Abstract
Background
Long‐acting reversible contraception (LARC), including intrauterine devices (IUDs) and contraceptive implants, are highly effective, reversible methods of contraception. Providing LARC methods during the postpartum period is important to support contraceptive choice, and to prevent unintended pregnancy and short interpregnancy intervals. Delaying offering contraception to postpartum people until the first comprehensive postpartum visit, traditionally at around six weeks postpartum, may put some postpartum people at risk of unintended pregnancy, either due to loss to follow‐up or because of initiation of sexual intercourse prior to receiving contraception. Therefore, immediate provision of highly effective contraception, prior to discharge from hospital, has the potential to improve contraceptive use and prevent unintended pregnancies and short interpregnancy intervals.
Objectives
To compare the initiation rate, utilization rates (at six months and 12 months after delivery), effectiveness, and adverse effects of immediate versus delayed postpartum insertion of implants and IUDs for contraception.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and POPLINE for eligible studies up to December 2020. We examined review articles and contacted investigators. We checked registers of ongoing clinical trials, citation lists of included studies, key textbooks, grey literature, and previous systematic reviews for potentially relevant studies.
Selection criteria
We sought randomized controlled trials (RCTs) that compared immediate postpartum versus delayed insertion of contraceptive implant and IUDs for contraception.
Data collection and analysis
Two review authors (JS, SK) independently screened titles and abstracts of the search results, and assessed the full‐text articles of potentially relevant studies for inclusion. They extracted data from the included studies, assessed risk of bias, compared results, and resolved disagreements by consulting a third review author (PL, SA or PP). We contacted investigators for additional data, where possible. We computed the Mantel‐Haenszel or inverse variance risk ratio (RR) with 95% confidence interval (CI) for binary outcomes and the mean difference (MD) with 95% CI for continuous variables.
Main results
In this updated review, 16 studies met the inclusion criteria; five were studies of contraceptive implants (715 participants) and 11 were studies of IUDs (1894 participants). We identified 12 ongoing studies. We applied GRADE judgements to our results; the overall certainty of the evidence for each outcome ranged from moderate to very low, with the main limitations being risk of bias, inconsistency, and imprecision.
Contraceptive implants
Immediate insertion probably improves the initiation rate for contraceptive implants compared with delayed insertion (RR 1.48, 95% CI 1.11 to 1.98; 5 studies, 715 participants; I2 = 95%; moderate‐certainty evidence).
We are uncertain if there was a difference between the two groups for the utilization rate of contraceptive implants at six months after delivery (RR 1.16, 95% CI 0.90 to 1.50; 3 studies, 330 participants; I2 = 89%; very low‐certainty evidence) or at 12 months after insertion (RR 0.98, 95% CI 0.93 to 1.04; 2 studies, 164 participants; I2 = 0%; very low‐certainty evidence).
People who received an immediate postpartum contraceptive implant insertion may have had a higher mean number of days of prolonged vaginal bleeding within six weeks postpartum (mean difference (MD) 2.98 days, 95% CI ‐2.71 to 8.66; 2 studies, 420 participants; I2 = 91%; low‐certainty evidence) and a higher rate of other adverse effects in the first six weeks after birth (RR 2.06, 95% CI 1.38 to 3.06; 1 study, 215 participants; low‐certainty evidence) than those who received a delayed postpartum insertion. We are uncertain if there was a difference between the two groups for prolonged bleeding at six months after delivery (RR 1.19, 95% CI 0.29 to 4.94; 2 studies, 252 participants; I2 = 0%; very low‐certainty evidence).
There may be little or no difference between the two groups for rates of unintended pregnancy at six months (RR 0.20, 95% CI 0.01 to 4.08; one study, 205 participants; low‐certainty evidence). We are uncertain whether there was a difference in rates of unintended pregnancy at 12 months postpartum (RR 1.82, 95% CI 0.38 to 8.71; 1 study, 64 participants; very low‐certainty evidence). There may be little or no difference between the two groups for any breastfeeding rates at six months (RR 0.97, 95% CI 0.92 to 1.01; 2 studies, 225 participants; I2 = 48%; low‐certainty evidence).
IUDs
Immediate insertion of IUDs probably improves the initiation rate compared with delayed insertion, regardless of type of IUD (RR 1.27, 95% CI 1.07 to 1.51; 10 studies, 1894 participants; I2 = 98%; moderate‐certainty evidence). However, people who received an immediate postpartum IUD insertion may have had a higher expulsion rate at six months after delivery (RR 4.55, 95% CI 2.52 to 8.19; 8 studies, 1206 participants; I2 = 31%; low‐certainty evidence) than those who received a delayed postpartum insertion.
We are uncertain if there was a difference between the two groups in the utilization of IUDs at six months after insertion (RR 1.02, 95% CI 0.65 to 1.62; 6 studies, 971 participants; I2 = 96%; very low‐certainty evidence) or at 12 months after insertion (RR 0.86, 95% CI 0.5 to 1.47; 3 studies, 796 participants; I2 = 92%; very low‐certainty evidence).
Immediate IUDs insertion may reduce unintended pregnancy at 12 months (RR 0.26, 95% CI 0.17 to 0.41; 1 study, 1000 participants; low‐certainty evidence). We are uncertain whether there was difference in any breastfeeding rates at six months in people receiving progestin‐releasing IUDs (RR 0.90, 95% CI 0.63 to 1.30; 5 studies, 435 participants; I2 = 54%; very low‐certainty evidence).
Authors' conclusions
Evidence from this updated review indicates that immediate postpartum insertion improves the initiation rate of both contraceptive implants and IUDs by the first postpartum visit compared to delayed insertion. However, it is not clear whether that there are differences in utilization rates at six and 12 months postpartum. We are uncertain whether there is any difference in the unintended pregnancy rate at 12 months. Provision of progestin‐releasing implants and IUDs immediately postpartum may have little or no negative impact on breastfeeding. However, the expulsion rate of IUDs and prolonged vaginal bleeding associated with immediate implants appears to be higher.
Plain language summary
Is it better to insert an implant or intrauterine device (coil) for contraception within days of childbirth or wait 4 to 6 weeks?
When we use the term 'people' in this summary, we mean individuals with a current ability to become pregnant.
Key messages
‐ Insertion of contraceptive implants or intrauterine devices (IUDs) within days of childbirth (immediate insertion, while in hospital), rather than waiting 4 to 6 weeks for insertion (delayed insertion), increases the number of people in whom they are inserted.
‐ Timing of insertion makes little or no difference to the number of people who use these methods of contraception 6 or 12 months after childbirth.
‐ Expulsion of IUDs seems to occur more frequently in people who have immediate insertion.
‐ Further research is needed about the rates of unintended pregnancy with both immediate insertion and delayed insertion of implants and IUDs.
What are contraceptive implants or intrauterine devices?
Contraceptive implant and intrauterine devices (IUDs) are highly effective methods of birth control that are reversible and safe for use shortly after childbirth. Implants are inserted into the upper arm, and IUDs into the womb (uterus) by doctors or nurses. People who use these methods of contraception use either an implant or an IUD.
Appropriate spacing of pregnancies is good for the health of both pregnant people and newborn babies. Normally, contraception is provided at the first comprehensive health visit after childbirth (usually around six weeks after giving birth). However, some people have sex before this visit, or do not attend it, which increases the risk of unplanned pregnancy. Insertion of a contraceptive implant or IUD within days of childbirth, before discharge from hospital, is convenient for patients and medical staff. The person having the implant or IUD is known not to be pregnant, and this practice may increase the number of people who are able to use these contraception methods.
What did we want to find out?
We wanted to find out if inserting contraceptive implants or IUDs within days of childbirth was better than waiting 6 to 8 weeks for:
‐ the number of people who agreed to insertion (insertion rate);
‐ the number of people who continued to use these contraception methods (utilization rate);
‐ preventing pregnancy; and
‐ whether insertion of contraceptive implants or IUDs within days of childbirth was associated with any unwanted effects.
What did we do?
We searched for studies that investigated insertion of contraceptive implants or IUDs in people within days of childbirth ('immediate insertion') compared to insertion 6 to 8 weeks after childbirth ('delayed' insertion).
We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 16 studies that involved a total of 2609 people (715 people in contraceptive implant studies, and 1894 people in IUD studies). All studies were conducted in hospitals. Most took place in the USA, but others took place in Uganda, Eygpt, Brazil and Sri Lanka. The studies included people who had just given birth, most of whom were 18 years old or older, though 1 study included younger people. The studies investigated different types of contraceptive implants and IUDs.
Main results
Contraceptive implants
People were 48% more likely to have contraceptive implants inserted when they could be inserted within days of childbirth compared to delayed insertion.
The timing of insertion made little or no difference to the number of people using contraceptive implants 6 or 12 months after childbirth.
Vaginal bleeding seemed to last longer in people who had implants inserted a few days after childbirth compared to delayed insertion (3 days more bleeding), but there was no difference between groups for bleeding 6 months after childbirth.
We are uncertain if there was a difference in rates of unintended pregnancy between the groups at 6 and 12 months after childbirth.
IUDs
People were 27% more likely to have IUDs inserted when they could be inserted within days of childbirth compared to delayed insertion.
It was unclear whether the timing of insertion made any difference to the number of people using IUDs 6 or 12 months after childbirth.
Six months after childbirth, expulsion of IUDs from the womb seemed to occur more frequently in people who had had an IUD inserted within days of childbirth.
We are uncertain if there was a difference in rates of unintended pregnancy between the groups at 6 and 12 months after childbirth.
What are the limitations of the evidence?
Our confidence in the evidence for the different results ranges from moderate to uncertain. This is because people in the studies were aware of when their implants or IUDs were inserted, which may have affected reporting of some outcomes, and because of the dropout rate from the studies. Also, not all of the studies provided data about everything in which we were interested, which meant some results are based on a lower number of people.
How up to date is this evidence?
The evidence is up to date to December 2020.
Summary of findings
Summary of findings 1. Immediate compared to delayed postpartum insertion of contraceptive implants for contraception.
| Immediate compared to delayed postpartum insertion of contraceptive implants for contraception | ||||||
|
Participant or population: postpartum people who desire a contraceptive implant for contraception Settings: tertiary hospitals Intervention: immediate postpartum insertion Comparison: delayed insertion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with delayed postpartum insertion of contraceptive implants | Risk with immediate postpartum insertion of contraceptive implants | |||||
| Rate of initiation of contraceptive implants | Study population | RR 1.48 (1.11 to 1.98) | 715 (5 studies) | ⊕⊕⊕⊝ Moderate1 | ||
| 633 per 1000 | 936 per 1000 (702 to 1000) | |||||
| Utilization rate at 6 months postpartum | Study population | RR 1.16 (0.90 to 1.50) | 330 (3 studies) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 712 per 1000 | 826 per 1000 (641 to 1000) | |||||
| Utilization rate at 12 months postpartum | Study population | RR 0.98 (0.93 to 1.04) | 164 (2 studies) | ⊕⊝⊝⊝ Very low2,3,4 | ||
| 922 per 1000 | 904 per 1000 (858 to 959) | |||||
| Adverse effect: mean days of prolonged vaginal bleeding within 6 weeks postpartum | Mean 17.8 days | MD 2.98 higher (2.71 less to 8.66 more) | MD 2.98 (2.71 to 8.66) | 420 (2 studies) | ⊕⊕⊝⊝ Low1,2 | |
| Adverse effects other than prolonged vaginal bleeding | Study population | RR 2.06 (1.38 to 3.06) | 215 (1 study) | ⊕⊕⊝⊝ Low3,5 | ||
| 229 per 1000 | 472 per 1000 (317 to 702) | |||||
| Unintended pregnancy rate at 12 months postpartum | Study population | RR 1.82 (0.38 to 8.71) | 64 (1 study) | ⊕⊝⊝⊝ Very low2,3,4 | ||
| 74 per 1000 | 135 per 1000 (28 to 645) | |||||
| Any breastfeeding at six months postpartum | Study population | RR 0.97 (0.92 to 1.01) | 225 (2 studies) | ⊕⊕⊝⊝ Low2,3 | ||
| 775 per 1000 | 751 per 1000 (713 to 782) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level owing to inconsistency across studies (I2 > 50%).
2Downgraded by one level owing to serious imprecision (e.g. 95% CI includes both appreciable appreciable benefit and harm, or because of low number of participants (total number of participants < 400))
3Downgraded by one level owing to serious imprecision (low number of events (total number of events < 300)).
4Downgraded by one level owing to serious risk of attrition bias.
5Downgraded by one level owing to serious risk of assessment bias.
Summary of findings 2. Immediate compared to delayed postpartum insertion of IUDs for contraception.
| Immediate compared to delayed postpartum insertion of IUDs for contraception | ||||||
| Patient or population: postpartum people who desire IUDs for contraception Setting: tertiary hospitals Intervention: immediate postpartum insertion Comparison: delayed postpartum insertion of IUDs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with delayed postpartum insertion of IUD | Risk with immediate insertion of IUD | |||||
| Rate of initiation of IUDs | Study population | RR 1.27 (1.07 to 1.51) | 1894 (10 studies) | ⊕⊕⊕⊝ Moderate1 | ||
| 614 per 1000 | 780 per 1000 (657 to 927) | |||||
| Expulsion by 6 months | Study population | RR 4.55 (2.52 to 8.19) | 1206 (8 studies) | ⊕⊕⊝⊝ Low2,3 | ||
| 21 per 1000 | 94 per 1000 (52 to 170) | |||||
| Utilization rate at 6 months postpartum | Study population | RR 1.02 (0.64 to 1.62) | 971 (6 studies) | ⊕⊝⊝⊝ Very low1,3,4 | ||
| 822 per 1000 | 838 per 1000 (534 to 1000) | |||||
| Utilization rate at 12 months postpartum | Study population | RR 0.86 (0.50 to 1.49) | 796 (3 studies) | ⊕⊝⊝⊝ Very low1,3,4 | ||
| 781 per 1000 | 672 per 1000 (391 to 1000) | |||||
| Unintended pregnancy at 12 months postpartum | Study population | RR 0.26 (0.17 to 0.41) | 1000 (1 study) | ⊕⊕⊝⊝ Low2,3 | ||
| 168 per 1000 | 44 per 1000 (29 to 69) | |||||
| Any breastfeeding at 6 months (hormonal IUD) | Study population | RR 0.90 (0.63 to 1.30) | 435 (5 studies) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 468 per 1000 | 421 per 1000 (295 to 608) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level owing to inconsistency across studies (I2 > 50%).
2Downgraded by one level owing to serious imprecision (low number of events (total number of events < 300)).
3Downgraded by one level owing to serious risk of attrition bias.
4Downgraded by one level owing to serious imprecision (e.g. 95% CI includes both appreciable appreciable benefit and harm, or because of low number of participants (total number of participants < 400)).
5Downgraded by one level owing to serious risk of assessment bias.
Background
Note: when we use the term 'people' in this summary, we mean individuals with presumed current or past ability to become pregnant.
Description of the condition
Traditionally, the comprehensive postpartum visit is scheduled around six weeks after childbirth (Speroff 2008). At this visit, healthcare providers typically assess recovery after childbirth and address contraceptive needs. Although the postpartum visit is an ideal time to discuss and provide family planning services, there is a notably poor attendance at postpartum appointments, particularly among adolescents. This results in delayed or missed opportunities to counsel about contraception (Moore 2015; Nkwabong 2015). In addition, nearly half of people who have given birth report unprotected vaginal sexual intercourse before attending a six‐week postpartum visit (Brito 2009; Chaovisitsaree 2012). Delayed initiation of contraception after childbirth and having unprotected sexual intercourse before attending the postpartum visit can put people at risk of unintended pregnancy (Wilson 2011), as well as short interpregnancy intervals ‐ defined as a pregnancy within two years of the previous pregnancy (WHO 2005). As unintended pregnancy and short interpregnancy intervals have a negative impact on maternal and infant health (Finer 2011; Fraser 1995; Singh 2010; WHO 2005), designing effective contraceptive practices to reduce undesired pregnancy among people who are at high risk of becoming pregnant is of utmost importance.
Description of the intervention
Provision of contraception usually occurs around six weeks postpartum. However, a study conducted in the USA indicated that only 41% of birthing people received contraceptives within 90 days of delivery (Thiel de Bocanegra 2013). This is similar to results reported from low‐ and middle‐income countries (Moore 2015; Nkwabong 2015). Therefore immediate provision of effective contraception is an important public health concern. According to ACOG 2016, the term 'immediate postpartum' refers to the period between delivery and hospital discharge.
Long‐acting reversible contraception (LARC) methods include contraceptive implants and intrauterine devices (IUDs), which are extremely effective and safe methods of contraception (ACOG 2017). Currently, two types of progestins are used in contraceptive implants, levonorgestrel and etonogestrel.
The primary mechanism of action of the implant is to suppress ovulation by altering the hypothalamic–pituitary–ovarian axis; secondary mechanisms include thickening of cervical mucous and altering the endometrial lining (ACOG 2017).
With regard to IUDs, hormonal and non‐hormonal IUDs are available. The primary mechanism of action for both types of IUDs is prevention of fertilization. The progestogenic effects of hormonal IUDs cause the thickening of cervical mucous that hinders sperm transport and alterations of the endometrium that inhibit fertilization (Ortiz 1996; Stanford 2002). Non‐hormonal IUDs inhibit sperm motility.
These contraceptive methods offer between three to 12 years of reliable pregnancy prevention once inserted in people with a cervix. The typical use failure rate is less than 1% for both implants and IUDs (ACOG 2017). The satisfaction rates for implants and IUDs are reported to be high, and are reflected in high continuation rates (Hubacher 2018).
Based on their high efficacy and safety, LARC methods are suitable for nearly all people with a cervix who need contraception (ACOG 2018; WHO 2015). The common adverse effects of contraceptive implants include amenorrhea (absence of menstrual bleeding), irregular unpredictable menstrual bleeding or spotting, and headache. However, most adverse effects are mild, well‐tolerated, and some resolve within the first few months of use; potential users should receive counseling in order to understand the adverse effects. Common adverse effects associated with non‐hormonal IUDs include heavy and prolonged menstruation and pain, while hormonal IUDs have progestin‐related adverse effects that include decreases in menstrual bleeding and irregular menstrual bleeding or spotting.
How the intervention might work
There is an increased risk of unintended pregnancy among people who have not initiated contraception after childbirth and have unprotected sexual intercourse before attending a postpartum visit to discuss contraception. Immediate postpartum provision of highly effective contraception, including IUDs and contraceptive implants, has been proposed as an intervention to prevent unintended pregnancy and short interpregnancy intervals (ACOG 2017).
Based on a previous Cochrane systematic review, immediate postpartum insertion of IUDs appears to be safe and effective (Lopez 2015). Advantages of this practice include high motivation and convenience for both postpartum people and providers. However, the expulsion rate of IUDs inserted immediately postpartum is slightly higher than in people who have delayed insertion of IUDs (Lopez 2015).
Immediate postpartum provision of the contraceptive implant has not been shown to have any significant negative impacts on maternal health, breastfeeding or infant health, but data are limited (Phillips 2016). The World Health Organization's (WHO's) medical eligibility criteria for contraceptive use supports contraceptive implant insertion prior to hospital discharge regardless of lactation status (WHO 2015; Whaley 2015). The adverse effects of immediate postpartum insertion of contraceptive implants also appear to be similar to those of delayed insertion (Ireland 2014). Additionally, the three‐year continuation rate of this method is as high as 66% when contraceptive implants are inserted immediately postpartum, which suggests a high level of satisfaction with this practice (Wilson 2014).
Why it is important to do this review
Immediate postpartum insertion of a LARC device could be a promising choice for timing and location of contraceptive method initiation. The postnatal hospital stay provides a timely opportunity to offer contraception to postpartum people. However, IUD expulsion has been shown to be higher in the immediate postpartum period so IUD continuation rates may be affected (Jatlaoui 2018). It is generally accepted that people using IUDs need to check their IUD string regularly to avoid being at risk of unintended pregnancy. In addition, the natural postpartum decline in serum progesterone after delivery has been proposed as a trigger for lactogenesis (Rodriguez 2009). Therefore, administration of progestin‐containing contraceptives such as implants and LNG‐IUS immediately postpartum could, theoretically, have a negative impact on breastfeeding initiation.
This is an update to the Sothornwit 2017 review. In this previous Cochrane Review, we found and included only three studies and concluded that immediate insertion of contraceptive implants improves initiation rate compared to delayed insertion. However, evidence about utilization was lacking. In order to make a more comprehensive review, we broadened our scope in this update to cover immediate versus delayed postpartum LARCs, including both implants and IUDs.
Objectives
To compare the initiation rate, utilization rates (at six months and 12 months after delivery), effectiveness, and adverse effects of immediate versus delayed postpartum insertion of implants and IUDs for contraception.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), irrespective of blinding, language, publication status, or sample size. We did not include controlled clinical trials (CCTs) that used non‐random methods of assigning participants to treatment, such as by alternation, by birth date, or by medical record number, as they may be subject to high risk of bias.
Types of participants
Postpartum people who requested a long‐acting reversible contraceptive method and were recruited before hospital discharge.
Types of interventions
Contraceptive implants:
immediate postpartum insertion of contraceptive implants (after delivery and before hospital discharge) compared to delayed postpartum insertion (during a postpartum visit after hospital discharge), which is often referred to as standard, or interval, insertion.
IUDs:
immediate postpartum insertion, including both immediate postplacental insertion (within 10 minutes of placenta delivery, following vaginal or cesarean delivery) and early postpartum insertion (10 minutes after delivery to hospital discharge), compared to delayed postpartum insertion (during a postpartum visit four or more weeks after hospital discharge), which is often referred to as standard, or interval, insertion.
Types of outcome measures
Primary outcomes
Rate of insertion of contraceptive implants and IUDs at the first postpartum visit (four to six weeks postpartum).
Secondary outcomes
Expulsion rate of IUDs at 6, 12, and 24 months after delivery.
Utilization rate at 6, 12, and 24 months after delivery.
-
Adverse effects:
for contraceptive implants: bleeding, including mean days of bleeding and proportion of people experiencing irregular bleeding, and adverse effects or harms other than bleeding;
for IUDs: perforation, and infection.
Participant satisfaction.
Unintended pregnancy rate at 6, 12, and 24 months after delivery.
Rapid repeat pregnancy (pregnancy within 18 months of delivery).
Breastfeeding at six months postpartum.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (via Ovid EBM Reviews, CENTRAL, 2020, issue 12);
MEDLINE ALL (Ovid) (1946 to December week 2, 2020);
Embase.com (1980 to December 2020);
POPLINE (1970 to December 2020).
We have provided the search strategies in Appendix 1.
Searching other resources
We checked the citation lists of included studies, key textbooks, and systematic reviews for potentially relevant references. We searched the WHO International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en) and ClinicalTrials.gov to identify ongoing trials. We applied modified versions of the same search strategy to check the following databases for grey literature: OpenGrey, GreyNet, Scirus, Social Care Online, National Research Register, NIHR portfolio database, and Index to theses.
Data collection and analysis
Selection of studies
Before examining the identified trials for possible inclusion, we developed and piloted a data collection form, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We downloaded all titles and abstracts retrieved by electronic searching. After removal of duplicates, all references were transferred to Covidence. Two review authors, JS and SK, independently screened the titles and abstracts of the remaining studies. We excluded studies that clearly did not meet the inclusion criteria. We obtained full‐text copies of potentially relevant studies. Two review authors, JS and SK, independently assessed the eligibility of the retrieved reports and publications. We resolved any disagreement through discussion or, if required, we consulted a third review author (PL, SA or PP). We identified and intended to collate multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009), and a.'Characteristics of excluded studies table.
Data extraction and management
Two review authors, JS and SK, independently extracted study characteristics and outcome data from the included studies using a piloted data collection form. When the included trials did not report outcome data in a usable way, we noted in this the Characteristics of included studies table. We then contacted the trial authors for further information. We resolved disagreements by consensus or by involving a third review author (PL, SA or PP). One review author, PP, entered data into Review Manager 5 (RevMan 5) (RevMan 2014). We double‐checked whether data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (PL or SA) checked all study characteristics for accuracy against the trial report.
For included studies, we extracted the following data:
author, year of publication, and journal citation (including language);
country;
setting;
inclusion and exclusion criteria;
study design and methodology;
study population;
study outcomes and their related summary statistics.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), which recommends an assessment is made for each of the following domains for RCTs:
selection bias: random sequence generation and allocation concealment;
performance bias: blinding of participants and personnel (participants and treatment providers);
detection bias: blinding of outcome assessors;
attrition bias: incomplete outcome data;
reporting bias: selective reporting of outcomes.
Two review authors, JS and SA, independently applied the risk of bias tool (ROB 1) to each included study and resolved differences by discussion or by consulting a third review author (PL or PP). SA was a study author on two studies, so other review authors assessed these studies for risk of bias (JS and PL). We judged each item as at being at either 'high’, 'low’, or 'unclear’ risk of bias, as set out in the criteria provided by Higgins 2019, and provided a quotation from the study report or a statement as justification for the judgement for each item in the risk of bias table. We summarized the results in both a risk of bias graph and a risk of bias summary. When we interpreted treatment effects and meta‐analyses, we took into account the risk of bias for the studies that contributed to that outcome. Where information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the risk of bias table.
Measures of treatment effect
We used the following measures of the effect of treatment.
For dichotomous outcomes, such as the rates of contraceptive implantation and IUD initiation, we used number of events and number of participants assessed for both the intervention and comparison groups to calculate the risk ratio (RR) and 95% confidence interval (CI).
For continuous outcomes, such as satisfaction rate, we used mean, standard deviation (SD), and the number of participants assessed for both the intervention and comparison groups to calculate mean difference (MD) with 95% CI.
Unit of analysis issues
We intended to include studies where postpartum people were randomized individually and also cluster‐randomized studies where, for example, the hospital was the unit of randomization. However, we did not find any relevant cluster‐randomized studies. In future updates of this review, if we identify studies that use a cluster‐randomized design but do not have any information related to the design effect, we will estimate the design effect based on a fairly large assumed intra‐cluster correlation of 0.10. We will base this assumption by analogy on studies about implementation research (Campbell 2000; Ukoumunne 1999).
Dealing with missing data
We did not impute any missing outcome data and we tried to contact the trial authors for missing data.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots. We also assessed statistical heterogeneity in each meta‐analysis using the I² statistic and Chi² test. We regarded heterogeneity as substantial if the I² statistic value was greater than 50%, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity (Deeks 2001).
Assessment of reporting biases
We considered all 16 studies to be at low risk of selective reporting bias, since all relevant outcomes were reported by all included studies.
Data synthesis
We undertook meta‐analysis for all the outcomes where suitable data were available. We described skewed data reported as medians and interquartile ranges narratively.
Using RevMan 5, we performed statistical analyses (RevMan 2014). We used a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect, that is where studies were examining the same intervention, and we judged the studies' populations and methods to be sufficiently similar. If there was unexplained clinical heterogeneity sufficient to expect that the underlying treatment effects differed between studies, or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary, but only where it was possible to derive a meaningful meta‐analysis. For example, population differences (high‐income and low‐ and middle‐income countries), type of IUDs (non‐hormonal IUDs, hormonal IUDs, and any other type of IUD). When we used random‐effects meta‐analysis, we treated the pooled treatment effect as the average range of possible treatment effects, and discussed the clinical implications of treatment effects differing between studies. When we used random‐effects analyses, we presented the results as the pooled treatment effect with 95% CIs, and estimates of the T² and I² statistic (DerSimonian 1986). We prepared a summary of findings table to present the results of meta‐analysis, based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). For the data that we were unable to pool for meta‐analysis, we conducted a narrative synthesis of the results.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses according to:
age of participants (teens (13‐19 years old) versus non‐teens);
type of IUDs (non‐hormonal IUDs versus hormonal IUDs); and
population differences (high‐income versus low‐ and middle‐income countries, according to World Bank 2020).
We performed subgroup analyses for the primary outcome of rate of insertion at first postpartum visit (six to eight weeks postpartum) to assess:
population differences for contraceptive implants, and
types of IUDs.
We did not perform subgroup analyses according to age as the number of included studies that would allow this subgroup analysis was limited.
Sensitivity analysis
We performed the following sensitivity analyses in order to determine the impact of the following factors on effect size:
repeating the analysis excluding unpublished studies (if any);
repeating the analysis excluding trials rated as 'high' or 'unclear' for risk of selection bias.
Summary of findings and assessment of the certainty of the evidence
Two review authors working independently prepared a summary of findings table, and disagreements were resolved by consensus. The following outcomes were reported in the table reporting on contraceptive implants:
rate of initiation;
utilization rate at six months postpartum;
utilization rate at 12 months postpartum;
adverse effects of contraceptive implants: prolonged vaginal bleeding within six weeks postpartum;
adverse effects other than prolonged vaginal bleeding;
unintended pregnancy rate at 12 months postpartum; and
any breastfeeding at six months postpartum.
The following outcomes were reported in the table reporting on IUDs:
rate of initiation;
expulsion by six months postpartum;
utilization rate at six months postpartum;
utilization rate at 12 months postpartum;
unintended pregnancy rate at 12 months postpartum; and
any breastfeeding at six months postpartum.
We used the GRADE approach (consideration of study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the evidence (GRADEproGDT 2014). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We justified all decisions to downgrade the certainty of evidence, using footnotes.
Results
Description of studies
Results of the search
We identified 4275 references from the combined searches for this updated review. After removing 792 duplicated references, we screened the titles and abstracts of 3483 references and discarded 3429 as it was obvious that they did not meet the inclusion criteria. The 54 references that potentially met the inclusion criteria, included the three studies that had been included in the original review, as well as 13 studies that we excluded because they did not meet the inclusion criteria (see the 'Characteristics of excluded studies' table), 12 ongoing studies (see the 'Characteristics of ongoing studies'), and 13 new studies (26 records) that we included; see the 'Characteristics of included studies' table. We checked the reference lists of the included studies, key textbooks, and systematic reviews for additional relevant references but found no other relevant studies. Figure 1 displays the PRISMA flow diagram.
1.
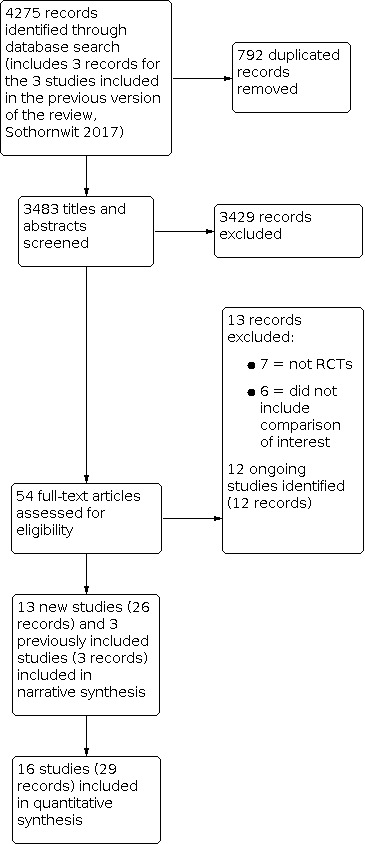
Study flow diagram
Included studies
Study design and setting
We included 16 RCTs, and presented the details in the Characteristics of included studies table. Two of the trials were multi‐centre studies (Turok 2017; Whitaker 2014), while the others were single‐centre studies. All were conducted in tertiary or university hospitals: 11 in the USA (Bryant 2017; Chen 2010; Dahlke 2011; Gurtcheff 2011; Levi 2015; Ogburn 2013; Phemister 1995; Soon 2018; Stuart 2015; Turok 2017; Whitaker 2014), two in Uganda (Averbach 2017; Lester 2015); and one each in Egypt (Bayoumi 2020), Brazil (Carmo 2017), and Sri Lanka (Dias 2015). Dahlke 2011 and Soon 2018 were pilot studies.
Participants
Most studies included participants aged 18 and above. The five studies that investigated contraceptive implants included 361 people in the intervention groups (immediate postpartum insertion) and 354 people in the control groups (delayed insertion). Only Bryant 2017 focused on postpartum adolescents (aged 14 to 24 years).
The 11 studies that investigated IUDs included 954 people in the intervention groups that received non‐hormonal IUDs or hormonal IUDs immediately postpartum, and 940 people in the comparator groups. Soon 2018 was the only trial that included teenagers (14‐ to 19‐years old).
Details of the inclusion and exclusion criteria for each trial are found in Characteristics of included studies.
Intervention
Five studies reported outcomes pertaining to LNG contraceptive implants (Averbach 2017; Phemister 1995), or etonogestrel‐releasing (ENG) implants (Bryant 2017; Carmo 2017; Gurtcheff 2011), while 11 studies reported outcomes pertaining to IUDs, including five studies of hormonal IUDs (Chen 2010; Dahlke 2011; Soon 2018; Stuart 2015; Turok 2017), and five studies of non‐hormonal IUDs (Bayoumi 2020; Dias 2015; Lester 2015; Ogburn 2013; Whitaker 2014). Levi 2015 included both types of IUD. We included studies where immediate postpartum IUDs were inserted after vaginal or cesarean delivery.
Five studies investigated insertion of IUDs after vaginal delivery (Chen 2010; Dahlke 2011; Soon 2018; Stuart 2015; Turok 2017).
Four studies investigated insertion of IUDs after cesarean delivery (Bayoumi 2020; Lester 2015; Levi 2015; Whitaker 2014).
Ogburn 2013 investigated insertion after either vaginal or cesarean birth.
Dias 2015 did not specify the route of delivery.
Primary outcomes
Initiation rate
Contraceptive implants
All five of the included contraceptive implant studies reported the initiation rate. Initiation rates in the delayed groups were measured at four to eight weeks after delivery.
IUDs
All 11 IUD studies reported this outcome. Five studies reported initiation rate of non‐hormonal IUDs (Bayoumi 2020; Dias 2015; Lester 2015; Ogburn 2013; Whitaker 2014), while five studies reported this outcome for hormonal IUDs (Chen 2010; Dahlke 2011; Soon 2018; Stuart 2015; Turok 2017). Levi 2015 included both types of IUDs.
Secondary outcomes
Utilization rate
Contraceptive implants
Four studies reported utilization rate (Averbach 2017; Bryant 2017; Carmo 2017; Gurtcheff 2011), of which three reported rates at six months postpartum (Averbach 2017; Bryant 2017; Gurtcheff 2011), and two at 12 months postpartum (Bryant 2017; Carmo 2017).
IUDs
Seven studies reported utilization rate (Bayoumi 2020; Chen 2010; Dahlke 2011; Lester 2015; Levi 2015; Ogburn 2013; Whitaker 2014), of which five reported rates at six months postpartum (Chen 2010; Dahlke 2011; Lester 2015; Levi 2015; Whitaker 2014), two at 12 months postpartum (Ogburn 2013; Whitaker 2014), and one at both six and 12 months postpartum (Bayoumi 2020).
Adverse effects
Contraceptive implants
Two studies reported mean number of days of prolonged vaginal bleeding within six weeks postpartum (Averbach 2017; Phemister 1995). Three studies reported prolonged vaginal bleeding at three, six, and 12 months postpartum (Averbach 2017; Bryant 2017; Carmo 2017). Only Phemister 1995 reported adverse effects other than vaginal bleeding.
IUDs
No included study reported on adverse effects of IUDs.
Expulsion
IUDs
Eight studies reported expulsion rate of IUDs. Most studies reported the rate at six months (Bayoumi 2020; Chen 2010; Dahlke 2011; Lester 2015; Levi 2015; Stuart 2015; Turok 2017; Whitaker 2014).
Participant satisfaction
Contraceptive implants
Averbach 2017 reported satisfaction rate at six months postpartum and Carmo 2017 reported the rate at 12 months postpartum.
IUDs
Levi 2015 reported satisfaction rate at six months postpartum and Bayoumi 2020 reported the rate at 12 months postpartum.
Breastfeeding
Contraceptive implants
Two studies reported exclusive breastfeeding rates (Averbach 2017; Carmo 2017), and two studies reported any breastfeeding rates at six months (Averbach 2017; Bryant 2017).
LNG‐IUS
Five studies reported breastfeeding rates at six months postpartum (Chen 2010; Dahlke 2011; Levi 2015; Stuart 2015; Turok 2017). Only Chen 2010 reported rates of exclusive breastfeeding.
Unintended pregnancy
Contraceptive implants
Averbach 2017 reported unintended pregnancy rates at six months postpartum and Bryant 2017 reported the rates at 12 months postpartum.
IUDs
Only Bayoumi 2020 reported the rates at 12 months postpartum.
Excluded studies
After assessing the full texts of potentially eligible studies, we excluded 13 studies because of the following main reasons (see the Characteristics of excluded studies table):
six studies were not RCTs or CCTs (Bryant 2013; Gariepy 2015; Ireland 2014; Taneepanichkul 2001; Tocce 2012; Wilson 2014);
seven studies did not compare immediate postpartum insertion with delayed insertion of LARC (Baldwin 2014; Braga 2015; Brito 2009; Brito 2012; Pentickly 2013; Shabaan 1985; VanDerPas 1980).
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias items presented as percentages across all included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation and allocation concealment
Fourteen included studies randomly allocated participants to the comparison groups using adequate methods for sequence generation and allocation concealment (Averbach 2017; Bayoumi 2020; Bryant 2017; Carmo 2017; Chen 2010; Dahlke 2011; Gurtcheff 2011; Lester 2015; Levi 2015; Phemister 1995; Soon 2018; Stuart 2015; Turok 2017; Whitaker 2014). We therefore judged these studies as being at low risk of selection bias. Two studies did not provide enough information about randomization process and we judged these studies to be at unclear risk of bias (Dias 2015; Ogburn 2013)
Blinding
Although the timing of contraceptive implant and IUD insertion could not be blinded, we judged all 16 included studies as being at low risk of performance and detection biases for the primary outcomes, because blinding is unlikely to affect these outcome measures. However, as knowledge of group assignment may affect self‐report of satisfaction and adverse effects, we considered this domain to be at high risk of bias for these outcomes.
Incomplete outcome data
Nine included studies had rates of incomplete outcome data of less than 20% (Averbach 2017; Carmo 2017; Chen 2010; Dahlke 2011; Gurtcheff 2011; Lester 2015; Levi 2015; Phemister 1995; Turok 2017). Thus, we judged all these studies to be at low risk of attrition bias (Figure 3). We judged five studies to be at high risk of attrition bias: two because they had more than 20% of incomplete outcome data in at least one arm (Bayoumi 2020; Bryant 2017), and three because they were stopped early by the Data and Safety Monitoring Board or because of slow recruitment (Soon 2018; Stuart 2015; Whitaker 2014). Two studies did not report loss to follow‐up clearly (Dias 2015; Ogburn 2013), so we deemed these to be at unclear risk of attrition bias.
Selective reporting
The trial investigators of all included studies reported all relevant outcomes for their objectives, so we judged this domain as being at low risk of reporting bias for all included studies.
Other potential sources of bias
We judged six studies to be at unclear risk of bias for other sources of bias for the following reasons:
in two studies, the trial investigators excluded people after randomization (Phemister 1995; Turok 2017);
in three studies the trial investigators did not state their definitions for prolonged vaginal bleeding, which was an outcome of interest for these contraceptive implants studies (Bryant 2017; Gurtcheff 2011; Phemister 1995); and
the two pilot studies were not powered for the outcomes of interest (Dahlke 2011; Soon 2018).
Effects of interventions
See Table 1.
Primary outcome
Rate of initiation of contraceptive implant
All included studies reported the rate of initiation of contraceptive implants by the first postpartum visit (four to six weeks postpartum in Bryant 2017, Carmo 2017 and Phemister 1995; and four to eight weeks postpartum in Averbach 2017 and Gurtcheff 2011). There was a higher rate of contraceptive implant initiation among people assigned to the immediate insertion group compared with those in the delayed insertion group (risk ratio (RR) 1.48, 95% confidence interval (CI) 1.11 to 1.98; 5 studies, 715 participants; I2 = 95%; moderate‐certainty evidence; Analysis 1.1; Figure 4; Table 1). The evidence suggests that if the initiation rate with delayed insertion is assumed to be 63%, the initiation rate with immediate insertion would be between 70% and 100%.
1.1. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 1: Rate of initiation of contraceptive implants
4.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.1 Rate of initiation of contraceptive implants
We detected substantial heterogeneity, and, therefore, performed subgroup analysis for HICs versus LMICs, as planned, for this comparison. The test for subgroup differences did not indicate a difference in the effect by the study setting for this comparison (P = 0.82), but this may have been due to the small number of studies available for the comparison (n = 5).
We performed sensitivity analysis and restricted the analysis to the two studies with low risk of bias (Averbach 2017; Carmo 2017). The estimated effect increased slightly, but the confidence interval was wider and contained the null; heterogeneity remained substantial (RR 1.57, 95% CI 0.61 to 4.05; 2 studies, 305 participants; I2 = 98%).
Rate of initiation of IUDs
Immediate insertion of IUDs likely improves the initiation rate compared with delayed insertion (RR 1.27, 95% CI 1.07 to 1.51; 11 studies, 1894 participants; I2 = 98%; moderate‐certainty evidence; Analysis 2.1; Figure 5; Table 2). The evidence suggests that the chance of initiation of IUDs with immediate insertion is between 66% and 93%, while the chance of initiation of IUDs with delayed insertion is 61%. We performed a planned subgroup analysis by IUD type. Immediate insertion may improve the initiation rate when compared to delayed insertion for both the non‐hormonal IUDs subgroup (RR 1.38, 95% CI 0.96 to 1.97; 5 studies, 1329 participants; I2= 95%) and the hormonal subgroup (RR 1.11, 95% CI 1.05 to 1.18; 5 studies, 453 participants; I2= 0%). The heterogeneity may be partially explained by the difference in IUD type although it remained substantial (test for subgroup difference: P = 0.005, I2 = 78.7%).
2.1. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 1: Rate of initiation of IUDs
5.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.4 Other adverse effects
Findings were not influenced by sensitivity analysis restricting to the three studies with low risk of bias; one hormonal IUDs study (Lester 2015), one LNG‐IUS (Chen 2010), and one mostly hormonal IUDs study (Levi 2015). The estimated effect increased slightly but the confidence interval was wider (RR 1.43,95% CI 1.02 to 2.01; 3 studies, 282 participants; I2 =88%; forest plot not shown).
Secondary outcome: utilization rate
It is unclear whether there was a difference in utilization rates of both contraceptive implants and IUDs at six and 12 months after delivery.
Contraceptive implants
Utilization rate at six months postpartum
We are uncertain whether immediate insertion of contraceptive implants improves utilization rate at six months (RR 1.16, 95% CI 0.9 to 1.5; 3 studies, 330 participants; I2 = 89%; very low certainty evidence; Analysis 1.2; Figure 6; Table 1). The evidence suggests that if the utilization rate with delayed insertion is assumed to be 71%, the utilization rate with immediate insertion would be between 64% and 100%.
1.2. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 2: Utilization rate
6.

Forest plot of comparison: 1 Immediate versus delayed postpartum insertion of contraceptive implants, outcome: 1.2 Utilization rate
Utilization rate at 12 months postpartum
We are uncertain whether immediate insertion of contraceptive implants improves utilization rate at 12 months (RR 0.98, 95% CI 0.93 to 1.04; 2 studies, 164 participants; I2 = 0%; very low‐certainty evidence; Analysis 1.2; Figure 6; Table 1). The evidence suggests that if the utilization rate with delayed insertion is assumed to be 92%, the utilization rate with immediate insertion would be between 86% and 96%.
Utilization rate at 24 months postpartum
No studies reported this outcome.
IUDs
Utilization rate at six months postpartum
We are uncertain whether immediate postpartum insertion of IUDs improves utilization rate at six months compared with delayed insertion (RR 1.02, 95% CI 0.65 to 1.62; 6 studies, 971 participants; I2 = 96%; very low‐certainty evidence; Analysis 2.2; Table 2). This suggests that if the utilization rate at six months with delayed insertion is assumed to be 82%, the utilization rate with immediate insertion would be between 52% and 100%.
2.2. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 2: Utilization rate
Utilization rate at 12 months postpartum
We are uncertain whether immediate insertion improves utilization rate at 12 months (RR 0.86, 95% CI 0.5 to 1.47; 3 studies, 796 participants; I2 = 91%; very low‐certainty evidence; Analysis 2.2; Figure 6; Table 2). The evidence suggests that if the utilization rate with delayed insertion is assumed to be 78%, the utilization rate with immediate insertion would be between 39% and 100%.
Utilization rate at 24 months after delivery
No studies reported this outcome.
Secondary outcome: expulsion rate of IUDs
Expulsion rate at six months postpartum
Expulsion rate for IUDs at six months postpartum may be higher with immediate insertion than with delayed insertion (RR 4.55, 95% CI 2.52 to 8.19; 9 studies, 1269 participants; I2 = 31%; low‐certainty evidence; Analysis 2.3; Table 2). The evidence suggests that if the expulsion rate with delayed insertion is assumed to be 2%, the rate with immediate insertion would be between 5% and 17%.
2.3. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 3: Expulsion
Expulsion rate at 12 months postpartum
No studies reported this outcome.
Expulsion rate at 24 months postpartum
No studies reported this outcome.
Secondary outcome: adverse effects
Mean days of vaginal bleeding within six weeks postpartum with contraceptive implants
For contraceptive implants, the duration of prolonged vaginal bleeding among participants in the immediate insertion group may have been longer than for participants in the delayed insertion group (mean difference (MD) 2.98 days, 95% CI ‐2.71 to 8.66; 2 studies, 420 participants; I2 = 91%; low‐certainty evidence; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 3: Adverse effect: mean days of duration of vaginal bleeding
Prolonged bleeding at three months postpartum with contraceptive implants
Due to very low‐certainty evidence, we are uncertain whether immediate insertion was associated with a reduction in prolonged bleeding at three months (RR 0.39, 95% CI 0.10 to 1.47; 2 studies, 225 participants; I2 = not applicable; very low‐certainty evidence; Analysis 1.4; Figure 5). This suggests that if the rate of prolonged bleeding with delayed insertion is assumed to be 5%, the rate with immediate insertion would be between 0.5% and 7%.
1.4. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 4: Other adverse effects
Prolonged bleeding at six months postpartum with contraceptive implants
Due to very low‐certainty evidence, we are uncertain whether immediate insertion was associated with prolonged bleeding at six months (RR 1.19, 95% CI 0.29 to 4.94; 2 studies, 252 participants; I2 = 0%; very low‐certainty evidence; Analysis 1.4; Figure 5). This suggests that if the rate of prolonged bleeding with delayed insertion is assumed to be 3%, the rate with immediate insertion would be between 0.8% and 14%.
Other vaginal bleeding or associated severe cramping within 12 months postpartum with contraceptive implants
One included study reported the rate of heavy, irregular bleeding, or associated cramping evaluated at 12 months among participants who had immediate insertion compared to participants who had delayed insertion of contraceptive implant (Bryant 2017). It was unclear whether there was any difference between the two groups (RR 1.01, 95% CI 0.72 to 1.44; 1 study, 64 participants; very low‐certainty evidence; Analysis 1.4; Figure 5). The evidence suggests that the risk of having heavy, irregular vaginal bleeding or associated severe cramping within 12 months of immediate insertion was between 48% and 96%, while the risk with delayed insertion was 67%.
Other adverse effects
Phemister 1995 reported that participants in the immediate insertion group experienced a higher rate of other adverse effects including nausea, headache, and acne than reported among participants in the delayed insertion group (RR 2.06; 95% CI 1.38 to 3.06; 1 study, 215 participants; low‐certainty evidence; Analysis 1.4; Figure 5; Table 1). This suggests that if the risk of having other adverse effects with delayed insertion is assumed to be 23%, the risk with immediate insertion would be between 32% and 70%.
Secondary outcome: participant satisfaction
Contraceptive implants
Participant satisfaction at six months postpartum
In Averbach 2017, the trial investigators reported an overall high satisfaction rate during the six‐month follow‐up. There was probably little or no difference between the immediate and delayed groups (RR 0.97, 95% CI 0.90 to 1.04; 1 study, 152 participants; very low‐certainty evidence; Analysis 1.5). This suggests that if the rate of satisfaction with delayed insertion is assumed to be 97%, then with immediate insertion, the satisfaction rate would be between 87% and 100%.
1.5. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 5: Participant satisfaction
Participant satisfaction at 12 months postpartum
One RCT undertaken in Brazil reported on this outcome (Bryant 2017). We are uncertain whether there was any difference in satisfaction rate at 12 months between the groups (RR 1.01, 95% CI 0.77 to 1.31; 1 study, 64 participants; very low‐certainty evidence; Analysis 1.5). This suggests that if the rate of satisfaction with delayed insertion is assumed to be 78%, then for people with the immediate insertion, the satisfaction rate would be between 60% and 100%.
IUDs
Participant satisfaction at six months postpartum
Levi 2015 found that there was little or no difference between immediate and delayed insertion of IUDs for participant satisfaction at six months postpartum (RR 0.93, 95% CI 0.83 to 1.03; 1 study, 69 participants; very low‐certainty evidence; Analysis 2.4). The evidence from this one included trial suggested that participants' satisfaction with immediate insertion was 92% (36/39), and was 100% (30/30) for delayed insertion.
2.4. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 4: Participant satisfaction
Participant satisfaction at 12 months postpartum
Bayoumi 2020 found that there was little or no difference between immediate and delayed insertion of IUDs (RR 1.05, 95% CI 0.98 to 1.12; 1 study, 598 participants; low‐certainty evidence; Analysis 2.4; Table 1). The evidence from the one included trial suggested that participants' satisfaction with immediate insertion was 90% (357/396), and was 86% (174/202) for delayed insertion.
Secondary outcome: unintended pregnancy rates
Contraceptive implants
Unintended pregnancy rates at six months postpartum
Only one included study reported the unintended pregnancy rate at six months postpartum in people who used contraceptive implants (Averbach 2017). No pregnancies occurred during the follow‐up period in the immediate insertion group (0/103), compared to 1% (2/102) in the delayed insertion group. We are uncertain whether there was a difference in unintended pregnancy rates between the groups (RR 0.20, 95% CI 0.01 to 4.08; one study, 205 participants; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 6: Unintended pregnancy rate
Unintended pregnancy rates at 12 months postpartum
Bryant 2017 reported that the rates of unintended pregnancy within one year of delivery were 13.5% and 7.0% among participants allocated to immediate and delayed insertion groups, respectively. It was unclear whether there was any difference between the groups (RR 1.82, 95% CI 0.38 to 8.71; 1 study, 64 participants; very low‐certainty evidence; Analysis 1.6; Table 1).
Unintended pregnancy rates at 24 months postpartum
No studies reported this outcome.
IUDs
Unintended pregnancy rates at six months postpartum
Three studies reported pregnancy rate in people who used IUDs for contraception, however, no pregnancies occurred during the follow‐up period of these studies. We were, therefore, unable to estimate the effect of the intervention for this outcome.
Unintended pregnancy rates at 12 months postpartum
Only one included trial reported the unintended pregnancy rate at 12 months postpartum (Bayoumi 2020). The rates of unintended pregnancy were 4.4% and 16.8% among participants allocated to the immediate and delayed insertion groups, respectively. The rate of unintended pregnancies may have been lower in the immediate insertion group (RR 0.26, 95% CI 0.17 to 0.41; 1 study, 1000 participants; low‐certainty evidence; Analysis 2.5; Table 2).
2.5. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 5: Unintended pregnancy rate
Unintended pregnancy rates at 24 months postpartum
No studies reported this outcome.
Secondary outcome: short interpregnancy interval (defined as pregnancy onset within two years of the previous pregnancy)
No studies reported this outcome.
Secondary outcome: breastfeeding at six months postpartum
Contraceptive implants
Exclusive breast feeding
There was little or no difference between the two groups in exclusive breastfeeding rate between immediate and delayed insertion of contraceptive implants (RR 0.89, 95% CI 0.66 to 1.21; 2 studies, 261 participants; I2 = 27%; low‐certainty evidence; Analysis 1.7). This suggests that if the exclusive breastfeeding rate with delayed insertion is assumed to be 34%, the rate with immediate insertion would be between 23% and 42%.
1.7. Analysis.

Comparison 1: Immediate versus delayed postpartum insertion of contraceptive implants, Outcome 7: Breastfeeding at 6 months postpartum
Any breastfeeding
Averbach 2017, Bryant 2017, and Gurtcheff 2011 reported any breastfeeding rate at six months. Gurtcheff 2011, however, presented Kaplan‐Meier curves to evaluate the impact of immediate postpartum insertion of contraceptive implants on exclusive or any breastfeeding compared to that of delayed postpartum insertion. The rates between the two groups were not significantly different at any time period. However, numerical data for this outcome were insufficient to allow us to determine the relative effect at each time point. Attempts to obtain additional data from the investigators were unsuccessful since the trial authors did not reply to our requests.
There may be little or no difference between the two groups for any breastfeeding (RR 0.97, 95% CI 0.92 to 1.01; 2 studies, 225 participants; I2= 48%; low‐certainty evidence; Analysis 1.7; Table 1). The evidence suggested that if the breastfeeding rate with delayed insertion is assumed to be 78%, the rate with immediate insertion would be between 71% and 78%.
Hormonal IUDs
Exclusive breast feeding
Chen 2010 and Turok 2017 reported exclusive breastfeeding rate at six months. There may be little or no difference between the two groups in exclusive breastfeeding rate between immediate and delayed insertion of hormonal IUDs (RR 0.52, 95% CI 0.08 to 3.19; 2 studies, 297 participants; I2 = 68%; low‐certainty evidence; Analysis 2.6). This suggests that if the exclusive breastfeeding rate with delayed insertion is assumed to be 20%, the rate with immediate insertion would be between 2% and 64%.
2.6. Analysis.

Comparison 2: Immediate versus delayed postpartum insertion of IUDs, Outcome 6: Breastfeeding (LNG‐IUS)
Any breastfeeding
We are uncertain whether there was any difference in the breastfeeding rate between immediate and delayed insertion of hormonal IUDs (RR 0.90, 95% CI 0.63 to 1.30; 5 studies, 435 participants; I2 = 54%; very low‐certainty evidence; Analysis 2.6; Table 2). This suggests that if the rate for any breastfeeding with delayed insertion is assumed to be 47%, the rate with immediate insertion would be between 30% and 61%.
Discussion
Summary of main results
Sixteen studies conducted in five countries from both low‐to‐middle‐income and high‐income settings were included in our analysis. Moderate‐certainty evidence demonstrated that immediate insertion at the first postpartum visit likely increases the rate of initiation of both contraceptive implant and IUDs. However, the certainty of the evidence was very low for estimating the utilization rate at six and 12 months postpartum. It is uncertain whether immediate postpartum insertion has an effect on utilization at these time points. Low‐certainty evidence suggests the immediate insertion of IUDs may be associated with a higher expulsion rate. The mean prolonged vaginal bleeding among people receiving immediate insertion of contraceptive implants was longer than that noted among people receiving delayed insertion, however, this difference is unlikely to be clinically meaningful, as the mean difference in duration of days of bleeding was only three days. Additionally, rates of prolonged bleeding between the two comparison groups were comparable regardless of the severity of bleeding. Because the timing of LARC insertion cannot be blinded, the difference in the rate of self‐reported adverse effects between the immediate and delayed postpartum insertion of contraceptive implant and IUDs remains inconclusive. Based on the limited data available, we are uncertain whether there are any differences between the two groups in terms of participant satisfaction, unintended pregnancy rate at 12 months, and rate of breastfeeding at six months postpartum for both contraceptive implants and IUDs.
Overall completeness and applicability of evidence
This review included 16 RCTs. Of these, five trials evaluated immediate versus delayed postpartum insertion of contraceptive implants for contraception in 715 participants while 11 trials evaluated the IUDs in 1894 participants. The study participants were recruited from four countries, but mainly from the USA. Additionally, the study settings were large hospitals. Only three of the included studies were mainly conducted in low‐ to middle‐income countries (LMICs), which often have low postpartum contraceptive prevalence (UN 2013). Thus, generalization of these findings to LMICs settings should be done with caution since studies in these populations are lacking.
Contraceptive implants
For contraceptive implants, five included studies reported the initiation rate of contraceptive implants by the first postpartum follow‐up visit, which was the primary outcome of this review. For this update, two studies were added, allowing us to perform planned subgroup analysis based on the resource setting. The results, however, did not affect interpretation of the main analysis. Only three included studies reported utilization rate at six months after delivery. Moreover, a limited number of included studies reported adverse effects which meant that only one or two studies could be included in the analysis for each effect. Administration of progestogen‐releasing modes of contraception may be delayed because of concerns about the effect on lactation. Two studies reported data regarding the impact of the insertion time of contraceptive implant on exclusive breastfeeding at six months postpartum (Averbach 2017; Carmo 2017). Three trials reported on the outcome of any breastfeeding, Averbach 2017; Bryant 2017; and Gurtcheff 2011. However, we could not include results from Gurtcheff 2011 in our analysis since the report did not provide data regarding the number of people breastfeeding in each comparison group. More studies focusing on both types of breastfeeding are needed. Data regarding long‐term utilization rate, rate of unintended pregnancies or short‐interval pregnancies, and participant satisfaction were limited as only one or two included studies reported these data.
IUDs
For IUDs, 11 studies assessed our primary objective. Studies of hormonal IUDs were all conducted in the USA, but studies of non‐hormonal IUDs included one each from the USA, Sri Lanka, Egypt, and Uganda. The Levi 2015 study, conducted in the USA, included both types of IUDs. Due to limited number of included studies, we were unable to perform planned subgroup analyses based on the setting (high‐income versus LMICs). However, we performed subgroup analyses based on the type of IUDs (non‐hormonal IUDs versus hormonal IUDs). The result showed that immediate insertion seems to have a beneficial effect on utilization at the first follow‐up visit regardless of the type of IUD. The expulsion rate at six months postpartum was reported by eight included studies. A higher expulsion rate was observed in the immediate insertion group with low heterogeneity. Three studies reported on the unintended pregnancy rate at six months postpartum, but the effect of the intervention could not be estimated as there were no unintended pregnancies in either group. The unintended pregnancy rate at 12 months postpartum seemed to be lower in the immediate insertion group according to one included study.
Insertion of hormonal IUDs may be delayed because of concerns about the effect on lactation. Five included studies did provide data on lactation with hormonal IUDs use. The exclusive breastfeeding rates, however, were addressed by only two included studies.
Quality of the evidence
Although it was not possible to blind the timing of the intervention, blinding was unlikely to affect the rate of initiation and utilization rate of contraceptive implant or IUDs. However, the awareness of the intervention assigned may have affected self‐reporting of treatment‐related adverse effects, thus indicating a high risk of performance and assessment biases for this outcome measures. This compromises the internal validity of the included studies when determining self‐reported subjective outcomes, such as adverse effects and participant satisfaction. The higher risk of adverse effects among people who received an immediate postpartum insertion versus those who received a delayed insertion should be viewed cautiously. It is not known whether this was due to participants knowing which intervention had been administered, or the timing of the insertion itself. We extracted data regarding participant satisfaction and unintended pregnancies from a single study for each outcome for both contraceptive implants and IUDs. Most of the studies that provided data for the analysis regarding breastfeeding were secondary analyses which may have had insufficient power.
We determined the certainty of evidence using the GRADE approach for each outcome. We downgraded the certainty of the evidence to moderate certainty for rate of initiation of contraceptive implants and IUDs. We downgraded the evidence to low‐ or very low‐certainty for the utilization rate, prolonged vaginal bleeding, adverse effects other than vaginal bleeding, participant satisfaction, rates of unintended pregnancy, and breastfeeding at six months (see Table 1; Table 2).
Thus, the certainty of the evidence ranged from moderate to very low. The main limitations were imprecision, inconsistency, and risk of bias (related to lack of blinding and to attrition). There is substantial heterogeneity of the pooled effect estimate of the primary outcome, which can be accounted for in part by differences in study populations and types of IUDs.
Potential biases in the review process
With assistance from the Information Specialist of the Cochrane Fertility Regulation Group, we made every attempt to include all potentially relevant studies through a thorough search of the standard databases, grey literature, conference proceedings, and ongoing trials. However, since we were only able to include, at most, nine studies for each outcome, we cannot exclude the possibility of publication bias.
None of the review authors has any links to pharmaceutical companies or a financial interest in the prescription of the drugs under assessment. One of the review authors, SA, is an author of two included studies (Averbach 2017; Lester 2015). However, she was not involved in the selection process or judgements about risk of bias for these studies. We followed Cochrane guidelines to extract data and assess the certainty and potential risks of different types of biases in all included studies. Thus, there were no issues related to conflicts of interests in this review.
Agreements and disagreements with other studies or reviews
To date, there are no other systematic reviews that compare immediate versus delayed postpartum insertion of contraceptive implants. For IUDs, a Cochrane Review that assessed the effectiveness of immediate postplacental (within 10 minutes of placenta delivery) insertion of IUDs for contraception compared to early postpartum insertion (after 10 minutes to 48 hours) and the standard insertion (during the postpartum visit) demonstrated that there was no significant difference in the rates of IUD use among people who received an immediate insertion compared to those who received an early postpartum insertion (odds ratio (OR) 0.46, 95% CI 0.04 to 5.75) (Lopez 2015). In this updated review, however, immediate insertion was associated with a slight increase in the rate of initiation by the first postpartum visit compared to delayed insertion for both contraceptive implants and IUDs (RR 1.48; 95% CI 1.11 to 1.98 and RR 1.27; 95% CI 1.07 to 1.51, respectively).
In the previous version of this review, IUD use at six months was more likely with immediate insertion than with delayed insertion (OR 2.04, 95% CI 1.01 to 4.09) (Lopez 2015), but in this update, the meta‐analysis revealed little to no difference in the rate of both contraceptive implant and IUD utilization among people assigned to receive immediate insertion compared with delayed insertion at six and 12 months after delivery. For IUDs, the higher expulsion rate in the immediate insertion group may contribute to this similar rate of continuation, despite higher initiation (RR 4.55; 95% CI 2.52 to 8.19). Immediate postpartum insertion of IUDs can be subcategorized as immediate postplacental (within 10 minutes of placenta delivery) or early postpartum insertion (10 minutes to hospital discharge). This difference makes it challenging to make comparisons across studies. A recent systematic review also suggested that immediate and early postpartum IUD placement was associated with increased risk of expulsion compared with interval placements (adjusted RR 8.33; 95% CI 4.32 to 16.08 and adjusted RR 5.27; 95% CI 2.56 to 10.85, respectively) (Averbach 2017). A preliminary result from a recent large Food and Drugs Agency (FDA)‐mandated retrospective cohort multi‐center study utilizing Kaiser Permanente's observational clinical data also found the highest risk of expulsion occurred with immediate postpartum insertion (0 to 3 days postpartum) compared to insertion after 52 weeks or more postpartum or with no evidence of delivery (adjusted hazard ratio 5.34, 95% CI 4.47 to 6.39) (Reed 2020).
For contraceptive implants, the inability to blind the timing of the intervention assignment in the included studies in this review precluded a reliable assessment of the difference in the rate of self‐reported adverse effects between the immediate and delayed postpartum insertion of contraceptive implants. A two‐year observational study conducted in the USA with 414 people showed no difference in removal rate due to prolonged vaginal bleeding among people who received immediate and delayed postpartum insertion of contraceptive implants (19.3% versus 18.4%, respectively) (Ireland 2014). In a prospective cohort from Thailand that evaluated the safety of immediate postpartum insertion of contraceptive implants among 88 asymptomatic, HIV type‐1 positive people, 62.5% of participants who had immediate insertion reported having irregular bleeding at six months, which appeared to be comparable to people who received a standard insertion (Taneepanichkul 2001). Likewise, our review demonstrated that the prolonged vaginal bleeding among people in the immediate insertion group was comparable to that noted among people who received delayed insertion of contraceptive implants (mean difference (MD) 2.98 days; 95% CI ‐2.71 to 7.81; 2 studies, 420 participants). Although immediate insertion has higher initiation and does not appear to be associated with prolonged bleeding, the continuation rate did not differ according to placement timing. Greater numbers of adverse effects other than vaginal bleeding observed by Phemister 1995 may explain this finding.
Lopez 2015 reported no difference in pregnancy rate between immediate postplacental and standard insertion, which is consistent with the results of this review for both contraceptive implant and IUDs. However, the number of studies assessing pregnancy are limited, which means we also lack important information about the effect of immediate postpartum insertion of LARC on pregnancy. For instance, conditions such as lactational amenorrhea were not adequately measured and reported. Moreover, the rate of unintended pregnancy differs between populations on the basis of age and sexual activity. Further prospective data are needed to enable us to understand the association between immediate initiation of LARC methods and subsequent risk of unintended and short interval pregnancies better.
As the postpartum decrease in the progesterone level is supposed to trigger the onset of lactogenesis, theoretically, immediate postpartum administration of progestin contraception could have a negative impact on initiation of breastfeeding. However, we found that rates of exclusive and any breastfeeding in this review were similar between the groups at six months. A previous systematic review was also unable to identify a detrimental effect of progestin‐only contraception on breastfeeding (Phillips 2016).
Authors' conclusions
Implications for practice.
Evidence from this review indicates that the rate of initiation of long‐acting reversible contraception (LARC; i.e. contraceptive implants and intra‐uterine devices) was higher for people randomized to receive it after delivery and before hospital discharge compared to those randomized to receive it four to six weeks postpartum. This practice is a very appealing choice in areas with low postpartum follow‐up rates. Due to a high risk of performance bias and unclear risk of detection bias, the different profiles of the self‐reported adverse effects between immediate and delayed postpartum insertion of contraceptive implants remain inconclusive. People who desire immediate postpartum insertion of intra‐uterine devices (IUDs) should be aware of the higher expulsion rate for IUDs with immediate placement and be advised to check their IUD strings regularly to check they are still in place.
Implications for research.
While the evidence available in this review indicates that in‐hospital postpartum insertion of LARC could improve the initiation rate of contraceptive implants and IUDs by the first postpartum follow‐up visit, short inter‐pregnancy interval was not reported by any of the included studies. More trials are needed, particularly in the adolescent population, to strengthen the volume and consistency of the evidence generated on this topic, so that future updates of this review can provide more rigorous pooled estimates and subgroup explorations.
Trials on contraceptive implants
Further large‐scale RCTs with long follow‐up periods (more than 12 months) to determine rates of utilization and unintended pregnancy are required. In addition, further study to investigate whether immediate postpartum contraceptive implant insertion increases the risk of adverse effects and breastfeeding compared with standard insertion is warranted.
Trials on IUDs
More trials conducted in low‐ and middle‐income countries are needed, especially for hormonal IUDs. Study with longer follow‐up periods to evaluate utilization rate, unintended pregnancy, and satisfaction are required.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2022 | New search has been performed | New search December 2020 |
| 27 October 2022 | New citation required and conclusions have changed | Included IUDs and changed conclusion for pregnancy rate |
History
Protocol first published: Issue 10, 2015 Review first published: Issue 4, 2017
Acknowledgements
We thank the Cochrane Fertility Regulation Review Group for their contributions to the editorial process and clinical advice, Robin Paynter for designing the search strategies and running the search, and the referees for their useful suggestions and comments during the prepublication editorial process.
Appendices
Appendix 1. Search strategy for 2022 update of this review
Cochrane Central Register of Controlled Trials (Ovid EBM Reviews, December 2020)
1 ((after or afterward or before or day or days or delay* or during or early or follow* or hour or hours or immediat* or insert* or initiat* or late or later or month or months or placement* or subsequent* or timing or uptake or week or weeks or while or within) adj5 (birth or cesarean or delivery or deliveries or delivered or discharg* or childbirth or child‐birth or interpregnancy or inter‐pregnancy or interval or intracesarean or intra‐cesarean or intrapartum or intra‐partum or parturition or peripartum or peri‐partum or placenta or postcesarean or post‐cesarean or postdelivery or post‐delivery or postnatal* or post‐natal* or postpartum or post‐partum or postplacental or post‐placental or puerperium)).ti,ab. (52350)
2 (((contracept* or etonogestel or ENG or levonorgestrel or LNG or progest*) adj5 (implant or implants or rod or rods or subcutaneous* or sub‐cutaneous* or subdermal* or sub‐dermal*)) or LARC or LARCs or (long‐acting adj2 reversible)).ti,ab. (1062)
3 (Femplant or Jadelle or Implanon or Nexplanon or Norplant or Sino‐implant* or Sinoimplant* or Trust or Zarin).ti,ab. (2652)
4 (IUC or IUCs or IUCD or IUCDs or IUD or IUDs or IUS or IUSs or LNGIUC or LNGIUCs or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs or PPIUC or PPIUCs or PPIUCD or PPIUCDs or PPIUD or PPIUDs or PPIUS or PPIUSs).ti,ab. (1951)
5 ((intrauterine or intra‐uterine) adj3 (coil or coils or contraceptive or contraception or device or devices or system or systems)).ti,ab. (2182)
6 (Kyleena or Liletta or Mirena or Skyla).ti,ab. (144)
7 or/2‐6 (6029)
8 and/1,7 (647)
MEDLINE ALL (Ovid) (1946 to December 8, 2020)
1 Postpartum Period/ or Cesarean Section/ or Delivery, Obstetric/ or Peripartum Period/ (89558)
2 ((after or afterward or before or day or days or delay* or during or early or follow* or hour or hours or immediat* or insert* or initiat* or late or later or month or months or placement* or subsequent* or timing or uptake or week or weeks or while or within) adj5 (birth or cesarean or delivery or deliveries or delivered or discharg* or childbirth or child‐birth or interpregnancy or inter‐pregnancy or interval or intracesarean or intra‐cesarean or intrapartum or intra‐partum or parturition or peripartum or peri‐partum or placenta or postcesarean or post‐cesarean or postdelivery or post‐delivery or postnatal* or post‐natal* or postpartum or post‐partum or postplacental or post‐placental or puerperium)).ti,ab,kf. (466801)
3 or/1‐2 (520939)
4 Contraception/ or Contraceptive Agents, Female/ or Contraception Behavior/ or Contraceptive Devices, Female/ or Long‐Acting Reversible Contraception/ (30737)
5 (((contracept* or etonogestel or ENG or levonorgestrel or LNG or progest*) adj5 (implant or implants or rod or rods or subcutaneous* or sub‐cutaneous* or subdermal* or sub‐dermal*)) or LARC or LARCs or (long‐acting adj2 reversible)).ti,ab,kf. (4310)
6 (Femplant or Jadelle or Implanon or Nexplanon or Norplant or Sino‐implant* or Sinoimplant* or Trust or Zarin).ti,ab,kf. (32476)
7 Intrauterine Devices/ or Intrauterine Devices, Medicated/ (10061)
8 (IUC or IUCs or IUCD or IUCDs or IUD or IUDs or IUS or IUSs or LNGIUC or LNGIUCs or LNGIUCD or LNGIUCDs or LNGIUD or LNGIUDs or LNGIUS or LNGIUSs or PPIUC or PPIUCs or PPIUCD or PPIUCDs or PPIUD or PPIUDs or PPIUS or PPIUSs).ti,ab. (9905)
9 ((intrauterine or intra‐uterine) adj3 (coil or coils or contraceptive or contraception or device or devices or system or systems)).ti,ab,kf. (9040)
10 (Kyleena or Liletta or Mirena or Skyla).ti,ab,kf. (319)
11 or/4‐10 (75317)
12 Randomized Controlled Trials as Topic/ or Equivalence Trials as Topic/ or Pragmatic Clinical Trials as Topic/ (127182)
13 ("adaptive clinical trial" or "clinical trial" or "equivalence trial" or "pragmatic clinical trial" or "randomized controlled trial").pt. (794538)
14 (blind* or mask* or random* or trial*).ti,ab. (1792992)
15 drug therapy.fs. (2144092)
16 or/12‐15 (3795982)
17 16 not ((exp animals/ not humans/) or (bovine or canine or capra or cat or cats or cattle or cow or cows or dog or dogs or equine or ewes or feline or goat or goats or horse or mice or mouse or ovine or pig or pigs or porcine or rabbit or rabbits or rat or rats or rattus or sheep or sow or sows).ti.) (3374377)
18 and/3,11,17 (721)
Appendix 2. Search strategies for the 2017 version of this review
CENTRAL
(postpartum OR post‐partum) AND ( implant* OR (contracept* AND levonorgestrel) OR norplant OR norplants OR (keto AND desogestrel) OR etonorgestrel OR implanon OR ((subdermal OR subcutaneous) AND implant*) OR (subcutaneous AND implant) OR jadelle)
MEDLINE
(postpartum OR puerperium OR postcesarean OR delivery OR cesarean section) AND ((contracept* AND implant*) OR levonorgestrel OR norplant* OR (keto AND desogestrel) OR etonorgestrel OR implanon OR (subdermal AND implant) OR (subcutaneous AND implant) OR jadelle) AND insert*
Embase
(postpartum OR puerperium OR postcesarean OR delivery OR cesarean section) AND ((contracept* AND implant*) OR (contraceptive devices, female AND implant) OR (contracept* AND (levonorgestrel OR norplant* OR (keto AND desogestrel) OR etonorgestrel OR implanon OR (subdermal AND implant) OR (subcutaneous AND implant) OR jadelle))) AND (insert OR inserting OR inserted OR insertion)
POPLINE
("contraceptive implants" OR levonorgestrel OR norplant OR norplants OR "keto desogestrel" OR etonorgestrel OR implanon OR jadelle OR "subdermal implant" OR "subdermal implants")AND insertion AND (postpartum OR puerperium OR postcesarean OR "post cesarean")
Data and analyses
Comparison 1. Immediate versus delayed postpartum insertion of contraceptive implants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Rate of initiation of contraceptive implants | 5 | 715 | Risk Ratio (IV, Random, 95% CI) | 1.48 [1.11, 1.98] |
| 1.1.1 High‐income countries | 3 | 410 | Risk Ratio (IV, Random, 95% CI) | 1.41 [1.28, 1.55] |
| 1.1.2 Middle‐ to low‐income countries | 2 | 305 | Risk Ratio (IV, Random, 95% CI) | 1.57 [0.61, 4.05] |
| 1.2 Utilization rate | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 At 6 months postpartum | 3 | 330 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.90, 1.50] |
| 1.2.2 At 12 months postpartum | 2 | 164 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.93, 1.04] |
| 1.3 Adverse effect: mean days of duration of vaginal bleeding | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Mean days of prolonged vaginal bleeding within 6 weeks postpartum | 2 | 420 | Mean Difference (IV, Random, 95% CI) | 2.98 [‐2.71, 8.66] |
| 1.4 Other adverse effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Prolonged bleeding at 3 months postpartum | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.10, 1.47] |
| 1.4.2 Prolonged bleeding at 6 months postpartum | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.29, 4.94] |
| 1.4.3 Other vaginal bleeding or associated severe cramping within 12 months | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.72, 1.44] |
| 1.4.4 Adverse effects other than prolonged vaginal bleeding | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.38, 3.06] |
| 1.5 Participant satisfaction | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 At 6 months postpartum | 1 | 152 | Risk Ratio (IV, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 1.5.2 At 12 months postpartum | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.77, 1.31] |
| 1.6 Unintended pregnancy rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 At 6 months postpartum | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.08] |
| 1.6.2 At 12 months postpartum | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.38, 8.71] |
| 1.7 Breastfeeding at 6 months postpartum | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Exclusive breastfeeding | 2 | 261 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.66, 1.21] |
| 1.7.2 Any breastfeeding | 2 | 225 | Risk Ratio (IV, Fixed, 95% CI) | 0.97 [0.92, 1.01] |
Comparison 2. Immediate versus delayed postpartum insertion of IUDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Rate of initiation of IUDs | 11 | 1894 | Risk Ratio (IV, Random, 95% CI) | 1.27 [1.07, 1.51] |
| 2.1.1 Copper IUDs | 5 | 1329 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.96, 1.97] |
| 2.1.2 Levonorgestrel IUDs | 5 | 453 | Risk Ratio (IV, Random, 95% CI) | 1.11 [1.05, 1.18] |
| 2.1.3 Any types of IUDs | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.25, 1.94] |
| 2.2 Utilization rate | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 At 6 months postpartum | 6 | 971 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.65, 1.62] |
| 2.2.2 At 12 months postpartum | 3 | 796 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.50, 1.47] |
| 2.3 Expulsion | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Expulsion by 6 months postpartum | 8 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [2.52, 8.19] |
| 2.4 Participant satisfaction | 2 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 At 6 months postpartum | 1 | 69 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.83, 1.03] |
| 2.4.2 At 12 months postpartum | 1 | 598 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.98, 1.12] |
| 2.5 Unintended pregnancy rate | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 at 6 months postpartum | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.5.2 at 12 months postpartum | 1 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.17, 0.41] |
| 2.6 Breastfeeding (LNG‐IUS) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.6.1 Exclusive breastfeeding at 6 months | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.08, 3.19] |
| 2.6.2 Any breastfeeding at 6 months | 5 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.63, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Averbach 2017.
| Study characteristics | ||
| Methods | Location: tertiary hospital in Kampala, Uganda Design: randomized controlled trial Study duration: June 2015 to May 2016 Sample size: 96 participants were recruited to detect a 20% difference in contraceptive implant use at 6 months postpartum among peripartum women assigned to receive immediate postpartum insertion (prior to hospital discharge) and delayed postpartum insertion (6 weeks) of levonorgestrel contraceptive implant with a study power of 80% and an alpha of 0.05. |
|
| Participants | Women who were 18 years old and older, who spoke English or Luganda, had had a vaginal or cesarean delivery at Mulago Hospital within the past 5 days, and could demonstrate that they had a working cellular telephone Exclusion criteria included women who had a medical contraindication to progestin‐only contraceptives or were taking efavirenz medication as part of their HIV antiretroviral treatment. |
|
| Interventions | Intervention arm: participants received levonorgestrel contraceptive implant within 48 hours of delivery. Control arm: participants received the same contraceptive method 6 weeks after delivery. | |
| Outcomes | Primary outcome: proportion of women using a contraceptive implant at 6 months after delivery Other outcome measures: utilization of the implant at 3 months, vaginal bleeding, adverse effects, and satisfaction. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using blocks of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Sequential, numbered, opaque envelopes containing a card with computer‐generated assignment information were prepared. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, continuation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Blinding of the participants was not possible. However, other outcomes were measured objectively. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was low rate of loss to follow‐up (93% completed the 6‐month follow‐up visit) and no difference in follow‐up between groups at 3 or 6 months. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Bayoumi 2020.
| Study characteristics | ||
| Methods | Location: large hospital in Egypt Design: randomized controlled trial Study duration: February 2016 to December 2018 Sample size: 1000 participants were recruited to detect 6% difference in IUDs expulsion after intra‐caesarean insertion among women assigned to receive immediate insertion (post‐placental insertion) and delayed postpartum insertion (6 weeks) of Cu (non‐hormonal) IUDs with a study power of 80% and an alpha of 0.05. |
|
| Participants | Pregnant women, aged 20 to 45 years, undergoing caesarean delivery at the hospital and wishing to use an IUDs as a method of contraception. Exclusion criteria included one or more of the following.
|
|
| Interventions | Intervention arm: participants received intra‐caesarean insertion of an non‐hormonal IUDs (Cu IUDs) after placental delivery. Control arm: participants received the same contraceptive method 6 weeks after delivery. |
|
| Outcomes | IUDs expulsions and the proportion of women using an IUDs at 6 months, and pregnancy rate after 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using blocks of 4 or 8. |
| Allocation concealment (selection bias) | Low risk | A researcher not involved in the study prepared sealed envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, expulsion rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Although blinding of the participants was not possible, pregnancy rate was unlikely to be affected by performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding of the participants was not possible, expulsion rate was unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were high rates of loss to follow‐up in both groups (20% (104/500) and 59% (298/500) in the immediate insertion and delayed insertion groups respectively). |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Bryant 2017.
| Study characteristics | ||
| Methods | Location: large hospital in North Carolina, USA Design: randomized controlled trial Study duration: August 2012 to April 2014 Sample size: 96 participants were recruited to detect a 30% difference in contraceptive implant use at 1 year postpartum among peripartum women assigned to receive immediate postpartum insertion (prior to hospital discharge) and delayed postpartum insertion (4 to 6 weeks postpartum) of etonogestrel contraceptive implant with a study power of 80% and an alpha of 0.05. |
|
| Participants | Adolescents and young women aged 14 to 24 years, who had given birth to a healthy infant, spoke English or Spanish, and wanted to use the contraceptive implant.
Exclusion criteria included one or more of the following.
The participants were of many different ethnicities; the largest group were Hispanic/Latino (40% of women assigned to both the immediate and delayed postpartum insertion groups). |
|
| Interventions | Intervention arm: participants received etonogestrel contraceptive implant within 48 hours of delivery. Control arm: participants received the same contraceptive method 4 to 6 weeks after delivery. | |
| Outcomes | Primary outcome: contraceptive implant use at 12 months postpartum Other outcome measures: satisfaction, vaginal bleeding patterns, breastfeeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computer‐generated random numbers in blocks of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | The assignments were enclosed in sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | High risk | Blinding of the participants was not possible which may have affected the self‐reported side‐effects and satisfaction. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a high rate of loss to follow‐up in both groups (23% (11/48) and 44% (21/48) in the immediate insertion and delayed insertion groups, respectively). |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | Many outcomes in this study were not analyzed following an intention‐to‐treat basis. |
Carmo 2017.
| Study characteristics | ||
| Methods | Location: university hospital in São Paulo, Brazil Design: randomized controlled trial Study duration: June to August 2015 Sample size: 100 participants were recruited to detect 10% difference in infant weight at 12 months of age among peripartum women assigned to receive immediate postpartum insertion (prior to hospital discharge) and delayed postpartum insertion (6 weeks) of etonogestrel contraceptive implant with a study power of 80% and an alpha of 0.05. |
|
| Participants | Participants had to reside in Ribeirão Preto and be willing to breastfeed their infants for at least 3 months. Exclusion criteria included one or more of the following.
|
|
| Interventions | Intervention arm: participants received etonogestrel contraceptive implant within the first 48 hours after delivery (before discharge) Control arm: participants received the etonogestrel contraceptive 6 weeks after delivery. |
|
| Outcomes | Primary outcome: average infant weight at 12 months of age. Secondary outcomes: infant height and head and arm circumferences. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program generated sequence in blocks of four. |
| Allocation concealment (selection bias) | Low risk | Randomization numbers placed in sealed and opaque envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, infant weight was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Blinding of the participants was not possible. However, the secondary outcomes were measured using objective methods. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding of the participants was not possible, infant weight was unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low rate of loss to follow‐up in both groups (10% (5/50) and 6% (3/50) in immediate insertion and delayed insertion groups, respectively). |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Chen 2010.
| Study characteristics | ||
| Methods | Location: university hospital at Pittsburgh, PA (USA) Design: randomized controlled trial Study duration: May 2007 to October 2008 Sample size: expected 40% of standard insertion participants would not return for insertion, 4% of inserted IUDs would be discontinued in 6 months because of removal requests, and expulsion would be 11% in the immediate insertion group and 2% in the standard insertion group; estimated proportions continuing with IUDs at 6 months were 0.850 for immediate insertion and 0.564 for standard insertion; 92 participants (46 per group) for 80% power to detect 34% difference in use at 6 months and significance level 0.05. Increased to 51 per group to allow a 10% loss to follow‐up. Expected 40% would not be eligible for insertion because of post‐enrollment ineligibility criteria, so increased the total to 168. |
|
| Participants | 124 postpartum women were randomized; 102 remained eligible after randomization Inclusion criteria: 18 years or older; ≥ 24 0/7 weeks pregnant at enrollment; with anticipated vaginal delivery; and wanting to use hormonal IUDs for postpartum contraception Exclusion criteria: scheduled cesarean section; allergy to polyethylene or levonorgestrel or other contraindication to hormonal IUDs use; exposure to or treatment for gonorrhea, chlamydia, or trichomoniasis during the pregnancy without subsequent negative test‐of‐cure result; leiomyomata (fibroids) > 3 cm diameter; uterine anomaly (other than repaired septate uterus); current cervical cancer or carcinoma in situ; desire for a repeat pregnancy within 1 year of delivery Post‐enrollment ineligibility: cesarean delivery; clinical diagnosis of chorioamnionitis; sexually transmitted infection without negative test‐of‐cure result; membranes ruptured for > 24 hours; postpartum hemorrhage; rupture of membranes at < 34 weeks gestation; participant no longer desired IUD; precipitous delivery so investigators could not begin IUD insertion within 10 minutes; investigators not notified of labor and delivery |
|
| Interventions | Intervention arm: immediate insertion of hormonal IUDs (within 10 minutes postplacental) Control arm: standard insertion of hormonal IUDs (6 to 8 weeks postpartum) |
|
| Outcomes | Insertion, IUD use, continuation of IUD, expulsion, pregnancy (tested), safety (infection and perforation), pain with insertion, quality of life, contraceptive use Assessment times: visit 6 to 8 weeks post delivery, phone interview at 3 months, and visit at 6 months |
|
| Notes | Among women randomized to immediate insertion, 5 had the IUD inserted from 11 to 15 minutes postplacental. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Study staff performing 3‐ and 6‐month evaluations were blinded to randomization assignment. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Study staff performing 3‐ and 6‐month evaluations were blinded to randomization assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding of the participants was not possible, continuation rate was unlikely to be affected by performance bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 12% (5 immediate and 7 standard) Ineligible after randomization: 18% each group |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Dahlke 2011.
| Study characteristics | ||
| Methods | Location: Portsmouth, VA (USA) Study duration: August 2009 to January 2010 Design: randomized controlled trial, pilot study Sample size calculation: the Discussion notes that the study was not powered to detect difference in utilization at 6 months. This pilot study was planned to determine the feasibility of a later trial. |
|
| Participants | 53 women in a single medical centre. Inclusion criteria: desired hormonal IUDs for postpartum contraception at presentation for admission to labor and delivery, and had no contraindication Exclusion criteria: congenital or acquired uterine anomaly including fibroids that distorted uterine cavity; known or suspected uterine or cervical neoplasia or unresolved abnormal pap smear; untreated acute cervicitis or known vaginitis including bacterial vaginosis or other lower genital tract infection; acute liver disease or liver tumor; hypersensitivity to any product component; and known or suspected carcinoma of breast Intrapartum exclusion: postpartum hemorrhage (blood loss > 500 mL); chorioamnionitis; cesarean delivery |
|
| Interventions | Hormonal IUD insertion
|
|
| Outcomes | Insertion, utilization rate, pain at time of placement, breastfeeding status, blood loss, intrauterine infection rate, expulsion rate Assessment times: 3 and 6 months post delivery by phone (n = 36) or through review of medical records (n = 7) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Blinding of the participants was not possible, however, outcome measures may not have been affected by awareness of intervention. |
| Blinding of participants and personnel (performance bias) secondary outcomes | High risk | Blinding of the participants was not possible which may have affected the pain perception of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 2% (1 in standard group) Excluded after randomization because of cesarean section, as per trial criteria: 13% (2 each from immediate and early groups, 3 from standard) |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | This was a pilot study. |
Dias 2015.
| Study characteristics | ||
| Methods | Location: university hospital in Sri Lanka Design: randomized controlled trial Study duration: November 2014 to May 2015 Sample size: 30 participants were recruited in the intervention arm (immediate postpartum insertion of IUDs) and 33 participants to the control arm (delayed insertion) |
|
| Participants | Women who were willing to start using an IUDs as a contraceptive method after childbirth. | |
| Interventions | Intervention arm: participants received postplacental non‐hormonal IUDs (Cu‐IUDs) Control arm: participants received delayed insertion of hormonal IUDs (Cu‐IUDs) at 6‐week postpartum visit | |
| Outcomes | Primary outcome: the distance from the IUDs to the internal os Other outcome measures: spontaneous expulsion and displacement at 6 weeks postpartum | |
| Notes | Information from conference abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Blinding of the participants was not possible, however, outcome measures may not have been affected by awareness of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mention. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Gurtcheff 2011.
| Study characteristics | ||
| Methods | Location: university hospital in Utah, USA Design: non‐inferiority randomized controlled trial Study duration: January to October 2009 Sample size: 34 participants were required in each group to compare the immediate and delayed postpartum insertion of the etonogestrel implant when a non‐inferiority margin of delay time to lactogenesis stage II was expected to be 8 hours at the study power of 80% and an alpha of 0.05. |
|
| Participants | Healthy peripartum women with healthy, term newborns who desired the etonogestrel implant for contraception. Exclusion criteria: onset of lactogenesis before randomization, hemorrhage requiring transfusion, severe pregnancy‐induced hypertension, prolonged hospitalization, coagulopathy, liver disease, undiagnosed genital bleeding, or other relative contraindication to etonogestrel implant insertion (known or suspected pregnancy; known, suspected, or history of breast cancer; or hypersensitivity to any of the components in the etonogestrel implant). Women taking inducers of hepatic enzymes were also excluded, including barbiturates, griseofulvin, rifampin, phenylbutazone, phenytoin, carbamazepine, felbamate, oxcarbazepine, topiramate, modafinil, protease inhibitors, and herbal products. Most study participants were Hispanic Whites (73% and 60% of women assigned to immediate and delayed postpartum insertion groups, respectively). |
|
| Interventions | Intervention arm: participants received etonogestrel contraceptive implant within 1 to 3 days after delivery. Control arm: participants received delayed insertion of etonogestrel contraceptive implant at 4 to 8 weeks postpartum visit | |
| Outcomes | Primary outcome: time to lactogenesis stage II documented by maternal perception, lactational failure Other outcome measures: lactational failure, postpartum contraceptive prevalence, participant‐reported vaginal bleeding, and mean creamatocrit value of breast milk at first postpartum visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computer‐generated random numbers in blocks of varying sizes of 2, 4, and 6. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by enclosing assignments in sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | High risk | Blinding of the participants was not possible which may have affected self‐reported adverse effects. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low rate of loss to follow‐up in both groups; (17% (6/35) and 17% (6/34) in the immediate insertion and delayed insertion groups, respectively.) |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | The trial authors did not provide definitions of the prolonged vaginal bleeding patterns applied in this study, which was an outcome of interest in this review. |
Lester 2015.
| Study characteristics | ||
| Methods | Location: Kampala, Uganda Design: randomized controlled trial; pilot study to determine feasibility of larger study Study duration: February 2011 to December 2011 Sample size calculation and outcome of focus: based on literature and postpartum return rates at Mulago where 40% do not return for delayed insertion and 10% discontinue use within 6 months. Expected that 90% of the immediate insertion group and 54% of the delayed insertion group would use an IUD at 6 months postpartum. Sample size of 30 per group for 84% power (β = 0.16) to detect difference of 36% between groups, using a significance level of 0.05. |
|
| Participants | 68 randomized in national referral hospital Inclusion criteria: pregnant at enrollment; scheduled for cesarean delivery > 8 hours after consent; desiring non‐hormonal IUDs (Cu IUDs); age ≥ 18 years; English or Luganda speaking; willing to be accompanied home from hospital and to have address recorded; willing to have home visit at 6 months postpartum if had not returned for a scheduled visit Exclusion criteria: allergy to copper; pelvic tuberculosis; severe thrombocytopenia; positive test for gonorrhea or Chlamydia; leiomyomata or other anomaly that would prevent IUD placement; cervical cancer or carcinoma in situ; desire for repeat pregnancy within 12 months; chorioamnionitis; preterm birth < 34 weeks; diagnosis of AIDS (HIV not an exclusion criterion); fetal demise; hemorrhage; ruptured uterus; eclampsia; evidence of severe anemia Post‐enrollment exclusion criteria: participant no longer desired non‐hormonal IUDs or
|
|
| Interventions | Non‐hormonal (Cu) IUDs inserted after cesarean section Intervention arm: immediate postplacental insertion (within 10 minutes) Control arm: standard insertion (6 weeks) |
|
| Outcomes | IUDs insertion and use at 6 months post delivery, time to IUD expulsion, time to pregnancy; safety measures (infection, uterine perforation), vaginal bleeding, satisfaction Assessment times: delivery, 6‐week postpartum visit, phone at 3 months, clinic visit at 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted‐block randomization scheme with block sizes of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes; opened after delivery of baby and placenta, if no intraoperative exclusion criteria developed |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Although blinding of the participants was not possible, continuation and expulsion rates were unlikely to be affected by performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: overall 10% (7/68); immediate 15% (5/34) and delayed 6% (2/34) |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Levi 2015.
| Study characteristics | ||
| Methods | Location: Chapel Hill, NC (USA) Study duration: February 2012 to June 2014 Design: randomized controlled trial, open label Sample size calculation and outcome of focus: not mentioned |
|
| Participants | 112 women who delivered by cesarean section at university hospital Inclusion criteria: age 18 to 45 years, pregnant ≥ 24 weeks estimated gestational age, live pregnancy, planning to use IUDs postpartum for contraception, planned cesarean delivery, intending to stay in Chapel Hill area for ≥ 6 months after birth, fluent in English or Spanish Exclusion criteria: known uterine anomalies, allergies to component of IUDs of their choice, known or suspected carcinoma of breast, known acute liver disease or liver tumor, known or suspected uterine or cervical neoplasia, active pelvic inflammatory disease, genital bleeding of unknown etiology, history of solid organ transplantation, positive test for gonorrhea or chlamydia during this pregnancy |
|
| Interventions | Hormonal IUDs or non‐hormonal IUDs (Cu) Treatment: immediate insertion (cesarean delivery, postplacental) Comparison: standard insertion (4 to 8 weeks post cesarean) |
|
| Outcomes | Primary outcome was IUDs use at 6 months postpartum Secondary outcomes included IUDs expulsion, IUDs discontinuation, IUDs strings visible at 6 months postpartum, and women's satisfaction with the IUDs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random allocation in randomly permutated blocks of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Used numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, continuation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Although blinding of the participants was not possible, secondary outcomes were unlikely to be affected by performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 12.5% overall (14/112); immediate insertion group 14.3% (8/56) and delayed insertion group 10.7% (6/56) |
| Selective reporting (reporting bias) | Low risk | The trialists reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Ogburn 2013.
| Study characteristics | ||
| Methods | Location: not mentioned; investigator at university in Albuquerque, NM (USA) Design: RCT Study duration: not mentioned Sample size calculation: not mentioned |
|
| Participants | 156 postpartum women Inclusion criteria: term vaginal or cesarean delivery Exclusion criteria: not mentioned |
|
| Interventions | Non‐hormonal (Cu) IUD insertion after vaginal or cesarean delivery Intervention arm: immediate insertion (within 10 minutes of placenta delivery) Control arm: standard insertion (4 to 12 weeks postpartum) |
|
| Outcomes | IUDs insertion and continuation, reasons for discontinuation, complications Assessment times: 6‐week IUD check and contacted 3, 6, and 12 months after insertion |
|
| Notes | Information from conference abstract only 24 June 2020: investigator communicated the work was not published yet. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Blinding of the participants was not possible, however, outcome measures may not have been affected by awareness of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Phemister 1995.
| Study characteristics | ||
| Methods | Location: large hospital in North Carolina, USA Design: randomized controlled trial Study duration: June 1992 to February 1993 Sample size: 250 participants were recruited to detect 10% difference of prevalence of prolonged vaginal bleeding occurring among peripartum women assigned to receive immediate postpartum insertion (1 to 3 days) and delayed postpartum insertion (4 to 6 weeks) of a levonorgestrel contraceptive implant with a study power of 80% and an alpha of 0.05. |
|
| Participants | Peripartum women were offered inclusion in this trial if they intended to use a contraceptive implant for contraception, and had: vaginal births; predelivery gestational age of 34 weeks or more; a spontaneous delivery of a grossly intact placenta; a predelivery hemoglobin value of 8.0 g/dL or higher; and no contraindications to Norplant insertion as listed by the manufacturer, which include acute liver disease, acute thromboembolic disorder, abnormal genital bleeding, pregnancy, breast cancer, or an allergy to silicone or levonorgestrel. Exclusion criteria included women who intended to breastfeed; experienced postpartum hemorrhage (defined as total estimated blood loss > 500 mL) or infection; were receiving heparin or warfarin sodium; were massively obese (postpartum weight > 250 pounds); or had a history of hypertension, seizures, or bleeding disorders. 250 non‐breastfeeding postpartum women who met the study inclusion were enrolled. Most women who participated in this study were Black (75.2% and 72.5% of women in the immediate and delayed postpartum insertion groups, respectively) |
|
| Interventions | Intervention arm: participants received levonorgestrel contraceptive implant within 48 hours of delivery. Control arm: participants received the same contraceptive method 4 to 6 weeks after delivery. | |
| Outcomes | Primary outcome was postpartum bleeding evaluated by the change of hemoglobin level and participants’ self‐reported vaginal bleeding patterns
Secondary outcomes included blood pressure and weight changes and participants’ self‐reports for other adverse effects including nausea, hair loss, hirsutism, headaches and acne All outcomes were evaluated during the visit scheduled at 4 to 6 weeks after delivery |
|
| Notes | The trial investigators excluded 9 women after randomization. The included study did not state how vaginal bleeding patterns were defined, although this was the primary safety concern of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was computer generated and sequences were numbered sequentially. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by enclosing assignments in opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, initiation rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | High risk | Blinding of the participants was not possible, which may have affected self‐reported adverse effects. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up rates were 12% and 9% of participants assigned to the immediate and delayed insertion groups, respectively. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes.I |
| Other bias | Unclear risk | The trial investigators excluded 9 women after randomization. In addition, no well‐defined terminology of prolonged vaginal bleeding was applied in this trial. |
Soon 2018.
| Study characteristics | ||
| Methods | Location: large hospital in Honolulu, Hawai‘i, USA Design: randomized controlled trial; pilot study Study duration: November 2013 to June 2015 Sample size: 11 participants were recruited, in order to be 68% confident that the estimates would be accurate to within 8 percentage points. |
|
| Participants | Pregnant adolescents (14 to 19 years old) who planned to use an hormonal IUDs after delivery. Exclusion criteria: women with an allergy to the hormonal IUDs; chlamydia or gonorrhea during pregnancy without a negative test‐of‐cure result; an anomaly distorting the uterine cavity; current cervical cancer; a desire for a repeat pregnancy within one year; plans to move from Oahu < 6 months after delivery; a planned cesarean delivery; or delivery at < 34 weeks gestation, chorioamnionitis, postpartum hemorrhage, unanticipated cesarean delivery, or delivery at a time when a study investigator was unavailable. |
|
| Interventions | Intervention arm: participants received hormonal IUDs within 10 minutes of delivery of the placenta Control arm: participants received hormonal IUD at 4‐ to 6‐week postpartum visit | |
| Outcomes | Primary outcome was to identify methodological challenges and the percent attrition in both study groups. Secondary outcomes included patient satisfaction, expulsion, bleeding patterns, and breastfeeding rates. |
|
| Notes | The study was discontinued prior to meeting the sample size goal (30 subjects in total) due to suboptimal enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician used random number generator to develop 1:1 randomization scheme using block sizes of 6. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Although blinding of the participants was not possible, expulsion rate was unlikely to be affected by performance bias. |
| Blinding of participants and personnel (performance bias) secondary outcomes | High risk | Blinding of participants was not possible, which may have affected self‐reported adverse effects such as satisfaction. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study was discontinued prior to meeting the sample size goal. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | This was a pilot study. |
Stuart 2015.
| Study characteristics | ||
| Methods | Location: large hospital in North Carolina, USA Design: randomized controlled trial Study duration: March 2012 to June 2013 Sample size: 172 participants were recruited to detect 20% difference of breastfeeding at 6 months among peripartum women assigned to receive immediate postpartum insertion (1 to 3 days) and delayed postpartum insertion (4 to 6 weeks) of hormonal IUD with a study power of 80% and an alpha of 0.05. Finally, 290 women were recruited to accommodate loss to follow‐up. |
|
| Participants | 35 women were randomized. Authors mentioned that eligibility was similar to other studies including Chen 2010. |
|
| Interventions | Treatment: early insertion of hormonal IUDs (6‐48 hours after delivery) Comparison: standard insertion of hormonal IUDs (postpartum visit) |
|
| Outcomes | Primary outcome was breastfeeding at 6 months. Secondary outcomes included breastfeeding at 6 weeks, 3 and 6 months postpartum. |
|
| Notes | The study was stopped early because the expulsion rate of hormonal IUDs reached 50%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was computer generated with varied block sizes of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Persons unrelated to the study prepared the randomization list and allocation envelopes. |
| Blinding of participants and personnel (performance bias) primary outcome | Unclear risk | Blinding of the participants was not possible, which may have affected breastfeeding intention. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Unclear risk | Blinding of the participants was not possible, which may have affected breastfeeding intention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study was discontinued prior to meeting the sample size goal. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Turok 2017.
| Study characteristics | ||
| Methods | Location: two university hospitals in USA Study duration: February 2014 to March 2016. Design: randomized controlled trial, open label Sample size calculation: 264 participants were recruited to detect 60% of women with delayed initiation of hormonal contraception to continue breast‐feeding at 8 weeks’ postpartum with a study power of 80% and alpha of 0.05. Finally, 317 women were recruited to accommodate a 20% loss to follow‐up |
|
| Participants | 285 healthy pregnant women who were fluent in English or Spanish, aged 18 to 40 years, who desired the hormonal IUDs as their postpartum method of contraception, planned to breastfeed, and agreed to randomization of hormonal IUDs placement timing. Exclusion criteria: delivery < 37.0 weeks’ gestational age, clinical chorioamnionitis, postpartum hemorrhage, preeclampsia with severe features, prolonged antepartum hospitalization, coagulopathy, hepatic disease, undiagnosed genital bleeding, or relative contraindications to hormonal IUD insertion |
|
| Interventions | Treatment: immediate insertion of hormonal IUDs (within 30 minutes following placental delivery) Comparison: standard insertion of hormonal IUDs (4 to 12 weeks postpartum) |
|
| Outcomes | Primary outcome was continuation of any breastfeeding at 8 weeks’ postpartum. Secondary outcomes included lactogenesis, contraceptive method use, including dates of expulsions and reinsertions if they occurred, method satisfaction, and adverse effects including any hospitalizations or events related to IUD use. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized block randomization with alternating block sizes of 4, 6, and 8 |
| Allocation concealment (selection bias) | Low risk | Assignments were stored in secure spreadsheet software files. |
| Blinding of participants and personnel (performance bias) primary outcome | Unclear risk | Blinding of participants was not possible, which may have affected breastfeeding intention. |
| Blinding of participants and personnel (performance bias) secondary outcomes | Unclear risk | Blinding of participants was not possible, which may have affected breastfeeding intention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up rates were 10.4% (13/125) and 0.9% (1/103) of participants assigned to immediate and delayed insertion groups, respectively. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Unclear risk | 7 women in the immediate insertion group and 24 women in the delayed insertion group did not receive the assigned intervention. |
Whitaker 2014.
| Study characteristics | ||
| Methods | Locations and durations: Chicago, IL (USA), from May 2007 to July 2010; Evanston, IL (USA), from April 2009 to January 2011 Design: randomized safety and efficacy study Sample size calculation: assumed 60% of standard insertion would return for follow‐up, 20% of each group would discontinue, estimated 80% of the postplacental group and 48% of the standard group would use IUDs within 12 months; 46 per group for 90% power to detect difference at alpha = 0.05; assumed 15% loss to follow‐up; planned to recruit 108 participants in order to have 92 participants complete trial; outcome of focus was use at 12 months. |
|
| Participants | 42 women in 2 hospitals (20 postplacental and 22 standard group) Inclusion criteria: pregnant; 18 years or older; planning a scheduled cesarean delivery; desiring hormonal IUDs for contraception; English speaker Exclusion criteria: allergy to polyethylene or levonorgestrel or other contraindication to hormonal IUDs use; positive test for gonorrhea, chlamydia, or trichomoniasis during pregnancy without treatment and subsequent test‐of‐cure result confirming negative result; leiomyomata distorting uterine cavity; uterine anomaly precluding IUD placement; current cervical cancer or carcinoma in situ; wanting a repeat pregnancy within 12 months; history of postabortal or postpartum sepsis; randomized to postplacental IUD placement and could not undergo insertion within 10 minutes because of hemorrhage or surgical complication Exclusion at delivery: clinical evidence of infection; prolonged rupture of membranes; or no longer desiring hormonal IUD |
|
| Interventions | Intervention arm: immediate postplacental insertion of hormonal IUDs after cesarean delivery (within 10 minutes) Control arm: standard insertion of hormonal IUDs, 4 to 8 weeks after cesarean delivery |
|
| Outcomes | Use of hormonal IUDs at 12 months after delivery; proportion with IUS inserted per protocol, expulsion, satisfaction with IUD, complications Assessment times: 3 and 6 months after delivery by telephone and 12 months after delivery in clinic (by phone for those with IUD inserted off protocol, expelled without replacement, or removed) |
|
| Notes | Slow recruitment, early termination, small number of participants (42 women) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked‐randomization scheme, stratified by site |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) primary outcome | Low risk | Research staff members conducting follow‐up assessments were blinded to participant allocation. ClinicalTrials.gov listing states "single blind (investigator)" |
| Blinding of participants and personnel (performance bias) secondary outcomes | Low risk | Research staff members conducting follow‐up assessments were blinded to participant allocation. ClinicalTrials.gov listing states "single blind (investigator)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 33% (30% immediate insertion group; 36% standard insertion group) |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all potentially relevant outcomes. |
| Other bias | Low risk | None |
Abbreviations
Cu: copper IUD: intrauterine device
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baldwin 2014 | Did not compare immediate versus delayed IUD insertion. |
| Braga 2015 | Did not compare immediate versus delayed contraceptive implant insertion. |
| Brito 2009 | Did not compare immediate versus delayed contraceptive implant insertion. |
| Brito 2012 | Did not compare immediate versus delayed contraceptive implant insertion. |
| Bryant 2013 | The outcome was feasibility to conduct RCTs. |
| Gariepy 2015 | Not a randomized controlled trial. |
| Ireland 2014 | Not a randomized controlled trial. |
| Pentickly 2013 | Did not compare immediate versus delayed contraceptive implant insertion. |
| Shabaan 1985 | Did not compare immediate versus delayed contraceptive implant insertion. |
| Taneepanichkul 2001 | Not a randomized controlled trial. |
| Tocce 2012 | Not a randomized controlled trial. |
| VanDerPas 1980 | Did not compare immediate versus delayed IUD insertion. |
| Wilson 2014 | Not a randomized controlled trial. |
Abbreviation
IUD: intrauterine device
Characteristics of ongoing studies [ordered by study ID]
EUCTR2017‐001945‐29‐SE.
| Study name | Immediate postpartum LNG‐IUS insertion or standard insertion procedure after childbirth. An open‐label, randomized, multicenter study ‐ early insertion of LNG‐IUS after childbirth |
| Methods | Randomized controlled trial conducted in university hospital (Sweden) |
| Participants | Inclusion criteria
•Women opting for hormonal IUD for contraception postpartum
•> 18 and < 36 years old at the time of inclusion
•Delivery at ≥ 37 weeks according to ultrasound dating
•Vaginal delivery or uncomplicated instrumental delivery as judged by nurse‐midwife or physician
•Written informed consent
•Hormonal IUD insertion possible within 48 hours postpartum Exclusion criteria •Complicated instrumental delivery (as judged by nurse‐midwife or physician) or caesarean section delivery •Known hypersensibility/allergy to levonorgestrel or any of the substances added to the hormonal IUD •Known abnormal uterine cavity •Preterm delivery (< 37 weeks) •Chorioamnionitis •Delivery associated bleeding > 1000 mL •Uterine atony postpartum •Placental retention •Therapeutic antibiotic treatment during delivery, (antibiotics used only for prophylaxis accepted) •History of breast cancer • If any of the following conditions are present an evaluation and decision by a physician must be done before inclusion:
|
| Interventions | Treatment: immediate insertion of hormonal IUD (48 hours postpartum) Comparison: standard insertion of hormonal IUD (6‐8 weeks postpartum) |
| Outcomes | Number of abortions within one year after insertion of hormonal IUD, efficacy, safety, and acceptability of immediate insertion of hormonal IUD postpartum |
| Starting date | 20 September 2017 |
| Contact information | Principal Investigator: Jan Brynhildsen Linköping University Hospital, Sweden |
| Notes |
NCT01161095.
| Study name | A multicenter analysis of levonorgestrel‐intrauterine system (LNG‐IUS) use in the postpartum period |
| Methods | Randomized controlled trial conducted in 2 university hospitals (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of hormonal IUD (72 hours postpartum) Comparison: standard insertion of hormonal IUD (6 weeks postpartum) |
| Outcomes | Continuation rates at 6 months, pain at the time of placement, postpartum depression, breastfeeding status, postpartum weight retention, expulsion rates, bleeding profile, uterine infection (endometritis) |
| Starting date | August 2010 |
| Contact information | Principal Investigator: Joshua D. Dahlke MD United States Navy |
| Notes |
NCT01272960.
| Study name | Mirena intrauterine system timing of insertion: a randomized controlled trial (MISTIC) |
| Methods | Randomized controlled trial conducted in university hospital (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of hormonal IUD (within 10 minutes of delivery of placenta ) Comparison: standard insertion of hormonal IUD (4‐8 weeks postpartum) |
| Outcomes | Continuation rates at 6 months, expulsion rates at 6 weeks, perforation rates at 6 months, intrauterine Infection at 6 months |
| Starting date | October 2010 |
| Contact information | Principal Investigator: Lorie M Harper Washington University School of Medicine |
| Notes |
NCT01666912.
| Study name | Postpartum etonogestrel implant for adolescents (PPImplant) |
| Methods | Randomized controlled trial conducted in university hospital (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of contraceptive implant (prior to leaving the hospital postpartum) Comparison: standard insertion of contraceptive implant (6 weeks postpartum) |
| Outcomes | Continuation, satisfaction, rapid repeat pregnancy Assessment time: 12 months postpartum |
| Starting date | 16 August 2012 |
| Contact information | Principal Investigator: Amy Bryant University of North Carolina, Chapel Hill |
| Notes |
NCT01767285.
| Study name | A randomized controlled trial of immediate postpartum etonogestrel implant versus six‐week postpartum etonogestrel implant: a pilot study |
| Methods | Open label, randomized controlled trial conducted in university hospital (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of etonogestrel contraceptive implant (before discharge home) Comparison: standard insertion of etonogestrel contraceptive implant (6 weeks postpartum) |
| Outcomes | Continuation, rate of Intercourse, rapid repeat pregnancy, continuation of breastfeeding, pregnancy rate, satisfaction Assessment times: 6, and 12 months postpartum |
| Starting date | 19 December 2016 |
| Contact information | Principal Investigator: Jessica Morse Duke University Medical Center |
| Notes |
NCT02674139.
| Study name | Comparison between intrauterine contraceptive device insertion during cesarean section vs conventional application |
| Methods | Open label, randomized controlled trial conducted in university hospital (Egypt) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of copper T 380A IUD (within 10 minutes of delivery of the placenta during caesarean section) Comparison: standard insertion of copper T 380A IUD (6 weeks postpartum) |
| Outcomes | Expulsion, uterine perforation, pelvic pain, strings not visible, pelvic infection, satisfaction, pregnancy Assessment time: 6 months postpartum |
| Starting date | 4 February 2016 |
| Contact information | Principal Investigator: Sara AA Mohamed Beni‐Suef University |
| Notes |
NCT02866279.
| Study name | Immediate postpartum contraceptive implant placement and breastfeeding success in women at risk for low milk supply: a non‐inferiority trial |
| Methods | Open label, randomized controlled trial conducted in academic medical center (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: postplacental insertion of etonogestrel contraceptive implant (within 30 minutes of placental delivery) Treatment: immediate postpartum insertion of etonogestrel contraceptive implant (1‐3 days postpartum) Comparison: delayed insertion of etonogestrel contraceptive implant (6 or more weeks postpartum) |
| Outcomes | Duration of breastfeeding, time to lactogenesis II, vaginal bleeding, satisfaction Assessment times: 1‐5 days postpartum for lactogenesis II, and 6 months postpartum for other outcomes |
| Starting date | 15 August 2016 |
| Contact information | Principal Investigator: Erika Levi Montefiore Medical Center |
| Notes |
NCT03353012.
| Study name | Acceptability and tolerance of immediate versus delayed postpartum contraceptive implant |
| Methods | Open label, randomized controlled trial conducted in university hospital (Thailand) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate postpartum insertion of levonorgestrel implant or an etonogestrel implant (1‐3 days postpartum) Comparison: delayed insertion of levonorgestrel implant or an etonogestrel implant (6 or more weeks postpartum) |
| Outcomes | Treatment‐related adverse events, removal rate, satisfaction, breastfeeding status, child weight, child height Assessment time: 12 weeks postpartum |
| Starting date | 24 November 2017 |
| Contact information | Principal Investigator: Sitanan Lertsiripanich Chulalongkorn University |
| Notes |
NCT03404622.
| Study name | Immediate post‐placental insertion of IUD during cesarean delivery versus 6 week post‐cesarean insertion |
| Methods | Open label, randomized controlled trial conducted in university hospital (Egypt) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: postplacental insertion of intrauterine devices (postplacental removal during Cesarean section) Comparison: delayed insertion of intrauterine devices (6 weeks post Cesarean section delivery) |
| Outcomes | Expulsion, displacement, infection, bleeding, perforation Assessment time: 3 months postpartum |
| Starting date | 19 January 2018 |
| Contact information | Principal Investigator: Alshaimaa A Ali Ain Shams Maternity Hospital |
| Notes |
NCT03492034.
| Study name | IUDs insertion during cesarean section |
| Methods | Open label, randomized controlled trial conducted in university hospital (Egypt) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of intrauterine device (during Cesarean section) Comparison: insertion of intrauterine device after puerperium |
| Outcomes | IUD expulsion rate, bleeding, degree of pain, dyspareunia, satisfaction Assessment time: 6 weeks postpartum |
| Starting date | 10 April 2018 |
| Contact information | Principal Investigator: Mohamed S Sweed Ain Shams Maternity Hospital |
| Notes |
NCT03585504.
| Study name | Immediate versus delayed insertion of Implanon in postpartum adolescents (Implanon) |
| Methods | Open label, randomized controlled trial conducted in university hospital (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate etonogestrel contraceptive implant insertion (prior to hospital discharge) Comparison: delayed etonogestrel contraceptive implant insertion (6 weeks postpartum) |
| Outcomes | Continuation, satisfaction with bleeding, satisfaction with method of contraception Assessment time: 6 months postpartum |
| Starting date | 13 July 2018 |
| Contact information | Principal Investigator: Angela Dempsey Medical University of South Carolina |
| Notes |
NCT03978598.
| Study name | Effect of immediate versus standard postpartum insertion of the contraceptive implant on breastfeeding outcomes |
| Methods | Open label, randomized controlled trial conducted in university hospital (USA) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment: immediate insertion of etonogestrel contraceptive implant (first 24 hours after delivery) Comparison: delayed insertion of etonogestrel contraceptive implant (4 to 6 weeks postpartum) |
| Outcomes | Continuation of breastfeeding, time to lactogenesis, exclusive breastfeeding, factors associated with breastfeeding discontinuation, satisfaction with postpartum contraception counseling, postpartum mood, sexual function, postpartum bleeding days, satisfaction with timing of implant insertion Assessment time: first 8 weeks after delivery for continuation of breastfeeding, first 7 days for lactogenesis, up to 24 weeks postpartum for other outcomes |
| Starting date | 7 June 2019 |
| Contact information | Principal Investigator: Jamie Krashin University of New Mexico |
| Notes |
Abbreviations
EBL: estimated blood loss
Hb: hemoglobin concentration
HCG: human chorionic gonadotropin
Differences between protocol and review
Primary review outcome
In our protocol the primary outcome was 'Postpartum contraceptive prevalence at the first postpartum check‐up visit'. This has been rephrased as 'Rate of contraceptive implant initiation at the first postpartum visit (four to eight weeks postpartum)'. 'Continuation rate' has been rephrased as 'utilization rate'.
Contributions of authors
Jen Sothornwit initiated the review topic.
Jen Sothornwit and Srinaree Kaewrudee screened and selected the studies.
Jen Sothornwit and Sarah Averbach made risk of bias judgements.
Jen Sothornwit and Srinaree Kaewrudee extracted data.
Porjai Pattanittum entered data into RevMan 5.
Pisake Lumbiganon or Sarah Averbach checked all study characteristics for accuracy against the trial reports.
Porjai Pattanittum and Jen Sothornwit performed the meta‐analysis.
Jen Sothornwit, Sarah Averbach, Porjai Pattanittum, Srinaree Kaewrudee, and Pisake Lumbiganon drafted the review.
All authors reviewed and approved the final version of the review.
Sources of support
Internal sources
Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Thailand
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand
External sources
Thailand Research Fund (Distinguished Professor Awards), Thailand
Cochrane Thailand, Thailand
Long‐term Institutional Development HUBs (LID‐HUBs), the Human Reproduction Programme (HRP) Alliance for Research Capacity Strenghtening, Department of Reproductive Health and Research, World Health Organization, Switzerland
-
National Institutes of Health, USA
Dr. Averbach is supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) physician scientist award (K12HD001259).
Declarations of interest
Jen Sothornwit: none known
Srinaree Kaewrudee: none known
Pisake Lumbiganon: none known
Porjai Pattanittum: none known
Sarah Averbach: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Averbach 2017 {published data only}
- Averbach S, Kakaire O, Kayiga H, Lester F, Sokoloff A, Byamugisha J, et al. Immediate versus delayed postpartum use of levonorgestrel contraceptive implants: a randomized controlled trial in Uganda. American Journal of Obstetrics and Gynecology 2017;217(5):568.e1-e7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbach S, Kakaire O, McDiehl R, Dehlendorf C, Lester F, Steinauer J. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception 2019;99(2):87-93. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiehl RP, Kakaire O, Lester F, Steinauer J, Averbach S. The effect of immediate postpartum levonorgestrel implant use on breastfeeding and infant outcomes among women in Kampala, Uganda. Contraception 2017;96(4):268. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayoumi 2020 {published data only}
- Bayoumi YA, Dakhly DM, Bassiouny YA, Gouda HM, Hassan MA, Hassan AA. Post-placental intrauterine device insertion vs puerperal insertion in women undergoing caesarean delivery in Egypt: a 1 year randomised controlled trial. European Journal of Contraception & Reproductive Health Care 2020;25(6):439-44. [DOI] [PubMed] [Google Scholar]
Bryant 2017 {published data only}
Carmo 2017 {published data only}
- Carmo LS, Braga GC, Ferriani RA, Quintana SM, Vieira CS. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstetrics and Gynecology 2017;130(1):100-7. [DOI: 10.1097/AOG.0000000000002092] [DOI] [PubMed] [Google Scholar]
- Nadai M, De Melo Pereira do Carmo LS, Braga G, Fregonesi Infante B, Stifani BM, Ferriani RA, et al. Immediate postpartum insertion of the etonogestrel implant and bleeding pattern: a RCT. International Journal of Gynecology and Obstetrics 2018;143:378. [Google Scholar]
- Vieira CS, Nadai MN, Melo Pereira do Carmo LS, Braga GC, Infante BF, Stifani BM, et al. Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: a randomized controlled trial. Contraception 2019;100(4):258-63. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen BA, Hayes JL, Hohmann HL, Perriera LK, Reeves MF, Creinin MD. A randomized trial of postplacental compared to delayed insertion of the levonorgestrel-releasing intrauterine device after vaginal delivery (abstract). Contraception 2009;80(2):205. [Google Scholar]
- Chen BA, Reeves MF, Creinin MD, Schwarz EB. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception 2011;84(5):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstetrics and Gynecology 2010;116(5):1079-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlke 2011 {published data only}
- Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel--intrauterine system at three time periods: a prospective randomized pilot study. Contraception 2011;84(3):244-8. [DOI] [PubMed] [Google Scholar]
Dias 2015 {published data only}
- Dias TD, Wijekoon D, Ganeshamoorthy P, Abeykoon S, Liyanage G, Padeniya T. Post-placental and interval intrauterine contraceptive device (IUD) insertion: does timing matter? BJOG: An International Journal of Obstetrics and Gynaecology 2015;122:395-7. [Google Scholar]
Gurtcheff 2011 {published data only}
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstetrics and Gynecology 2011;117(5):1114-21. [DOI] [PubMed] [Google Scholar]
Lester 2015 {published data only (unpublished sought but not used)}
- Averbach S, Lester F, Fortin J, Byamugisha J, Goldberg A, Kakaire O. Acceptability of the IUD among women who opted out of a randomized controlled trial of intracesarean insertion of the copper-T 380A in Kampala, Uganda. Contraception 2012;86:318. [Google Scholar]
- Lester F, Kakaire O, Byamugisha J, Averbach S, Fortin J, Maurer R, et al. Intracesarean insertion of the Copper T380A versus 6 weeks post-cesarean: a randomized clinical trial. Contraception 2015;91(3):198-203. [DOI] [PubMed] [Google Scholar]
- Lester F, Kakaire O, Byamugisha J, Fortin J, Averbach S, Maurer R, et al. Intracesarean insertion of the Copper T 380A vs. 6 week post-cesarean insertion: an RCT. International Journal of Gynaecology and Obstetrics 2012;119:S3. [DOI: ] [Google Scholar]
Levi 2015 {published data only}
- Levi EE, Findley MK, Avila K, Bryant AG. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeeding Medicine 2018;13(10):674–9. [DOI: 10.1089/bfm.2018.0060] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi EE, Stuart GS, Zerden ML, Garrett JM, Bryant AG. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstetrics and Gynecology 2015;126(1):5-11. [DOI: 10.1097/AOG.0000000000000882.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogburn 2013 {published data only (unpublished sought but not used)}
- Ogburn T, Espey E, Leeman L, Singh R, Pereda B, Carr S. A randomized trial of immediate postpartum versus interval insertion of an intrauterine device. Contraception 2013;88(3):455. [Google Scholar]
Phemister 1995 {published data only}
- Phemister DA, Laurent S, Harrison FN Jr. Use of Norplant contraceptive implants in the immediate postpartum period: safety and tolerance. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 1):175-9. [DOI] [PubMed] [Google Scholar]
Soon 2018 {published data only}
- Soon R, McGuire K, Salcedo J, Kaneshiro B. Immediate versus delayed insertion of the levonorgestrel intrauterine device in postpartum adolescents: a randomized pilot study. Hawai'i Journal of Medicine & Public Health 2018;77(3):60–5. [PMC free article] [PubMed] [Google Scholar]
Stuart 2015 {published data only}
- Stuart GS, Lesko C, Stuebe A, Bryant A. Breastfeeding and postpartum insertion of the levonorgestrel intrauterine system: a randomized trial. Obstetrics and Gynecology 2014;123 Suppl 1:15S. [Google Scholar]
- Stuart GS, Lesko CR, Stuebe AM, Bryant AG, Levi EE, Danvers AI. A randomized trial of levonorgestrel intrauterine system insertion 6 to 48 h compared to 6 weeks after vaginal delivery; lessons learned. Contraception 2015;91(4):284-8. [DOI] [PubMed] [Google Scholar]
Turok 2017 {published data only}
- Espey E, Turok DK, Sanders J, Singh RH, Thaxton L, Leeman L. Breastfeeding continuation in postplacental versus interval postpartum IUD insertion: the breastfeeding levonorgestrel IUD study (BLIS): a randomized controlled trial. Contraception 2016;94(4):407. [DOI: ] [Google Scholar]
- Turok D, Espey E, Sanders JN, Eggebrotten J, Bullock H, Gawron L. The effect of postplacental versus interval postpartum IUD insertion on lactogenesis: the breastfeeding levonorgestrel IUD study (BLIS): a randomized controlled trial. Contraception 2016;94(4):390. [Google Scholar]
- Turok DK, Leeman L, Sanders JN, Thaxton L, Eggebroten JL, Yonke N, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;217(6):665.e1-8. [DOI: 10.1016/j.ajog.2017.08.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turok DK, Leeman L, Sanders JN, Thaxton L, Eggebroten JL, Yonke N, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Obstetrical and Gynecological Survey 2018;73(1):30-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitaker 2014 {published data only}
- Whitaker AK, Endres LK, Mistretta SQ, Gilliam ML. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception 2014;89(6):534-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baldwin 2014 {published data only}
- Baldwin MK, Edelman AB, Lim JY, Nichols MD, Bednarek PH, Jensen JT. Intrauterine device placement at 3 versus 6 weeks postpartum: a randomized trial. Contraception 2016;93(4):356-63. [DOI] [PubMed] [Google Scholar]
Braga 2015 {published data only}
- Braga GC, Ferriolli E, Quintana SM, Ferriani RA, Pfrimer K, Vieira CS. Immediate postpartum initiation of etonogestrel-releasing implant: a randomized controlled trial on breastfeeding impact. Contraception 2015;92(6):536-42. [DOI] [PubMed] [Google Scholar]
Brito 2009 {published data only}
- Brito MB, Ferriani RA, Quintana SM, Yazlle ME, Silva de Sá MF, Vieira CS. Safety of the etonogestrel-releasing implant during the immediate postpartum period: a pilot study. Contraception 2009;80(6):519-26. [DOI] [PubMed] [Google Scholar]
Brito 2012 {published data only}
- Brito MB, Ferriani RA, Meijers JC, Garcia AA, Quintana SM, Silva de Sá MF, et al. Effects of the etonogestrel-releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: a randomized controlled trial. Thrombosis Research 2012;130(3):355-60. [DOI] [PubMed] [Google Scholar]
Bryant 2013 {published data only}
- Bryant AG, Kamanga G, Stuart GS, Haddad LB, Meguid T, Mhango C. Immediate postpartum versus 6-week postpartum intrauterine device insertion: a feasibility study of a randomized controlled trial. African Journal of Reproductive Health 2013;17(2):72-9. [PubMed] [Google Scholar]
Gariepy 2015 {published data only}
- Gariepy AM, Duffy JY, Xu X. Cost-effectiveness of immediate compared with delayed postpartum etonogestrel implant insertion. Obstetrics and Gynecology 2015;126(1):47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ireland 2014 {published data only}
- Ireland LD, Goyal V, Raker CA, Murray A, Allen RH. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception 2014;90(3):253–8. [DOI] [PubMed] [Google Scholar]
Pentickly 2013 {published data only}
- Pentickly S, Ratcliffe SJ, Schreiber C. The impact of progestin-only contraceptives on postpartum weight loss (POPP): a year-long randomized controlled study. Contraception 2013;88(3):434. [Google Scholar]
Shabaan 1985 {published data only}
- Shaaban MM, Salem HT, Abdullah KA. Influence of levonorgestrel contraceptive implants, NORPLANT, initiated early postpartum upon lactation and infant growth. Contraception 1985;32(6):623-35. [DOI] [PubMed] [Google Scholar]
Taneepanichkul 2001 {published data only}
- Taneepanichskul S, Tanprasertkul C. Use of Norplant implants in the immediate postpartum period among asymptomatic HIV-1-positive mothers. Contraception 2001;64(1):39-41. [DOI] [PubMed] [Google Scholar]
Tocce 2012 {published data only}
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? American Journal of Obstetrics and Gynecology 2012;206(6):481.e1-7. [DOI] [PubMed] [Google Scholar]
VanDerPas 1980 {published data only}
- Van Der Pas MT, Delbeke L, Van Dets H. Comparative performance of two copper-wired IUDs (ML Cu 250 and T Cu 200: immediate postpartum and interval insertion. Contraceptive Delivery Systems 1980;1:27-35. [PubMed] [Google Scholar]
Wilson 2014 {published data only}
- Wilson S, Tennant C, Sammel MD, Schreiber C. Immediate postpartum etonogestrel implant: a contraception option with long-term continuation. Contraception 2014;90(3):259-64. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2017‐001945‐29‐SE {published data only}
- EUCTR2017-001945-29-SE. Immediate post partum LNG-IUS insertion or standard insertion procedure after childbirth An open-label, randomized, multicenter study. ww.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2017-001945-29 (first received 14 July 2017).
NCT01161095 {published data only}
- NCT01161095. A multicenter analysis of levonorgestrel-intrauterine system (LNG-IUS) use in the postpartum period. clinicaltrials.gov/ct2/show/study/NCT01161095 (first received 13 July 2010).
NCT01272960 {published data only}
- NCT01272960. Mirena intrauterine system timing of insertion: a randomized controlled trial (MISTIC). linicaltrials.gov/ct2/show/NCT01272960 (first received 10 January 2011).
NCT01666912 {published data only}
- NCT01666912. Postpartum etonogestrel implant for adolescents (PPImplant) [Etonogestrel-releasing subdermal implant for adolescents in the postpartum period: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT01666912 (first received 16 August 2012).
NCT01767285 {published data only}
- NCT01767285. A randomized controlled trial of immediate postpartum etonogestrel implant versus six-week postpartum etonogestrel implant: a pilot study [Immediate vs. delayed postpartum etonogestrel implant]. clinicaltrials.gov/ct2/show/NCT01767285 (first received 19 December 2016).
NCT02674139 {published data only}
- NCT02674139. Comparison between intrauterine contraceptive device insertion during cesarean section vs conventional application [Comparative study of intrauterine contraceptive device insertion during caesarean section versus conventional application]. clinicaltrials.gov/ct2/show/NCT02674139 (first received 4 February 2016).
NCT02866279 {published data only}
- NCT02866279. Immediate postpartum contraceptive implant placement and breastfeeding success in women at risk for low milk supply: a non-inferiority trial. clinicaltrials.gov/ct2/show/NCT02866279 (first received 15 August 2016).
NCT03353012 {published data only}
- NCT03353012. Acceptability & tolerance of immediate versus delayed postpartum contraceptive implant. clinicaltrials.gov/ct2/show/NCT03353012 (first received 24 November 2017).
NCT03404622 {published data only}
- NCT03404622. Immediate post-placental insertion of the intrauterine contraceptive device during cesarean delivery versus 6 week post-cesarean insertion. clinicaltrials.gov/ct2/show/NCT03404622 (first received 19 January 2018).
NCT03492034 {published data only}
- NCT03492034. Insertion of intrauterine contraceptive device during cesarean section: randomized clinical trial. linicaltrials.gov/ct2/show/NCT03492034 (first received 10 April 2018).
NCT03585504 {published data only}
- NCT03585504. Early insertion of a hormonal intrauterine device after childbirth [A randomized controlled trial of immediate versus delayed insertion of Implanon in postpartum adolescents]. clinicaltrials.gov/ct2/show/NCT03585504 (first received 13 July 2018).
NCT03978598 {published data only}
- NCT03978598. Effect of immediate versus standard postpartum insertion of the contraceptive implant on breastfeeding outcomes [Breastfeeding etonogestrel implant study (LACTO-Rod)]. clinicaltrials.gov/ct2/show/NCT03978598 (first received 7 June 2019).
Additional references
ACOG 2016
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstetrics and Gynecology 2016;128(2):e32-7. [DOI] [PubMed] [Google Scholar]
ACOG 2017
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstetrics and Gynecology 2017;130(5):e251-69. [DOI] [PubMed] [Google Scholar]
ACOG 2018
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 735: Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstetrics and Gynecology 2018;131(5):e130-9. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell MJ. Cluster randomised trials in general (family) practice research. Statistical Methods in Medical Research 2000;9(2):81-94. [DOI] [PubMed] [Google Scholar]
Chaovisitsaree 2012
- Chaovisitsaree S, Noi-um S, Kietpeerakool C. Review of postpartum contraceptive practices at Chiang Mai University Hospital: implications for improving quality of service. Medical Principles and Practice 2012;21(2):145-9. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed November 2019. Melbourne, Australia: Veritas Health Innovation, Available at covidence.org.
Deeks 2001
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Finer 2011
- Finer LB, Kost K. Unintended pregnancy rates at the state level. Perspectives on Sexual and Reproductive Health 2011;43(2):78-87. [DOI] [PubMed] [Google Scholar]
Fraser 1995
- Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine 1995;332(17):1113-7. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2014 [Computer program]
- GRADEpro GDT. Version accessed 11 November 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Hubacher 2018
- Hubacher D, Spector H, Monteith C, Chen PL. Not seeking yet trying long-acting reversible contraception: a 24-month randomized trial on continuation, unintended pregnancy and satisfaction. Contraception 2018;97(6):524-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jatlaoui 2018
- Jatlaoui TC, Whiteman MK, Jeng G, Tepper NK, Berry-Bibee E, Jamieson DJ, et al. Intrauterine device expulsion after postpartum placement: a systematic review and meta-analysis. Obstetrics and Gynecology 2018;132(4):895-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2015
- Lopez LM, Bernholc A, Hubacher D, Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD003036. [DOI: 10.1002/14651858.CD003036.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore Z, Pfitzer A, Gubin R, Charurat E, Elliott L, Croft T. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 2015;92(1):31-9. [DOI: 10.1016/j.contraception.2015.03.007] [DOI] [PubMed] [Google Scholar]
Nkwabong 2015
- Nkwabong E, Ilue EE, Bisong CE. Factors associated with poor attendance at the postpartum clinic six weeks after delivery in Cameroon. International Journal of Gynaecology and Obstetrics 2015;129(3):248-50. [DOI] [PubMed] [Google Scholar]
Ortiz 1996
- Ortiz ME, Croxatto HB, Bardin CW. Mechanisms of action of intrauterine devices. Obstetrical and Gynecological Survey 1996;51(12 Suppl):S42-51. [DOI] [PubMed] [Google Scholar]
Phillips 2016
- Phillips SJ, Tepper NK, Kapp N, Nanda K, Temmerman M, Curtis KM. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception 2016;94(3):226-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reed 2020
- Reed S, Getahun D, Gatz J, Lynen R, Asiimwe A, Anthony M. 78 Postpartum timing of IUD insertion is associated with risk of uterine perforation: results from APEX IUD. Contraception 2020;102(4):302. [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez 2009
- Rodriguez MI, Kaunitz AM. An evidence-based approach to postpartum use of depot medroxyprogesterone acetate in breastfeeding women. Contraception 2009;80(1):4-6. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Singh 2010
- Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Studies in Family Planning 2010;41(4):241-50. [DOI] [PubMed] [Google Scholar]
Speroff 2008
- Speroff L, Mishell DR Jr. The postpartum visit: it's time for a change in order to optimally initiate contraception. Contraception 2008;78(2):90-8. [DOI] [PubMed] [Google Scholar]
Stanford 2002
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. American Journal of Obstetrics and Gynecology 2002;187(6):1699-708. [DOI] [PubMed] [Google Scholar]
Thiel de Bocanegra 2013
- Thiel de Bocanegra H, Chang R, Menz M, Howell M, Darney P. Postpartum contraception in publicly-funded programs and interpregnancy intervals. Obstetrics and Gynecology 2013;122(2 Pt 1):296-303. [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii-92. [PubMed] [Google Scholar]
UN 2013
- United Nations (UN), Department of Economic and Social Affairs, Population Division. World contraceptive patterns 2013. www.un.org/en/development/desa/population/publications/family/contraceptive-wallchart-2013.shtml (accessed 28 October 2016).
Whaley 2015
- Whaley N, Burke A. Contraception in the postpartum period: immediate options for long-acting success. Women's Health (London, England) 2015;11(2):97-9. [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Report of a WHO TechnicalConsultation on Birth Spacing 2005 Jun 13-15; Geneva. apps.who.int/iris/bitstream/handle/10665/69855/WHO_RHR_07.1_eng.pdf?sequence=1&ua= (accessed 2 February 2020).
WHO 2015
- Department of Reproductive Health, World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf?ua=1&ua=1 (accessed 2 February 2020).
Wilson 2011
- Wilson EK, Samandari G, Koo HP, Tucker C. Adolescent mothers' postpartum contraceptive use: a qualitative study. Perspectives on Sexual and Reproductive Health 2011;43(4):230-7. [DOI] [PubMed] [Google Scholar]
World Bank 2020
- World Bank Country and Lending Groups. available from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 13 April 2021).
References to other published versions of this review
Sothornwit 2015
- Sothornwit J, Werawatakul Y, Kaewrudee S, Lumbiganon P, Laopaiboon M. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD011913. [DOI: 10.1002/14651858.CD011913] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sothornwit 2017
- Sothornwit J, Werawatakul Y, Kaewrudee S, Lumbiganon P, Laopaiboon M. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD011913. [DOI: 10.1002/14651858.CD011913.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


