Purpose of review
The purpose of this review is to describe acute kidney injury (AKI) phenotypes in children.
Recent findings
AKI is a heterogenous disease that imposes significant morbidity and mortality on critically ill and noncritically ill patients across the age spectrum. As our understanding of AKI and its association with outcomes has improved, it is becoming increasingly apparent that there are distinct AKI subphenotypes that vary by cause or associated conditions. We have also learned that severity, duration, and repeated episodes of AKI impact outcomes, and that integration of novel urinary biomarkers of tubular injury can also reveal unique subphenotypes of AKI that may not be otherwise readily apparent.
Summary
Studies that further delineate these unique AKI subphenotypes are needed to better understand the impact of AKI in children. Further delineation of these phenotypes has both prognostic and therapeutic implications.
Keywords: acute kidney injury, hospital-acquired acute kidney injury, pediatrics, precision biomarkers
INTRODUCTION
Acute kidney injury (AKI) is a heterogenous disease that imposes significant morbidity and mortality on critically ill and noncritically ill patients across the age spectrum. Epidemiologic studies among neonates and children report varying risk factors for AKI development and associated outcomes [1,2▪,3]. In each of the three major epidemiology studies, AKI was associated with increased mortality, and in some settings, increased hospital resource utilization characterized by longer duration of ventilation and longer time in the ICU and hospital.
As our understanding of AKI and its association with outcomes has improved, it is becoming increasingly apparent that there are distinct AKI subphenotypes that vary by cause or associated conditions. We have also learned that severity, duration, and repeated episodes of AKI impact outcomes, and that integration of novel urinary biomarkers of tubular injury can also reveal unique subphenotypes of AKI that may not be otherwise readily apparent [4]. Pediatric AKI incidence, severity, and outcomes are further complicated by variables such as patient age, nephron endowment, and kidney development.
The purpose of this review is to summarize the clinical subphenotypes of pediatric hospital-acquired AKI. Importantly, the role of prognostic and predictive enrichment in defining AKI phenotypes will be discussed.
Box 1.
no caption available
HOSPITAL-ACQUIRED ACUTE KIDNEY INJURY
Nephrotoxic medication-associated acute kidney injury
Nephrotoxic medication-associated AKI (NTMx-AKI) is common in hospitalized children. Approximately 30% of children exposed to a nephrotoxic medication while hospitalized will develop AKI [5]. The risk is greater for children who receive at least three concomitant nephrotoxins or prolonged intravenous aminoglycoside antibiotics [5]. Particular patient groups as described below frequently develop AKI where nephrotoxin exposure is a primary contributing factor [6–9].
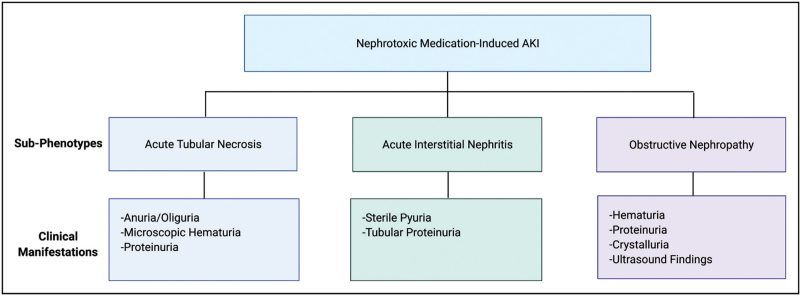
The NTMx-AKI subphenotype includes several mechanisms of injury calling for further characterization (Fig. 1). Nephrotoxicity from medications most commonly presents as three subtypes, including acute tubular necrosis (ATN), acute interstitial nephritis (AIN), and/or obstructive nephropathy [10,11]. Drugs such as aminoglycosides, vancomycin, and amphotericin B are frequently implicated in causing a direct nephrotoxic effect leading to ATN [10]. Studies in children show a relatively low rate of AKI associated with vancomycin use alone, but an increased risk of AKI in patients receiving vancomycin concomitantly with other medications [5,12]. In a cohort of 5686 pediatric critically ill patients, it was piperacillin/tazobactam and not vancomycin that was independently associated with increased AKI risk [12]. Alternatively, exposure to a medication can result in an idiosyncratic effect as occurs with AIN [11]. Antiviral medications are commonly implicated in obstructive nephropathy, which can occur when medications crystallize in the urinary system [13,14]. The clinical manifestations of the different subtypes of NTMx-AKI phenotype can differ (Fig. 1) [15,16].
FIGURE 1.
Phenotypes of nephrotoxic medication-associated acute kidney injury.
Early recognition of patients at risk for NTMx-AKI is paramount. Program-directed nephrotoxic medication surveillance has been shown to be impactful in decreasing the burden of AKI in children. An example of this is seen with the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) collaborative through which an alert is delivered to a pharmacist who notifies a care team of nephrotoxin exposure [17]. The NINJA collaborative has demonstrated the impact of focusing attention on a single AKI subphenotype, with a 37% reduction in AKI rates per exposure and 24% reduction in AKI prevalence rates [17].
Cardiac surgery-associated acute kidney injury
An extremely large proportion of children undergoing congenital heart surgery experience AKI in the postoperative period. There appears to be a substantial variation in the rate of AKI that is affected by patient age, the degree of heterogeneity in the population studied, center differences in intraoperative and postoperative management strategies, timing of diagnosis, and which AKI definition is applied. This has made direct comparisons of multiple studies particularly challenging. Of the 20 studies included in a recent meta-analysis describing strategies to prevent AKI after cardiac surgery, the timing, severity, and duration of AKI were not similar between more than two studies [18]. Adjudication of AKI was also dissimilar across studies [18]. The heterogeneity in AKI rates was reported in a recent report from the multicenter Neonatal and Pediatric Heart and Renal Outcomes Network, including 2240 neonates undergoing cardiac surgery with or without cardiopulmonary bypass [19]. AKI rates across centers ranged from 27 to 86%, of which 0–31% were quantified as severe (KDIGO ≥2). The vast majority of AKI was diagnosed by urine output and occurred early in the postoperative course. In this study, only stage 3 AKI was associated with mortality. Another example of discrepant AKI rates across centers was reported by Blinder et al.[20], in a secondary analysis of the Safe Pediatric Euglycemia after Cardiac Surgery trial. The difference in AKI rates between the two centers was 51%. Although there was an overall association of longer duration of AKI with longer time spent on the ventilator and time in the ICU, when the variables were compared between centers, the median duration of ventilation and length of stay at the center with the highest AKI rate was significantly shorter ventilation duration and length of stay. Both studies have reinforced the notion that not all creatinine elevation, or decrements in urine output are because of tubular dysfunction. Indeed, creatinine elevation may be affected by significant hemoconcentration resulting from intra-operative ultrafiltration.
These studies have prompted investigators to evaluate the contribution of AKI duration for delineation of subphenotypes and the associations with outcomes as recommended by the 16th Acute Disease Quality Initiative (ADQI) [4]. Gist et al.[21] compared the association of transient (≤48 h) and persistent (>48 h) in a two-center study of children with hypoplastic left heart syndrome. Mortality was four times higher (41 vs. 12%) in those with persistent AKI, although this association was not significant on multivariable analysis. However, severe persistent AKI was associated with a 59% increase in the expected duration of ventilation. Lobasso and colleagues classified a heterogenous cohort of 3620 children in groups by recovery. The overall AKI rate was 19%, only 3.4% were characterized as persistent (3–7 days) or acute kidney disease (>7 days). In this study, there was a graded increase in the odds of mortality with transient, persistent, and acute kidney disease (AKD) phenotypes as compared with no AKI [22▪▪].
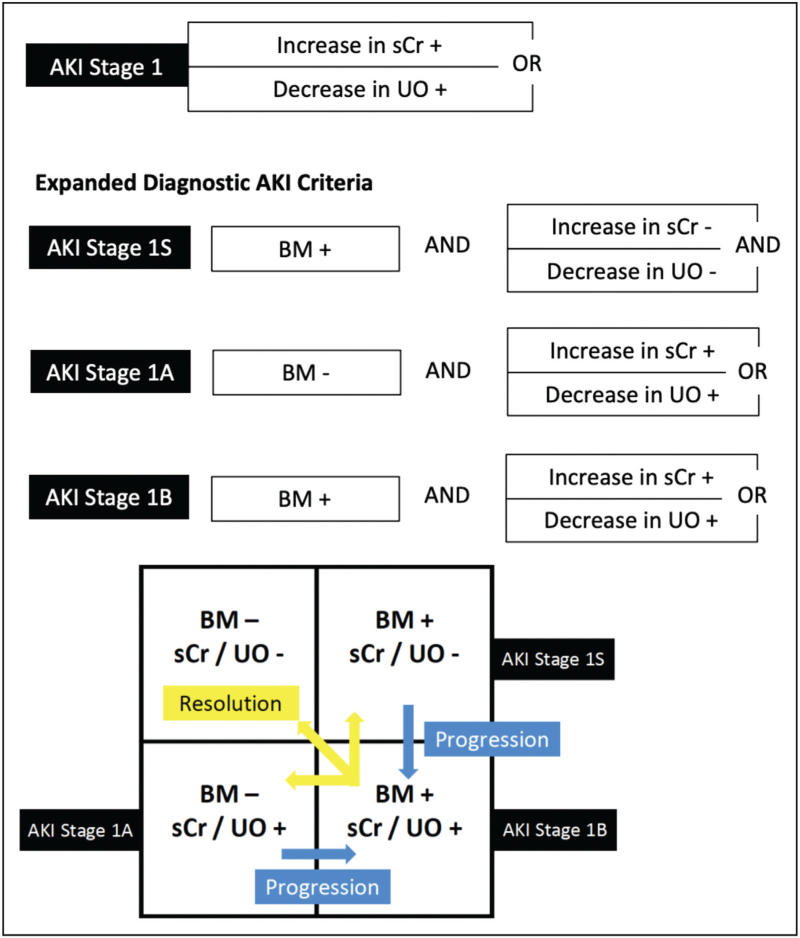
There are hundreds of studies describing novel urinary AKI biomarkers for early detection of AKI after cardiac surgery that are beyond the scope of this review. Unfortunately, few have made it to the clinical space, and thus integration into clinical care has not occurred. The 23rd ADQI consensus conference recommended integration of biomarkers into the AKI definition to delineate sub-AKI phenotypes in which patients can either be creatinine/urine output positive or negative with or without biomarker elevation (Fig. 2) [23]. Among a cohort of critically ill children without cardiac disease, patients with both creatinine and biomarker elevation had the highest odds for predicting day 3 severe AKI compared with only elevation of one of the parameters. Furthermore, the need for kidney replacement therapy (KRT) and mortality were highest among patients with both creatinine and biomarker positivity [24]. There are no studies evaluating this in children following cardiac disease. Demonstrating differences in AKI prediction and associations with outcomes by delineating AKI subphenotypes by creatinine/urine output and biomarker elevation following congenital heart surgery has the potential to enhance prognostic and predictive enrichment, and even stratify patients into specific care pathways.
FIGURE 2.
Acute kidney injury phenotypes. Patients with a biomarker of injury positivity without elevation/decline in serum creatinine and not reaching urine output criteria should be classified as 1S. Reassessment should be performed according to patient clinical context and temporal trends. Patients reaching SCr/UO criteria, and no elevation on biomarker are defined as 1A, and those reaching SCr/UO criteria with elevated biomarker are reclassified as 1B. Biomarker positivity should be based on its mechanism and defined threshold. BM, biomarker; sCr, serum creatinine; UO, urine output. Reproduced with permission from Acute Disease Quality Initiative 23 (https://www.ADQI.org).
Sepsis-associated acute kidney injury
Sepsis-associated acute kidney injury (S-AKI) is a unique subphenotype of AKI with heterogeneous underlying pathobiology that is in large part driven by the host-dysregulated immune response to infection [25,26]. Although historically described as a consequence of renal hypoperfusion in the setting of shock, our current understanding leverages what we have learned about sepsis heterogeneity to recognize S-AKI as a complex, multifactorial disorder of altered macrocirculatory and microcirculatory blood flow, metabolic derangements, and disordered inflammation that is quite variable at the individual patient level [25–27]. Recognizing this variability, several groups have begun to identify unique subphenotypes of S-AKI by incorporating demographic, clinical, and biomarker data and using cluster analysis and/or machine-learning methodologies [28–30]. These unique subphenotypes have been shown to be associated with differences in inflammatory patterns, markers of endothelial dysfunction, response to therapy, and outcomes [28–30]. In children specifically, one group recently identified differences in outcomes for patients subgrouped by AKI severity and duration, with those with severe (≥KDIGO stage 2) and/or persistent (present for ≥48 h) AKI suffering higher rates of mortality and fewer ICU-free days compared with those with mild and/or transient AKI [31▪▪]. Highlighting the importance of the septic inflammatory response in the development of S-AKI, another group recently demonstrated the association between validated biomarkers of the pediatric septic inflammatory response and the development of S-AKI and incidence of renal recovery [32]. Although none of these subphenotyping strategies have been widely applied to clinical practice at the bedside, this preliminary work has made it clear that S-AKI is a heterogeneous disorder that requires improved diagnostic precision in order to identify effective therapies and improve outcomes.
Onconephrology
There has been significant advancement in the diagnosis and treatment of children with oncologic diseases. The survival rates for childhood cancers have substantially increased in recent years with over 80% of children and adolescents diagnosed with cancer surviving at least 5 years between 2008 and 2014 [33]. The reported incidence of AKI in children with cancer is 11–84% [34,35]. In a prospective study of 1047 children admitted to the ICU, the most common admission diagnoses in AKI cases were hemolytic uremic syndrome and oncologic diseases [6].
The direct infiltration of the urinary system by cancer cells or exposures encountered during cancer therapy are well known risk factors for AKI [36]. Notable exposures that increase the risk for AKI include a diagnosis of tumor lysis syndrome, use of contrast for computed tomography scans, chemotherapeutic agents and hematopoietic stem cell transplantation [36–38]. In a multicenter study of children hospitalized with cancer, the administration of purine analogs carried the highest rate of AKI when compared with other types of chemotherapy [39].
With the advent of newer therapies such as CD19-targeted chimeric antigen receptor T-cell therapy and vascular endothelial growth factor-targeted therapy, there is a need for increased attention to the study and prevention of AKI in children with cancer. Embryonal tumors of the hemopoietic system and the central nervous system are more common in children, whereas tumors occurring in solid organs are more common in adults [38]. Given that the types of cancer diagnoses and treatments differ substantially in children when compared with adults, the future study of AKI prevention and treatment requires a focus on the unique subphenotype of AKI in pediatric oncology patients.
Neonatal acute kidney injury
AKI is common in critically ill neonates and adversely impacts outcomes. Similar to other age groups, AKI in neonates is a heterogeneous syndrome consisting of distinct phenotypes based on unique neonatal factors like gestational age and postnatal age (early vs. late AKI) in addition to cause and underlying diseases [3,40–42]. The current diagnosis and staging of AKI severity in neonates uses the neonatal modified KDIGO definition, which is based on the rise in creatinine from a previous trough or decrements in urine output [43]. It is possible that future definitions of neonatal AKI may incorporate gestational age, varying creatinine thresholds, and other metrics like fluid balance and urinary biomarkers to enhance the definition, and better delineate these phenotypes [44,45].
AKI in extremely low-gestational-age neonates is often associated with nephrotoxin medication exposure. In term or near-term neonates, AKI is often multifactorial and may be related to other associated conditions like hypoxic–ischemic encephalopathy, congenital cardiac disease and/or surgery, and multiorgan dysfunction [3]. Early-onset neonatal AKI, defined as AKI diagnosed on postnatal days 2–7 is associated with resuscitation with epinephrine, inborn errors of metabolism, or need for surgery at admission [40]. On the other hand, late AKI (occurring >7 days after birth) is associated with oligohydramnios and polyhydramnios, presence of congenital mild–moderate renal anomalies, diagnoses of congenital heart disease, necrotizing enterocolitis, and exposure to nephrotoxic medications [41]. Recognition of different phenotypes can improve AKI screening and lead to work to mitigate the consequences of AKI.
PRECISION BIOMARKERS AS A WAY TO ELUCIDATE PATHOPHYSIOLOGY, TREATMENT, AND PROGNOSIS
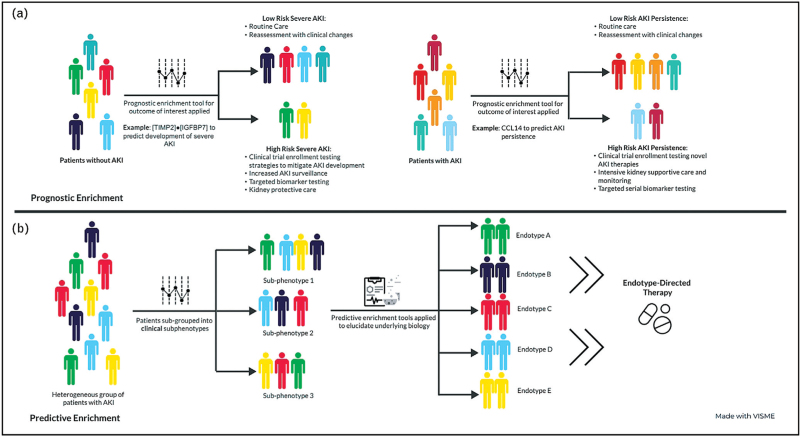
As outlined above, AKI is a heterogeneous disorder with multiple inciting etiologies and underlying pathophysiologies, whose development is informed by unique patient-level susceptibilities. Because of this heterogeneity, a ‘one size fits all’ approach to its diagnosis and management is unlikely to be successful, as has been demonstrated by a number of failed clinical trials examining therapies and interventions for AKI [46–51]. As such, a precision medicine approach that leverages biomarkers (and other clinically available data) to identify strategies for both prognostic (i.e. identifying patients at high risk for an outcome of interest) and predictive (i.e. identifying patients with shared underlying biology more likely to respond to a specific therapy) enrichment is required to improve the care of children with AKI [27]. Furthermore, as biomarkers associated with different phenotypes of AKI are identified, it is important to consider their potential role in disease pathophysiology, as this could inform future development of novel therapeutics. A framework for a precision medicine approach to the study, diagnosis and management of pediatric AKI is outlined below and in Fig. 3.
FIGURE 3.
Prognostic and predictive enrichment in acute kidney injury.
Prognostic enrichment in acute kidney injury: what to predict and why?
Before prognostic enrichment strategies can be identified, one must first answer two questions: what is a clinical outcome of interest worth predicting, and how will predicting that outcome be useful? Table 1 outlines proposed relevant outcomes of interest for prognostic enrichment in AKI, potential use cases for such tools, and some existing examples, whenever applicable. Notably, there has been an appropriate focus on identifying tools to predict who will develop severe and/or persistent AKI, as there is a growing body of evidence suggesting an association between these outcomes and morbidity and mortality in both adults and children [1,4,21,31▪▪]. Importantly, utilization of prognostic enrichment tools designed to predict these outcomes may facilitate enrichment of future clinical trials aimed at preventing AKI (as has been done successfully in some adult studies) [52,53], and testing novel therapeutics, reducing the number of patients needed to enroll, and increasing the likelihood of seeing a benefit if one exists. The former is of particular importance in pediatrics, given the relatively small patient population compared with adults. Finally, additional work is needed to identify and validate prognostic enrichment tools to better predict who will need KRT, and which patients are at highest risk of developing chronic kidney disease (CKD) following an episode of AKI (Table 1). Having the ability to reliably identify these populations could inform care at the bedside (i.e. identify those who need nephrology follow-up after discharge), and similarly enrich future clinical trials in these patient populations.
Table 1.
Proposed outcomes of interest for prognostic enrichment, their potential utility, and some existing examples in patients with and without AKI
| Outcomes of interest | Proposed use | Examples | |
| Patient without AKI | Development of any AKI | Increased surveillance for AKI Consideration of alternatives to nephrotoxic medications or procedures Closer monitoring of drug levels (i.e. vancomycin), when applicable Patient/family education Targeted novel biomarker testing | Various machine learning algorithms, including EHR-embedded [51,54,55] |
| Development of severe and/or persistent AKI | In addition to above: Clinical trial enrichment for studies examining standardized AKI prevention strategies | The Renal Angina Indexa[56,57,59▪] [TIMP2]·[IGFBP7] [65] | |
| Patient with AKI | Development of severe and/or persistent AKI | Clinical trial enrichment for studies examining novel therapies for AKI Intensive kidney supportive care (i.e. strict urine output monitoring, frequent laboratory monitoring) Patient/family counseling Targeted serial novel biomarker testing | The Renal Angina Indexa[56,57,58,59▪,60,61,62] CCL14 [64,63▪] Furosemide stress testa[60–62] |
| Need for KRT | Clinical trial enrichment for studies examining timing of KRT initiation Informed clinical decision-making Patient/family counselling | Furosemide stress testa[60–62] | |
| Development of CKD | Clinical trial enrichment for studies examining therapies to prevent AKI/AKD to CKD transition Identify patients appropriate for outpatient nephrology follow-up Patient/family counselling | No validated tools available | |
[TIMP2]·[IGFBP7], the product of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7; AKD, acute kidney disease; AKI, acute kidney injury; CCL14, C-C motif chemokine ligand 14; CKD, chronic kidney disease; KRT, kidney replacement therapy.
Tools that are well studied in children.
Predictive enrichment in acute kidney injury: tying clinical phenotypes to biological endotypes
As increasing numbers of clinically relevant AKI phenotypes are identified, it is important to characterize the biological underpinnings (i.e. endotypes) of these unique subsets of patients in order to identify novel treatment strategies. Currently, the most common clinically available AKI biomarkers ([TIMP2]·[IGFBP7], CCL14, NGAL) are all markers of tubular stress and/or direct tubular injury – as opposed to modifiable targets in AKI pathogenesis – and therefore, have limited utility for predictive enrichment [64–66]. Thus, identification of biomarkers elucidating underlying patient biology is sorely needed to help develop novel therapeutics or identify subsets of patients who may respond to existing therapies. A recent and promising example of this concept can be found in serum renin levels. Recent post hoc analyses of the ATHOS-3 trial examining the use of the novel vasoactive medication angiotensin II in adults with vasoplegic shock demonstrated that patients with AKI had higher serum renin levels and those with AKI and elevated serum renin levels who received angiotensin II had increased rates of renal recovery compared with placebo [67–69]. Importantly, the investigators were also able to tie elevation in serum renin (an upstream molecule in the renin–angiotensin–aldosterone pathway that is easily measured in the serum) to increases in angiotensin I/angiotensin II ratios, suggesting a relative deficiency of angiotensin II in these patients and providing strong biological plausibility for their findings [67]. Similar work is needed in children with AKI to begin delivering the right therapy to the right patients to improve outcomes.
THE ROLE OF DEVELOPMENT AS A BIOLOGICAL VARIABLE IN ACUTE KIDNEY INJURY SUBPHENOTYPES
Traditionally, AKI was considered a self-resolving condition with no long-term implications. There is now better understanding of kidney recovery after AKI, and how it depends on AKI severity, cause, duration, and baseline kidney function. Various phenotypes of recovery after AKI have been identified: early sustained AKI reversibility, late sustained AKI reversibility, relapse AKI and recovery, relapse AKI without recovery and never recovered AKI [70]. Each of these correlates differently with long-term outcomes. Despite increasing awareness of the link between AKI and chronic kidney disease (CKD), numerous knowledge gaps persist. The progression from AKI to CKD likely involves maladaptive regeneration after tubulointerstitial injury, fibrosis, and glomerulosclerosis [71]. More details on these mechanisms, and strategies to halt or reverse them are still being studied. Unique to pediatrics, the timing, duration, and severity of AKI and how it interacts with the patient's nephron endowment and development, likely play a critical role in long-term outcomes. However, data supporting this hypothesis are lacking and future studies are warranted to investigate the interplay of development as a biological variable and pediatric AKI outcomes. Although we have ample evidence of the high rates of CKD and hypertension after pediatric AKI, there are no protocols or guidelines regarding follow-up of these patients. This stems from lack of clear data on which patients are most likely to develop complications, how long should children with AKI be followed post discharge, and what evaluation is needed during follow-up.
CONCLUSION
Pediatric hospital-acquired AKI subphenotypes are informed by numerous factors and are distinct from hospitalized adult patients. The risk of developing AKI as well as the chance for renal recovery and/or progression to CKD are also informed by the patient's nephron endowment and renal development at the time of AKI. This concept of development as a biological variable is unique to pediatrics; however, portends significance throughout the patient's lifespan.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017; 376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Alten JA, Cooper DS, Blinder JJ, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the Multicenter Neonatal and Pediatric Heart and Renal Outcomes Network. Crit Care Med 2021; 49:e941–e951. [DOI] [PubMed] [Google Scholar]; In this large 22-center epidemiology study, only stage 3 AKI, which was mostly defined by urine output criteria was associated with mortality. AKI was not associated with other clinically relevant outcomes.
- 3.Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 2017; 1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13:241–257. [DOI] [PubMed] [Google Scholar]
- 5.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 2011; 6:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey D, Phan V, Litalien C, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med 2007; 8:29–35. [DOI] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294:813–818. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 2011; 39:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoops C, Stone S, Evans E, et al. Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr 2019; 215:223.e6–228.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downes KJ, Hayes M, Fitzgerald JC, et al. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother 2020; 75:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Awdishu L, Davenport A, et al. Phenotype standardization for drug-induced kidney disease. Kidney Int 2015; 88:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce EL, Kane-Gill SL, Priyanka P, et al. Piperacillin/tazobactam and antibiotic-associated acute kidney injury in critically ill children. J Am Soc Nephrol 2019; 30:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischer R, Johnson M. Acyclovir nephrotoxicity: a case report highlighting the importance of prevention, detection, and treatment of acyclovir-induced nephropathy. Case Rep Med 2010; 2010:602783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly RF, Tray K, Perazella MA. Indinavir nephropathy revisited: a pattern of insidious renal failure with identifiable risk factors. Am J Kidney Dis 2001; 38:E23. [DOI] [PubMed] [Google Scholar]
- 15.John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol 2009; 62:505–515. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta 2005; 351:31–47. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein SL, Dahale D, Kirkendall ES, et al. A prospective multicenter quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 2020; 97:580–588. [DOI] [PubMed] [Google Scholar]
- 18.Van den Eynde J, Cloet N, Van Lerberghe R, et al. Strategies to prevent acute kidney injury after pediatric cardiac surgery: a network meta-analysis. Clin J Am Soc Nephrol 2021; 16:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alten JA, Cooper DS, Blinder JJ, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the Multicenter Neonatal and Pediatric Heart and Renal Outcomes Network. Crit Care Med 2021; 49:e941–e951. [DOI] [PubMed] [Google Scholar]
- 20.Blinder JJ, Asaro LA, Wypij D, et al. Acute kidney injury after pediatric cardiac surgery: a secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med 2017; 18:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gist KM, Borasino S, SooHoo M, et al. Transient and persistent acute kidney injury phenotypes following the Norwood operation: a retrospective study. Cardiol Young 2022; 32:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.LoBasso M, Schneider J, Sanchez-Pinto LN, et al. Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol 2022; 37:659–665. [DOI] [PubMed] [Google Scholar]; In this multicenter investigation of children undergoing cardiac surgery, persistence of acute kidney injury was associated with worse outcomes.
- 23.Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative Consensus Conference: a consensus statement. JAMA Netw Open 2020; 3:e2019209. [DOI] [PubMed] [Google Scholar]
- 24.Stanski N, Menon S, Goldstein SL, Basu RK. Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care 2019; 53:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol 2015; 35:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019; 96:1083–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 2020; 16:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatraju PK, Zelnick LR, Herting J, et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 2019; 199:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhary K, Vaid A, Duffy A, et al. Utilization of deep learning for subphenotype identification in sepsis-associated acute kidney injury. Clin J Am Soc Nephrol 2020; 15:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiersema R, Jukarainen S, Vaara ST, et al. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit Care 2020; 24:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪▪.Basu RK, Hackbarth R, Gillespie S, et al. Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr Res 2021; 90:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study of 757 children with sepsis, temporality and severity of AKI correlated with unique survival patterns.
- 32.Stanski NL, Stenson EK, Cvijanovich NZ, et al. PERSEVERE biomarkers predict severe acute kidney injury and renal recovery in pediatric septic shock. Am J Respir Crit Care Med 2020; 201:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 34.Kist-van Holthe JE, Goedvolk CA, Brand R, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr Nephrol 2002; 17:1032–1037. [DOI] [PubMed] [Google Scholar]
- 35.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol 2004; 19:91–95. [DOI] [PubMed] [Google Scholar]
- 36.Rosner MH, Perazella MA. Acute kidney injury in patients with cancer. N Engl J Med 2017; 376:1770–1781. [DOI] [PubMed] [Google Scholar]
- 37.Hingorani S. Renal complications of hematopoietic-cell transplantation. N Engl J Med 2016; 374:2256–2267. [DOI] [PubMed] [Google Scholar]
- 38.Park PG, Hong CR, Kang E, et al. Acute kidney injury in pediatric cancer patients. J Pediatr 2019; 208:243.e3–250.e3. [DOI] [PubMed] [Google Scholar]
- 39.Xiong M, Wang L, Su L, et al. Acute kidney injury among hospitalized children with cancer. Pediatr Nephrol 2021; 36:171–179. [DOI] [PubMed] [Google Scholar]
- 40.Charlton JR, Boohaker L, Askenazi D, et al. Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol 2019; 14:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charlton JR, Boohaker L, Askenazi D, et al. Late onset neonatal acute kidney injury: results from the AWAKEN Study. Pediatr Res 2019; 85:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Askenazi DJ, Heagerty PJ, Schmicker RH, et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr Nephrol 2020; 35:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zappitelli M, Ambalavanan N, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res 2017; 82:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Askenazi D, Abitbol C, Boohaker L, et al. Optimizing the AKI definition during first postnatal week using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort. Pediatr Res 2019; 85:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta C, Massaro AN, Ray PE. A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr Nephrol 2016; 31:1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Askenazi DJ, Heagerty PJ, Schmicker RH, et al. The impact of erythropoietin on short- and long-term kidney-related outcomes in neonates of extremely low gestational age. Results of a multicenter, double-blind, placebo-controlled randomized clinical trial. J Pediatr 2021; 232:65.e6–72.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bove T, Zangrillo A, Guarracino F, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA 2014; 312:2244–2253. [DOI] [PubMed] [Google Scholar]
- 48.Bagshaw SM, Wald R, et al. STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 2020; 383:240–251. [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Shim JK, Song JW, et al. Effect of erythropoietin on the incidence of acute kidney injury following complex valvular heart surgery: a double blind, randomized clinical trial of efficacy and safety. Crit Care 2013; 17:R254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickkers P, Mehta RL, Murray PT, et al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 2018; 320:1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson FP, Martin M, Yamamoto Y, et al. Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. BMJ 2021; 372:m4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gocze I, Jauch D, Gotz M, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the Prospective Randomized BigpAK Study. Ann Surg 2018; 267:1013–1020. [DOI] [PubMed] [Google Scholar]
- 53.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017; 43:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong J, Feng T, Thapa-Chhetry B, et al. Machine learning model for early prediction of acute kidney injury (AKI) in pediatric critical care. Crit Care 2021; 25:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandokji I, Yamamoto Y, Biswas A, et al. A time-updated, parsimonious model to predict AKI in hospitalized children. J Am Soc Nephrol 2020; 31:1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 2014; 85:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gist KM, SooHoo M, Mack E, et al. Modifying the renal angina index for predicting aki and related adverse outcomes in pediatric heart surgery. World J Pediatr Congenit Heart Surg 2022; 13:196–202. [DOI] [PubMed] [Google Scholar]
- 58.Ortiz-Soriano V, Kabir S, Claure-Del Granado R, et al. Assessment of a modified renal angina index for AKI prediction in critically ill adults. Nephrol Dial Transplant 2022; 37:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Stanski NL, Wong HR, Basu RK, et al. Recalibration of the renal angina index for pediatric septic shock. Kidney Int Rep 2021; 6:1858–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recalibration of the renal angina index for children with septic shock using platelet count and a higher cutoff restored sensitivity and improved specificity.
- 60.Borasino S, Wall KM, Crawford JH, et al. Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr Crit Care Med 2018; 19:310–317. [DOI] [PubMed] [Google Scholar]
- 61.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol 2015; 26:2023–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penk J, Gist KM, Wald EL, et al. Furosemide response predicts acute kidney injury in children after cardiac surgery. J Thorac Cardiovasc Surg 2019; 157:2444–2451. [DOI] [PubMed] [Google Scholar]
- 63▪.Bagshaw SM, Al-Khafaji A, Artigas A, et al. External validation of urinary C-C motif chemokine ligand 14 (CCL14) for prediction of persistent acute kidney injury. Crit Care 2021; 25:185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among adults, CCL14 was able to predict persistence of AKI. In addition, increasing tertiles of CCL14 were able to predict 90-day mortality and/or need for renal replacement therapy.
- 64.Hoste E, Bihorac A, Al-Khafaji A, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med 2020; 46:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singer E, Marko L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013; 207:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellomo R, Forni LG, Busse LW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock. A clinical trial. Am J Respir Crit Care Med 2020; 202:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 2017; 377:419–430. [DOI] [PubMed] [Google Scholar]
- 69.Tumlin JA, Murugan R, Deane AM, et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit Care Med 2018; 46:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 2016; 27:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]