Abstract
A 25-year-old man presented to the emergency department with a 3-day history of fever, anorexia, jaundice, and a generalized skin eruption. His liver function tests showed marked cholestatic and cytolytic abnormalities without liver insufficiency. A liver biopsy was performed, and morphology with routine stains was considered non-specific. Because of the dermatological findings, the non-specific biopsy morphology, and the absence of an identified infectious etiology, a diagnosis of Kawasaki disease was presumed. However, additional colorations on liver biopsy with Warthin–Starry stain revealed multiple thin and coiled microorganisms compatible with spirochetes. His serology for leptospirosis was found to be positive for IgM, supporting the diagnosis of acute leptospirosis with liver involvement. Our case illustrates the diagnostic challenge of leptospirosis and highlights the utility of conventional laboratory tests to confirm the diagnosis. Exceptionally, Warthin–Starry stain allowed the identification of leptospires in liver biopsy and confirmed liver involvement of systemic leptospirosis.
Key words: leptospirosis, pathology, travel medicine, zoonosis
Abstract
Un homme de 25 ans a consulté à l’urgence parce qu’il faisait de la fièvre depuis trois jours, de l’anorexie, un ictère et une éruption cutanée généralisée. Les tests de fonction hépatique ont révélé des anomalies cholestatiques et cytolytiques marquées, sans insuffisance hépatique. La coloration standard de la biopsie hépatique a révélé une morphologie cellulaire considérée comme non spécifique. Compte tenu des observations dermatologiques, de la morphologie non spécifique de la biopsie et de l’absence d’étiologie infectieuse établie, un diagnostic de maladie de Kawasaki a été présumé. Cependant, l’ajout d’une coloration de Warthin-Starry a révélé de multiples microorganismes minces et torsadés compatibles avec des spirochètes. La sérologie pour la leptospirose s'est avérée positive pour les anticorps IgM, appuyant un diagnostic de leptospirose aiguë avec atteinte hépatique. Ce cas illustre les difficultés diagnostiques de la leptospirose et fait ressortir l’utilité des tests de laboratoire traditionnels pour confirmer le diagnostic. Exceptionnellement, la coloration de Warthin–Starry a permis d’observer des leptospires à la biopsie hépatique et confirmé la leptospirose systémique avec atteinte hépatique.
Mots-clés : leptospirose, pathologie, médecine des voyages, zoonose
Case Presentation
A 25-year-old Caucasian man from Québec, Canada, presented to the emergency department with a 3-day history of fever, anorexia, cough, and a generalized skin eruption. His past medical history was significant for tinea versicolor and psoriasis. He had worked as a construction worker for the past 3 years in Alberta, Canada, and had recently returned to the province of Québec 1 month prior to visit his parents. His parents owned a dog, with which he had occasional contact. He slept in the home’s basement, which had recently been infested with rodent feces prior to his return. One week prior to his illness, he had gone hiking and trekking in a wooded area behind his home, during which he encountered a deer carcass; however, he denied direct contact with it.
On physical exam, he appeared unwell, with a body temperature of 39.9°C, a normal heart rate of 86 beats per minute, respiratory rate of 20 breaths per minute, oxygen saturation of 98% on room air, and blood pressure of 111/72 mm Hg. Cardiopulmonary and abdominal examinations were unremarkable. A generalized maculopapular eruption was notable, as well as a desquamative palmoplantar rash, cheilitis, and bilateral conjunctival erythema (Figure 1).
Figure 1:
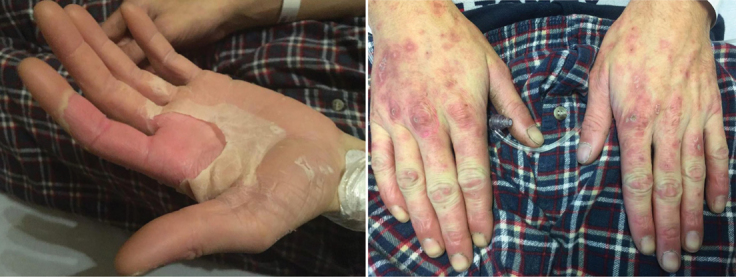
Desquamative and maculopapular rash involving both palms
The patient’s bloodwork showed a normal leukocyte count of 7.8 × 109/L, a low hemoglobin level of 114 g/L, and a normal platelet count of 212 × 109/L. Laboratory findings showed a normal creatinine value of 61 µmol/L, an elevated C-reactive protein level of 291 mg/L, an international normalized ratio (INR) of 1.0, and a low albumin level of 28 g/L. His liver function tests showed both a cholestatic and cytolytic process with an alkaline phosphatase level of 1,059 IU/L, a gamma-glutamyl transferase level of 1,159 IU/L, a total bilirubin level of 137 µmol/L, and an alanine aminotransferase level of 539 IU/L.
Blood cultures, serology for HIV, hepatitis A, B, and C, Epstein–Barr virus, and cytomegalovirus were all negative. Chest radiography was normal, and an abdominal computed tomography (CT) scan showed mild hepatosplenomegaly without focal lesions.
A liver biopsy was performed, and histopathology showed moderate neutrophilic and eosinophilic portal space infiltration with mild lobular necroinflammatory changes and moderate hepatocellular and canalicular cholestasis. These findings were considered non-specific (Figure 2).
Figure 2:
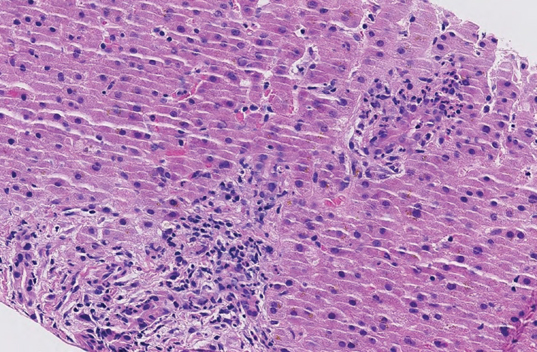
Liver biopsy with portal tracts showing mild and mixed inflammation with mild bile duct damage; there is canalicular cholestasis with mild ballooning of hepatocytes (hematoxylin and eosin stain, 20×)
Diagnosis
The patient received intravenous ceftriaxone for suspected zoonosis with initial defervescence over 72 hours, followed by a second phase of febrile illness which lasted 4 additional days. Given the presence of a generalized exanthema, a presumed diagnosis of Kawasaki disease with liver involvement was made, and the patient was treated with intravenous immunoglobulins, Aspirin, and supportive care. He eventually improved before discharge. Upon follow-up, the leptospirosis IgM antibody was found to be positive, although the microagglutination test performed at the Centers for Disease Control and Prevention (CDC) did not group with any serovars included in the panel. With this new information, a revision of the liver biopsy with additional Warthin–Starry stains revealed multiple thin and coiled microorganisms compatible with spirochetes (Figure 3), supporting the diagnosis of acute leptospirosis with liver involvement. At the 3-month follow-up, he was clinically well with slowly resolving cholestasis.
Figure 3:
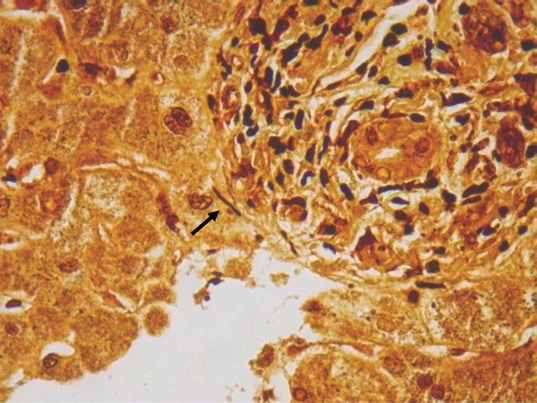
Liver biopsy in which multiple thin and coiled microorganisms compatible with spirochetes can be identified (Warthin–Starry stain, 63×)
Discussion
Leptospirosis is a zoonosis caused by pathogenic spirochetes of the genus Leptospira (1). Leptospirosis has a worldwide distribution but is more frequently reported from tropical areas, particularly during the rainy season. In temperate climates, leptospirosis is less frequently encountered (2). Between 2009–2017, 61 possible and proven cases of leptospirosis were reported in Canada at the National Microbiology Laboratory (NML), which performs testing nationally. Four of these cases were from the Atlantic region, 19 were from Québec, 18 were from Ontario, and 20 cases were from the Prairies and the Western region (3). Often, leptospirosis is imported from endemic areas by returning travellers, but it is unclear whether these cases were acquired locally, in Canada, or abroad (4). We report a locally acquired case of leptospirosis in Québec.
Human infection usually occurs following exposure to water contaminated with the urine of an infected animal, predominantly rodents, with an incubation period of 2 to 20 days (2). A detailed questionnaire on occupational and recreational exposure to contaminated water or animals is key in raising a clinical suspicion. Our patient had possible exposure through contact with rodent feces, contact with a dog, potential contact with a deer carcass, and trekking in a muddy wooded area. Since he had been in Québec for the past month, and considering the incubation period, we believe he acquired leptospirosis locally.
Typically, patients present with acute onset of fever, myalgia, and headache. Disease severity ranges from mild, self-limited febrile illness to life-threatening disease that can involve any organ such as the kidney, liver, lung, or brain. Classic presentations include Weil’s syndrome characterized by jaundice and renal failure, icterohemorrhagic manifestation, and severe pulmonary hemorrhage syndrome (1,2). Given its protean manifestation, the diagnosis of leptospirosis is challenging and often missed. Exanthema, such as seen in our patient, is observed in 8%–58% of patients with leptospirosis (5,6).
Diagnosis is usually based on laboratory results. Direct microscopic observation and leptospire culture are insensitive, unreliable, and time consuming. Diagnosis is usually performed by serology with enzyme-linked immunoassay and microagglutination test (MAT), which is restricted to reference laboratories and has a slow turnaround time for results (1). In recent years, rapid lateral flow assays and real-time polymerase chain reaction assays have become available; however, they are not easily accessible and are mostly useful in the acute bacteremic phase of illness (1).
The main histological features of acute leptospirosis with hepatic involvement remain non-specific and consist of hepatocyte damage, including apoptotic bodies, liver regeneration, canalicular cholestasis, and mild portal or sinusoidal mononuclear inflammation. The histological differential diagnosis is broad and includes acute liver injury due to viral infections, drugs, or toxic products, large bile duct obstruction or inflammation and, given the clinical presentation, Kawasaki disease (2,7,8). Leptospires are not seen on routine stain and are difficult to detect with ancillary techniques, including histochemical staining (silver and Warthin–Starry stains), immunofluorescence and immunohistochemical studies, and in situ hybridization (ISH) (9,10). Very few studies have addressed the issue of sensitivity and specificity of these techniques in human subjects, whereas limitations have been highlighted in animal studies.
We report a case of endemically acquired leptospirosis in Québec, missed by conventional histopathology examination, but subsequently diagnosed with Warthin–Starry stain and serological testing. Our case illustrates the diagnostic challenge of leptospirosis and highlights the utility of conventional laboratory tests to confirm the diagnosis. Exceptionally, Warthin–Starry stain allowed the identification of leptospires in liver biopsy and confirmed liver involvement of systemic leptospirosis.
Competing Interests:
The authors have nothing to disclose.
Ethics Approval:
N/A
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Animal Studies:
N/A
Funding:
No funding was received for this work.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001; 14(2):296–326. 10.1128/cmr.14.2.296-326.2001. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. 10.1007/978-3-662-45059-8_5. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassim SS, Dibernardo A, Lindsay LR, Wuerz TC. Locally acquired leptospirosis in expedition racer, Manitoba, Canada. Emerg Infect Dis. 2018;24(12):2386–8. 10.3201/eid2412.181015. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung V, Luong ML, Libman M. Leptospirosis: pulmonary hemorrhage in a returned traveller. CMAJ. 2011;183(7):E423–7. 10.1503/cmaj.092203. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abgueguen P, Delbos V, Blanvillain J, et al. Clinical aspects and prognostic factors of leptospirosis in adults. Retrospective study in France. J Infect. 2008;57(3):171–8. 10.1016/j.jinf.2008.06.010. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Zaki SA, Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010;38(4):285–91. 10.1007/s15010-010-0030-3. Medline: [DOI] [PubMed] [Google Scholar]
- 7.MacSween RN, Burt AD, Portmann B, Ferrell LD. MacSween's pathology of the liver. New York: Churchill Livingstone/Elsevier; 2012. [Google Scholar]
- 8.Wysocki J, Liu Y, Shores N. Leptospirosis with acute liver injury. Proc (Bayl Univ Med Cent). 2014;27(3): 257–8. 10.1080/08998280.2014.11929130. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Brito T, Menezes LF, Lima DM, Lourenço S, Silva AM, Alves VA. Immunohistochemical and in situ hybridization studies of the liver and kidney in human leptospirosis. Virchows Arch. 2006;448(5):576–83. 10.1007/s00428-006-0163-z. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245–52. 10.1016/j.jmii.2013.03.001. Medline: [DOI] [PubMed] [Google Scholar]


