Abstract
OBJECTIVES
The aim of this study was to investigate the effect of management on the risk for recurrent events among patients with cryptogenic ischemic stroke or transient ischemic attack.
BACKGROUND
The combination of patent foramen ovale (PFO) and hypercoagulability may greatly increase the risk for paradoxical embolism. However, previous randomized controlled trials evaluating the efficacy of PFO closure excluded these potential high-risk patients.
METHODS
Patients diagnosed with PFO attributable cryptogenic embolism were prospectively, without randomization, recruited from January 2005 to March 2018. The relationship between thrombophilia and recurrent events was evaluated in overall patients. Multivariate Cox regression was conducted to assess the relative risk for recurrence in PFO closure and medical therapy groups.
RESULTS
A total of 591 patients with cryptogenic embolism with PFO were identified. The median duration of follow-up was 53 months, and thrombophilia significantly increased the risk for recurrent events (hazard ratio [HR]: 1.85; 95% confidence interval [CI]: 1.09 to 3.16; p = 0.024). PFO closure was superior to medical therapy in overall patients (HR: 0.16; 95% CI: 0.09 to 0.30; p < 0.001). Of the 134 patients (22.7%) with thrombophilia, there was a difference in the risk for recurrence events between the PFO closure (6 of 89) and medical therapy (15 of 45) groups (HR: 0.25; 95% CI: 0.08 to 0.74; p = 0.012). There was no potential heterogeneity in the further subgroup analysis.
CONCLUSIONS
Patients with cryptogenic stroke with PFO and hypercoagulable state had increased risk for recurrent stroke or transient ischemic attack. PFO closure provided a lower risk for recurrent events compared with medical therapy alone.
Keywords: closure, cryptogenic stroke, patent foramen ovale, secondary prevention, thrombophilia
Previous studies have shown that inherited or acquired thrombophilia is associated with higher risk for venous thromboembolism and ischemic stroke (1–6) and that patent foramen ovale (PFO) is a potential etiology for cryptogenic stroke (7–10). A combination of hypercoagulable state and PFO is not rare in clinical practice, with a prevalence of 5% to 31% (11–15), and the presence of the 2 conditions may increase the risk for stroke (14).
Advances in randomized controlled trials (RCTs) and clinical observation studies showed that PFO closure was superior to medical therapy in reducing recurrent stroke or transient ischemic attack (TIA) (16–23). However, rigorous RCT criteria excluded many potentially high-risk patients with thrombophilia. Thus far, there has been little beside clinical experience to guide treatment of patients with PFO stroke with thrombophilia. One study showed that in 72 patients who underwent PFO closure, patients with PFO with thrombophilia (n = 20) had a higher risk for recurrence and derived similar benefit compared with those without thrombophilia (n = 52) (14). However, the study is a retrospective single-arm investigation of PFO closure only. We aimed to prospectively evaluate the potential benefit of PFO closure compared with medical therapy in patients with cryptogenic embolism and thrombophilia.
METHODS
PATIENT POPULATION.
Patients with PFO diagnosed at the Massachusetts General Hospital from January 1, 2005, to March 1, 2018, were prospectively, without randomization, enrolled in the PFO Registry Study with the following criteria: 1) neurological deficits of acute onset suggestive of stroke or TIA and confirmed by at least 2 vascular neurologists and supported by brain magnetic resonance imaging or computed tomography; 2) patients with any identified potential cause of ischemic stroke or TIA other than the PFO were excluded, such as significant carotid artery stenosis, atrial fibrillation (AF), significant left ventricular dysfunction, infective endocarditis, trauma, and so on, per published literature on cryptogenic stroke (24–26); 3) PFO was confirmed by transthoracic echocardiography with a bubble study and/or transesophageal echocardiography; and 4) patients with oral contraceptive use, active cancer, and other disease that may have effects on thrombophilia tests were also excluded. The Massachusetts General Hospital Investigational Review Board approved the study and all patients gave informed consent.
HYPERCOAGULATION TEST.
The following hypercoagulation tests were evaluated in all patients: protein C and S, antithrombin III, factor V Leiden, homocysteine, the anticardiolipin antibodies (immunoglobulin G and immunoglobulin M), lupus anticoagulant, and prothrombin G20210A mutation. Factor VIII levels were also measured, when the functional assay for protein S was decreased. For patients with deficiencies in protein C, protein S, antithrombin III, positive anticardiolipin antibodies, and/or lupus anticoagulant at their onset time, repeat tests were required at least 3 months later. Patients were assigned to the normal group if the initial “deficiencies” subsequently normalized after the presumed acute-phase response abated. Similarly, patients with initially positive anticardiolipin antibodies and/or lupus anticoagulant were considered to exhibit thrombophilia only if results of repeat tests were positive after 3 months.
ECHOCARDIOGRAPHIC ASSESSMENT.
A PFO with intracardiac right-to-left shunting was characterized by the appearance of microbubbles in the left atrium within 3 beats of right atrial opacification at rest or with release of the Valsalva maneuver. Shunt size was defined as small for the presence of 3 to 9 microbubbles in the left atrium, moderate for 10 to 30 microbubbles, and large if more than 30 microbubbles appeared (27,28). Atrial septal aneurysm (ASA) was diagnosed when septal excursion exceeded 10 mm into the left and/or right atrium, and a hypermobile atrial septum was defined if the hypermobility did not meet the criteria for ASA (27,28).
TREATMENT STRATEGIES.
Patients with suspected paradoxical embolism were evaluated in a multidisciplinary clinic including specialists in cardiology, vascular neurology, vascular medicine, and hematology. Patients were discussed at a weekly meeting of the interdisciplinary PFO committee. On the basis of adequate assessment, including brain imaging, at least 24-h Holter cardiac monitoring, transthoracic echocardiography and/or transesophageal echocardiography, thrombophilia tests, and pelvic venography on either computed tomography or magnetic resonance imaging, the committee considered the appropriateness of PFO closure. Patients received aspirin (81 or 325 mg/day) and/or clopidogrel (75 mg/day) at the discretion of the operator. Patients who had thrombophilia and a single embolism were anticoagulated with warfarin for 3 months with a target international normalized ratio between 2 and 3 and then switched to aspirin. Patients with 2 or more embolic events were anticoagulated with lifelong warfarin therapy.
FOLLOW-UP.
Follow-up visits with the specialists typically occurred at 1 month, 3 months, 6 months, and annually for 5 years or more. Echocardiographic and medication data were recorded at each follow-up visit. The primary endpoint was the composite of recurrence of TIA or stroke. Secondary endpoints were recurrence of TIA and stroke individually. Recurrent events were clinically evaluated by vascular neurologists and by full stroke workup including lab, cardiac, and imaging studies.
STATISTICAL ANALYSES.
Categorical variables are expressed as count (percentage) and were compared using the chi-square test. Continuous variables are represented as mean ± SD and were compared using Student’s t-test or the Fisher exact test, as appropriate. Kaplan-Meier survival analysis was used to assess the relationship between thrombophilia and recurrent events in overall patients. Multivariate Cox regression was conducted to evaluate the relative risk for recurrence in PFO closure and medical therapy groups. Further subgroup analyses were designed to explore the interaction of treatment options effect. A 2-sided p value <0.05 was considered to indicate statistical significance.
RESULTS
STUDY PATIENTS.
We enrolled 591 patients with cryptogenic embolism attributed to PFO, and a total of 134 patients (22.7%) were identified with at least 1 thrombophilia abnormality. Distribution of coagulation abnormalities in our study is shown in Supplemental Table 1.
THROMBOPHILIA AND RISK FOR RECURRENCE IN OVERALL PATIENTS.
The baseline characteristics of patients with and without thrombophilia are shown in Table 1. The median duration of follow-up was 53 months (interquartile range: 24 to 86 months) in overall patients. The main outcome occurred in 21 patients (15.7%) among those with thrombophilia and in 38 patients (8.3%) among those without thrombophilia (hazard ratio [HR] for patients with thrombophilia vs. without thrombophilia: 1.85; 95% confidence interval [CI]: 1.09 to 3.16; p = 0.024) (Figure 1).
TABLE 1.
Baseline Characteristics of the 591 Patients With and Without Thrombophilia
| With Thrombophilia (n = 134) | Without Thrombophilia (n = 457) | p Value | |
|---|---|---|---|
| Age, yrs | 51.7 ± 14.4 | 49.8 ± 13.3 | 0.140 |
|
| |||
| Female | 77 (57.5) | 205 (44.9) | 0.010 |
|
| |||
| BMI, kg/m2 | 27.3 ± 7.6 | 26.9 ± 8.5 | 0.615 |
|
| |||
| Medical history | |||
| Hypertension | 49 (36.6) | 161 (35.2) | 0.776 |
| Diabetes mellitus | 12 (9.0) | 40 (8.8) | 0.942 |
| Hypercholesterolemia | 48 (35.8) | 176 (38.5) | 0.572 |
| CAD history | 9 (6.7) | 23 (5.0) | 0.449 |
| Smoking status | |||
| Current | 14 (10.4) | 72 (15.8) | 0.126 |
| Former | 31 (23.1) | 17 (3.7) | <0.001 |
| Family history of stroke | 25 (18.7) | 94 (20.6) | 0.627 |
|
| |||
| Interatrial septal mobility | |||
| Atrial septal aneurysm | 24 (17.9) | 81 (17.7) | 0.960 |
| Hypermobility | 26 (19.4) | 74 (16.2) | 0.383 |
|
| |||
| Interatrial right-to-left shunt size | |||
| Small | 42 (31.3) | 167 (36.5) | 0.268 |
| Medium | 34 (25.4) | 102 (22.3) | 0.460 |
| Large | 58 (43.3) | 188 (41.1) | 0.658 |
Values are mean ± SD or n (%).
BMI = body mass index; CAD = coronary artery disease; RoPE = Risk of Paradoxical Embolism.
FIGURE 1. Thrombophilia and Risk for Recurrence in Overall Patients.
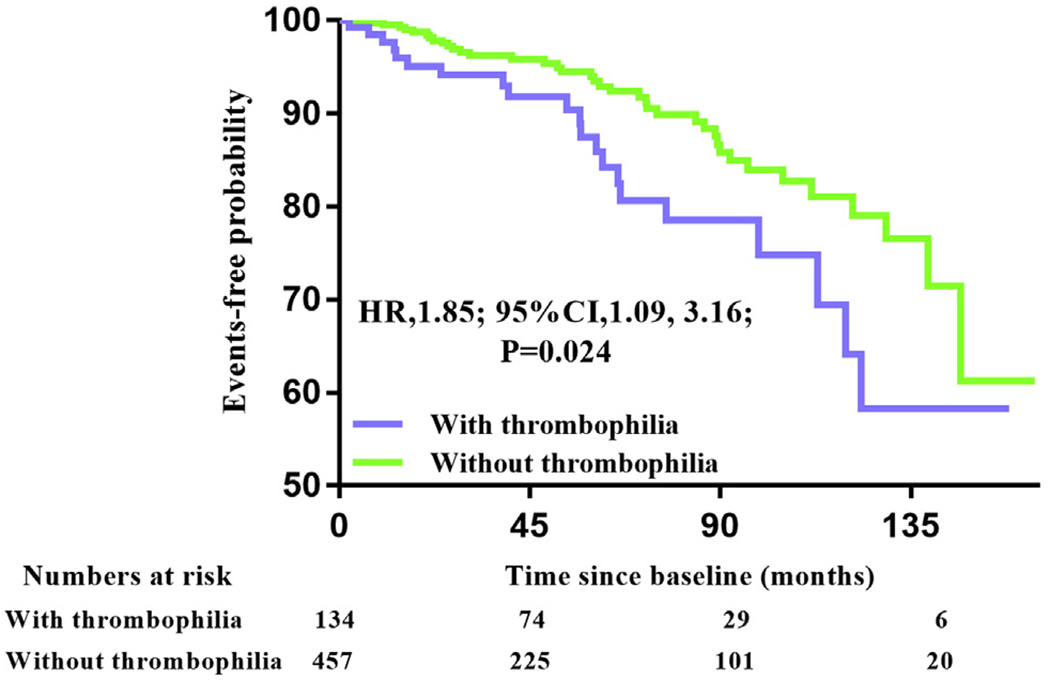
Kaplan-Meier cumulative estimates indicated that the combination of thrombophilia and patent foramen ovale (PFO) increased the risk for recurrent events in patients with cryptogenic PFO stroke or transient ischemic attack. CI = confidence interval; HR = hazard ratio.
OUTCOMES IN THE PFO CLOSURE GROUP COMPARED WITH THE MEDICAL THERAPY GROUP.
Of 591 patients with cryptogenic stroke, 383 underwent PFO closure and 208 received medical therapy only. The comparison of the baseline characteristics of the 2 groups is shown in Table 2. Patients who underwent PFO closure were younger and presented more frequently with ASA and moderate to large shunt size at baseline. Patients who received medical therapy presented more frequently with traditional risk factors for cerebrovascular disease. Recurrent events occurred in 15 patients (3.9%) in the PFO closure group and 44 patients (21.2%) in the medical therapy group (HR for PFO closure vs. medical therapy: 0.22; 95% CI: 0.12 to 0.39; p < 0.001) (Figure 2). Multivariate Cox regression showed that the results remained significant (HR: 0.16; 95% CI: 0.09 to 0.30; p < 0.001) when adjusted by age, sex, traditional risk factors (hypertension, diabetes, hypercholesterolemia, and smoking history) and interatrial characteristics (moderate to large shunt size and ASA).
TABLE 2.
Baseline Characteristics of Patients Who Underwent PFO Closure and Patients Who Received Medical Therapy
| PFO Closure (n = 383) | Medical Therapy (n = 208) | p Value | |
|---|---|---|---|
| Age, yrs | 48.6 ± 12.1 | 53.2 ± 15.4 | <0.001 |
|
| |||
| Female | 171 (44.6) | 111 (53.4) | 0.043 |
|
| |||
| BMI, kg/m2 | 27.0 ± 7.7 | 27.0 ± 9.2 | 0.974 |
|
| |||
| Medical history | |||
| Hypertension | 117 (30.5) | 93 (44.7) | 0.001 |
| Diabetes mellitus | 23 (6.0) | 29 (13.9) | 0.001 |
| Hypercholesterolemia | 134 (35.0) | 90 (43.3) | 0.047 |
| CAD history | 12 (3.1) | 20 (9.6) | 0.001 |
| Smoking status | |||
| Current | 56 (14.6) | 30 (14.4) | 0.948 |
| Former | 22 (5.7) | 26 (12.5) | 0.004 |
| Family history of stroke | 83 (21.7) | 36 (17.3) | 0.206 |
|
| |||
| Interatrial septal mobility | |||
| Atrial septal aneurysm | 82 (21.4) | 23 (11.1) | 0.002 |
| Hypermobility | 70 (18.3) | 30 (14.4) | 0.233 |
|
| |||
| Interatrial right-to-left shunt size | |||
| Small | 104 (27.2) | 105 (50.5) | <0.001 |
| Medium | 98 (25.6) | 38 (18.3) | 0.044 |
| Large | 181 (47.3) | 65 (31.3) | <0.001 |
|
| |||
| Thrombophilia | 89 (23.2) | 45 (21.6) | 0.657 |
Values are mean ± SD or n (%).
PFO = patent foramen ovale; other abbreviations as in Table 1.
FIGURE 2. Primary Endpoint in the PFO Closure Group Versus the Medical Therapy Group.
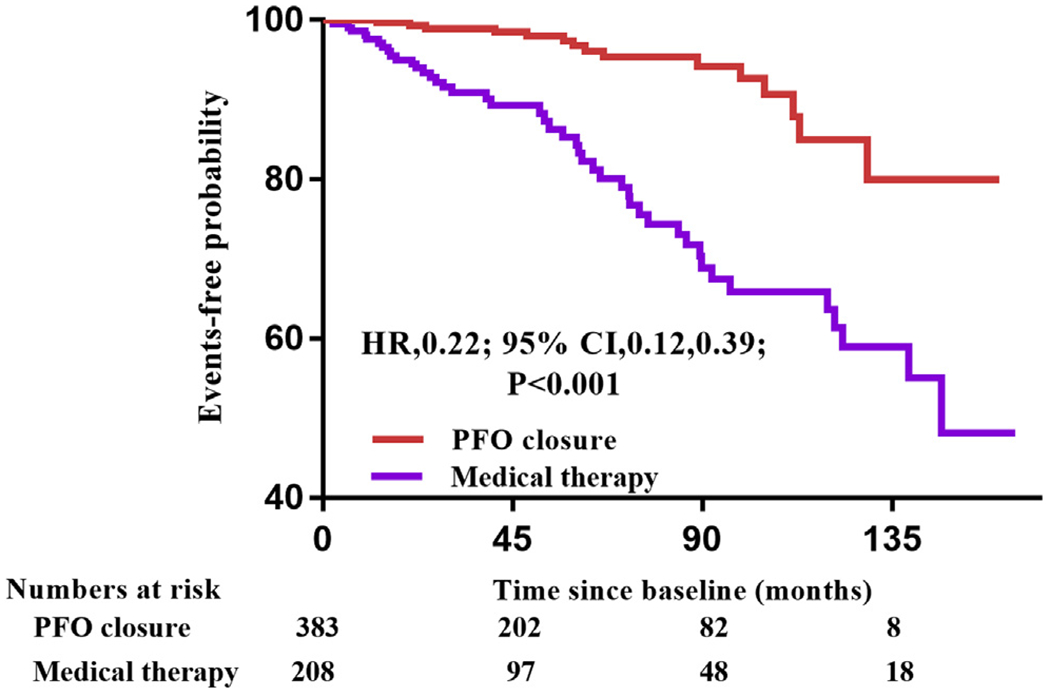
Kaplan-Meier cumulative estimates indicated that patent foramen ovale (PFO) closure significantly reduced recurrent events of stroke or transient ischemic attack compared with medical therapy. CI = confidence interval; HR = hazard ratio.
Kaplan-Meier curves of treatments in various groups are shown in the Central Illustration.
CENTRAL ILLUSTRATION.
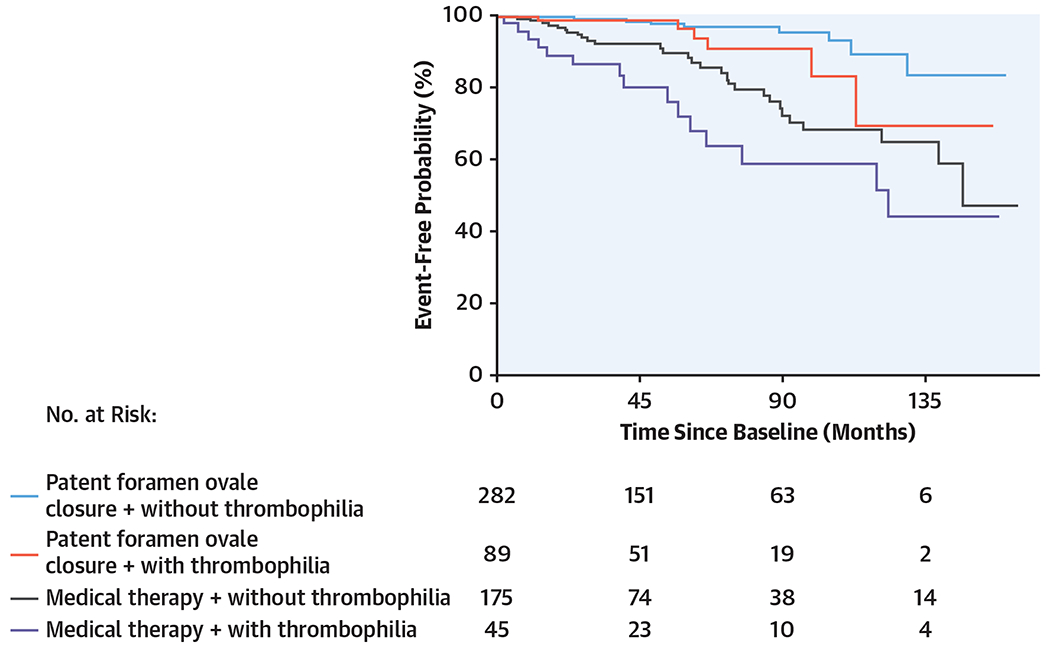
Kaplan-Meier Cumulative Estimates of the Rate of the Primary Endpoint in Different Groups
TREATMENT CHOICE AND OUTCOMES IN PATIENTS WITH THROMBOPHILIA.
A total of 88 patients underwent PFO closure, with successful device implantation in all patients, and 46 patients received medical therapy only. The baseline characteristics of the 2 groups are shown in Supplemental Table 2. The median duration of follow-up was 54 months in the closure group and 53 months in the medical therapy group. The primary endpoint occurred in 6 patients (6.7%) in the closure group and 15 patients (33.3%) in the medical therapy group (HR for PFO closure vs. medical therapy: 0.23; 95% CI: 0.09 to 0.61; p = 0.003). The results remained significant in multivariate Cox regression (Table 3). Further subgroup analyses (Figure 3) to determine potential heterogeneity in relation to baseline characteristics showed no interactions across age, sex, traditional risk factors (hypertension, hypercholesterolemia, and smoking history), PFO characteristics (shunt size and ASA/hypermobile), and number of hypercoagulation abnormalities.
TABLE 3.
Multivariate Cox Regression for the Primary and Secondary Endpoints Between PFO Closure and Medical Therapy in the 134 Patients With Thrombophilia
| Outcomes | PFO Closure (n = 89) | Medical Therapy (n = 45) | Model 1* |
Model 2† |
Model 3‡ |
|||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Recurrent stroke or TIA | 6 (6.7) | 15 (33.3) | 0.21 (0.08–0.57) | 0.002 | 0.21 (0.08–0.57) | 0.002 | 0.25 (0.08–0.74) | 0.012 |
|
| ||||||||
| Recurrent stroke | 1 (1.1) | 6 (13.3) | 0.09 (0.01–0.83) | 0.033 | 0.08 (0.01–0.75) | 0.027 | 0.06 (0.01–0.62) | 0.019 |
|
| ||||||||
| Recurrent TIA | 5 (5.6) | 9 (20.0) | 0.29 (0.09–0.92) | 0.036 | 0.30 (0.09–0.97) | 0.043 | 0.48 (0.13–1.74) | 0.262 |
Model 1 adjusted for age, sex, and therapy options.
Model 2 adjusted for age, sex, therapy options and traditional risk factors (hypertension, diabetes, hypercholesterolemia, and smoking history).
Model 3 adjusted for age, sex, therapy options, traditional risk factors (hypertension, diabetes, hypercholesterolemia, and smoking history) and PFO characteristics (large shunt and atrial septal aneurysm).
CI = confidence interval; HR = hazard ratio; PFO = patent foramen ovale; TIA = transient ischemic attack.
FIGURE 3. Subgroup Analyses of the Primary Endpoint in the 134 Patients With Thrombophilia.
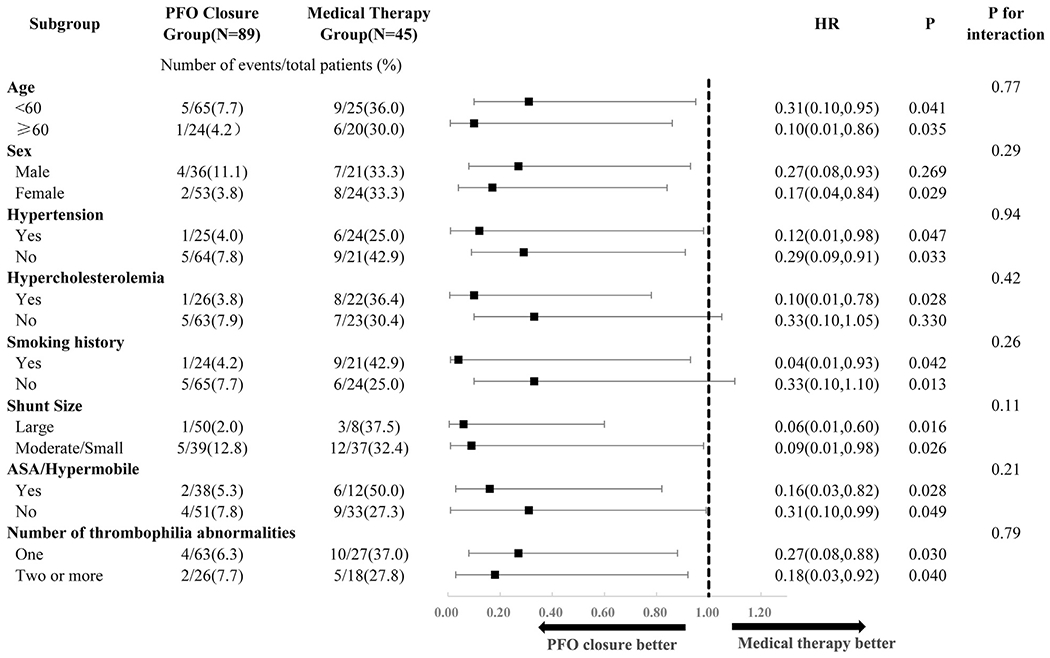
Hazard ratios (HRs) were calculated with the use of a Cox proportional hazards model. ASA = atrial septal aneurysm; PFO = patent foramen ovale.
When the individual components of the primary endpoint were analyzed, stroke occurred in 1 patient (1.1%) in the closure group and in 6 patients (13.3%) in the medical therapy group (HR: 0.09; 95% CI: 0.01 to 0.77; p = 0.028). Between the closure and medical therapy groups, TIAs occurred in 5 patients (5.6%) and 9 patients (20.0%), respectively (HR: 0.33; 95% CI: 0.11 to 1.00; p = 0.051). The results were similar in multivariate Cox regression analysis (Table 3).
Of the 46 patients who received medical therapy only, 31 (67.4%) received anticoagulation therapy, including 14 patients who received short-term therapy (<3 months) and 17 patients who received lifelong therapy, and 15 patients received antiplatelet therapy. The individual recurrence rates were 28.6% (4 of 14), 17.6% (3 of 17), and 53.3% (8 of 15). There was a beneficial trend for anticoagulation therapy (22.6% vs. 53.3%; p = 0.080) in reducing the recurrent events.
DISCUSSION
In this study, we found that the combination of thrombophilia and PFO increased the risk for recurrent stroke or TIA. Endovascular PFO closure significantly reduced the rate of recurrent events compared with medical therapy.
Previous studies demonstrated that a hypercoagulable state may be associated with cryptogenic stroke (29–31). Similar to a retrospective previous study (14), our prospective study showed that thrombophilia may be associated with a higher risk for recurrent events. At a median follow-up of 53 months, the rate of recurrent events was 15.7% (21 of 134) across patients with thrombophilia in our study, while it was 6.0% (207 of 3,440) in 5 published RCTs with a mean follow-up duration of about 48 months (32,33). Thus, comprehensive hypercoagulation testing should be considered in patients with PFO-attributable cryptogenic embolism, which may identify patients with high risk for recurrence.
RCTs and meta-analysis of PFO closure to prevent the recurrent events demonstrated that PFO closure was superior to medical therapy in the secondary prevention of cryptogenic stroke. However, high-risk patients with the combination of PFO and thrombophilia were excluded from most RCTs, such as CLOSURE I, PC, Gore REDUCE, and RESPECT (17,18,34–36), and few observational studies have explored optimal treatment for secondary prevention in these patients. Consistent with encouraging RCTs (16–18), our study provided evidence favoring PFO closure for patients with PFO and thrombophilia, as shown by a reduction of 78% in the risk for embolic events, and the results remained significant when adjusted for possible cofounding factors such as age, sex, traditional risk factors (hypertension, diabetes, hypercholesterolemia, and smoking history) and interatrial characteristics (moderate to large shunt size and ASA).
The optimal antithrombotic strategy for secondary prevention with PFO-attributable cryptogenic embolism is still unknown. A previous meta-analysis including multiple observational studies demonstrated inconsistent results. Our study indicated that anticoagulation therapy showed a beneficial trend compared with antiplatelet therapy. Patients who are not suitable for PFO closure may gain more benefit from long-term anticoagulation therapy when carefully weighed against the risk for hemorrhagic events. However, a recent trial in embolic stroke of undetermined source did not show the superiority of anticoagulant agents but revealed an increased risk for bleeding compared with aspirin (37). The issue may arise from the lack of hypercoagulability testing to isolate subgroups of patients who may derive the most benefit from anticoagulation. In severe cases, patients with multiple thrombophilic conditions may need additional protection from anticoagulation even after PFO closure.
To the best of our knowledge, this is the largest prospective study on recurrent events in hypercoagulable patients with PFO associated embolism. We prospectively enrolled all patients with PFO-attributable cryptogenic embolism with full hypercoagulable testing and provide new information regarding thrombophilia for patients who did not fit the criteria of published RCTs. Our data indicated that older patients (age >60 years) with thrombophilia also derived benefit from PFO closure. We speculate that older adults may harbor more procoagulable conditions with higher annual risk for paradoxical embolism (38), while they may not derive as many years of benefit from PFO closure. More research on the efficacy of PFO closure in older patients is required to provide clinical evidence for these high-risk patients who heretofore have been excluded from RCTs.
STUDY LIMITATIONS.
This was a nonrandomized, single-center study from a prospective registry. Thus, potential selection bias is unavoidable. There were differences in certain baseline characteristics between the PFO closure group and the medical therapy group, which may have confounded the results. Thus, we conducted multivariate Cox regression, which adjusted all potential confounders in the study population and subgroup analysis to make our findings reliable. Patients with 2 or more thrombophilic abnormalities derived similar benefit as those with 1 abnormal test result in our study. But we suspect that the roles of different thrombophilic abnormalities in the risk for recurrent events are not the same. However, our sample size may limit our ability to detect a potential difference. Last, all patients in our study completed at least 24-h Holter cardiac monitoring to exclude AF before PFO closure, but systematic methods of screening for AF were not routinely performed after PFO closure.
CONCLUSIONS
A hypercoagulable state was associated with a higher rate of recurrent stroke or TIA in patients with PFO-related cryptogenic embolism. In accordance with previous RCTs, among patients who had cryptogenic embolism with thrombophilia, closure of a PFO was superior to medical therapy alone with regard to the secondary prevention of recurrent events.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Previous RCTs evaluating the efficacy of PFO closure have excluded high-risk patients with the combination of PFO and hypercoagulability. There has been little beside clinical experience to guide treatment of patients with PFO-attributable embolism with thrombophilia.
WHAT IS NEW?
Patients with hypercoagulable state have increased risk of PFO-associated stroke recurrence. In agreement with previous RCTs, such patients respond to PFO closure for secondary stroke prevention.
WHAT IS NEXT?
Extensive blood testing for thrombophilia should be considered in patients with PFO-attributable embolism to individualize therapy.
AUTHOR DISCLOSURES
This study was funded by the National Institutes of Health (grant NS051588 to Dr. Ning). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- ASA
atrial septal aneurysm
- CI
confidence interval
- HR
hazard ratio
- PFO
patent foramen ovale
- RCT
randomized controlled trial
- TIA
transient ischemic attack
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009;113: 5314–22. [DOI] [PubMed] [Google Scholar]
- 2.Croles FN, Nasserinejad K, Duvekot JJ, Kruip MJ, Meijer K, Leebeek FW. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and bayesian meta-analysis. BMJ (Clin Res Ed) 2017;359:j4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol 2005;25:1992–7. [DOI] [PubMed] [Google Scholar]
- 4.Kenet G, Lutkhoff LK, Albisetti M, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation 2010;121:1838–47. [DOI] [PubMed] [Google Scholar]
- 5.Carod-Artal FJ, Nunes SV, Portugal D, Silva TV, Vargas AP. Ischemic stroke subtypes and thrombophilia in young and elderly Brazilian stroke patients admitted to a rehabilitation hospital. Stroke 2005;36:2012–4. [DOI] [PubMed] [Google Scholar]
- 6.Pahus SH, Hansen AT, Hvas AM. Thrombophilia testing in young patients with ischemic stroke. Thromb Res 2016;137:108–12. [DOI] [PubMed] [Google Scholar]
- 7.Mas JL, Zuber M. for the French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. Am Heart J 1995;130:1083–8. [DOI] [PubMed] [Google Scholar]
- 8.Comess KA, DeRook FA, Beach KW, Lytle NJ, Golby AJ, Albers GW. Transesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: prevalence of findings and recurrent stroke risk. J Am Coll Cardiol 1994;23:1598–603. [DOI] [PubMed] [Google Scholar]
- 9.De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000;31:2407–13. [DOI] [PubMed] [Google Scholar]
- 10.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med 1992;117:461–5. [DOI] [PubMed] [Google Scholar]
- 11.Florez JC, Ay H, Van Cott EM, Buonanno FS. Patent foramen ovale and hypercoagulability as combined risk factors for stroke. J Stroke Cerebrovasc Dis 2003;12:114–8. [DOI] [PubMed] [Google Scholar]
- 12.Dearani JA, Ugurlu BS, Danielson GK, et al. Surgical patent foramen ovale closure for prevention of paradoxical embolism-related cerebrovascular ischemic events. Circulation 1999;100 19 suppl:II171–5. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi S Coagulation abnormalities in adults with cryptogenic stroke and patent foramen ovale. J Neurol Sci 1998;160:158–60. [DOI] [PubMed] [Google Scholar]
- 14.Giardini A, Donti A, Formigari R, et al. Comparison of results of percutaneous closure of patent foramen ovale for paradoxical embolism in patients with versus without thrombophilia. Am J Cardiol 2004;94:1012–6. [DOI] [PubMed] [Google Scholar]
- 15.Lim ST, Murphy SJX, Smith DR, et al. Clinical outcomes and a high prevalence of abnormalities on comprehensive arterial and venous thrombophilia screening in TIA or ischaemic stroke patients with a patent foramen ovale, an inter-atrial septal aneurysm or both. J Neurol Sci 2017;377:227–33. [DOI] [PubMed] [Google Scholar]
- 16.Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017;377:1011–21. [DOI] [PubMed] [Google Scholar]
- 17.Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017;377:1033–42. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017;377:1022–32. [DOI] [PubMed] [Google Scholar]
- 19.Casaubon L, McLaughlin P, Webb G, Yeo E, Merker D, Jaigobin C. Recurrent stroke/TIA in cryptogenic stroke patients with patent foramen ovale. Can J Neurol Sci 2007;34:74–80. [DOI] [PubMed] [Google Scholar]
- 20.Schuchlenz HW, Weihs W, Berghold A, Lechner A, Schmidt R. Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol 2005;101:77–82. [DOI] [PubMed] [Google Scholar]
- 21.Thanopoulos BV, Dardas PD, Karanasios E, Mezilis N. Transcatheter closure versus medical therapy of patent foramen ovale and cryptogenic stroke. Catheter Cardiovasc Interv 2006;68:741–6. [DOI] [PubMed] [Google Scholar]
- 22.Weimar C, Holle DN, Benemann J, et al. Current management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shunt. Cerebrovasc Dis 2009;28:349–56. [DOI] [PubMed] [Google Scholar]
- 23.Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004; 44:750–8. [DOI] [PubMed] [Google Scholar]
- 24.Saver JL, Mattle HP, Thaler D. Patent foramen ovale closure versus medical therapy for cryptogenic ischemic stroke: a topical review. Stroke 2018;49:1541–8. [DOI] [PubMed] [Google Scholar]
- 25.Ning M, Gonzalez RG. Case records of the Massachusetts General Hospital. Case 34-2013. A 69-year-old man with dizziness and vomiting. N Engl J Med 2013;369:1736–48. [DOI] [PubMed] [Google Scholar]
- 26.Saver JL. Clinical practice. Cryptogenic stroke. N Engl J Med 2016;374:2065–74. [DOI] [PubMed] [Google Scholar]
- 27.Martin F, Sanchez PL, Doherty E, et al. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation 2002;106:1121–6. [DOI] [PubMed] [Google Scholar]
- 28.Inglessis I, Elmariah S, Rengifo-Moreno PA, et al. Long-term experience and outcomes with transcatheter closure of patent foramen ovale. J Am Coll Cardiol Intv 2013;6:1176–83. [Google Scholar]
- 29.Pezzini A, Del Zotto E, Magoni M, et al. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke 2003;34:28–33. [DOI] [PubMed] [Google Scholar]
- 30.Botto N, Spadoni I, Giusti S, Ait-Ali L, Sicari R, Andreassi MG. Prothrombotic mutations as risk factors for cryptogenic ischemic cerebrovascular events in young subjects with patent foramen ovale. Stroke 2007;38:2070–3. [DOI] [PubMed] [Google Scholar]
- 31.Karttunen V, Hiltunen L, Rasi V, Vahtera E, Hillbom M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis 2003;14:261–8. [DOI] [PubMed] [Google Scholar]
- 32.Mojadidi MK, Elgendy AY, Elgendy IY, et al. Transcatheter patent foramen ovale closure after cryptogenic stroke: an updated meta-analysis of randomized trials. J Am Coll Cardiol Intv 2017;10: 2228–30. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad Y, Howard JP, Arnold A, et al. Patent foramen ovale closure vs. medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J 2018;39:1638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366: 991–9. [DOI] [PubMed] [Google Scholar]
- 35.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083–91. [DOI] [PubMed] [Google Scholar]
- 36.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368: 1092–100. [DOI] [PubMed] [Google Scholar]
- 37.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378: 2191–201. [DOI] [PubMed] [Google Scholar]
- 38.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007;357: 2262–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


