ABSTRACT
Objective: To evaluate differences in perioperative clinical outcomes in men undergoing artificial urinary sphincter (AUS) implantation in primary versus replacement settings. Secondarily, we aimed to identify patient-related factors contributing to complications associated with AUS placement.
Materials and Methods: A review of the American College of Surgeons-National Surgical Quality Improvement Program was performed between 2010 and 2018 identifying males undergoing AUS implantation. Subjects were further subdivided into primary implantation or removal/replacement of AUS simultaneously via current procedural terminology codes 53445 and 53447, respectively. 30-Day postoperative outcomes were compared between cohorts using t-test and Fisher’s exact test. The relationship between patient factors and complications was evaluated using logistic regression.
Results: A total of 1,892 patients were identified: 1,445 primary AUS placement and 447 AUS replacement procedures. Patients undergoing AUS replacement were statistically older than those undergoing primary implantation (71.4 vs 69.7 years, P < .001). AUS replacement procedures were associated with an increased rate of superficial surgical site infection (SSI) compared to primary procedures (1.3% vs 0.4%, P = .042). There were no differences identified between cohorts for deep SSI, cardiopulmonary complications, reoperation, operative time, or length of stay. Logistic regression demonstrated that higher body mass index was found to be independent risk factors for any complications, and diabetes mellitus was associated with increased risk of AUS-related readmission.
Conclusion: Within the perioperative period, patients undergoing replacement AUS have an increased risk of superficial SSI compared to primary AUS implantation. These findings can assist with appropriate perioperative counseling of patients undergoing primary and replacement AUS implantations.
Keywords: Artificial urinary sphincter, male, urinary bladder, urinary incontinence.
Introduction
The artificial urinary sphincter (AUS) was first introduced in 1972 as the preferred surgical treatment for severe male stress urinary incontinence (SUI).1 Since that time, additional modalities for the treatment of male incontinence such as urethral bulking agents and male urethral slings have been developed; however, the AUS has remained the gold standard treatment for male SUI.2
As of 2012, over 150,000 AUS procedures have been performed worldwide. In the most recent series, AUS implantation was used in just over 50% of males who had incontinence procedures.3,4 The AUS has been adapted and proven to be effective in a wide range of situations, which include postprostatectomy incontinence, traumatic urethral injury, radical pelvic surgery, neurogenic causes, and as a salvage procedure when other modalities have failed.5,6
Though the AUS remains the gold standard therapy to decrease SUI in male patients, it is not without its necessary surveillance and maintenance. In a pooled analysis of outcomes in patients undergoing primary AUS placement, over 25% of patient’s required surgical reintervention after initial device placement either due to medically induced failure or device fatigue.7,8 The leading causes for evaluation and potential replacement of an AUS include erosion (8.5-10%), infection (7%), and mechanical failure (7.9% cuff and 6.3% pump failure).9,10 Additionally, patients with predisposed risk factors for AUS compromise, namely, a history of pelvic radiotherapy, have been noted to have an increased rate of necessary AUS replacement as compared to nonirradiated patients.11,12
In 1,082 patients with primary AUS placement over a 28-year period, 74 and 57% were revision free at 5 and 10 years, respectively.7 With several decades of use and implantation, many patients may be representing with complaints focused on their AUS device necessitating revision. Since many patients may require device revision, it would be prudent to understand the risk of complications compared to primary implantation to provide improved patient counseling. This study aims to evaluate differences in perioperative clinical outcomes during the 30-day early postsurgical period in men who underwent AUS implantation in a primary versus replacement setting utilizing the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) database. Additional outcomes included identifying patient-related factors that increase risk of perioperative outcomes.
Materials and Methods
We conducted a cohort study of patients receiving either a primary or replacement AUS implantation from 2010 to 2018 in the NSQIP database. The NSQIP database is comprised of greater than 150 individual perioperative data elements compiled from 600 participating hospitals and institutions. Nearly 95% of all patient perioperative outcomes are reported within this database. We accumulated data related to two procedures via current procedural terminology (CPT™) coding and insertion (53445) or removal and replacement (53447) of an inflatable urinary sphincter, including pump, reservoir, and cuff. Patients with concern for active infection (alternative CPT code 53448) were excluded from analysis. Due to the publicly available and deidentified nature of the NSQIP database, no ethic committee review was required for the completion of this project.
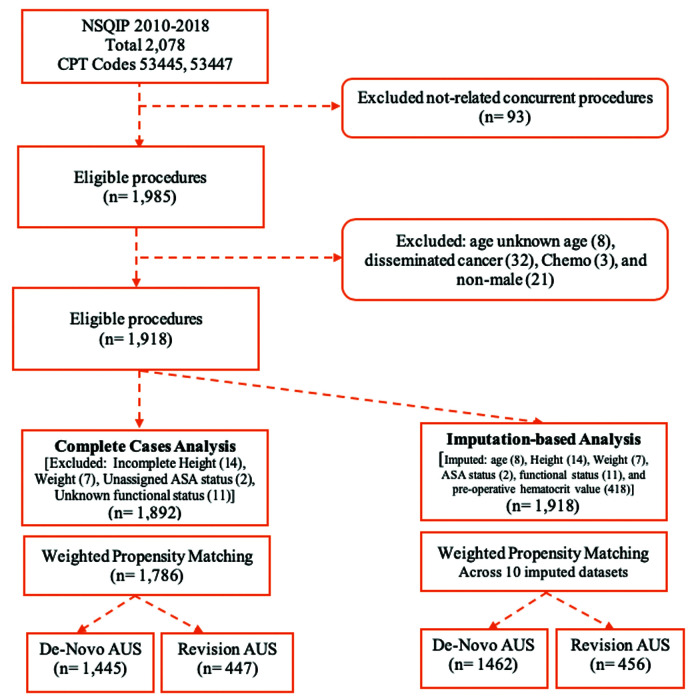
Patients 18 years of age and older who underwent the specified procedure between 2010 and 2018 were included. Figure 1 illustrates patient selection through a flowchart. Among these patients, grouping into primary versus replacement AUS was made. Patients with evidence of a systemic infection, local infection near, involving or prompting implant removal, advanced cancer burden, or incomplete data sets (missing perioperative height, weight, unknown ASA, and functional status) were excluded. No known past surgical or radiation history was available or included within the analysis.
Figure 1.
Patient selection in a flow diagram, no definitions or units to define. NSQIP, National Surgical Quality Improvement Project; CPT, current procedural terminology; AUS, artificial urinary sphincter.
Patient specific data points collected from this selection of patients included general demographic details such as their age, race/ethnicity, body mass index (BMI), smoking status, diabetes mellitus (DM) status, dyspnea, history of severe chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), hypertension (HTN) on medication, acute renal failure preoperatively, dialysis status, steroid use, weight loss, bleeding disorders, American Society of Anesthesiology (ASA) physical status, and overall functional status. Functional status was outlined as dependent versus independent.
Postoperative outcomes were categorized as infectious, wound disruption, respiratory complications, renal complications, cardiovascular complications, perioperative transfusion, and hospital-related outcomes, including readmission rate, return to operating room, operative duration, and length of stay (LOS).
Statistical Analysis
Descriptive statistics are reported as means with standard deviations. 30-Day postoperative outcomes were compared between primary AUS and replacement AUS cohorts using the Fisher’s exact test and Student’s t-test for categorical and continuous variables, respectively.
We performed multivariable regression (MVR) analyses in a complete dataset and an imputed dataset (Figure 1). Preoperative hematocrit values were used in the imputation analysis, while they were excluded from the complete data analysis because of the large number of missing values. Imputation was performed utilizing the R mice package where 10 imputed datasets were generated. Weighted propensity score matching (PSM) was performed utilizing the WeightIt and MatchThem packages in the imputation analyses. PSM is a statistical technique that can reduce confounding by matching similar patients based upon patient covariates, such as age, race, and comorbidities. By matching patients and achieving balance of covariates, we can compare differences in outcome variables between the groups. In our analysis, propensity scores were conducted using all previously mentioned patient variables as inputs with AUS device as the outcome variable (primary vs replacement) to balance covariates between these groups.
The multiple imputation MVR analysis was performed after PSM to provide double robustness of estimating associations between input variables and output variables. The survey package was used for developing the MVR analyses via its function “syvglm.” The results of the MVR analyses across the 10 weighted and imputed datasets were pooled utilizing the “pool” function from the mice package. Separate MVR models were created for the dependent outcome variables: any complications and AUS-related readmissions. MVR models were generated step-wise, beginning with AUS presence and all patient variables as independent variables and removing patient variables with P > .20 to limit the effect of multicollinearity.
All statistical analyses were performed using (R Foundation of Statistical Computing, Vienna, Austria) R 4.0.2. P values <.05 were considered statistically significant. Studies from the NSQIP database at our institution are considered exempt by the institutional review board.
Results
Of the 1,445 patients presented for primary AUS placement, 75.8% were Caucasian with comorbidities, including HTN (66.8%), DM (23.5%), COPD (3.3%), chronic steroid use (2.9%), and bleeding disorders (3.1%) (Table 1). Among the 447 patients who underwent replacement AUS procedures, 76.1% were Caucasian presented with similar comorbidity profiles including HTN (65.8%), DM (19.7%), COPD (3.8%), chronic steroid use (2%), and bleeding disorders (4.5%) (Table 1).
Table 1.
Patient Characteristics
| Overall | Primary AUS | Replacement AUS | P | |
|---|---|---|---|---|
| n | 1,892 | 1,445 | 447 | |
| Age (years): mean (SD) | 70.1 (8.91) | 69.7 (8.71) | 71.4 (9.40) | <.001 |
| BMI: mean (SD) | 29.7 (4.85) | 29.7 (4.90) | 29.7 (4.68) | .731 |
| Race (%) | .508 | |||
| White | 1436 (75.9) | 1096 (75.8) | 340 (76.1) | |
| Black or African American | 166 (8.8) | 122 (8.4) | 44 (9.8) | |
| Other/unknown | 290 (15.3) | 227 (15.7) | 63 (14.1) | |
| Current smoker (%) | 151 (8.0) | 121 (8.4) | 30 (6.7) | .274 |
| Diabetes mellitus (%) | 427 (22.6) | 339 (23.5) | 88 (19.7) | .105 |
| Congestive heart failure (%) | 8 (0.4) | 7 (0.5) | 1 (0.2) | .689 |
| Dyspnea (%) | 83 (4.4) | 59 (4.1) | 24 (5.4) | .237 |
| Functional health status (%) | 16 (0.8) | 12 (0.8) | 4 (0.9) | 1 |
| Hx. severe COPD (%) | 65 (3.4) | 48 (3.3) | 17 (3.8) | .656 |
| Hypertensive on meds (%) | 1259 (66.5) | 965 (66.8) | 294 (65.8) | .689 |
| Chronic steroid use (%) | 51 (2.7) | 42 (2.9) | 9 (2.0) | .403 |
| ASA class ≥3 (%) | 1062 (56.1) | 795 (55.0) | 267 (59.7) | .081 |
| Bleeding disorders (%) | 65 (3.4) | 45 (3.1) | 20 (4.5) | .181 |
Values are presented as the number of patients with SD (standard deviation) as well as % (percentages). In total, 1,892 patients met inclusion criteria and were gathered for this analysis. Primary AUS represents patients with virgin AUS implantation versus replacement who had undergone primary placement in the years preceding this analysis.
Postprocedural outcomes are illustrated in Table 2. Complications were noted in 59 (3.1%) patients overall, and there was no statistical difference between clinical complication rates outlined within the NSQIP database between primary and replacement AUS complications (46 (3.2%) vs 13 (2.9%), P = .877). However, among all identifiable infectious outcomes (superficial surgical site infections (SSIs), deep incisional infections, organ infections, UTIs, and sepsis), superficial SSI appeared to demonstrate modest but significant occurrence (6 (0.4%) vs 6 (1.3%), P = .042). There were no statistically significant differences found between either cohorts regarding disruption of their operative wound, respiratory, renal, or cardiovascular complications. Additional readmission, reoperation, operative time, and LOS are similar between cohorts.
Table 2.
Postprocedural Outcomes
| Overall | Primary AUS | Revision AUS | P | |
|---|---|---|---|---|
| n | 1,892 | 1,445 | 447 | |
| Any complication (%) | 59 (3.1) | 46 (3.2) | 13 (2.9) | .877 |
| Infections | ||||
| Superficial surgical site infection (SSI) (%) | 12 (0.6) | 6 (0.4) | 6 (1.3) | .042 |
| Deep incisional SSI (%) | 6 (0.3) | 5 (0.3) | 1 (0.2) | 1 |
| Organ/space SSI (%) | 12 (0.6) | 11 (0.8) | 1 (0.2) | .315 |
| Urinary tract infection (%) | 19 (1.0) | 15 (1.0) | 4 (0.9) | 1 |
| Sepsis (%) | 8 (0.4) | 8 (0.6) | 0 (0.0) | .21 |
| Septic shock (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1 |
| Wound disruption (%) | 6 (0.3) | 6 (0.4) | 0 (0.0) | .346 |
| Respiratory complications | ||||
| Pneumonia (%) | 3 (0.2) | 3 (0.2) | 0 (0.0) | 1 |
| Unplanned intubation (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1 |
| Pulmonary embolism (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1 |
| Ventilator > 48 hour (%) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1 |
| Renal complications | ||||
| Progressive renal insufficiency (%) | 3 (0.2) | 3 (0.2) | 0 (0.0) | 1 |
| Acute renal failure (%) | 5 (0.3) | 5 (0.3) | 0 (0.0) | .598 |
| Cardiovascular complications | ||||
| Cardiac arrest requiring (CPR) (%) | 2 (0.1) | 2 (0.1) | 0 (0.0) | 1 |
| Myocardial infarction (%) | 4 (0.2) | 3 (0.2) | 1 (0.2) | 1 |
| DVT/thrombophlebitis (%) | 4 (0.2) | 3 (0.2) | 1 (0.2) | 1 |
| Composite outcomes | ||||
| All readmissions (%) | 62 (3.3) | 51 (3.5) | 11 (2.5) | .361 |
| AUS-related readmission (%) | 55 (2.9) | 45 (3.1) | 10 (2.2) | .421 |
| Return to OR (%) | 43 (2.3) | 36 (2.5) | 7 (1.6) | .282 |
| Operative time (minutes): mean (SD) | 91.7 (37.0) | 91.0 (35.4) | 94.0 (41.7) | .134 |
| Days from operation to discharge: mean (SD) | 0.94 (2.43) | 0.97 (2.74) | 0.84 (0.84) | .31 |
| Wound class ≥3 (%) | 13 (0.7) | 8 (0.6) | 5 (1.1) | .202 |
This table represents the same cohort of 1,892 patients having primary and revision AUS procedures and lists their postprocedural outcomes as listed within the NSQIP data base. Again, values are listed as number of patients and SD (standard deviations) and % (percentages).
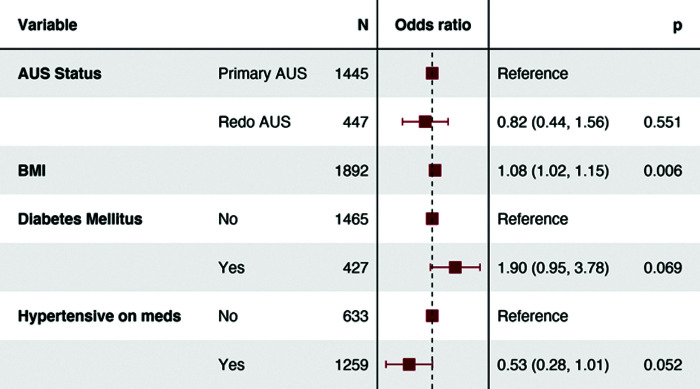
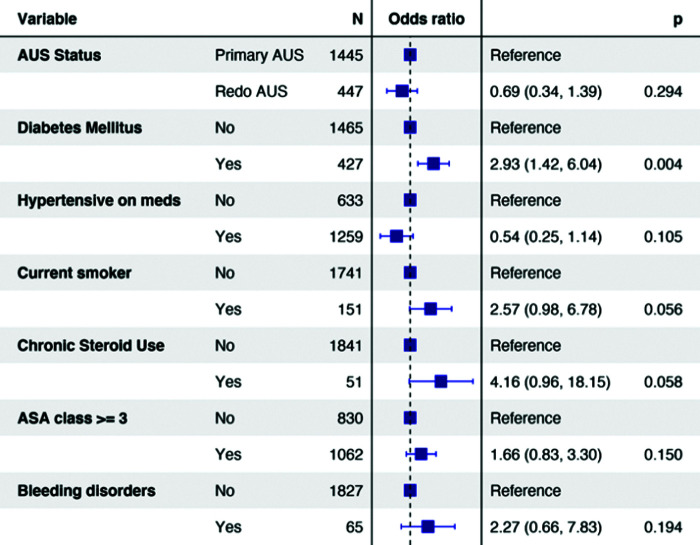
Logistic regression analysis was used to determine risk factors for any complication and for procedure-related readmission (Figures 2 and 3). On logistic regression analysis, replacement AUS placement had similar risk of any complication (OR0.440.821.56, P = .551) and readmission (OR0.340.691.39, P = .294). Regression analysis for AUS-related readmission demonstrated the presence of DM (OR1.422.936.04, P = .004) to be significant predictor. When utilizing a multiple imputation multivariable regression model, replacement AUS was not associated with increased risk of any complication (OR0.5231.0081.943, P = .981) or AUS-related readmissions (OR0.4220.8381.662, P = .613 (Table 3).
Figure 2.
Odds ratio (OR) is a statistic that quantifies the strength of the association between two events, A and B. OR does not incorporate a unit. AUS: artificial urinary sphincter; BMI: body mass index.
Figure 3.
Odds ratio (OR) is a statistic that quantifies the strength of the association between two events, A and B. OR does not incorporate a unit. AUS, artificial urinary sphincter; ASA, American Society of Anesthesiologists.
Table 3.
Multiple Imputation Multivariable Regression Analysis
| Term | OR | |||
|---|---|---|---|---|
| Outcome | Primary AUS | Reference | 95% CI | P value |
| Any complication | Revision AUS | 1.008 | 0.523-1.943 | .981 |
| AUS-related readmissions | Revision AUS | 0.838 | 0.422-1.662 | .613 |
This table represents the multivariable regression analysis of complications within AUS-implanted patients. OR, odds ratios; CI, confidence intervals.
Discussion
This nationwide analysis of AUS device implantations demonstrated comparable risk of postoperative complications and readmissions for both primary and repeat AUS procedures; however, it did show an association between repeat procedures with increased risk of superficial surgical site infections.
It was hypothesized that the scarring caused by prior urethral surgical procedures could enhance the difficulty of dissection and reduce the efficacy of repeat AUS surgery. Additionally, longer operative times and older, sicker patients in repeat procedures were proposed as a potential risk factor for complications or readmissions. Interestingly, our data did not support these hypotheses with the exception of superficial surgical site infections occurring more often in repeat AUS patients.
Data on AUS implantation have been collected since its introduction in 1972, and several longitudinal cohort studies have demonstrated excellent outcomes and patient satisfaction.5,7 With respect to repeat procedures, the data have been limited and heterogeneously reported, as documented by a recent systematic review.12 There has been a lack of prospective controlled trials on the topic, and poor consensus on important definitions such as that of SUI at varying levels of severity.12
There has been discordance in the literature on the comparison of primary and replacement AUS procedures. Several single institution retrospective studies, however, have attempted to answer the question over the past few decades. One such study from 2005 included 554 patients and found that 5-year incontinence and device durability outcomes were comparable between the two groups.13 Another study from 2012, reporting on 227 consecutive AUS operations, found that repeat implantation patients experienced a fourfold increased risk of cuff erosion.14 Similarly, a 2014 chart review of 704 patients found increased rates of infection, erosion, and explanation in repeat procedures.7 Finally, a recent multicenter study involving 892 AUS surgeries between 1989 and 2012 found an association between both previous incontinence surgery and low institutional caseload with higher complication rates.15
Recent research into AUS has revealed a more complex set of variables driving patient outcomes. An existing study also utilizing the ACS-NSQIP database evaluated 771 procedures and reported an association between patient frailty (as opposed to just age) and major complications, including device explantation.16 A similar study of 841 patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare population reported that those who underwent AUS surgery more than 15 months following prostatectomy experienced longer device survival and lower complications rates. Additionally, prior incontinence surgery was found to be predictive of earlier reoperation.17 These data suggest that factors such as frailty and surgical timing could play a confounding role in the relationship between primary and repeat AUS surgery.
While our results show that there are similar complication profiles between primary and replacement procedures, these results differ from those previously reported in the literature. This discrepancy suggests that the reported higher complication rates with AUS replacement procedures occur in a delayed fashion beyond the 30-day outcomes analysis in our study. Increased volume at tertiary referral centers (where the majority of these cases are performed) has thoroughly demonstrated consistent and gradually improved surgical outcomes despite employing very similar surgical principles over several decades of device existence within the market. Furthermore, innovations in the biomedical device space could also play a role in improved outcomes or a reduced disparity between primary and repeat surgery. The AMS 800 implant (a standard AUS device) was recently upgraded with an antibiotic coating, and newer technologies are consistently being brought to market.18 Additionally, novel surgical approaches have been introduced and shown improvements in safety and efficacy. For instance, transcorporal cuff placement is described as an alternative to tandem cuff placement (with a discrete CPT code 53444, not included within this study) after it was shown to reduce postoperative continence and device erosion.19
This national database analysis of over 1,600 AUS patients found no significant difference in complication or readmission rates between primary and repeat procedures. Superficial surgical site infections were the only complication found to be more associated with replacement AUS perioperatively, while increased BMI and the presence of DM were significant predictors of complication and readmissions, respectively.
The ACS-NSQIP database has the strength of being nationally validated, risk adjusted, and independently reviewed. It uses prospectively collected outcomes across a wide range of heterogeneous institutions across the United States to minimize bias.9 The ACS-NSQIP has been shown to be accurate, reliable, and on par with, if not superior to, several administrative claims datasets.10 , 11
There are a number of limitations within our study, many of them inherent to the use of the NSQIP database itself. The most significant limitation with regard to our study is that it does not capture outcomes beyond a 30-day operative period.20 Information regarding disease severity, indications for surgery, etiology of stress incontinence, device specific details, functional outcomes, or complications beyond 30-days is also not included.20 An additional important limitation to again note is the absence of notation or capture of radiotherapeutics prior to AUS implantation, which is a known significant risk factor for device morbidity. These limitations are especially notable with regard to AUS implants because many if not all postoperative issues do not present until well beyond this time period. Additionally, the deidentified nature of the database prevents analysis of patients’ relevant surgical or radiation history. While it is well-known that radiotherapy predisposes patients to higher-likelihood of AUS replacement, we are unable to capture this within the confines of the database. Scholars have commented on the great cost associated with participation in ACS-NSQIP and hypothesized a skew toward the patient populations of large academic hospitals. It is possible that only the most clinically severe cases are being captured in the dataset.20 Although the size of the present study provides potential insight into subtle correlations and associations, the retrospective design does not allow for commentary on causal relationships.
Patients who have already undergone one or more AUS implantations will have more information on their prospects regarding complications and readmissions, while surgeons may use these data to promote best practices, successful techniques, and approaches. Similarly, professional societies will find these data useful in the crafting of revised evidence-based guidelines. Finally, although this analysis did not compare devices, the standardized performance metrics will allow industry groups to pursue more targeted innovation and quality improvement.
Within the perioperative period, patients undergoing replacement AUS have an increased risk of superficial SSI compared to primary AUS implantation. These findings can assist with appropriate perioperative counseling of patients undergoing primary and replacement AUS implantations.
Main Points
No statistically significant difference was noted between complication and readmission rates among patients undergoing primary versus revision/secondary AUS implantation.
Superficial surgical site infections were the only complication found to be more associated with replacement AUS perioperatively.
Increased BMI and the presence of diabetes mellitus were significant predictors of complication and readmissions, respectively.
Revision AUS procedures are safe and effective as index AUS implantation.
Footnotes
Ethics Committee Approval: N/A
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.J., A.F., A.M.; Design - A.F., A.M., A.R.; Supervision - A.L., N.T., S.G.; Materials - W.M., T.J., T.C.; Data Collection and/or Processing - T.J., A.M.; Analysis and/or Interpretation - T.C., W.M., B.P., A.R.; Literature Search - M.C., W.M., A.R., B.P.; Writing Manuscript - T.J., A.F., M.C., W.M., B.P., A.R., H.E., M.A.; Critical Review - A.L., N.T., S.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1. Van Der Aa F, Drake MJ, Kasyan GR, Petrolekas A, Cornu JN. The artificial urinary sphincter after a quarter of a century: A critical systematic review of its use in male non-neurogenic ?A3B2 thyc?> incontinence. Eur Urol . 2013;63:(4):681–689.. 10.1016/j.eururo.2012.11.034) [DOI] [PubMed] [Google Scholar]
- 2. Crivellaro S, Morlacco A, Bodo G.et al. Systematic review of surgical treatment of post radical prostatectomy stress urinary incontinence. Neurourol Urodynam . 2016;35:(8):875–881.. 10.1002/nau.22873) [DOI] [PubMed] [Google Scholar]
- 3. Nambiar AK, Bosch R, Cruz F.et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. Eur Urol . 2018;73:(4):596–609.. 10.1016/j.eururo.2017.12.031) [DOI] [PubMed] [Google Scholar]
- 4. Poon SA, Silberstein JL, Savage C, Maschino AC, Lowrance WT, Sandhu JS. Surgical practice patterns for male urinary incontinence: Analysis of case logs from certifying American urologists. J Urol . 2012;188:(1):205–210.. 10.1016/j.juro.2012.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: A 10-year retrospective review from the University of Michigan. J Urol . 2008;179:(5):1912–1916.. 10.1016/j.juro.2008.01.048) [DOI] [PubMed] [Google Scholar]
- 6. Lai HH, Hsu EI, Teh BS, Butler EB, Boone TB. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol . 2007;177:(3):1021–1025.. 10.1016/j.juro.2006.10.062) [DOI] [PubMed] [Google Scholar]
- 7. Linder BJ, Rivera ME, Ziegelmann MJ, Elliott DS. Long-term outcomes following artificial urinary sphincter placement: An analysis of 1082 cases at Mayo Clinic. Urology . 2015;86:(3):602–607.. 10.1016/j.urology.2015.05.029) [DOI] [PubMed] [Google Scholar]
- 8. Yafi FA, DeLay KJ, Stewart C, Chiang J, Sangkum P, Hellstrom WJG. Device survival after primary implantation of an artificial urinary sphincter for male stress urinary incontinence. J Urol . 2017;197:(3):759–765.. 10.1016/j.juro.2016.08.107) [DOI] [PubMed] [Google Scholar]
- 9. Cohen ME, Liu Y, Ko CY, Hall BL. Improved surgical outcomes for ACS NSQIP hospitals over time: Evaluation of hospital cohorts with up to 8 years of participation. Ann Surg . 2016;263:(2):267–273.. 10.1097/SLA.0000000000001192) [DOI] [PubMed] [Google Scholar]
- 10. Lawson EH, Louie R, Zingmond DS.et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg . 2012;256:(6):973–981.. 10.1097/SLA.0b013e31826b4c4f) [DOI] [PubMed] [Google Scholar]
- 11. Enomoto LM, Hollenbeak CS, Bhayani NH, Dillon PW, Gusani NJ. Measuring surgical quality: A national clinical registry versus administrative claims data. J Gastrointest Surg . 2014;18:(8):1416–1422.. 10.1007/s11605-014-2569-2) [DOI] [PubMed] [Google Scholar]
- 12. Guachetá Bomba PL, Ocampo Flórez GM, Echeverría García F.et al. Effectiveness of surgical management with an adjustable sling versus an artificial urinary sphincter in patients with severe urinary postprostatectomy incontinence: A systematic review and network meta-analysis. Ther Adv Urol . 2019;11. DOI: 10.1177/1756287219875581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raj GV, Peterson AC, Khai LT, Webster GD. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol . 2005;173:(4):1242–1245.. 10.1097/01.ju.0000152315.91444.d0) [DOI] [PubMed] [Google Scholar]
- 14. Lai HH, Boone TB. Complex artificial urinary sphincter revision and reimplantation cases-how do they fare compared to virgin cases?. J Urol . 2012;187:(3):951–955.. 10.1016/j.juro.2011.10.153) [DOI] [PubMed] [Google Scholar]
- 15. Tutolo M, Cornu JN, Bauer RM.et al. Efficacy and safety of artificial urinary sphincter (AUS): Results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol Urodyn . 2019;38:(2):710–718.. 10.1002/nau.23901) [DOI] [PubMed] [Google Scholar]
- 16. Medendorp AR, Anger JT, Jin C.et al. The impact of frailty on artificial urinary sphincter placement and removal procedures. Urology . 2019;129:210–216.. 10.1016/j.urology.2019.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen AJ, Kuchta K, Park S, Milose J. Patterns and timing of artificial urinary sphincter failure. World J Urol . 2018;36:(6):939–945.. 10.1007/s00345-018-2203-0) [DOI] [PubMed] [Google Scholar]
- 18. Chung E, Ranaweera M, Cartmill R. Newer and novel artificial urinary sphincters (AUS): The development of alternatives to the current AUS device. BJU Int . 2012;110:5–11.. 10.1111/j.1464-410X.2012.11614.x) [DOI] [PubMed] [Google Scholar]
- 19. Guralnick ML, Miller E, Toh KL, Webster GD. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol . 2002;167:(5):2075–2079.. 10.1016/S0022-5347(05)65088-4) [DOI] [PubMed] [Google Scholar]
- 20. Tyson MD, Barocas DA. Improving quality through clinical registries in urology. Curr Opin Urol . 2017;27:(4):375–379.. 10.1097/MOU.0000000000000406) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a