V.; Literature Review – A.V., F.S.; Writing – A.V., F.S.; Critical Review – H.S., C.H.
Acknowledgment: The authors wish to acknowledge the support of the non-profit German Arthritis Society (Deutsche Arthrose-Hilfe e.V.) and its president Helmut H. Huberti, MD, by grant P365.
Declaration of interests: The authors have no conflicts of interest to declare.
Funding: The study was funded by the “Deutsche Arthrose-Hilfe e.V.”
References
- 1. Deer T, Sayed D, Michels J, Josephson Y, Li S, Calodney AK. A review of lumbar spinal stenosis with intermittent neurogenic claudication: disease and diagnosis. Pain Med. 2019;20(Suppl 2):S32 S44. 10.1093/pm/pnz161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim YU, Park JY, Kim DH.et al. The role of the ligamentum flavum area as a morphological parameter of lumbar central spinal stenosis. Pain Phys. 2017;20(3):E419 E424. [PubMed] [Google Scholar]
- 3. Kosaka H, Sairyo K, Biyani A.et al. Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine. 2007;32(25):2805 2811. 10.1097/BRS.0b013e31815b650f) [DOI] [PubMed] [Google Scholar]
- 4. Schräder PK, Grob D, Rahn BA, Cordey J, Dvorak J. Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur Spine J. 1999;8(4):323 328. 10.1007/s005860050181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeda H, Nagai S, Ikeda D, Kaneko S, Tsuji T, Fujita N. Collagen profiling of ligamentum flavum in patients with lumbar spinal canal stenosis. J Orthop Sci. 2021;26(4):560 565. 10.1016/j.jos.2020.06.006) [DOI] [PubMed] [Google Scholar]
- 6. Park JB, Lee JK, Park SJ, Riew KD. Hypertrophy of ligamentum flavum in lumbar spinal stenosis associated with increased proteinase inhibitor concentration. J Bone Joint Surg Am. 2005;87(12):2750 2757. 10.2106/JBJS.E.00251) [DOI] [PubMed] [Google Scholar]
- 7. Yamashita T, Minaki Y, Ozaktay AC, Cavanaugh JM, King AI. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine. 1996;21(5):538 543. 10.1097/00007632-199603010-00002) [DOI] [PubMed] [Google Scholar]
- 8. Claeson AA, Barocas VH. Planar biaxial extension of the lumbar facet capsular ligament reveals significant in-plane shear forces. J Mech Behav Biomed Mater. 2017;65:127 136. 10.1016/j.jmbbm.2016.08.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ban E, Zhang S, Zarei V, Barocas VH, Winkelstein BA, Picu CR. Collagen organization in facet capsular ligaments varies with spinal region and with ligament deformation. J Biomech Eng. 2017;139(7). 10.1115/1.4036019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zarei V, Liu CJ, Claeson AA, Akkin T, Barocas VH. Image-based multiscale mechanical modeling shows the importance of structural heterogeneity in the human lumbar facet capsular ligament. Biomech Model Mechanobiol. 2017;16(4):1425 1438. 10.1007/s10237-017-0896-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boszczyk BM, Boszczyk AA, Korge A.et al. Immunohistochemical analysis of the extracellular matrix in the posterior capsule of the zygapophysial joints in patients with degenerative L4-5 motion segment instability. J Neurosurg. 2003;99(1):27 33. 10.3171/spi.2003.99.1.0027) [DOI] [PubMed] [Google Scholar]
- 12. Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44(12):614 619. [PubMed] [Google Scholar]
- 13. Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9(4):216 224. 10.1038/nrrheum.2012.199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14(3):491 504. 10.1016/S0030-5898(20)31329-8) [DOI] [PubMed] [Google Scholar]
- 15. Tischer T, Aktas T, Milz S, Putz RV. Detailed pathological changes of human lumbar facet joints L1-L5 in elderly individuals. Eur Spine J. 2006;15(3):308 315. 10.1007/s00586-005-0958-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9(6):557 565. 10.1097/00007632-198409000-00005) [DOI] [PubMed] [Google Scholar]
- 17. Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106(3):591 614. 10.1097/00000542-200703000-00024) [DOI] [PubMed] [Google Scholar]
- 18. Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Pathogenesis and morphologic and immunohistochemical observation. Spine. 1992;17(11):1353 1360. 10.1097/00007632-199211000-00015) [DOI] [PubMed] [Google Scholar]
- 19. Aslan H, Kimelman-Bleich N, Pelled G, Gazit D. Molecular targets for tendon neoformation. J Clin Invest. 2008;118(2):439 444. 10.1172/JCI33944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujiwara A, Lim TH, An HS.et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25(23):3036 3044. 10.1097/00007632-200012010-00011) [DOI] [PubMed] [Google Scholar]
- 21. Munns JJ, Lee JY, Espinoza Orías AA.et al. Ligamentum flavum hypertrophy in asymptomatic and chronic low back pain subjects. PLOS ONE. 2015;10(5):e0128321. 10.1371/journal.pone.0128321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JO, Lee BH, Kang YM.et al. Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J Spinal Disord Tech. 2013;26(1):E6 E12. 10.1097/BSD.0b013e3182698501) [DOI] [PubMed] [Google Scholar]
- 23. Nachemson AL, Evans JH. Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J Biomech. 1968;1(3):211 220. 10.1016/0021-9290(68)90006-7) [DOI] [PubMed] [Google Scholar]
- 24. Kamita M, Mori T, Sakai Y.et al. Proteomic analysis of ligamentum flavum from patients with lumbar spinal stenosis. Proteomics. 2015;15(9):1622 1630. 10.1002/pmic.201400442) [DOI] [PubMed] [Google Scholar]
- 25. Specchia N, Pagnotta A, Gigante A, Logroscino G, Toesca A. Characterization of cultured human ligamentum flavum cells in lumbar spine stenosis. J Orthop Res. 2001;19(2):294 300. 10.1016/S0736-0266(00)00026-7) [DOI] [PubMed] [Google Scholar]
- 26. Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002;20(6):1380 1386. 10.1016/S0736-0266(02)00046-3) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a