Abstract
A better understanding of how human immunodeficiency virus (HIV) coinfection affects the course of hepatitis C virus (HCV) infection is required to select patients with HIV who would benefit from current HCV therapy. Between June 1996 and March 2000, HCV RNA levels were quantified for 1,279 patients at the Louisiana State University Health Sciences Center; 28 of these patients were coinfected with HIV. HCV loads were quantified by the Bayer branched-DNA assay with a lower limit of detection of 0.2 Meq/ml. We compared the median HCV RNA levels of for patients coinfected with HIV and HCV and patients infected only with HCV who were in the same age range (23 to 55 years). The median HCV load for the 28 patients coinfected with HCV and HIV (17.8 Meq/ml) was significantly greater (P < 0.05) than that for similarly aged patients infected only with HCV (6.1 Meq/ml). The HCV load did not correlate with age or sex for either group of patients. A significant (R = −0.4; P < 0.05) negative correlation was observed between HCV load and CD4 count in the coinfected group, for whom the CD4 counts at the time of HCV load analysis ranged from 6 to 1,773/mm3. The increased HCV load in patients coinfected with HCV and HIV compared to that in patients infected only with HCV and the inverse relationship of the HCV load to the CD4 count indicate that immunosuppression results in decreased control of HCV replication. In addition, we report significantly higher HCV loads among coinfected African Americans than Caucasians.
Many patients infected with human immunodeficiency virus (HIV) are also coinfected with hepatitis C virus (HCV) due to shared modes of transmission, namely, transfusion of blood products and intravenous drug use (IVDU). The prevalence of HCV-HIV coinfection ranges from 4 to 90% depending on the percentage of IVDUs in the population (10). With the improved rates of survival of HIV-infected patients since initiation of highly active antiretroviral therapy (HAART), the impact of HCV and other chronic diseases on the quality of life and survival is of concern (33; V. Soriano, E. Valencia, F. Laguna, and J. Gonzalez-Lahoz, Letter, Genitourin. Med. 70:355–356, 1994), such that the National Institutes of Health has recommended treatment for HCV infection in patients coinfected with HIV (23). A recent report shows that increased numbers of HIV patients are dying of liver failure. These deaths are not related to HIV, as more than half of these patients have undetectable HIV RNA and CD4 T-cell counts >200/mm3 (B. H. McGovern, I. Bica, D. Stone, and D. Snydman, Abstr. 37th Infect. Dis. Soc. Am., abstr. 205, 1999). Thus, a better understanding of how HIV affects the course of hepatitis C and the response to anti-HCV therapy is needed to identify patients who would benefit most from anti-HCV therapy.
The course of chronic HCV infection is accelerated in patients coinfected with HIV (12, 13, 30, 32, 35, 37, 41). The livers of coinfected patients have more fibrosis and more severe necroinflammatory changes (1, 3, 5, 14, 27). The frequencies of cirrhosis (5, 27, 32, 35) and hepatic decompensation (12, 22, 29, 37) are increased among coinfected patients compared to those among patients infected only with HCV.
The cause of accelerated HCV disease in the setting of HIV infection is unknown, but several studies report that increased HCV RNA levels are found in patients with dual HCV and HIV infections (7, 13, 35, 40). In some studies (13, 36), higher levels of liver enzymes correspond with higher HCV RNA levels, suggesting that the accelerated and more aggressive course of HCV infection is due to the cytopathic effects of the higher viral load. However, a direct relationship between the level of HCV viremia and either the severity of liver disease or transaminase levels is not universally accepted (9, 16, 18, 19, 20, 24).
In addition, the role of HIV-mediated immunodeficiency in accelerated HCV disease has not been clearly defined. One study suggests that a decrease in the CD4 T-cell count is associated with decreased portal inflammation and piecemeal necrosis (17). In contrast, others (1, 3, 11, 29) report worsened histopathology and increased frequency of hepatic decompensation with decreased CD4 counts. Furthermore, some investigators report an inverse correlation between HCV RNA load and CD4 T-cell count (2, 11, 13, 15), whereas others have found no such correlation (4, 6, 7, 26, 33, 38, 39, 40).
Additional potential correlates of HCV load, e.g., alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, age, race, sex, HCV genotype, hepatitis B surface antigen (HBsAg) positivity, duration of infection, mode of infection, and HIV load, have been investigated, with various results (4, 8, 15, 18, 25, 36, 39, 40). These discrepant findings reflect the difficulty of understanding a disease for which both the initial time of infection is often unknown and the course is characterized by a prolonged asymptomatic period. Better understanding of the pathogenesis of HCV in the setting of HIV is critical as we move into an era of treating HCV infection in patients coinfected with HCV and HIV.
We investigated whether patients coinfected with HCV and HIV have higher HCV loads than non-HIV-infected patients and, if so, whether this can be correlated to their immunocompromised state, as measured by CD4+ T-cell counts. We also explored the influence of other demographic characteristics such as age, sex, and race on HCV load, as these demographic factors may be significant in identifying those who should be targeted for treatment against HCV.
MATERIALS AND METHODS
Collection of patient data.
HCV RNA viral loads were assessed for a total of 1,279 patients at the Louisiana State University Health Sciences Center (LSUHSC) Diagnostic Virology Laboratory from June 1996 to March 2000. Of these, 28 were determined by enzyme-linked immunosorbent assay to be coinfected with HIV. This most likely is an underestimate of the number of patients coinfected with HCV and HIV as we could not verify the HIV status for all samples tested for HCV. At LSUHSC, all patients identified as HIV positive are referred to the Viral Disease Clinic for follow-up. However, we cannot determine how many do not present for further treatment. The test samples were submitted by a variety of primary care clinics within the LSUHSC system. The sampling is most likely biased to adult patients, as children are not treated for chronic HCV infection. Criteria for HCV RNA testing are not available; however, this test is recommended for patients who are known to be HCV antibody positive. As HCV antibody status could not be verified for the 1,251 patients infected only with HCV, those (n = 327) with HCV RNA levels below the limit of detection, <0.2 Meq (1 Meq is 106 genome equivalents)/ml, were excluded from our analysis. Among the patients in the coinfected group, three patients had HCV RNA loads <0.2 Meq/ml. Because these three patients were documented to have HCV antibodies, they were included for analysis and assigned viral loads of zero. The age range of all patients infected only with HCV and with HCV RNA loads >0.2 Meq/ml was 4 months to 89 years. In order to exclude variation due to age in our comparison of the group infected only with HCV with the coinfected group, we used a subgroup of patients infected only with HCV (n = 819) that were in the same age group as the coinfected patients (23 to 55 years). The age distributions in these two groups were both normally distributed. There was no statistical difference between the mean ages for the group infected only with HCV (41.2 years; standard deviation, 6.8 years) and the coinfected group (39.4 years; standard deviation, 8.1 years).
The ages and sexes of all patients were obtained from Diagnostic Virology Laboratory patient logs. Other information, such as CD4 count, ALT level, AST level, and race, for the patients coinfected with HCV and HIV were gathered by chart review. All 28 coinfected patients were monitored at the LSUHSC Viral Disease Clinic. If more than one viral load assay was performed, the highest value was chosen for analysis for patients who were HCV positive only, as the lower values might be a result of anti-HCV therapy. In contrast, the lower HCV RNA level was chosen in the coinfected group since none of these patients were being treated for HCV. In so doing, we believed that we were biased against rather than in favor of our hypothesis. In all cases, HCV RNA levels of >120 Meq/ml are given the value 120 Meq/ml.
Determination of HCV load.
HCV RNA loads were measured by the Quantiplex branched-DNA assay (version 2.0; Bayer Corporation, Norwood, Mass.) according to the manufacturer's instructions. The lower and upper limits of detection of this assay were 0.2 and 120 Meq/ml, respectively.
Statistical analysis.
Because of the observed nonnormality of the data on HCV loads and CD4 counts, nonparametric methods were used to analyze these two variables in our study. The medians for the two groups were compared by the Wilcoxon test. Associations between HCV load and CD4+ T-cell count and between HCV load and age were determined by the Spearman rank correlation coefficient. Since a log10 transformation of HCV load, age, and CD4 count resulted in their normalization, the Pearson correlation coefficients were also calculated to determine significant associations of log10 HCV RNA load with age and log10 CD4 count. Both methods yielded equivalent results. A covariance analysis on the ranks of HCV RNA level and CD4 count was used to assess the association of HCV load with race and CD4 count among the coinfected patients. A significance level of 0.05 was used for all statistical tests.
RESULTS
To test our hypothesis that patients coinfected with HCV and HIV have higher HCV RNA levels than patients infected only with HCV, we compared the median HCV RNA levels for these patients in our study (Table 1). The median HCV RNA level for the coinfected patients (17.8 Meq/ml) was approximately sixfold greater than the median for all patients infected only with HCV (2.8 Meq/ml) (P < 0.05). In an effort to exclude from our analysis any patient who might not be truly HCV infected, we compared the median HCV RNA level for the coinfected patients with the median HCV RNA level for those infected only with HCV and with detectable RNA. The median HCV RNA level for the coinfected patients (17.8 Meq/ml) was approximately threefold greater than the median for that subgroup of patients infected only with HCV (6.0 Meq/ml) (P < 0.05) (Table 1). To exclude the effect of age, we compared the median HCV RNA level for coinfected patients with that for a subgroup of patients who were infected only with HCV and who were in the same age group as the coinfected patients (ages 23 to 55). We also found a threefold greater difference in the HCV RNA load for the coinfected patients. The larger interquartile ranges (Table 1), reflecting data variability, of the HCV RNA levels in the coinfected group are most likely due to the smaller sample size.
TABLE 1.
Median age and HCV load for each patient group
| Patient group | Total
|
Female
|
Male
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size (no.) | Age (yr [IRa]) | HCV loadb (Meq/ml [IR]) | Sample size (no.) | Age (yr [IR]) | HCV loadb (Meq/ml [IR]) | Sample size (no.) | Age (yr [IR]) | HCV loadb (Meq/ml [IR]) | |
| HCV positivec | 1,251 | NCd | 2.8 (11.5) | NC | NC | NC | NC | NC | NC |
| HCV positive with detectable HCVe | 924 | 42 (10) | 6.0 (15) | 441 | 42 (13) | 4.5 (12.2) | 483 | 42 (9) | 78 (17.8) |
| HCV positive with detectable HCV, ages 23 to 55 yr | 819 | 41 (9) | 6.1 (15.4) | 369 | 41 (10) | 4.5 (12.4) | 450 | 42 (9) | 7.7 (17.2) |
| HCV-HIV coinfectionf | 28 | 38 (11) | 17.8 (35.6) | 7 | 35 (8) | 6.8 (61.0) | 21 | 41 (11) | 18 (23.1) |
IR, interquartile range.
HCV load as measured by branched DNA assay.
HCV-infected patients, excluding the 28 coinfected patients.
NC, not calculated.
HCV-infected patients with viral loads greater than or equal to 0.2 Meq/ml and excluding the 28 coinfected patients.
All coinfected patients were within the age range of 23 to 55 years.
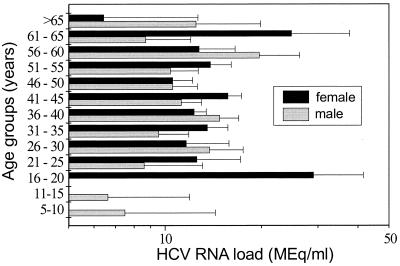
We also examined factors such as sex and age as potential correlates of HCV RNA level. Compared to the patients infected only with HCV, the coinfected patients had a predominance of men (75 versus 52 to 55%; Table 1). The median ages were similar for both HCV-infected and coinfected groups (Table 1). No significant correlation was found between HCV RNA level and age or sex in the HCV-infected group or the coinfected group (Table 1 and Fig. 1 and 2).
FIG. 1.
The 924 patients infected only with HCV and with detectable HCV RNA levels (greater than or equal to 0.2 Meq/ml) were divided into age groups of 5-year increments. Their mean HCV RNA levels were graphed for each age group by sex. No significant association was found between age or sex and HCV load.
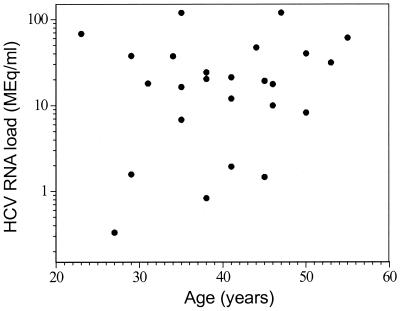
FIG. 2.
HCV RNA levels for patients coinfected with HCV and HIV were compared to their ages. No correlation (P = 0.2) was found between age and HCV RNA loads.
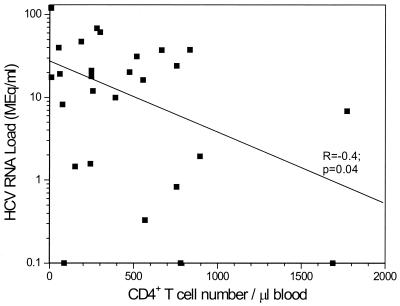
To determine potential determinants of HCV RNA levels in coinfected patients, we examined its association with the AST level, ALT level, CD4+ T-cell count, and race (Table 2). No liver biopsy specimens were available for comparison. AST and ALT levels, often used as markers of liver disease, had no significant association with HCV load (R = 0.3) [P = 0.1] and R = 0.14 [P = 0.5], respectively). The CD4+ T-cell counts at the time that the samples for determination of HCV loads were collected ranged from 6 to 1,773/mm3. Eleven patients had CD4 counts >500/mm3, 8 had CD4 counts between 200 and 500/mm3, and 9 had CD4 counts <200/mm3. We observed a significant inverse relationship between HCV RNA load and CD4+ T-cell count (R = −0.4 [P < 0.05]), as shown in Fig. 3.
TABLE 2.
Demographics and clinical features of patients coinfected with HCV and HIV
| Patient group | Sample size (no.) | Age (yr) | AST level (U/liter [no. of patients])a | ALT level (U/liter [no. of patients])b | CD4+ T-cell countc at diagnosis (no. of patients) | No. of patients by race
|
HCV level (Meq/ml) | |
|---|---|---|---|---|---|---|---|---|
| Caucasian | African American | |||||||
| Male | 21 | 41 | 74.9 (19) | 84.0 (17) | 247 (20) | 9 | 12 | |
| Female | 7 | 35 | 71.6 (7) | 77.5 (6) | 779 (6) | 4 | 3 | |
| Caucasian | 13 | 554 (12) | 10.0 | |||||
| African American | 15 | 248 (14) | 21.2 | |||||
Normal range, 20 to 57 U/liter means are reported.
Normal range, 21 to 72 U/liter; means are reported.
Normal range, 562 to 692/mm3; medians are reported.
FIG. 3.
HCV RNA levels for patients coinfected with HCV and HIV were compared to the CD4+ T-cell counts in peripheral blood. The CD4 counts were obtained at the same time as HCV RNA quantitation. A significant inverse relationship (P < 0.05) was found between HCV load and CD4+ T-cell count.
In addition to CD4 count, race also had a significant association with the HCV RNA level within the coinfected group. Fifteen African-American and 13 Caucasian patients were, coinfected with HCV and HIV (Table 2). The median HCV RNA level for the African-American patients (21.2 Meq/ml) was significantly higher than the median HCV RNA level for the Caucasian patients (10.0 Meq/ml; Table 2). The majority of the coinfected patients contracted HIV and HCV through IVDU (16 of 28; 57%) or male homosexual activity (12 of 28; 43%), or both (10 of 28; 36%). Equal numbers of African Americans and Caucasians admitted to IVDU and male homosexuality, and only one patient, a Caucasian female, was a hemophiliac. Only one patient, an African American, had detectable HBsAg, even though 12 patients (7 of whom were African Americans) were hepatitis B core antibody positive. We further examined whether the higher median HCV RNA load among African Americans was related to lower CD4 counts on initial diagnosis of HIV infection. We found that the median CD4 count for coinfected American-African patients was 248/mm3, whereas it was 554/mm3 for Caucasian patients (P < 0.05). However, when controlling for CD4 count, the difference in the median HCV RNA level between the two racial groups is no longer significant at a P level of 0.05, probably due to the resulting smaller sample sizes.
DISCUSSION
In the study described in this report we confirmed previous findings that HCV RNA levels in patients coinfected with HCV and HIV are higher than those in patients infected only with HCV (7, 13, 35, 39, 40). As reported by other investigators, this higher HCV RNA level does not correlate with age or sex (21, 39, 40). A recent study (39) of determinants of HCV RNA levels among a cohort of IVDUs found a strong correlation of HCV RNA level with age for the group infected only with HCV (n = 501) but no such correlation among the coinfected group (n = 468). This discrepancy in the relationship of HCV RNA load to age by patient group is not well explained. In our study, we found no correlation between age and HCV RNA level for either monoinfected or coinfected patients (Fig. 1 and 2).
We also examined the potential correlates of HCV RNA level in our coinfected group of patients. Like other investigators (4, 15, 39), we do not find a significant correlation between HCV RNA load and AST or ALT level. We believe this is because changes in AST and ALT levels do not correlate with the degree of liver damage (18). We, like others (2, 11, 13, 15), find that HCV RNA levels are inversely related to CD4+ T-cell count (P < 0.05), suggesting that immune status does play a role in controlling HCV replication. Whether the higher HCV load and the lower CD4 count result in more advanced liver disease. (1, 3, 11, 29) or to less severe liver histopathology (17) needs further study to help clarify the mechanism of hepatic injury in individuals with chronic HCV infection.
A unique finding in our study is the association of race with HCV RNA level among our coinfected population. The higher median HCV RNA level among African-American patients cannot be attributed to the mode of HCV acquisition, since equal numbers of African Americans and Caucasians admitted to being male homosexuals and intravenous drug abusers. Although the detection of HBsAg is associated with lower HCV RNA levels (39), hepatitis B virus infection status does not explain the higher level of HCV RNA among African Americans in our study. On the basis of the observed significant negative correlation between HCV load and CD4 count, we suspect that the higher HCV RNA levels among African Americans are most likely secondary to their lower CD4 counts. However, the small sample size precludes support of this association by covariance analysis. A major uncontrolled factor is the duration of HCV infection. The lower CD4 counts among African-American patients suggest more advanced HIV infection and probably a longer duration of infection with HIV and HCV at the time of presentation to our clinic. In addition, there may also be undefined genetic and immunologic factors that lead to differences in controlling HCV replication, since differential responses to anti-HCV combination therapy have been reported for African Americans and Caucasians (J. W. King, A. Shakashiro, D. R. Favrot, K. Kennedy, L. A. Balart, J. A. Kirkikis, W. E. Lyles, J. Hollier, D. Srinivas, J. Achord, Abstract, 36th Infect. Dis. Soc. Am., abstr. 292, 1998). We further speculate that HCV genotype differences may potentially contribute to higher HCV RNA levels among our coinfected African-American patients. Higher HCV RNA levels were reported for patients infected with genotype 1 strains (4).
Since the advent of HAART, HIV-infected patients have enjoyed improved survival rates but are experiencing increased rates of morbidity and mortality from other chronic diseases such as hepatitis C. Better understanding of the effect of HIV coinfection, determinants of increased HCV RNA levels, and the significance of high HCV RNA levels to the accelerated course of HCV infection are needed to better identify patients who would benefit most from treatment against HCV infection. In the study described in this report, we showed that CD4+ T-cell count and race, but not age or sex, are important determinants of the HCV RNA load. In light of recent reports suggesting that HAART results in a transient worsening of HCV infection (28, 31), further evaluation of the effect of HAART on the course of HCV infection and the response to treatment against HCV infection are also needed. Further attention should focus on racial differences in HCV genotype predominance, the course of HCV infection, and responses to anti-HCV therapy.
ACKNOWLEDGMENTS
NIH grant KO8 AI01438 and the LSUHSC Intramural Feist-Weiller Cancer Award of Wun-Ling Chang supported this work.
We thank Henri van der Heyde for thoughtful comments and criticisms.
REFERENCES
- 1.Allory Y, Charlotte F, Benhamou Y, Opolon P, Le Charpentier Y, Poynard T. Impact of human immunodeficiency virus infection on the histological features of chronic hepatitis C: a case-control study. The MULTIVIRC group. Hum Pathol. 2000;31:69–74. doi: 10.1016/s0046-8177(00)80201-4. [DOI] [PubMed] [Google Scholar]
- 2.Beld M, Penning M, Lukashov V, McMorrow M, Roos M, Pakker N, van den H A, Goudsmit J. Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology. 1998;244:504–512. doi: 10.1006/viro.1998.9130. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou Y, Bochet M, Martino V D, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus co-infected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 4.Berger A, von Depka P M, Doerr H W, Rabenau H, Weber B. Hepatitis C plasma viral load is associated with HCV genotype but not with HIV co-infection. J Med Virol. 1996;48:339–343. doi: 10.1002/(SICI)1096-9071(199604)48:4<339::AID-JMV7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Bierhoff E, Fischer H P, Willsch E, Rockstroh J, Spengler U, Brackmann H H, Oldenburg J. Liver histopathology in patients with concurrent chronic hepatitis C and HIV infection. Virchows Arch. 1997;430:271–277. doi: 10.1007/BF01092749. [DOI] [PubMed] [Google Scholar]
- 6.Chambost H, Gerolami V, Halfon P, Thuret I, Michel G, Sicardi F, Rousseau S, Perrimond H, Cartouzou G. Persistent hepatitis C virus RNA replication in haemophiliacs: role of co-infection with human immunodeficiency virus. Br J Haematol. 1995;91:703–707. doi: 10.1111/j.1365-2141.1995.tb05372.x. [DOI] [PubMed] [Google Scholar]
- 7.Cribier B, Rey D, Schmitt C, Lang J M, Kirn A, Stoll-Keller F. High hepatitis C viraemia and impaired antibody response in patients co-infected with HIV. AIDS. 1995;9:1131–1136. doi: 10.1097/00002030-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cribier B, Schmitt C, Rey D, Uhl G, Lang J M, Vetter D, Kirn A, Stoll-Keller F. HIV increases hepatitis C viraemia irrespective of the hepatitis C virus genotype. Res Virol. 1997;148:267–271. doi: 10.1016/s0923-2516(97)88363-x. [DOI] [PubMed] [Google Scholar]
- 9.De Moliner L, Pontisso P, De Salvo G L, Cavalletto L, Chemello L, Alberti A. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut. 1998;42:856–860. doi: 10.1136/gut.42.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieterich D T, Purow J M, Rajapaksa R. Activity of combination therapy with interferon alfa-2b plus ribavirin in chronic hepatitis C patients co-infected with HIV. Semin Liver Dis. 1999;19(Suppl. 1):87–94. [PubMed] [Google Scholar]
- 11.Dragoni F, Cafolla A, Gentile G, Mazzucconi M G, Vella S, Di Corpo U, Tosti M E, Pisani G, Donato V, Martino P. HIV-HCV RNA loads and liver failure in co-infected patients with coagulopathy. Haematologica. 1999;84:525–529. [PubMed] [Google Scholar]
- 12.Eyster M E, Diamondstone L S, Lien J M, Ehmann W C, Quan S, Goedert J J. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of co-infection with human immunodeficiency virus. The Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1993;6:602–610. [PubMed] [Google Scholar]
- 13.Eyster M E, Fried M W, Di Bisceglie A M, Goedert J J. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020–1023. [PubMed] [Google Scholar]
- 14.Garcia-Samaniego J, Soriano V, Castilla J, Bravo R, Moreno A, Carbo J, Iniguez A, Gonzalez J, Munoz F. Influence of hepatitis C virus genotypes and HIV infection on histological severity of chronic hepatitis C. The Hepatitis/HIV Spanish Study Group. Am J Gastroenterol. 1997;92:1130–1134. [PubMed] [Google Scholar]
- 15.Ghany M G, Leissinger C, Lagier R, Sanchez-Pescador R, Lok A S. Effect of human immunodeficiency virus infection on hepatitis C virus infection in hemophiliacs. Dig Dis Sci. 1996;41:1265–1272. doi: 10.1007/BF02088247. [DOI] [PubMed] [Google Scholar]
- 16.Gretch D, Corey L, Wilson J, dela Rosa C, Willson R, Carithers R, Jr, Busch M, Hart J, Sayers M, Han J. Assessment of hepatitis C virus RNA levels by quantitative competitive RNA polymerase chain reaction: high-titer viremia correlates with advanced stage of disease. J Infect Dis. 1994;169:1219–1225. doi: 10.1093/infdis/169.6.1219. [DOI] [PubMed] [Google Scholar]
- 17.Guido M, Rugge M, Fattovich G, Rocchetto P, Cassaro M, Chemello L, Noventa F, Giustina G, Alberti A. Human immunodeficiency virus infection and hepatitis C pathology. Liver. 1994;14:314–319. doi: 10.1111/j.1600-0676.1994.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 18.Haber M M, West A B, Haber A D, Reuben A. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol. 1995;90:1250–1257. [PubMed] [Google Scholar]
- 19.Hayashi J, Furusyo N, Ariyama I, Sawayama Y, Etoh Y, Kashiwagi S. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C viremia. J Infect Dis. 2000;181:1523–1527. doi: 10.1086/315431. [DOI] [PubMed] [Google Scholar]
- 20.Haydon G H, Jarvis L M, Blair C S, Simmonds P, Harrison D J, Simpson K J, Hayes P C. Clinical significance of intrahepatic hepatitis C virus levels in patients with chronic HCV infection. Gut. 1998;42:570–575. doi: 10.1136/gut.42.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau J Y, Davis G L, Kniffen J, Qian K P, Urdea M S, Chan C S, Mizokami M, Neuwald P D, Wilber J C. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993;341:1501–1504. doi: 10.1016/0140-6736(93)90635-t. . (erratum, 342:504.) [DOI] [PubMed] [Google Scholar]
- 22.Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas C M. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–1258. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health. 1997. Management of hepatitis C. Consensus statement. 15(3):1–41. [PubMed]
- 24.Negro F, Krawczynski K, Quadri R, Rubbia-Brandt L, Mondelli M, Zarski J P, Hadengue A. Detection of genomic- and minus-strand of hepatitis C virus RNA in the liver of chronic hepatitis C patients by strand-specific semiquantitative reverse-transcriptase polymerase chain reaction. Hepatology. 1999;29:536–542. doi: 10.1002/hep.510290223. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Gracia T, Galan F, Fernandez-Gutierrez C, Giron J A, Rodriguez-Iglesias M. Relationship of hepatitis C viremia to HIV state and to infection by specific hepatitis C genotypes. Liver. 1999;19:288–293. doi: 10.1111/j.1478-3231.1999.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 26.Picchio G R, Nakatsuno M, Boggiano C, Sabbe R, Corti M, Daruich J, Perez-Bianco R, Tezanos-Pinto M, Kokka R, Wilber J, Mosier D. Hepatitis C (HCV) genotype and viral titer distribution among Argentinean hemophilic patients in the presence or absence of human immunodeficiency virus (HIV) co-infection. J Med Virol. 1997;52:219–225. [PubMed] [Google Scholar]
- 27.Pol S, Lamorthe B, Thi N T, Thiers V, Carnot F, Zylberberg H, Berthelot P, Brechot C, Nalpas B. Retrospective analysis of the impact of HIV infection and alcohol use on chronic hepatitis C in a large cohort of drug users. Hepatol. 1998;28:945–950. doi: 10.1016/s0168-8278(98)80341-3. [DOI] [PubMed] [Google Scholar]
- 28.Puoti M, Gargiulo F, Roldan E Q, Chiodera A, Palvarini L, Spinetti A, Zaltron S, Putzolu V, Zanini B, Favilli F, Turano A, Carosi G. Liver damage and kinetics of hepatitis C virus and human immunodeficiency virus replication during the early phases of combination antiretroviral treatment. J Infect Dis. 2000;181:2033–2036. doi: 10.1086/315529. [DOI] [PubMed] [Google Scholar]
- 29.Rockstroh J K, Spengler U, Sudhop T, Ewig S, Theisen A, Hammerstein U, Bierhoff E, Fischer H P, Oldenburg J, Brackmann H H, Sauerbruch T. Immunosuppression may lead to progression of hepatitis C virus- associated liver disease in hemophiliacs co-infected with HIV. Am J Gastroenterol. 1996;91:2563–2568. [PubMed] [Google Scholar]
- 30.Roudot-Thoraval F, Bastie A, Pawlotsky J M, Dhumeaux D. Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6,664 patients. The Study Group for the Prevalence and the Epidemiology of Hepatitis C Virus. Hepatology. 1997;26:485–490. doi: 10.1002/hep.510260233. [DOI] [PubMed] [Google Scholar]
- 31.Rutschmann O T, Negro F, Hirschel B, Hadengue A, Anwar D, Perrin L H. Impact of treatment with human immunodeficiency virus (HIV) protease inhibitors on hepatitis C viremia in patients co-infected with HIV. Infect Dis. 1998;177:783–785. doi: 10.1086/517808. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Quijano A, Andreu J, Gavilan F, Luque F, Abad M A, Soto B, Munoz J, Aznar J M, Leal M, Lissen E. Influence of human immunodeficiency virus type 1 infection on the natural course of chronic parenterally acquired hepatitis C. Eur J Clin Microbiol Infect Dis. 1995;14:949–953. doi: 10.1007/BF01691375. [DOI] [PubMed] [Google Scholar]
- 33.Sherman K E, O'Brien J, Gutierrez A G, Harrison S, Urdea M, Neuwald P, Wilber J. Quantitative evaluation of hepatitis C virus RNA in patients with concurrent human immunodeficiency virus infections. J Clin Microbiol. 1993;31:2679–2682. doi: 10.1128/jcm.31.10.2679-2682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriano V, Garcia-Samaniego J, Valencia E, Rodriguez-Rosado R, Munoz F, Gonzalez-Lahoz J. Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. Eur J Epidemiol. 1999;15:1–4. doi: 10.1023/a:1007506617734. [DOI] [PubMed] [Google Scholar]
- 35.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo J A, Garcia-Bengoechea M, Hernandez-Quero J, Rey C, Abad M A, Rodriguez M, Sales G M, Gonzalez F, Miron P, Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 36.Tagariello G, Pontisso P, Davoli P G, Ruvoletto M G, Traldi A, Alberti A. Hepatitis C virus genotypes and severity of chronic liver disease in haemophiliacs. Br J Haematol. 1995;91:708–713. doi: 10.1111/j.1365-2141.1995.tb05373.x. [DOI] [PubMed] [Google Scholar]
- 37.Telfer P, Sabin C, Devereux H, Scott F, Dusheiko G, Lee C. The progression of HCV-associated liver disease in a cohort of haemophilic patients. Br J Haematol. 1994;87:555–561. doi: 10.1111/j.1365-2141.1994.tb08312.x. [DOI] [PubMed] [Google Scholar]
- 38.Telfer P T, Brown D, Devereux H, Lee C A, Dusheiko G M. HCV RNA levels and HIV infection: evidence for a viral interaction in haemophilic patients. Br J Haematol. 1994;88:397–399. doi: 10.1111/j.1365-2141.1994.tb05038.x. [DOI] [PubMed] [Google Scholar]
- 39.Thomas D L, Astemborski J, Vlahov D, Strathdee S A, Ray S C, Nelson K E, Galai N, Nolt K R, Laeyendecker O, Todd J A. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis. 2000;181:844–851. doi: 10.1086/315314. [DOI] [PubMed] [Google Scholar]
- 40.Thomas D L, Shih J W, Alter H J, Vlahov D, Cohn S, Hoover D R, Cheung L, Nelson K E. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 41.Zylberberg H, Pol S. Reciprocal interactions between human immunodeficiency virus and hepatitis C virus infections. Clin Infect Dis. 1996;23:1117–1125. doi: 10.1093/clinids/23.5.1117. [DOI] [PubMed] [Google Scholar]