ABSTRACT
Ureteral stents may induce complications that may disrupt the quality of life of patients. Several factors that may cause these symptoms are the design, material, diameter, length, and position of the stent. The impact of its diameter varies among current reports, thus we aimed to compare the symptoms between 6 Fr and 5 Fr or less ureteral stents. A systematic search and screening were performed in the Embase, Medline, and Scopus databases. Eligible studies included randomized controlled trials (RCTs). Cochrane risk of bias tool 2 was used to evaluate the studies. Seven RCTs were included in this review. Urinary symptoms were discussed qualitatively. From the included studies, the use of a relatively smaller stent diameter yielded an overall lower rate of Ureteral Stent Symptom Questionnaire score and urinary symptoms compared to a stent with a larger diameter. There was no significant difference in migration rate (OR: 1.55, 95% CI: 0.67–3.57, P = .31), visual analogue scale (MD: −0.42, 95% CI: −2.04 to 1.20, P = .61), analgesic use duration (MD: −0.06, 95% CI: −1.02 to 0.91, P = .91), and stone-free rate probability (OR: 1.29, 95% CI: 0.48–3.45, P = .62) between patients with 5 Fr or less and 6 Fr ureteral stents. Smaller ureteral stent size is suggested for reducing ureteral stent-related symptoms, without significant differences in the incidence of stent migration, pain, analgesic use, and stone-free rate.
Keywords: Stent diameter, Ureteral stent, urinary symptoms, USSQ score.
Main Points
Indwelling ureteral stents are routinely used in many urological procedures.
Ureteral stents may cause symptoms, which affect the quality of life of patients.
Among many other factors, stent size was reported to be associated with urinary symptoms and pain.
Patients with a smaller ureteral stent diameter have a lower incidence of urinary symptoms and overall USSQ score.
Ureteral stent size does not affect stent migration rate, pain, or stone-free rate.
Introduction
The development of endourological techniques has significantly increased the utilization of indwelling ureteral stents in urological procedures.1 One of the fundamental rationales of ureteral stents is to allow urine to escape the internal or external obstructions preventing it from draining properly.2 However, the use of ureteral stents is associated with several side effects that complicate their utilization, especially when used in a long-term period. The ureteral stent can cause localized inflammation, resulting in hematuria and pain.3,4 In addition, ureteral stents are associated with bladder irritation and urinary reflux, which affect urine flow and kidney pressure, causing patients to experience discomfort during urination. Furthermore, stent migration is also recognized as one of the major complications during the use of a ureteral stent.2 These issues may significantly impact the therapeutic outcomes, the quality of life of the patient, and healthcare-related costs.2 Therefore, being able to reduce stent-related symptoms is considered a significant advantage for patients. Several studies had analyzed various factors that were potentially associated with ureteral stent symptoms, including stent design, position, and size.5–8 Furthermore, a plethora of material composition and coatings are introduced in newer stents in an effort to reduce stent-related symptoms.1 Despite the fact that there is no perfect stent that is completely free of complications and failures, combining good material, design, and adjusting other associated factors would lead to an ideal stent placement.2 Recent studies demonstrated that the use of a smaller ureteral stent diameter is associated with the reduction of stent-related symptoms.8,9 This finding is crucial because reducing stent-related symptoms could translate to the improvement of the overall quality of life of the patients. On the other hand, various studies have shown conflicting results, and thus the evidence for prescribing different diameters of the ureteral stents has not been well established.7 Therefore, it is important to provide a systematic review and meta-analysis to gather evidence of the comparison of the different ureteral stent diameters on the stent-related symptoms.
Material and Methods
The protocol for this systematic review and meta-analysis was registered in the Prospero database (CRD42021230998). There were several changes in this review compared to the registered protocol. During this review, we only included randomized controlled trials (RCTs) evaluating postureterorenoscopy (URS) patients and excluded other study designs. The conduct of this review adhered to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline and synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline.10,11 The SWiM checklist for this article is available as a supplementary file.
Data Sources and Search Strategies
Online databases including MEDLINE, Embase, and Scopus were systematically searched for relevant RCTs based on the determined keywords on July 1, 2021. Studies reporting the comparison between ≤ 5 French (Fr) with 6 Fr ureteral stents for ureteral-related symptoms after ureterorenoscopy or endoscopic procedures. MeSH terms and keywords were utilized in the literature search, as shown in the Appendix Table. To widen the search results, we also conducted our search in the references of the obtained studies.
| Database | Keyword | Articles (n) |
|---|---|---|
| Medline | Ureteral stent OR ureter stent OR stent [All fields] OR Ureteral stent OR ureter stent OR stent [MeSH terms] AND stent diameter OR ureteral stent diameter OR ureter stent diameter OR diameter [All fields] OR Ureteral stent OR ureter stent OR stent AND stent diameter OR ureteral stent diameter OR ureter stent diameter OR diameter [MeSH terms] AND stent associated symptoms OR stent related symptoms OR ureteral stent symptoms OR urinary symptoms [All fields] OR stent associated symptoms OR stent related symptoms OR ureteral stent symptoms OR urinary symptoms [MeSH terms] | 108 |
| Scopus | Ureteral stent OR ureter stent OR stent AND stent diameter OR ureteral stent diameter OR ureter stent diameter OR diameter AND stent associated symptoms OR stent related symptoms OR ureteral stent symptoms OR urinary symptoms | 206 |
| Embase | Ureteral stent OR ureter stent OR stent [All fields] OR Ureteral stent OR ureter stent OR stent [MeSH terms] AND stent diameter OR ureteral stent diameter OR ureter stent diameter OR diameter [All fields] OR Ureteral stent OR ureter stent OR stent AND stent diameter OR ureteral stent diameter OR ureter stent diameter OR diameter [MeSH terms] AND stent associated symptoms OR stent related symptoms OR ureteral stent symptoms OR urinary symptoms [All fields] OR stent associated symptoms OR stent related symptoms OR ureteral stent symptoms OR urinary symptoms [MeSH terms] | 146 |
| Total | 460 | |
Inclusion Criteria and Literature Selection
Several inclusion criteria determined in grouping studies for synthesis include: (i) RCT study design, (ii) studies comparing stents ≤5 Fr and 6 Fr as the interventions, (iii) studies evaluating patients undergoing ureteral stent placement after ureterorenoscopy as the population, and (iv) studies evaluating unilateral procedures. Non-English articles, nonadult subjects, animal model experimental studies, unpublished articles, and abstract-only articles were excluded from this study.
Data Extraction and Quality Assessment
The extraction of data of included studies was performed by the three reviewers (AAN, YPK, FH) using a spreadsheet table. Several information were extracted, including article information, baseline characteristics, and different interventions used. Study quality was assessed by using Cochrane risk of bias (RoB) tool 2 for RCTs in the following domains: Randomization, allocation concealment, blinding (participants and outcome), incomplete outcome data, selective reporting of results, and any other bias. Funnel plot asymmetry was used to assess the publication bias of the outcomes.
Statistical Analysis and Qualitative Synthesis
Dichotomous data were displayed as odds ratio (OR) with a 95% confidence interval (CI) rate, whereas continuous data were presented as mean difference (MD). A P-value of less than .05 was considered to be statistically significant. Heterogeneity between studies was calculated using the I2 value. If the I2 value was more than 50%, the heterogeneity was considered to be statistically high and random-effect model was performed. If I2 value was less than 50%, a fixed-effect model was used. Forest plots were generated and interpreted accordingly. The outcomes measured in this review included: (i) urinary symptoms, (ii) stent migration incidence, (iii) visual analog scale (VAS), (iv) duration of analgesic use, and (v) stone-free rate (SFR). For outcomes that could not be analyzed quantitatively, a descriptive analysis was performed. The outcome for urinary symptoms was synthesized by vote counting based on the direction of effect. All analyses were performed using Review Manager (RevMan) (Version 5.4, The Cochrane Collaboration, Denmark, 2020).
Results
Search Result and Baseline Characteristics of Study
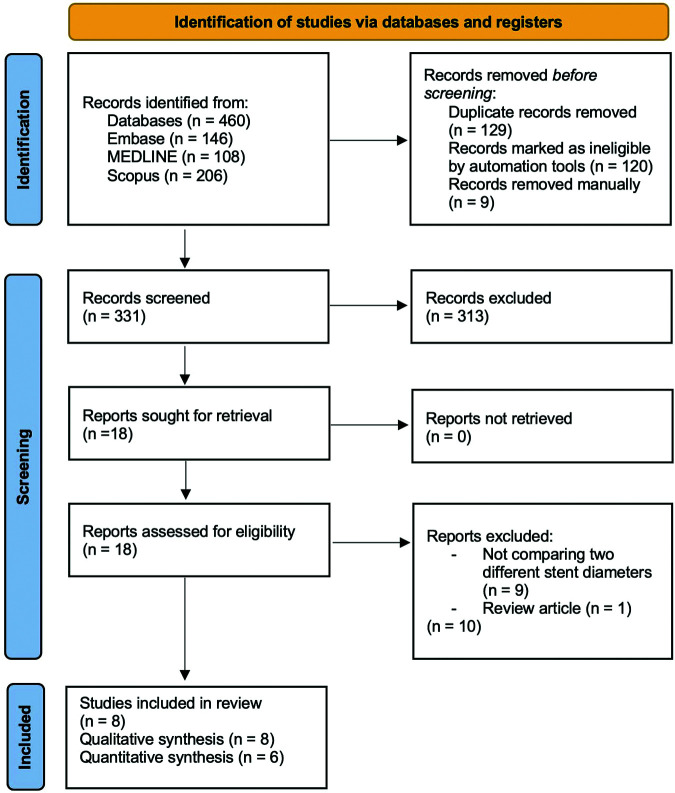
The systematic search and study screening were according to 2020 PRISMA flowchart guideline, as shown in Figure 1.10 Online database search generated potential 460 articles. After article duplication was excluded, we performed primary screening for 331 articles. Primary screening was based on abstract and title evaluation. From the primary screening, we excluded 313 articles in advance. Secondary screening evaluating complete article from each study was conducted for 18 articles. Eventually, six articles1,7–9,12,13 were eligible to be quantitatively analyzed. The baseline characteristics of the study was presented in Table 1. Quantitative data extracted in this study were presented in Table 2. Five outcomes were reported in this review. Urinary symptoms were assessed qualitatively due to the lack of sufficient data to perform a quantitative analysis using forest plots. The other four outcomes were evaluated quantitatively.
Figure 1.
Systematic search and screening based for eligible studies based on the PRISMA 2020 flowchart.
Table 1.
Baseline Characteristics of the Included Studies
| Study, Year | Design | Total Sample | Type of Intervention | Time of Evaluation | Type of Procedure | Type of Obstruction | Stent Design | Stent material (Brand) | Sample Distribution | Mean Age | Gender (Male/Female) | Mean Stone Size (mm) | Stone Free Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 5 Fr | 6 Fr | ≤ 5 Fr | 6 Fr | ≤ 5 Fr | 6 Fr | ≤ 5 Fr | 6 Fr | ≤ 5 Fr | 6 Fr | |||||||||
| Candela et al1 (1997) | RCT | 40 | 4.8 Fr with 6 Fr | 7-10 days | URS | Unilateral | Double pigtail | Polyurethane (Not reported) | 20 | 20 | 46.3 | 38.5 | 10/10 | 8/12 | - | - | - | - |
| Erturk et al8 2003 | RCT | 45 | 4.7 Fr with 6 Fr | 7 days | Rigid & Flexible URS | Unilateral | Double pigtail | Polyurethane (Bard Inlay®) | 24 | 21 | 51 | 53 | 13/11 | 6/15 | 10 | 14 | - | - |
| Damiano et al7 2005 | RCT | 34 | 4.8 Fr with 6 Fr | 7 days | URS & Cystoscopy | Unilateral | Double pigtail | Polyurethane (Not reported) | 17 | 17 | 47.3 | 50.1 | 7/10 | 6/11 | 9.4 ± 3.1 | 9.7 ± 2.7 | - | - |
| Prasanchaimontri et al13 2017 | RCT | 40 | 4.7 Fr with 6 Fr | 2 weeks | Semirigid URS | Unilateral | Double Pigtail | Polyurethane (Sof-flex®) | 20 | 20 | 57.4 ± 10.4 | 54.7 ± 11.3 | 9/11 | 19/1 | 8.8 ± 3.6 | 8.5 ± 2.7 | 17/20 (85%) | 17/20 (85%) |
| Cubuk et al14 2018 | RCT | 126 | 4.8 Fr with 6 Fr | 7 days | Semirigid & Flexible URS | Unilateral | Double J | Polyurethane (Not reported) | 63 | 63 | 41.9 ± 14.4 | 40.7 ± 12.7 | 46/17 | 38/25 | 11.6 ± 5.8 | 13.0 ± 6.8 | 58/63 (92.1%) | 56/63 (88.9%) |
| Nestler et al12 2019 | RCT | 114 | 4.7 Fr with 6 Fr | 7 days | Rigid & Flexible URS | Unilateral | Double J | Polyurethane (Uromed®) | 48 | 66 | 52 | 49 | 31/17 | 50/16 | - | - | - | - |
| Kim et al9 2019 | RCT | 106 | 5 Fr with 6 Fr | 2-3 weeks | Semirigid URS & RIRS | Unilateral | Double J | Polyurethane (Polaris Ultra®) | 55 | 51 | 60.0 ± 12.5 | 58.9 ± 12.5 | 31/24 | 29/22 | 10 ± 5.6 | 9.5 ± 4.4 | - | - |
Table 2.
Urinary Symptoms Findings From the Included Studies
| Study, Year | Total Sample | Stent Diameter | Symptoms | Instrument | Results | Conclusion |
|---|---|---|---|---|---|---|
| Candela and Bellman,1 1997 | 40 | ≤ 5 Fr | Hematuria, dysuria, urgency, incontinence, flank pain, frequency, nocturia | None | No significant difference in urinary symptoms between the groups | The authors reccomended a 6 Fr stent due to the ease of placement |
| 6 Fr | ||||||
| Erturk et al8 2003 | 45 | ≤ 5 Fr | Mean irritation score: 1.7 | None | Irritative symptoms are higher in the 6 Fr group, albeit the difference was insignificant | The authors recommend a 6 Fr stent due to the ease of placement, better drainage, and a lower stent migration incidence |
| 6 Fr | Mean irritation score: 2.2 | |||||
| Damiano et al7 2005 | 34 | ≤ 5 Fr | Dysuria (11), hematuria (11), urgency (14), frequency (14) | USSQ | No significant difference in urinary symptoms between the groups | The authors concluded that both diameters caused urinary symptoms |
| 6 Fr | Dysuria (12), hematuria (12), urgency(13), frequency (14) | |||||
| Prasanchaimontri et al13 2017 | 40 | 4.7 Fr | Increase OABSS score 20% | OABSS | No significant different in urinary symptoms (P = .185) | Irritative symptoms were fewer in smaller stents, albeit insignificant |
| 6 Fr | Increase OABSS score 45% | |||||
| Cubuk et al14 2018 | 126 | ≤ 4,8 Fr | USSQ total: 91.9 ± 27.9 (initial follow-up), USSQ total : 54.3 ± 5.2 (second follow-up) | USSQ | Significantly lower USSQ total score in patients with a 4.8 Fr ureteral stent at initial follow-up (P = .01) and second follow up (P < .001) | The use of a 4.8 Fr stent could decrease urinary symptoms. The authors recommended the use of 4.8 Fr stents. |
| 6 Fr | USSQ total: 103 ± 19.1 (initial follow-up), USSQ total: 58.7 ± 4.8 (second follow-up) | |||||
| Nestler et al12 2019 | 114 | ≤ 5 Fr | USSQ urinary symptoms: 19 | USSQ | Significantly lower urinary symptoms in patients with a 4.7 Fr compare with 6 Fr ureteral stents (P = .004). Significantly lower pain index, work performance and general health domain in patients with 4,7 Fr compare with 7 Fr ureteral stent. | Pain and discomfort increase with the increase of stent diameter. The authors recommended the use of 4.7 Fr stent to reduce urinary symptoms. |
| USSQ work performance: 5 | ||||||
| USSQ general Health: 9 | ||||||
| 6 Fr | USSQ urinary symptoms: 25 | |||||
| USSQ work performance: 7 | ||||||
| USSQ general Health: 12 | ||||||
| Kim et al9 2019 | 106 | ≤ 5 Fr | USSQ urinary symptoms: 27.0 ± 7.2 | USSQ | Milder urinary symptoms in patients with a 5 Fr stent compared to a 6 Fr stent (P = .014) | A 5 Fr stent generates fewer urinary symptoms than a 6 Fr stent. The authors recommended the use of 5 Fr stent for patients. |
| USSQ work performance: 7.8 ± 4.6 | ||||||
| USSQ general Health: 12.1 ± 4.7 | ||||||
| 6 Fr | USSQ urinary symptoms: 30.5 ± 7.4 | |||||
| USSQ work performance: 6.6 ± 4.2 | ||||||
| USSQ general health: 11.8 ± 4.4 |
Risk of Bias and Publication Bias Assessment
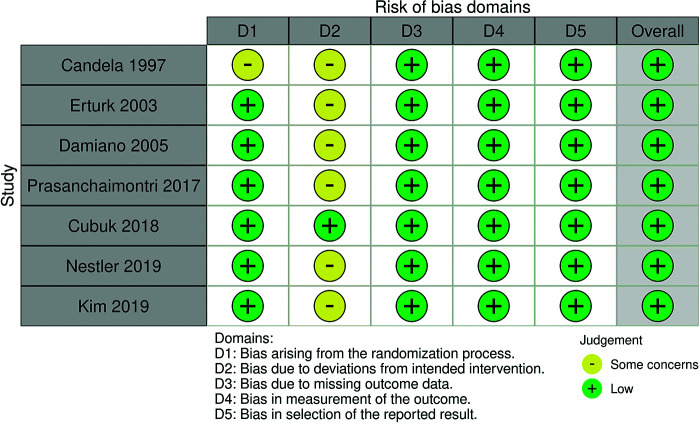
The quality assessment showed that the overall risk of bias was low, six trials did not report whether there was blinding of the researchers. According to the Cochrane Handbook for Systematic Reviews of Intervention, blinding during a trial can be difficult or impossible in some contexts. The lack of blinding on the researcher’s part in the context of this study would not have caused a major bias as the intervention is related to a surgical procedure.15 Even though Candela and Bellman1 did not specify how the randomization process in their study was performed, a randomization method was still conducted and mentioned in the manuscript. Figure 2 displayed the risk of bias assessment of each study. The inverted funnel plots’ shapes in Figure 3 are shown as cursory symmetries surrounding the vertical dashed lines, indicating no apparent publication bias.
Figure 2.
Risk of bias graph of the included trials.
Figure 3.
Funnel plot of studies reporting stent migration occurrence, VAS, duration of analgesic use, and SFR probability.
Qualitative Synthesis in Differences of Urinary Symptoms and Ureteral Stent Symptom Questionnaire (USSQ) Score Based on Stent Diameter
In this study, we found seven studies that reported urinary tract symptoms based on different stent diameters shown in Table 1. Several studies assessed urinary symptoms using the USSQ. This questionnaire was a routine instrument that was used to evaluate symptoms related to stent insertion which has been validated in many countries around the world. However, several studies did not use the USSQ to evaluate the patients, as the questionnaire was published in 2003.16 Due to the difference in reported findings, the analysis was conducted qualitatively in a descriptive review. Details regarding the urinary symptoms of patients from each included study are displayed in Table 2.
Candela and Bellman1 reported no significant difference for irritative symptoms between the patients with different stent diameters. However, the operator reported that a stent with a 4.8 Fr diameter was more difficult to be inserted compared to a larger stent in a case of the tortuous ureter and severe obstruction thus smaller stent was considered to be less rigid. Smaller stent size with hydrophilic material, which is less opaque, could lead to difficulties in visualization under fluoroscopy, especially in obese patients. Based on this reason, the study recommends 6 Fr stents. Erturk et al8 also reported more irritative symptoms and higher pain in patients receiving stent size of 6 Fr although the result was not significant (P = .37). Damiano et al7 evaluated a number of 34 patients were evaluated in this study. Patients with stents were divided into two groups mainly group using stent size of 4.8 Fr and group using stent size of 6 Fr. These two groups were also compared with 21 patients who experienced lower urinary tract symptoms (LUTS) due to nonstone cause and was assigned as control group. Symptom evaluation was performed using USSQ questionnaire modified into three domains including urinary symptoms, pain symptoms, and other symptoms. There were no significant differences found between these three groups and both patient groups. They concluded that stent diameter size did not affect patient symptoms. Research from the study reported that unpleasant sensation experienced by the patient could be reduced by providing sufficient information regarding stent-related symptoms. Prasanchaimontri et al13 use Overactive Bladder Symptom Score (OABSS) to assess the urinary symptoms of the included patients. They reported no statistical difference between the two groups. In conclusion, perioperative outcomes were similar between the groups. Irritative symptoms were fewer in patients with a smaller stent, albeit not statistically significant.
Cubuk et al14 reported that USSQ total score was found statistically significantly lower in a group of patients using stent size 4.8 Fr (91.9 ± 27.9) compared to 6 Fr (103 ± 19.1) group in first control (P = .01). In second control, the total score in two groups were decreasing but the patient with stent diameter size of 6 Fr (58.7 ± 4.8) had a higher score compared to a patient with stent diameter size of 4.8 Fr (54.3 ± 5.2) (P < .001). The domain regarding sexual function was also lower in a group of patients with stent size 4.8 Fr. The study stated that stent insertion with any size potentially lead to improvement of symptoms and USSQ score but the use of smaller stent contribute to a lower symptom compared to a larger stent. The use of 4.8 Fr stent after URS was recommended in this study. Nestler et al12 in their RCT, assessed the influence of stent size with symptom and quality of life based on USSQ instrument which several stent sizes were compared with each other mainly 4.7 Fr, 6 Fr, and 7 Fr. The group of patients using stent size of 4.7 Fr had lower score compared to a group of 6 Fr, but this significant difference was only found in the domain of urinary symptom (P = .004). The domain of pain, general health, and daily activity did not show any significant differences. However, a significant difference was found in pain, work performance, and general health index score in the comparison between a group with a stent size of 4.7 Fr and stent size of 7 Fr. In the study by Kim et al9 only urinary symptoms domain had significant differences between both groups (P = .014) from all USSQ score domains. There were no significant differences found on other evaluated domains such as pain, general health, and daily activity between both groups. The study also revealed that the use of stent size of 5 Fr had lower urinary symptoms compared to stent size of 6 Fr.
From the seven included articles, three studies recommended the use of a smaller stent diameter to reduce urinary symptoms, while four studies did not report a significant difference in urinary symptoms. However, the results from older studies had limitations, where not all USSQ domains were reported. Therefore, the results from the studies might not completely explain the influence of a smaller size stent objectively. The difference between the results reported by Candela and Bellman1 and Erturk et al8 also had to be evaluated according to the publication year of each respective study. The standard of stent placement to prevent symptoms caused by other factors was not performed, thus the results of the older articles, with a smaller sample size of 159 samples, were potentially less reliable compared to the newer studies. Other studies, which were published more recently in the past 3 years, with a larger sample size of 346 samples, recommended the smaller stent since it only led to mild lower urinary tract symptoms. Studies reporting urinary symptoms based on the USSQ reported significantly lower scores among patients with a smaller stent diameter, indicating that ureteral stent size does affect urinary symptoms.
Stent Migration Incidence Difference
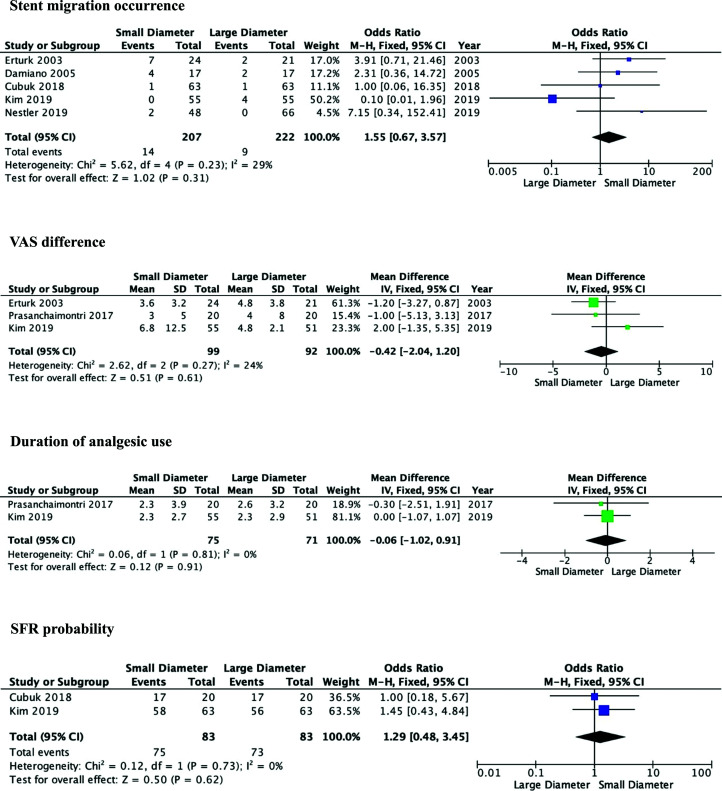
The result of analysis on stent migration in Figure 4 showed that the two groups did not reveal a significant difference (OR: 1.55, 95% CI: 0.67–3.57, P = .31). Five studies7–9,12,14 were included to analyze stone migration incidence outcome. This analysis used a fixed-effect model because of the homogeneity among the studies (I2 = 29%).
Figure 4.
Forest plot of stent migration occurrence, VAS difference, duration of analgesic use, and SFR probability between the groups.
Visual Analogue Scale Difference
Pain index score evaluation using VAS between ureteral stents ≤5 Fr and 6 Fr in size was reported by three studies8,9,13 with a total of 191 patients in Figure 4. The result revealed that there was no significant difference in VAS between the three groups (MD: −0.42, 95% CI: −2.04 to 1.20, P = .61). Fixed-effect model was used due to the homogeneity among the studies (I2 = 24%).
Analgesic Use Duration Difference
The analgesic use duration was reported by two studies in Figure 4.9,13 The result revealed that there was no significant difference in the duration of analgesic use between the two groups (MD: −0.06, 95% CI: −1.02 to 0.91, P = .91). The duration was reported in days. The analysis was performed using a fixed-effect model based on the homogeneity of the studies (I2 = 0%).
Stone-Free Rate (SFR) Difference
The SFR difference between the groups was reported by two studies in Figure 4.9,14 The difference between the probability of SFR was insignificant (OR: 1.29, 95% CI: 0.48–3.45, P = .62). using a fixed-effect model due to the homogeneity among the studies (I2 = 0%).
Discussion
Factors that may lead to ureteral stent related symptom had been evaluated since the use of silicon catheters for ureteral stents.17 They are installed to prevent kidney obstruction due to stone fragment residue, edema, hematoma, or ureteral stenosis caused by mass and urinary extravasation.18 Pain and unpleasant sensation experienced by the patient affected the quality of life of the patients. Ureteral stent led to approximately 80% patients complaining of unpleasant and painful symptoms, altering their daily activities.19 Around 32% patients also experienced sexual dysfunction and 58% complained of disturbances in work life that affects socioeconomic conditions.20 The etiology behind the presence of stent related symptoms is not fully understood. The current theory suggested that irritation occurs due to the distal stent loop contact with the bladder mucosa, ureteral smooth muscle spasm, inflammation, or urinary reflux to the kidney through the stent.21 Previous studies also believed that the symptom severity was influenced by several factors such as stent material, design, length, and diameter.21,22 To reduce the duration of physical discomfort, some studies even suggested the use of extraction strings on ureteric stents. Stents with extraction strings are easy for self-removal, thus reducing the stent dwell time and physical burden of patients. However, due to the risk of stent dislodgement, this option may not be suitable for all patients.23 The length of stent may worsen stent-related symptoms if the distal curl or end of the stent crosses the abdominal midline.24 The studies included in this review made sure that possible bias due to stent length did not occur by performing postoperative imaging examination ensuring that the distal end of the stent did not cross the midline.25
One of the factors that attracted the attention of the authors was the stent size diameter. With the increase in stone-related problems in the field of endourology, many user-friendly tools such as grading systems and tools have been published. One of which is the USSQ, created to evaluate a patient’s stent-related symptoms following procedures such as URS.26 In this review, we obtained seven relevant articles regarding the matter. Stents with a smaller diameter had smaller proximal and distal curvatures, thus the contact between the surface and bladder mucosa was minimal, leading to lower urinary tract symptom reduction.9 Moreover, a smaller stent size revealed a milder score based on the USSQ score based on the domains of work performance, pain and sexual function.12,14 Even though older studies published before the creation of the USSQ only reported insignificant difference in urinary symptoms based on ureteral stent size, studies reporting findings based on the USSQ claimed that ureteral stent size does affect urinary symptoms. A retrospective study by Taguchi et al27 also reported that the rate of urinary symptoms were significantly milder in a smaller ureteral stent based on the International Prostate Symptom Score (IPSS) and OABSS results.
Several studies reported the possibility of stent migration higher at a smaller stent size. Ureteral stent mobilization played a role in the development of pain, which is caused by the movement and irritation in ureteral lumen and bladder mucosa. Study reported by El-Nahas et al28 showed that ureteral stent moved approximately 2.5 cm during daily activity. Many trials and study designed ureteral stents, to reduce mobilization. Since 1978, routine stent use was accepted generally after the rise of ureteral stent designs, such as the double J and pigtail stents.29 Double J stent can retain its original shape, creating loops in proximal and distal tips after insertion. There was no significant difference in stent migration incident in the smaller stent size group (P = .58). Double J stent and a pigtail stent play a huge role to prevent stent migration. All five studies reported the use of these stent types. However, several studies did not clearly define stent migration in their respective studies, which hugely influence our analysis. Migration stent assessment should be performed after stent length measurement and confirming correct stent position.
The report of stent migration incident in double J stent was quite high with a 26.32% incidence rate in approximately 9 months.30 Proximal and distal loop designs contributed as a “fixation”, preventing stent migration upward or downward. Upper or proximal stent migrations are usually caused by the length of stent that was too short.31 Aside from the design and size, stent migration could also occur in obstructive ureters with severe hydroureter, inappropriate insertion position, the presence of mobile kidney and dynamic ureter, and ureteral peristalsis.
In certain circumstances, the contact between the stent and bladder mucosa may aggravate irritation that leads to pain. However, the results in this review based on three studies revealed that there was no significant difference in VAS in the use of a larger stent size (P = .61). Apart from the three analyzed studies, Damiano et al7 revealed the finding of a significantly higher pain score, based on the USSQ body pain domain, on patients with a 4.8 Fr ureteral stent (P = .031) and 6 Fr (P = .039) compared to patients without stent placement. However, the comparison of pain between the two groups with stents of both sizes were similar.7
This finding was supported from previous studies which stated that ureteral stent size did not significantly influence pain score.8 Another interesting result was reported by Nestler et al12 assessing pain using USSQ score questionnaire. They reported that although there was no significant difference found between the use of 4.7 Fr and 6 Fr stents, there was a significant difference between the 4.7 and 7 Fr stent (P = .03).12
Stent diameter defines the most outer size of stent, therefore a smaller ureteral stent will have a smaller outer diameter. Smaller stent size was estimated to be more flexible, semirigid, and able to generate smaller pressure in ureteral lumen. Moreover, smaller stent could reduce the degree of reflux from urine through the stent lumen.9
The use of ureteral stent significantly leads to pain, thus many studies have been performed to prevent or reduce the associated pain symptoms. Patients with ureteral stents generally require analgesics for approximately two to three days after insertion.9 Our results found that the duration of analgesic use in patients with smaller stent size was not significantly different compared to patients with a larger stent size (P = .91). Previous studies also reported that additional analgesic use was higher in patients with a larger stent size, although the difference was not significant (P = .223).14
Previous studies have been performed in order to reduce pain symptoms and unpleasant feelings from the use of stent. Alpha blockers may help dilate ureteral lumen especially in distal ureter, reducing ureteral spasms and peristaltic movements after stent insertion.22 Patients who received alpha blocker presented significantly reduced USSQ score in urinary symptoms and body pain.32 Anticholinergic may also help reduce urinary tract symptoms via muscarinic receptor in the bladder. Moreover, Solifenacin could significantly lower the LUTS, flank pain, abdominal pain, urethral pain and hematuria symptoms.33,34 Nonsteroidal anti-inflammatory drug could also provide significant benefits due to the presence of cyclooxygenase (COX-1) and COX-2 in ureteral urothelium, smooth muscle cell in ureteral muscularis layer thus COX-1 and COX-1 provide options as target in pharmacologically to manage pain due to stent insertion.35 Even though, statistically, the quality of pain was reported to be similar between the groups, some patients with a low tolerance for pain may benefit from an additional analgesic supplementation.
Several studies reported the impact of ureteral stent placement on SFR after lithotripsy. According to Kaygisiz et al36 intraoperative stent placement after a flexible URS procedure for kidney stone may improve SFR. On the contrary, Wang et al37 reported that stenting failed to improve SFR, and instead, it resulted in additional complications. A smaller stent diameter is expected to assist residual stone passage through the space between the stent and ureteral lumen. Chandhoke et al38 preferred a smaller stent size of 4.7 Fr compared to 7 Fr stents for patients undergoing extracorporeal shock wave lithotripsy. Stent placement is advised for patients with a high possibility of residual stones to prevent hospital readmission and unnecessary visits to the emergency room.38 Nevertheless, in this review, the SFR of both ureteral stent diameters were similar (P = .62).
In this review there are several limitations. We could not perform quantitative assessments of the USSQ score and urinary symptoms due to reporting differences in several studies. Several issues included: (i) different parameters of urinary symptoms, (ii) not all studies provided a complete total USSQ data with standard deviations, and (iii) not all studies reported data from each USSQ domain. This led us to perform a qualitative analysis only regarding the urinary symptoms. Some of the included studies did not explain the same definition regarding the time of assessment for stent migration. Several factors that may influence ureteral stent related symptom include stent design, material, length, and position. These factors may lead to bias in the findings of this review. However, all included studies used polyurethane stent material with specific designs, double J and pigtail, containing loop in the proximal and distal tips. Most studies had also ensured the stent position and measured the stent length in order to prevent the stent from crossing the bladder midline.
Conclusion
In this systematic review and meta-analysis, the authors suggest the use of a smaller ureteral stent diameter based on the lower incidence of urinary symptoms and overall USSQ score of patients. A smaller diameter also did not result in a higher migration rate. However, pain and SFR were similar between the two sizes. More multicenter studies with standardized and validated parameters are required for a further quantitative analysis on urinary symptoms.
Appendix Table: Search Keywords & Databases.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.A.N., M.A.S.; Design - A.A.N., F.H., M.A.S.; Supervision - T.D., M.A.S.; Resource - T.D., M.A.S; Materials - A.A.N, Y.P.K.; Data Collection and/or Processing - A.A.N., Y.P.K., F.H.; Analysis and/or Interpretation - A.A.N., Y.P.K., F.H., M.A.S., T.D.; Literature Search - A.A.N., Y.P.K.; Writing - A.A.N., Y.P.K., F.H., M.A.S.; Critical Reviews - A.A.N., Y.P.K., M.A.S., T.D.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1. Candela J, Bellman GC. Ureteral stents: Impact of diameter and composition on patient symptoms. J Endourol . 1997;11:(1):45–47.. 10.1089/end.1997.11.45) [DOI] [PubMed] [Google Scholar]
- 2. Mosayyebi A, Manes C, Carugo D, Somani BK. Advances in ureteral stent design and materials. Curr Urol Rep . 2018;19:(5):1–9.. 10.1007/s11934-018-0779-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lennon GM, Thornhill JA, Grainger R, McDermott TED, Butler MR. Double pigtail ureteric stent versus percutaneous nephrostomy: Effects on stone transit and ureteric motility. Eur Urol . 1997;31:24–29.. 10.1159/000474413) [DOI] [PubMed] [Google Scholar]
- 4. Stoller ML, Meng MV. Urinary Stone Disease: The Practical Guide to Medical and Surgical Management . Berlin: Springer Science & Business Media, 2007. [Google Scholar]
- 5. Rane A, Saleemi A, Cahill D, Sriprasad S, Shrotri N, Tiptaft R. Have stent-related symptoms anything to do with placement technique?. J Endourol . 2001;15:(7):741–745.. 10.1089/08927790152596352) [DOI] [PubMed] [Google Scholar]
- 6. Ai-Kandari A, Shaaban H. Effects of proximal and distal ends of double J ureteral stem position on postprocedural symptoms and quality of life: A randomized clinical trial. J Endourol . 2007;21:(7):698–702.. 10.1089/end.2007.9949) [DOI] [PubMed] [Google Scholar]
- 7. Damiano R, Autorino R, De Sio M.et al. Does the size of ureteral stent impact urinary symptoms and quality of life? A prospective randomized study. Eur Urol . 2005;48:(4):673–678.. 10.1016/j.eururo.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 8. Erturk E, Sessions A, Joseph JV. Impact of ureteral stent diameter on symptoms and tolerability. J Endourol . 2003;17:(2):59–62.. 10.1089/08927790360587342) [DOI] [PubMed] [Google Scholar]
- 9. Kim BS, Choi JY, Jung W. Does a ureteral stent with a smaller diameter reduce stent-related bladder irritation? A single-blind, randomized, controlled, multicenter study. J Endourol . 2020;34:(3):368–372.. 10.1089/end.2019.0482) [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM.et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ . 2021;372:n71. 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell M, McKenzie JE, Sowden A.et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ . 2020;368: l6890. 10.1136/bmj.l6890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nestler S, Witte B, Schilchegger L, Jones J. Size does matter: Ureteral stents with a smaller diameter show advantages regarding urinary symptoms, pain levels and general health. World J Urol . 2020;38:(4):1059–1063.. 10.1007/s00345-019-02829-0) [DOI] [PubMed] [Google Scholar]
- 13. Prasanchaimontri P, Nualyong C, Taweemonkongsap T, Chotikawanich E. Impact of ureteral stent size on Stone-Free rates in ureteroscopic lithotripsy for ureteral stones: Randomized controlled trial. J Med Assoc Thailand . 2017;100:(4):162. [Google Scholar]
- 14. Cubuk A, Yanaral F, Ozgor F.et al. Comparison of 4.8 Fr and 6 Fr ureteral stents on stent related symptoms following ureterorenoscopy: A prospective randomized controlled trial. Kaohsiung J Med Sci . 2018;34:(12):695–699.. 10.1016/j.kjms.2018.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thomas J, Chandler J.et al. Cochrane Handbook for Systematic Reviews of Interventions . New York: John Wiley & Sons, 2019. [Google Scholar]
- 16. Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX, Timoney AG. Ureteral stent symptom questionnaire: Development and validation of a multidimensional quality of life measure. J Urol . 2003;169:(3):1060–1064.. 10.1097/01.ju.0000049198.53424.1d) [DOI] [PubMed] [Google Scholar]
- 17. Donahue RP, Stamm AW, Gibbons RP.et al. Evolution of the ureteral stent: The pivotal role of the gibbons ureteral catheter. Urology . 2018;115:3–7.. 10.1016/j.urology.2018.02.007) [DOI] [PubMed] [Google Scholar]
- 18. Inn FX, Ahmed N, Hou LG, Abidin ZAZ, Yi LL, Zainuddin ZM. Intravesical stent position as a predictor of quality of life in patients with indwelling ureteral stent. Int Urol Nephrol . 2019;51:(11):1949–1953.. 10.1007/s11255-019-02262-7) [DOI] [PubMed] [Google Scholar]
- 19. Bosio A, Alessandria E, Dalmasso E.et al. How bothersome double-J ureteral stents are after semirigid and flexible ureteroscopy: A prospective single-institution observational study. World J Urol . 2019;37:(1):201–207.. 10.1007/s00345-018-2376-6) [DOI] [PubMed] [Google Scholar]
- 20. Mehra K, Manikandan R, Dorairajan LN, Kodakkattil SS, Kalra S. Effect of ureteral stent length and position of stent coil in bladder on stent-related symptoms and quality of life of patients. Cureus . 2020;12:(11):e11669. 10.7759/cureus.11669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koprowski C, Kim C, Modi PK, Elsamra SE. Ureteral stent-associated pain: A review. J Endourol . 2016;30:(7):744–753.. 10.1089/end.2016.0129) [DOI] [PubMed] [Google Scholar]
- 22. Fischer KM, Louie M, Mucksavage P. Ureteral stent discomfort and its management. Curr Urol Rep . 2018;19:(8):1–7.. 10.1007/s11934-018-0818-8) [DOI] [PubMed] [Google Scholar]
- 23. Oliver R, Wells H, Traxer O.et al. Ureteric stents on extraction strings: A systematic review of literature. Urolithiasis . 2018;46:(2):129–136.. 10.1007/s00240-016-0898-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Kandari AM, Al-Shaiji TF, Shaaban H, Ibrahim HM, Elshebiny YH, Shokeir AA. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: A randomized clinical trial. J Endourol . 2007;21:(7):698–702.. 10.1089/end.2007.9949) [DOI] [PubMed] [Google Scholar]
- 25. Ho C-H, Tai H-C, Chang H-C.et al. Predictive factors for ureteral double-J-stent-related symptoms: A prospective, multivariate analysis. J Formos Med Assoc . 2010;109:(11):848–856.. 10.1016/S0929-6646(10)60130-1) [DOI] [PubMed] [Google Scholar]
- 26. Jones P, Pietropaolo A, Chew BH, Somani BK. Atlas of scoring systems, grading tools and nomograms in Endourology: A comprehensive overview from the TOWER Endourological Society research group. J Endourol . 2021 Dec;35:(12):1863–1882.. 10.1089/end.2021.0124) [DOI] [PubMed] [Google Scholar]
- 27. Taguchi M, Yoshida K, Sugi M, Kinoshita H, Matsuda T. Effect of ureteral stent diameter on ureteral stent‐related symptoms. Low Urin Tract Sympt . 2019;11:(4):195–199.. 10.1111/luts.12259) [DOI] [PubMed] [Google Scholar]
- 28. El-Nahas A, El-Assmy A, Shoma A, Eraky I, El-Kenawy M, El-Kappany H. Self-retaining ureteral stents: Analysis of factors responsible for patients’ discomfort. J Endourol . 2006;32:(2):229. [DOI] [PubMed] [Google Scholar]
- 29. Finney RP. Experience with new double J ureteral catheter stent. J Urol . 1978;120:(6):678–681.. 10.1016/S0022-5347(17)57326-7) [DOI] [PubMed] [Google Scholar]
- 30. Ray RP, Mahapatra RS, Mondal PP, Pal DK. Long-term complications of JJ stent and its management: A 5 years review. Urol Ann . 2015;7:(1):41–45.. 10.4103/0974-7796.148599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breau RH, Norman RW. Optimal prevention and management of proximal ureteral stent migration and remigration. J Urol . 2001;166:(3):890–893.. 10.1016/S0022-5347(05)65858-2) [DOI] [PubMed] [Google Scholar]
- 32. Lamb AD, Vowler SL, Johnston R, Dunn N, Wiseman OJ. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int . 2011;108:(11):1894–1902.. 10.1111/j.1464-410X.2011.10170.x) [DOI] [PubMed] [Google Scholar]
- 33. Lee YJ, Huang KH, Yang HJ, Chang HC, Chen J, Yang TK. Solifenacin improves double-J stent-related symptoms in both genders following uncomplicated ureteroscopic lithotripsy. Urol Res . 2013;41:(3):247–252.. 10.1007/s00240-013-0554-y) [DOI] [PubMed] [Google Scholar]
- 34. Abdelhamid MH, Zayed AS, Ghoneima WE.et al. Randomized, double-blind, placebo-controlled trial to compare solifenacin versus trospium chloride in the relief of double-J stent-related symptoms. World J Urol . 2017;35:(8):1261–1268.. 10.1007/s00345-016-1988-y) [DOI] [PubMed] [Google Scholar]
- 35. Chaignat V, Danuser H, Stoffel MH, Z’Brun S, Studer UE, Mevissen M. Effects of a non-selective COX inhibitor and selective COX-2 inhibitors on contractility of human and porcine ureters in vitro and in vivo. Br J Pharmacol . 2008;154:(6):1297–1307.. 10.1038/bjp.2008.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaygisiz O, Özmerdiven G, Günseren KÖ, Kiliçarslan H. Stent placement after flexible ureterorenoscopy for renal stones can improve stone-free rate on final follow-up: A retrospective single center study. Arch Clin Exp Med . 2018;3:(2):67–70.. [Google Scholar]
- 37. Wang H, Man L, Li G, Huang G, Liu N, Wang J. Meta-analysis of stenting versus non-stenting for the treatment of ureteral stones. PLoS One . 2017;12:e0167670. 10.1371/journal.pone.0167670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chandhoke PS, Barqawi AZ, Wernecke C, Chee-Awai RA. A randomized outcomes trial of ureteral stents for extracorporeal shock wave lithotripsy of solitary kidney or proximal ureteral stones. J Urol . 2002;167:(5):1981–1983.. 10.1016/S0022-5347(05)65067-7) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a