Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused high inpatient mortality and morbidity throughout the world. COVID-19 convalescent plasma (CCP) has been utilized as a potential therapy for patients hospitalized with coronavirus disease 2019 (COVID-19) pneumonia. This study evaluated the outcomes of hospitalized patients with COVID-19 treated with CCP in a prospective, observational, multicenter trial.
Methods
From April through August 2020, hospitalized patients with COVID-19 at 16 participating hospitals in Colorado were enrolled and treated with CCP and compared with hospitalized patients with COVID-19 who were not treated with convalescent plasma. Plasma antibody levels were determined following the trial, given that antibody tests were not approved at the initiation of the trial. CCP-treated and untreated hospitalized patients with COVID-19 were matched using propensity scores followed by analysis for length of hospitalization and inpatient mortality.
Results
A total of 542 hospitalized patients with COVID-19 were enrolled at 16 hospitals across the region. A total of 468 hospitalized patients with COVID-19 were entered into propensity score matching with 188 patients matched for analysis in the CCP-treatment and control arms. Fine-Gray models revealed increased length of hospital stay in CCP-treated patients and no change in inpatient mortality compared with controls. In subgroup analysis of CCP-treated patients within 7 days of admission, there was no difference in length of hospitalization and inpatient mortality.
Conclusions
These data show that treatment of hospitalized patients with COVID-19 treated with CCP did not significantly improve patient hospitalization length of stay or inpatient mortality.
Keywords: COVID-19, convalescent plasma, hospitalization, mortality
A multicenter, prospective, cohort-controlled trial of COVID-19 convalescent plasma therapy in hospitalized patients with COVID-19 did not show benefit in time to discharge or inpatient mortality.
The ongoing pandemic due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 150 million cases and 3 million deaths worldwide as of April 2021. Since the onset of the pandemic, various therapeutic measures, including antivirals, immunomodulatory therapeutics, and passive antibody therapies, have been evaluated for efficacy in the treatment of coronavirus disease 2019 (COVID-19). Convalescent plasma therapy has been utilized for several viral and nonviral infectious diseases throughout history and was the only means of treating certain infectious diseases prior to the development of antimicrobial-specific therapy in the 1940s [1, 2]. Experience from prior outbreaks with other coronaviruses, such as SARS-CoV, shows that convalescent plasma contains neutralizing antibodies to the relevant virus and neutralizing antibodies may provide therapeutic benefit to acutely infected patients [3]. In the case of SARS-CoV-2, passive antibody therapy with convalescent plasma may mediate protection by viral neutralization or other mechanisms such as enhanced phagocytosis and antigen processing of virions.
Convalescent plasma, hyperimmune globulin, and monoclonal antibodies are different passive antibody therapeutics evaluated as possible treatments for COVID-19. Recent studies of COVID-19 convalescent plasma (CCP) therapy in several studies have shown potential benefit of treatment in hospitalized patients with COVID-19 [4–7]. Other studies including randomized, open-label trials have exhibited lack of a clear clinical benefit from CCP therapy in hospitalized patients with COVID-19 [8–10]. Because each study exhibits a range of strengths and weaknesses in study design, different targeted patient populations, and study power, additional studies of CCP therapy in hospitalized patients with COVID-19 are needed to inform appropriate use of CCP as a potential therapeutic alternative for COVID-19.
Our study utilized a multicenter trial design across 16 academic and nonacademic hospitals in Colorado in an open-label, prospective, observational, cohort-controlled trial to evaluate length of hospitalization and inpatient mortality rate in patients with COVID-19 treated with CCP.
METHODS
This was an open-label, prospective, multicenter cohort trial comparing hospitalized patients with COVID-19 who received CCP with hospitalized patients with COVID-19 who received standard-of-care treatment. The clinical study was conducted at 16 hospitals in Colorado, including UCHealth Metro Denver, UCHealth North, UCHealth South Hospitals, Denver Health Medical Center, Children’s Hospital of Colorado, and Sisters of Charity of Leavenworth (SCL) Health Hospitals. All human clinical trial work on COVID-19 was approved by the Colorado Multi-Institution Review Board (COMIRB, #20–0986, #20–0990) prior to the study opening. All hospitals within networks of UCHealth, Children’s Hospital of Colorado, and Denver Health Medical Center provided CCP through a University of Colorado Expanded Access program (Food and Drug Administration [FDA] IND#21426). SCL Health Hospitals provided CCP through the FDA Expanded Access program sponsored by Mayo Clinic. All patients or designated decision makers provided signed informed consent prior to enrollment into the expanded access programs to receive CCP. The study was conducted from April 2020 through August 2020.
Each site prospectively screened eligible patients for inclusion based on the following predetermined inclusion criteria: age 18 years or older, laboratory-confirmed diagnosis of SARS-CoV-2 infection by detection of nucleic acid from a respiratory sample, admission to a participating facility, COVID-19 pulmonary disease requiring hospitalization, sufficient CCP available for treatment, and informed consent provided by the patient or healthcare proxy. Exclusion criteria for the observational clinical trial included receipt of pooled immunoglobulin in the past 30 days, patients placed on extracorporeal membrane oxygenation, history of transfusion reaction, contraindication to receiving plasma products, and risk of transfusion exceeding potential benefit based on clinician determination.
Control patients were identified, and data were captured during each month of the study using a UCHealth database of hospitalized patients with COVID-19 for each month from April through August of 2020. Data were abstracted from all hospitalized patients with COVID-19 in the UCHealth system who met the inclusion and exclusion criteria above but who did not receive CCP therapy over the same time period. As previously described, all patient data were entered and maintained in a secure, Health Insurance Portability and Accountability Act (HIPAA)–compliant Research Electronic Data Capture (REDCap) database [11]. Baseline demographic data, clinical data, and visit data for the hospitalization were prospectively collected. COVID-19–related medications, remdesivir and dexamethasone, that received Emergency Use Authorization (EUA) and subsequent licensing for COVID-19 during the study were prospectively included in the analysis. Due to variable use of anticoagulation approaches throughout the study period at the 16 study sites and changing recommendations for anticoagulation for COVID-19 during the enrollment, anticoagulation treatment data were not prospectively captured.
Convalescent Plasma Procurement and Transfusion
FDA approval of the expanded access program for the use of convalescent plasma was obtained (IND#21426). Convalescent plasma was allocated from FDA-registered blood establishments (Children’s Hospital Colorado, Garth Englund Blood Center, Vitalant Center) to the treating hospitals using standardized procedures. COVID-19 convalescent plasma was supplied as an investigational blood product and was administered according to standard hospital procedures for plasma administration. Plasma was infused over 1–2 hours (rate of 100–250 mL/hour). Pre-medications, such as acetaminophen or diphenhydramine, were provided as indicated by the treating physician. One unit of CCP was administered to anyone weighing less than 90 kg, and 2 units were given to patients weighing over 90 kg. Levels of binding antibodies were assessed using VITROS (Ortho Clinical Diagnostics) anti–SARS-CoV-2 immunoglobulin G (IgG) assay. Some CCP units were provided prior to availability of antibody testing and frozen samples were each retrospectively tested for antibody. Of 375 units of CCP utilized in the study, 362 met FDA criteria for positive CCP antibody greater than 9 (S/C value, VITROS assay). Three tested units were negative for antibody and 10 units were not tested due to lack of frozen sample. The patients who received these units were included in the full intention-to-treat analysis. Ten patients enrolled through SCL Health had CCP units tested through the Mayo Clinic Expanded Access Program and data were not available for analysis.
Propensity Score Matching
Propensity score matching was performed to ensure that potential confounding factors were balanced between the CCP treatment group and the control group. Propensity score matching is an analysis approach utilized for nonrandomized trials to minimize bias in estimating the treatment effect [12, 13]. Propensity scores are calculated based on baseline criteria at admission that are expected to influence outcome. For example, age over 70 years is known to increase risk of a negative outcome for patients hospitalized with COVID-19 [14]. Thus, matching patients based on age in the treatment and control groups will help to decrease possible bias of enrolling patients of more advanced age in 1 group. The propensity score was estimated via logistic regression with no higher order or interaction terms. We conducted greedy-nearest-neighbor one-to-one propensity score matching via the R package MatchIt [15]. A caliper length of 0.2 multiplied by the standard deviation of the logit of the propensity score was used [16]. Covariate mean balance was assessed via standardized mean difference, with balance defined to be a standardized mean difference less than 0.1 [17]. The matching criteria included ethnicity, age (categorical by decade), admission month (continuous), sex, hypertension, lung disease, cancer, diabetes, obesity, smoking status, and immunosuppression. Lung disease was defined as an underlying lung disorder that requires treatment including asthma, chronic obstructive pulmonary disease, and interstitial lung disease. Immune suppression was defined as any condition that results in suppression of immune responses including primary and secondary immune deficiencies that result from treatment.
Statistical Analysis
We modeled the primary outcome (time to hospital discharge) in a competing risks framework, where in-hospital mortality is considered to be a competing risk [18]. All study subjects were observed until time to in-hospital mortality or discharge; thus, there was no censoring. There were a small number of patients who were discharged from the hospital, and who later returned to the hospital and experienced in-hospital mortality. The analysis treated the eventual in-hospital mortality for these patients as unobserved. Time to discharge and time to inpatient mortality are displayed by the nonparametric Aalen-Johansen curves. Inference is done via a Fine-Gray model for competing risks with robust variance estimation for the propensity-matched cohorts (http://github.com). In the Fine-Gray models, time to event is regressed on an indicator variable denoting treatment status with no other covariates.
RESULTS
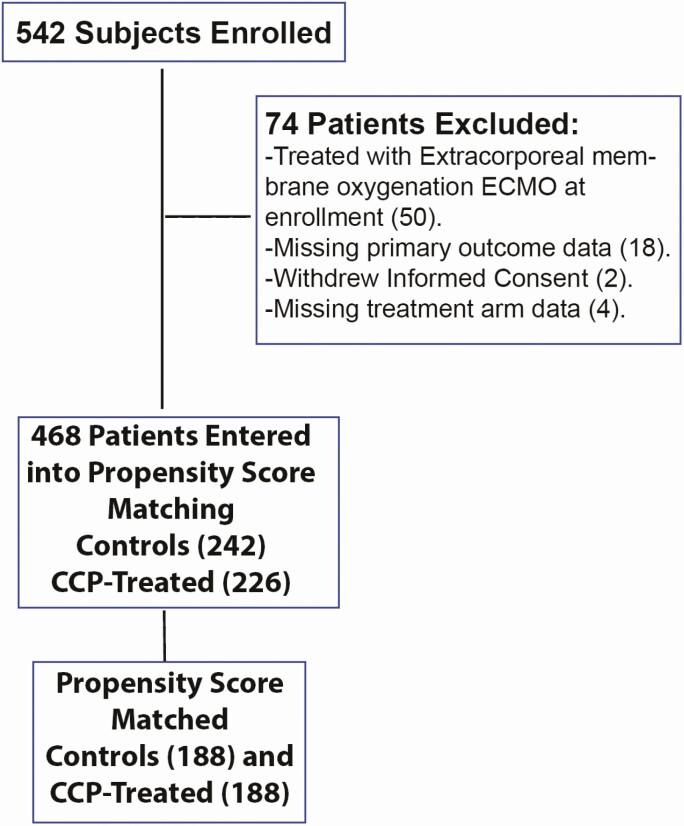
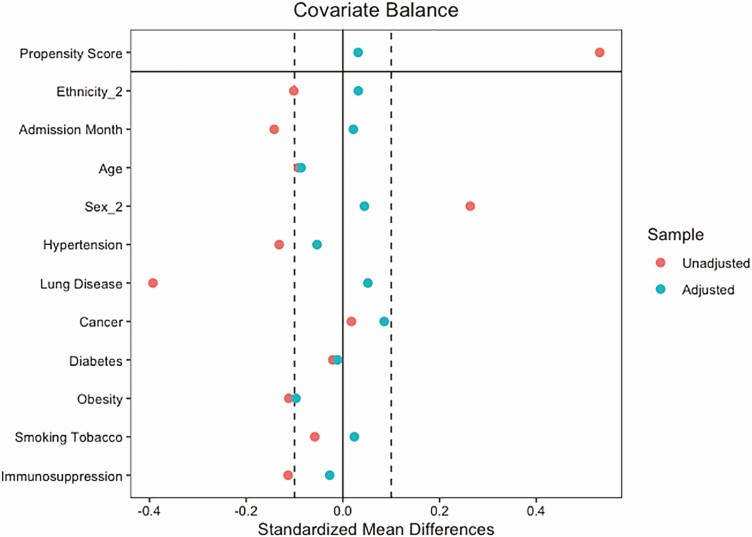
In our primary intention-to-treat analysis, 188 treatment and control subjects were retained after propensity score matching for 376 total patients included in the trial (Figure 1). The variance ratio was used to assess covariate variance balance, with balance defined to be a variance ratio less than 2 [19]. Covariate balance was achieved after propensity score matching for the intention-to-treat analysis (Figure 2). Propensity score matching was also performed for the subanalyses that limited the study population based on time to infusion. Covariate balance was achieved for the 7 or fewer days to infusion analysis and was nearly, but not, achieved for the 3 or fewer days to infusion analysis (Supplementary Figures 1 and 2). Subject characteristics for the propensity score–matched intention-to-treat analysis are displayed in Table 1. Overall, the cohorts enrolled represented the regional outbreak, with a higher proportion of older-adult patients (mean: 58.9 years), male patients (56.9%), and patients in the study who identified as having Hispanic ethnicity (50.8%). In the total cohort, 67.2% of CCP-treated patients identified as White or Caucasian, 15.7% identified as more than 1 race, 10.4% identified as Black or African American, and 4.8% identified as Asian. During the study, 38.3% of the patients were enrolled during May of 2020 and 26.3% of the patients were enrolled during July of 2020 (Table 1). Patients were matched based on month of enrollment due to the rapidly changing treatment approaches throughout the pandemic. Comorbid medical diagnoses, including hypertension, diabetes mellitus, and obesity, were found in 42–60% of the patients throughout the study and were matched at baseline for each individual. Of the 38 patients who were not matched, all of them were male and 63.2% were admitted in May. Most of the other characteristics were similar between the unmatched and the matched group (Supplementary Table 1). The use of remdesivir and dexamethasone was similar in both treatment and control groups (Supplementary Table 2).
Figure 1.
Flowchart of patient enrollment and propensity score matching. Abbreviation: CCP, COVID-19 [coronavirus disease 2019] convalescent plasma.
Figure 2.
Plot showing covariate balance in full dataset analysis before (red) and after (green) propensity score matching.
Table 1.
Demographic Data for Propensity Score–Matched Control and CCP-Treated Patients With COVID-19
| Control (n = 188) | CCP-Treated (n = 188) | Overall (N = 376) | ||||
|---|---|---|---|---|---|---|
| Age, years | ||||||
| Mean (SD) | 59.6 (16.8) | 58.2 (16.3) | 58.9 (16.5) | |||
| Median [min, max] | 58.7 [23.6, 93.2] | 58.6 [19.3, 91.9] | 58.7 [19.3, 93.2] | |||
| Sex, n (%) | ||||||
| Female | 83 (44.1) | 79 (42) | 162 (43.1) | |||
| Male | 105 (55.9) | 109 (58) | 214 (56.9) | |||
| Ethnicity, n (%) | ||||||
| Hispanic | 97 (51.6) | 94 (50) | 191 (50.8) | |||
| Not Hispanic | 91 (48.4) | 94 (50) | 185 (49.2) | |||
| Admission month (2020), n (%) | ||||||
| March | 1 (0.5) | 1 (0.5) | 2 (0.5) | |||
| April | 32 (17) | 19 (10.1) | 51 (13.6) | |||
| May | 65 (34.6) | 79 (42.0) | 144 (38.3) | |||
| June | 14 (7.4) | 24 (12.8) | 38 (10.1) | |||
| July | 57 (30.3) | 42 (22.3) | 99 (26.3) | |||
| August | 19 (10.1) | 23 (12.2) | 42 (11.2) | |||
| Yes | No | Yes | No | Yes | No | |
| Comorbid diagnosis, n (%) | ||||||
| Hypertension | 90 (47.9) | 98 (52.1) | 85 (45.2) | 103 (54.8) | 175 (46.5) | 201 (53.5) |
| Diabetes | 81 (43.1) | 107 (56.9) | 80 (42.6) | 108 (57.4) | 161 (42.8) | 215 (57.2) |
| Obesity | 83 (44.1) | 105 (55.9) | 74 (39.4) | 114 (60.6) | 157 (41.8) | 219 (58.2) |
| Immune suppression | 10 (5.3) | 178 (94.7) | 9 (4.8) | 179 (95.2) | 19 (5.1) | 357 (94.9) |
| Lung disease | 21 (11.2) | 167 (88.8) | 24 (12.8) | 164 (87.2) | 45 (12) | 331 (88) |
| Smoking tobacco | 11 (5.9) | 177 (94.1) | 12 (6.4) | 176 (93.6) | 23 (6.1) | 353 (93.9) |
Abbreviations: CCP, COVID-19 convalescent plasma; COVID-19, coronavirus disease 2019; max, maximum; min, minimum.
A total of 468 subjects, 226 in the treatment arm and 242 in the control arm, underwent propensity score matching (Figure 1). For the intention-to-treat analysis, 376 one-to-one matched CCP treatment and control subjects remained after propensity score matching. Analysis of patients who received CCP treatment at 7 days or less of hospitalization resulted in 358 total subjects after matching. For analysis of patients who received CCP treatment at 3 or fewer days of hospitalization, 322 total subjects remained after matching.
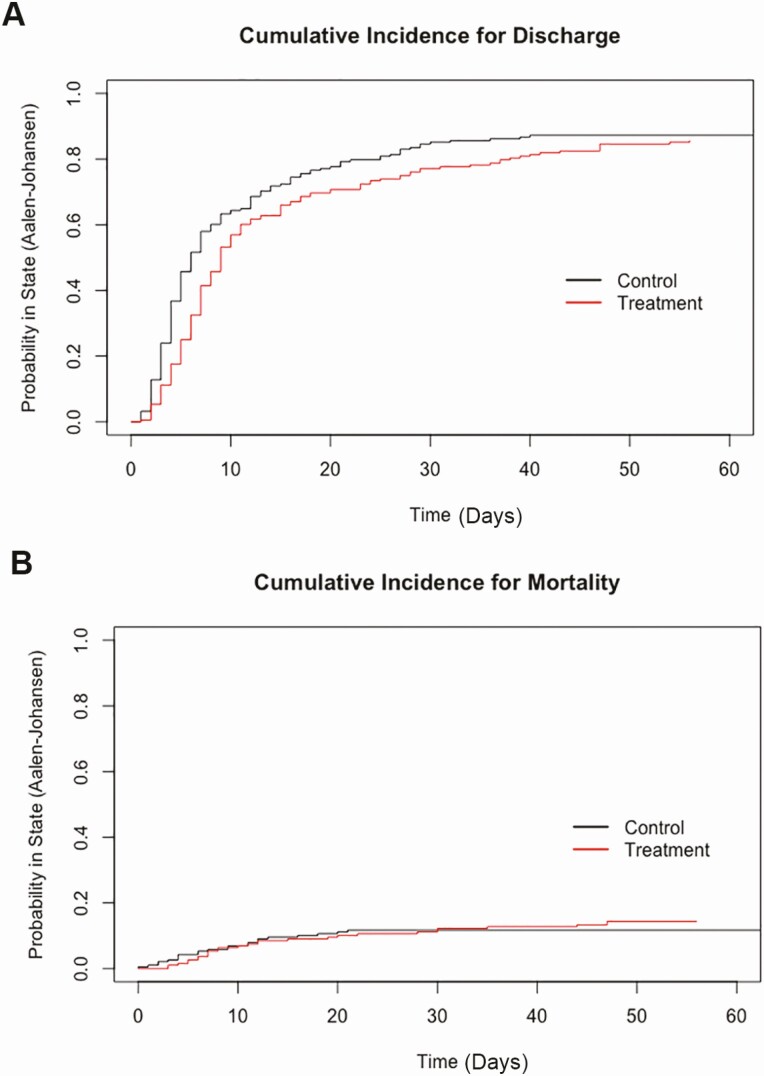
Overall, treatment with CCP of hospitalized patients with COVID-19 was associated with a longer hospitalization, calculated as a hazard ratio (0.67) for hospital discharge favoring control patients with COVID-19 compared with CCP-treated patients (P = .00053) (Table 2). As a secondary endpoint, there was no difference in inpatient mortality (P = .47). Column 4 of Table 2 displays the estimated hazard ratio of treatment status (compared with control) on hospital discharge and inpatient mortality. As these coefficients were estimated via Fine-Gray models, they should be interpreted as changes in the subdistribution hazard function for each event type [20]. Values less than 1 indicate that treatment status reduces the hazard of the corresponding event. The estimated Aalen-Johansen incidence curves for hospital discharge and in-hospital mortality for each matched cohort show the probability of hospital discharge or inpatient mortality over time (Figure 3).
Table 2.
Number of Patients in the Matched Treatment and Control Arms Who Experienced Primary (Hospital Discharge) or Secondary (Inpatient Mortality) Outcomes
| Outcome Measure | Control | CCP-Treated | Hazard Ratio (95% CI) | P |
|---|---|---|---|---|
| Propensity score–matched cohorts | ||||
| n | 188 | 188 | ||
| Hospital discharge | 165 | 161 | .67 (.54, .85) | .00053 |
| Inpatient mortality | 23 | 27 | .81 (.46, 1.43) | .47 |
| CCP infusion within 7 days of admission | ||||
| n | 179 | 179 | ||
| Hospital Discharge | 158 | 155 | .84 (.67, 1.04) | .11 |
| Inpatient Mortality | 21 | 24 | 1.08 (.59, 1.97) | .81 |
| CCP infusion within 3 days of admission | ||||
| n | 161 | 161 | ||
| Hospital discharge | 139 | 141 | .77 (.42, 1.42) | .41 |
| Inpatient mortality | 22 | 20 | .83 (.66, 1.06) | .13 |
Abbreviations: CCP, COVID-19 convalescent plasma; CI, confidence interval; COVID-19, coronavirus disease 2019.
Figure 3.
Cumulative incidence curves. A, Probability of hospital discharge over time in days. B, Probability of inpatient mortality over time in days. Estimated using Aalen-Johansen analysis.
Analysis of patients with COVID-19 who received CCP treatment within 7 or 3 days of admission revealed no significant difference in hospital discharge or inpatient mortality in compared with control patients (Table 2). The Aalen-Johansen curves for CCP-treated patients within 7 or 3 days of admission also showed no significant difference in hospital discharge or inpatient mortality when compared with control patients with COVID-19 (Supplementary Figures 3–6).
Four patients had documented adverse events associated with CCP infusion. Three patients had allergic reactions with pruritus, rash, or urticaria, which resolved following treatment with acetaminophen and antihistamines. One patient developed a febrile transfusion reaction, which improved with acetaminophen therapy. The transfusion was stopped in the patient after 60 mL, and the patient was included in the analysis.
DISCUSSION
In this prospective, open-label, multicenter, cohort-controlled study, we found that treatment of hospitalized patients with COVID-19 with CCP did not significantly improve time to hospital discharge or inpatient mortality compared with propensity score–matched controls. Given that hospital discharge is considered a positive endpoint, the fact that treatment reduces the hazard of hospital discharge is evidence that CCP treatment may have a negative effect on the prognosis of study participants. However, this statistically significant effect disappeared in the 2 subanalyses. This may be because study participants who received convalescent plasma more than 7 days after hospital admission differ systematically from the rest of the study population. For example, the patients who received CCP after 7 days may bias the treatment group to more severe disease. We also found that CCP treatment status does not have a statistically significant effect on the hazard of inpatient mortality. It would be difficult to detect an effect of convalescent plasma treatment on the hazard of inpatient mortality in this study given the relatively few numbers of inpatient deaths.
Previous, mostly noncontrolled, trials have suggested a potential benefit of CCP therapy in hospitalized patients with COVID-19. In a retrospective matched-cohort study, patients receiving CCP had decreased 7-day and 14-day mortality but no statistical difference in 28-day mortality. Similar to our study, the length of hospital stay was increased in the convalescent plasma group, suggesting possible selection bias [7]. A propensity score–matched study performed at Houston Methodist Hospital from March 2020 through September 2020 showed a significant decrease in mortality for patients transfused within 72 hours of admission with plasma containing anti-Receptor Binding Domain (RBD) IgG titer of more than 1:1350. No mortality benefit was noted in patients who received an RBD IgG titer of less than 1:1350 or who were intubated at the time of admission [6]. In a cohort of 3082 patients who received convalescent plasma through the Mayo Clinic–initiated Expanded Access Program, non–mechanically ventilated patients who received high-titer plasma had a lower relative risk for death as compared with patients in the low-titer group. However, this study did not have a control group [5].
Some randomized studies of CCP therapy in hospitalized patients with COVID-19 have shown no evidence of a clinical benefit. An open-label randomized controlled trial (RCT) conducted earlier in the pandemic in China enrolled 103 patients and had to be terminated early due to a decline in cases. This study did not show any benefit of convalescent plasma but was underpowered for the intended endpoints [21]. A smaller study performed in Argentina of older adults who received convalescent plasma within 72 hours of symptom onset showed reduced risk of progression in patients receiving convalescent plasma [22]. In the Convalescent Plasma in the management of moderate COVID-19 (PLACID) trial, an open-label multicenter RCT in India, 2 units of convalescent plasma was transfused. Neutralizing antibody was not found in 20% of the transfused plasma and the median neutralizing antibody ranged widely from 1:30 to 1:240. In a smaller subgroup of patients with pre-existing neutralizing antibody, no benefit was noted [23].
The Convalescent plasma for COVID (ConCOVID) study was an RCT comparing CCP with standard-of-care therapy. The study was discontinued early after enrollment of 86 patients (43 in each group) because a majority of enrolled patients had high titers of neutralizing antibody at the time of study enrollment, and it was considered futile to continue further with the study. In this small subset of patients, no significant difference was noted in mortality or improvement in disease severity [24].
Similar to prior studies, we found that convalescent plasma transfusion was well tolerated with rare adverse events [22, 25, 26]. In our study, we did not document any major transfusion reactions, including transfusion-associated acute lung injury or hemolytic reactions. Minor transfusion reactions were noted in 4 of 239 patients (0.017%). The therapeutic recommendations and regimens changed rapidly during our study period. In the earlier half of this trial, there were no approved standard therapies, including remdesivir or dexamethasone. Remdesivir received EUA in May 2020 and dexamethasone was more widely used after the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial results in June 2020. The use of both of these therapeutic interventions was similar across both groups.
This study has several strengths, including the multicenter design that included 16 academic and nonacademic community hospitals throughout the Colorado Front Range region. This resulted in recruitment of hospitalized patients with COVID-19 who represented the demographics of the regional COVID-19 pandemic with inclusion and analysis of a diverse cohort of patients. The size of the cohorts analyzed and the matched control cohort were also strengths of the study. Weaknesses of the study include the open-label, nonrandomized study design and lack of a placebo control group. While all CCP was evaluated for the presence of SARS-CoV-2–specific antibody, many of the units were retrospectively tested through individual regional plasma donation centers, resulting in variability in the data. However, this approach also represented CCP treatment and distribution in the community during the pandemic.
Conclusions
The risks of CCP treatment in hospitalized patients with COVID-19 are minimal, and this study shows that treatment with CCP for hospitalized patients with COVID-19 provides no significant improvement in length of hospitalization or inpatient mortality. Ongoing multicenter RCTs of CCP treatment for hospitalized patients and outpatient patients with COVID-19 are critical to define a potential role for CCP therapy in the SARS-CoV-2 pandemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported in part by a grant provided through Colorado Clinical and Translational Sciences Institute (CTSA grant UL1 TR002535 subaward to J. D. B.). The study was also supported by institutional funds from the University of Colorado Anschutz Medical Campus, Department of Medicine (to J. D. B.). L. D. reports employment with Vitalant Research Institute and funding from Biomedical Advanced Research and Development Authority (BARDA; funding through contract 75A50120C00094), during the conduct of the study.
Potential conflicts of interest. K. A. reports receiving speaker honoraria and support to attend meetings as a speaker from Terumo BCT, outside the submitted work. V. K. reports travel support to attend a workshop in primary immunodeficiencies, Melbourne, Australia, January 2020 (Chair and co-organizer; travel and accommodation covered: $8000.00); travel support to attend the College of American Pathologists (CAP) committee meetings (2018 and 2019, approximately $8000 for 3 meetings in 2018 and 3 meetings in 2019); travel support to attend the Clinical Immunology Society, Primary Immunodeficiencies Diagnostic School (Boston, MA) (faculty 2018 and 2019; travel and accommodation covered [approximately $4000 for 2 meetings]); and reports a leadership role with the Association of Medical Laboratory Immunologists (President, 2018–2019; past President, 2020–2021 [unpaid]). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Lakshmi Chauhan, Department of Medicine, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; University of Colorado School of Medicine, Aurora, Colorado, USA.
Jack Pattee, Center for Innovative Design and Analysis, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Joshay Ford, University of Colorado School of Medicine, Aurora, Colorado, USA.
Chris Thomas, University of Colorado School of Medicine, Aurora, Colorado, USA.
Kelsey Lesteberg, Department of Medicine, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Eric Richards, University of Colorado Hospital System, Denver, Colorado, USA.
Carl A Bernas, University of Colorado Hospital System, Denver, Colorado, USA.
Michele Loi, Children’s Hospital Colorado, Aurora, Colorado, USA.
Larry Dumont, University of Colorado School of Medicine, Aurora, Colorado, USA; Vitalant Research Institute, Denver, Colorado, USA.
Kyle Annen, Children’s Hospital Colorado, Aurora, Colorado, USA.
Mary Berg, University of Colorado Hospital System, Denver, Colorado, USA.
Mercedes Zirbes, Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Vijaya Knight, Children’s Hospital Colorado, Aurora, Colorado, USA.
Amanda Miller, Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Timothy C Jenkins, Denver Health Medical Center, Denver, Colorado, USA.
Tellen D Bennett, Children’s Hospital Colorado, Aurora, Colorado, USA; Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; Department of Pediatrics, Section of Informatics and Data Science, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Daniel Monkowski, University of Colorado Hospital System, Denver, Colorado, USA.
Rebecca S Boxer, Institute for Health Research, Kaiser Permanente of Colorado, Aurora, Colorado, USA.
J David Beckham, Department of Medicine, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; University of Colorado School of Medicine, Aurora, Colorado, USA; Colorado Clinical and Translational Sciences Institute, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
References
- 1. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695–703. [DOI] [PubMed] [Google Scholar]
- 2. Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis 1995; 21:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang JS, Chen JT, Liu YX, et al. . A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol 2005; 77:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenbaum U, Klein K, Martinez F, et al. . High levels of common cold coronavirus antibodies in convalescent plasma are associated with improved survival in COVID-19 patients. Front. Immunol. 2021; 12:675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joyner MJ, Carter RE, Senefeld JW, et al. . Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med 2021; 384:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salazar E, Christensen PA, Graviss EA, et al. . Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol 2021; 191:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shenoy AG, Hettinger AZ, Fernandez SJ, Blumenthal J, Baez V. Early mortality benefit with COVID-19 convalescent plasma: a matched control study. Br J Haematol 2021; 192:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021; 397:2049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Zhang W, Hu Y, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonovich VA, Burgos Pratx LD, Scibona P, et al. ; PlasmAr Study Group. . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021;384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pattanayak CW, Rubin DB, Zell ER. Propensity score methods for creating covariate balance in observational studies. Rev Esp Cardiol 2011; 64:897–903. [DOI] [PubMed] [Google Scholar]
- 13. Rubin DB. Propensity score methods. Am J Ophthalmol 2010; 149:7–9. [DOI] [PubMed] [Google Scholar]
- 14. Ciceri F, Castagna A, Rovere-Querini P, et al. . Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol 2020; 217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Kim HJ, Lonjon G, Zhu Y; AME Big-Data Clinical Trial Collaborative Group. . Balance diagnostics after propensity score matching. Ann Transl Med 2019; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol 2011; 11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36:4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L, Zhang W, Hu Y, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama 2020; 324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Libster R, Perez Marc G, Wappner D, et al. . Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators. . Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial). BMJ 2020; 371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. . Convalescent plasma for COVID-19: a randomized clinical trial. medRxiv [Preprint]. 2020. [cited 2021 Feb 1]. Available from: https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1. [Google Scholar]
- 25. Joyner MJ, Bruno KA, Klassen SA, et al. . Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc 2020; 95:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joyner MJ, Wright RS, Fairweather D, et al. . Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 2020; 130:4791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.