ABSTRACT
The epicardium, the outermost layer of the heart, is an important regulator of cardiac regeneration. However, a detailed understanding of the crosstalk between the epicardium and myocardium during development requires further investigation. Here, we generated three models of epicardial impairment in zebrafish by mutating the transcription factor genes tcf21 and wt1a, and ablating tcf21+ epicardial cells. Notably, all three epicardial impairment models exhibited smaller ventricles. We identified the initial cause of this phenotype as defective cardiomyocyte growth, resulting in reduced cell surface and volume. This failure of cardiomyocyte growth was followed by decreased proliferation and increased abluminal extrusion. By temporally manipulating its ablation, we show that the epicardium is required to support cardiomyocyte growth mainly during early cardiac morphogenesis. By transcriptomic profiling of sorted epicardial cells, we identified reduced expression of FGF and VEGF ligand genes in tcf21−/− hearts, and pharmacological inhibition of these signaling pathways in wild type partially recapitulated the ventricular growth defects. Taken together, these data reveal distinct roles of the epicardium during cardiac morphogenesis and signaling pathways underlying epicardial-myocardial crosstalk.
Keywords: Cardiomyocytes, Cell growth, Epicardium, Heart development, Inter-tissue crosstalk, Zebrafish
Summary: The epicardium is required to promote cardiomyocyte growth during ventricular development at least in part via the FGF and VEGF signaling pathways.
INTRODUCTION
The epicardium is the last layer to incorporate into the heart during development. Epicardial cells (EpiCs) delaminate from the extra-cardiac proepicardial organ (PEO) and attach to the naked myocardium as free-floating cells owing to the physical properties of the pericardial fluid (Rodgers et al., 2008; Peralta et al., 2013). The epicardium forms a mesothelial layer that completely envelops the heart, then undergoes epithelial-to-mesenchymal transition (EMT) and gives rise to various epicardial derived cells (EPDCs) (Smith et al., 2011; Acharya et al., 2012; Smits et al., 2018). Although the epicardium becomes dormant after undergoing EMT, it reactivates after cardiac injury and upregulates developmental genes as well as new gene regulatory networks (Weinberger et al., 2021 preprint), and subsequently regenerates (van Wijk et al., 2012; Masters and Riley, 2014; Cao and Poss, 2018).
The epicardium has received great attention because of its ability to differentiate into a multitude of cell types during cardiac repair and its role as a source of paracrine signals that promote wound healing (Masters and Riley, 2014; Cao and Poss, 2018). However, a detailed understanding of the epicardial-myocardial crosstalk has proven more elusive. As the factors involved in epicardial-myocardial signaling identified to date, including components of the fibroblast growth factor (FGF) and insulin growth factor (IGF) signaling pathways, are expressed in both developmental and regenerative contexts (Pennisi et al., 2003; Lavine et al., 2005; Brade et al., 2011; Li et al., 2011; Vega-Hernandez et al., 2011), identifying the processes underlying epicardial-myocardial crosstalk during development has important implications for cardiac repair.
Defects in epicardial coverage consistently result in a common myocardial phenotype – small, underdeveloped ventricles. Ablation of the PEO in chicken embryos causes reduced cardiac size and occasional ventricular bulging (Manner, 1993; Pennisi et al., 2003; Manner et al., 2005; Takahashi et al., 2014). Similarly, in mouse embryos, mutations in several epicardial-enriched genes, including those encoding the transcription factors TCF21 and WT1, abrogate epicardial coverage, leading to a reduction in ventricular size (Moore et al., 1999; Acharya et al., 2012). Most studies to date have concluded that the major role of the epicardium is to promote cardiomyocyte (CM) proliferation (Pennisi et al., 2003; Lavine et al., 2005; Li et al., 2011) and to contribute to the ventricular mass by giving rise to EPDCs such as fibroblasts (Mahtab et al., 2009; Acharya et al., 2012). Notably, a few studies have started to challenge the view that the sole function of the epicardium is to regulate the CM cell cycle (Eid et al., 1992; Kastner et al., 1994; Takahashi et al., 2014), but they have so far been limited to using in vitro explants or fixed tissue sections. Deeper investigation of epicardial function in promoting myocardial growth requires a model in which the cellular phenotypes can be experimentally followed in four dimensions.
Here, we generated three models of epicardial impairment in zebrafish larvae by mutating the transcription factor genes tcf21 and wt1a, and by ablating EpiCs. Leveraging the advantages of these newly established models and the amenability of zebrafish to live imaging at a three-dimensional (3D) resolution, we identified a novel role for the epicardium in promoting CM growth and determined the time window when this epicardium-to-myocardium interaction occurs. We also generated a transcriptomic dataset of epicardial-enriched factors to identify molecules important for this crosstalk. Focusing on fgf24 and vegfaa, we provide evidence that they are epicardial-enriched regulators of ventricular growth.
RESULTS AND DISCUSSION
Generation of three zebrafish epicardial impairment models
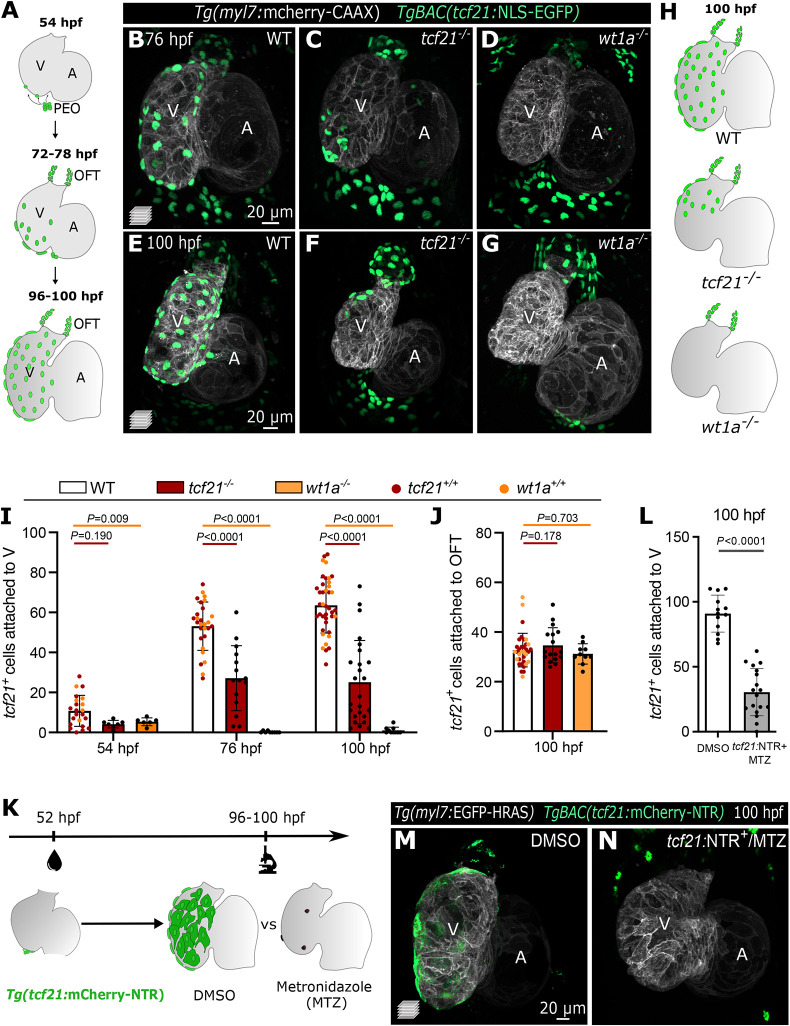
In zebrafish, EpiCs start adhering to the myocardial wall around 52-56 h post fertilization (hpf), and cover the entire ventricle by 96-120 hpf (Fig. 1A) (Peralta et al., 2014). To study the epicardial-myocardial crosstalk, we generated three different zebrafish models with impaired epicardial coverage. First, we mutated tcf21 and wt1a (Fig. S1C), two transcription factor genes enriched in the epicardium (Fig. S1A,B) (Serluca, 2008; Liu and Stainier, 2010; Peralta et al., 2014)). Our tcf21 deletion allele contains a frameshift in the coding sequence, leading to a predicted truncated protein with an incomplete DNA-binding domain (Fig. S1C), whereas the mutation did not affect the stability of the mutant mRNA (Fig. S1D). Conversely, our wt1a promoter deletion led to a complete absence of wt1a mRNA (Fig. S1C,E). In mouse, the lack of either transcription factor leads to impaired epicardial coverage, but its impact on myocardial development is poorly understood (Moore et al., 1999; Acharya et al., 2012). We observed that in zebrafish, mutations in tcf21 and wt1a also led to a reduction of TgBAC(tcf21:NLS-EGFP)+ (hereafter referred to as tcf21+) EpiCs on the ventricular wall, as evidenced at 54 hpf and even more prominently at 76 and 100 hpf (Fig. 1B-H). However, whereas wt1a mutants exhibited a complete absence of tcf21+ EpiCs on the ventricular wall, the epicardial coverage reduction in tcf21 mutants was variable (Fig. 1I). This phenotypic variability is likely not due to the tcf21 mutation leading to a hypomorphic allele, as non-cardiac phenotypes previously identified in tcf21 mutants, including the lack of head muscles (Nagelberg et al., 2015; Burg et al., 2016), were observed with complete penetrance in our tcf21 mutants (n>300 larvae). Notably, the number of outflow tract (OFT) tcf21+ EpiCs appeared to be unaffected in both mutants (Fig. 1J), likely owing to the different origin of this epicardial population (Perez-Pomares et al., 2003; Weinberger et al., 2020). Next, to establish a model in which epicardial coverage could be impaired in a specific time window, we used the previously described nitroreductase/metronidazole (NTR/MTZ) system (Curado et al., 2007, 2008; Pisharath et al., 2007). By treating TgBAC(tcf21:mCherry-NTR) (Wang et al., 2015) embryos (tcf21:NTR+) with MTZ before the epicardium covered the ventricle (52-100 hpf; Fig. 1K), we could ablate a majority of the tcf21+ EpiCs (∼67%; Fig. 1L-N), thereby establishing an inducible system that complements our two mutant models. Using the pan-epicardial marker caveolin 1 (Cav1) (Cao et al., 2016), we confirmed that TgBAC(tcf21:NLS-EGFP) expression was a reliable marker for all EpiCs, and that the loss of tcf21+ cells in our models was due to an absence of EpiCs and not the loss of TgBAC(tcf21:NLS-EGFP) expression. Cav1 immunostaining was only present in ‘escaper’ ventricular tcf21+ EpiCs in tcf21−/− hearts, and around the OFT in all three models (Fig. S1F-I′). Altogether, these three distinct epicardial impairment models constitute a complementary set of reagents to study the effects of epicardial impairment on cardiac morphogenesis. Moreover, the genetic models generated here will be useful to further investigate how Wt1a and Tcf21 regulate epicardial development before the onset of EMT.
Fig. 1.
The transcription factors Tcf21 and Wt1a are required for epicardial attachment to the ventricle. (A) Schematic representation of the epicardial coverage of the zebrafish embryonic and larval heart. (B-G) Confocal images of 76 hpf (B-D) and 100 hpf (E-G) Tg(myl7:mCherry-CAAX) TgBAC(tcf21:NLS-EGFP) larvae. (H) Schematics of the epicardial coverage in 100 hpf WT, tcf21−/− and wt1a−/− larvae. Gray, myocardium; green, EpiCs. (I-J) Quantification of tcf21+ EpiCs attached to the ventricular myocardium (I) and OFT (J). The colors of WT dots refer to tcf21+/+ (red) and wt1a+/+ (orange) siblings. Data show the mean±s.d. P-values were determined from unpaired two-tailed t-test or Mann–Whitney test (following normality test) compared with +/+ siblings of each genotype. (K) Epicardial ablation protocol using the NTR/MTZ system. (L) Quantification of tcf21+ EpiCs attached to the ventricular myocardium following epicardial ablation. Data show the mean±s.d.; P-values were determined from unpaired two-tailed t-test. Controls are pooled tcf21:NTR+ DMSO-treated and tcf21:NTR− MTZ-treated larvae (see Materials and Methods). (M,N) Confocal images of 100 hpf Tg(myl7:EGFP-HRAS); TgBAC(tcf21:mCherry-NTR) hearts showing the absence of EpiCs post MTZ treatment (N), compared with DMSO-treated larvae (M). WT, wild type; A, atrium; V, ventricle; OFT, outflow tract; PEO, proepicardial organ.
Impairment in cardiomyocyte growth becomes evident before reduced cardiomyocyte proliferation when epicardial cells are lost
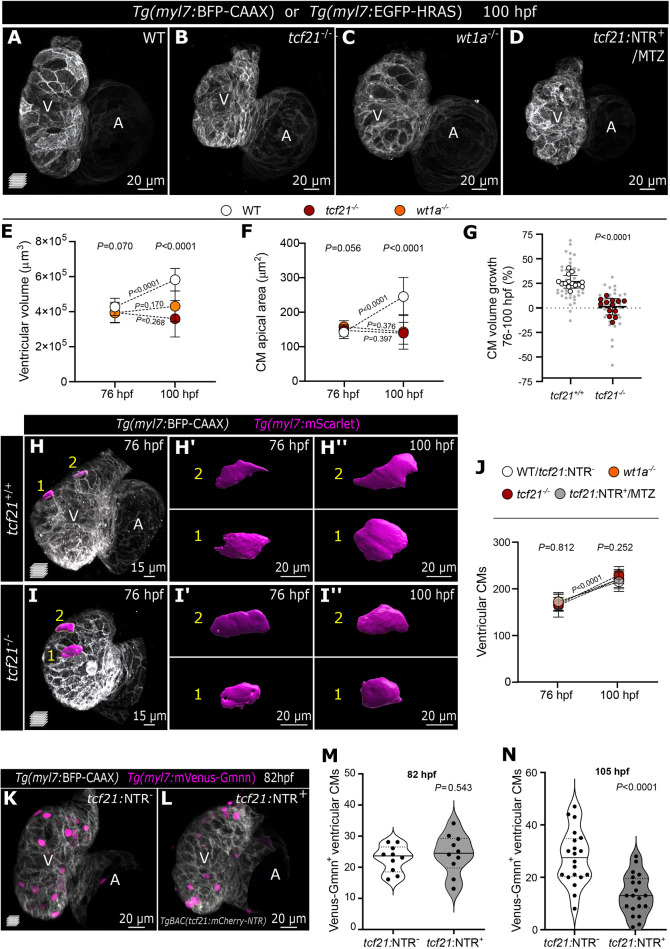
We next aimed to determine how epicardial impairment affects ventricular morphogenesis. Starting at 96 hpf, we observed pericardial edema in tcf21−/−, wt1a−/− and tcf21:NTR+ MTZ-treated larvae (Fig. S1J-M), together with impaired ventricular fractional shortening starting at 100 hpf (Fig. S2D). The cardiac ventricle in all epicardial-impairment models was also approximately 30% smaller than in wild type (WT; Fig. 2A-D; Fig. S2A-E). Interestingly, we observed that, from 76 to 100 hpf, the WT ventricle grew on average by 36.4% in volume, whereas the mutant ventricle started with a comparable volume at 76 hpf but failed to enlarge over time (Fig. 2E).
Fig. 2.
Impaired epicardial coverage affects ventricular cardiomyocyte size increase and ventricular growth. (A-D) Confocal images of 100 hpf WT, tcf21−/−, wt1a−/− and TgBAC(tcf21:mCherry-NTR)+ (tcf21:NTR+) MTZ-treated larvae, exhibiting reduced ventricular size. (E,F) Change in ventricular volume (E) and CM apical area (F) from 76 to 100 hpf in WT, tcf21−/− and wt1a−/− larvae. (G) Percentage increase of individual CM volume between 76 and 100 hpf, measured by the myl7:mScarlet signal. Large dots indicate the average per larva and small dots indicate individual CMs. P-values were determined from unpaired two-tailed t-test comparing the averages per larva. (H-I″) Confocal images of Tg(myl7:BFP-CAAX); tcf21+/+ or tcf21−/− larva at 76 and 100 hpf (same larvae shown), injected with myl7:mScarlet DNA to label individual CMs. (H′-I″) 3D surface rendering of individual CMs at the two time points. (J) Changes in ventricular CM numbers from 76 to 100 hpf in WT (or tcf21:NTR−), tcf21−/−, wt1a−/− and tcf21:NTR+-MTZ treated larvae. (K-N) Confocal images and quantification of myl7:mVenus-Gmnn+ CMs in 82 hpf (K-M) and 105 hpf (N) control (tcf21:NTR−) and tcf21:NTR+ MTZ-treated larvae. P-values (M,N) were determined by unpaired two-tailed t-test. Data show the mean±s.d. (E,F,G,J), and the solid and dotted lines (M,N) indicate the median and quartiles, respectively. P-values from one-way ANOVA among the three different genotypes at the same time point are shown above the graph (E,F,J), or from unpaired two-tailed t-test comparing the two different time points within the same genotype are shown on the dotted lines (E,F,J). Single data points are shown in Fig. S2. WT, wild type; A, atrium; V, ventricle.
Increase in organ size is driven by hypertrophic (increase in cell size) and hyperplastic (increase in cell number) growth. The first phenotype we observed between control and epicardial-impairment models was in the CM apical area. Although the average apical area of compact-layer CMs was comparable in WT and mutant larvae at 76 hpf, it was significantly smaller in mutant CMs compared with WT at 100 hpf (Fig. 2F; Fig. S2F). To assess the volumetric growth of individual compact-layer CMs over time, we tracked single CMs in tcf21+/+ and tcf21−/− hearts by mosaic expression of Tg(myl7:mScarlet). Strikingly, tcf21+/+ CM volume increased by 26.8% between 76 and 100 hpf, whereas tcf21−/− CM volume did not significantly change (+1.4%) (Fig. 2G-I). Our data, possibly for the first time, clearly reveal a correlation between increasing CM cell volume and ventricular growth and uncover a requirement for the epicardium in promoting CM cell growth during development.
Although the epicardium has not been previously linked with CM hypertrophic growth, it has been implicated in promoting CM proliferation (Pennisi et al., 2003; Lavine et al., 2005; Li et al., 2011). We therefore assessed the number of proliferating CMs at 82 hpf, a time point before the growth defects can be observed, by counting the number of Venus-Gmnn+ CMs (i.e. CMs in the S/G2/M phases; Sugiyama et al., 2009; Choi et al., 2013). We observed no significant difference between control and tcf21:NTR+ MTZ-treated larvae (Fig. 2K-M). We also counted the number of ventricular CMs and they increased by a similar proportion (∼30%) in WT larvae and in larvae from all three models between 76 and 100 hpf (Fig. 2J), resulting in comparable numbers of CMs at all time points analyzed (Fig. S2G). In addition, the loss of the epicardium did not affect the number of CMs in the trabecular layer as assessed in 100 hpf tcf21−/− hearts (P=0.307, unpaired two-tailed t-test; Fig. S2H). These data support the hypothesis that the impaired ventricular growth observed in the absence of the epicardial layer is not caused by defects in CM numbers or differences in their trabeculation potential. However, at 105 hpf, a time point subsequent to the appearance of CM size defects, we observed a severe reduction in the number of Venus-Gmnn+ CMs in tcf21:NTR+ MTZ-treated larvae compared with controls (∼48.5%; Fig. 2N). These defects in CM proliferation might be a consequence of the initial impairment in CM growth. In support of this interpretation, eukaryotic cell cycle progression is known to depend on cell growth (Jorgensen and Tyers, 2004), and a multitude of cell types, including CMs, expand in size prior to dividing (Son et al., 2015; Zlotek-Zlotkiewicz et al., 2015; Uribe et al., 2018). In addition, we observed CM extrusion away from the cardiac lumen in the three epicardial impairment models (Fig. S3A-F′), consistent with previous reports (Manner et al., 2005; Rasouli et al., 2018). The extrusion of these CMs does not appear to be caused by increased cell death, as evidenced by their intact nuclei (Fig. S3G-G‴), nor from a change in their fate to epicardial cells (Fig. S4E, n>30 at 76 and 100 hpf), as recently reported in a model of wt1 expressing CMs (Marques et al., 2022). CM extrusion has also been observed in zebrafish snai1b mutants, in which extruding CMs eventually detach and are found in the pericardial cavity (Gentile et al., 2021); whether epicardial cells also play a role in this model remains to be determined. Notably, we observed a reduced internuclear distance between CMs in our epicardial impairment models (Fig. S3H-J), which has not been reported in other models of CM extrusion. Therefore, we postulate that in the absence of the epicardium, excessive cellular density in the ventricular myocardium drives the aberrant extrusion of a few (i.e. fewer than ten) CMs. Altogether, our observations uncover a previously unidentified role for the epicardium in promoting the initial stages of CM growth, which affects ventricular growth as well as CM proliferation.
Epicardial cells are required and sufficient for ventricular cardiomyocyte growth in a restricted early time window
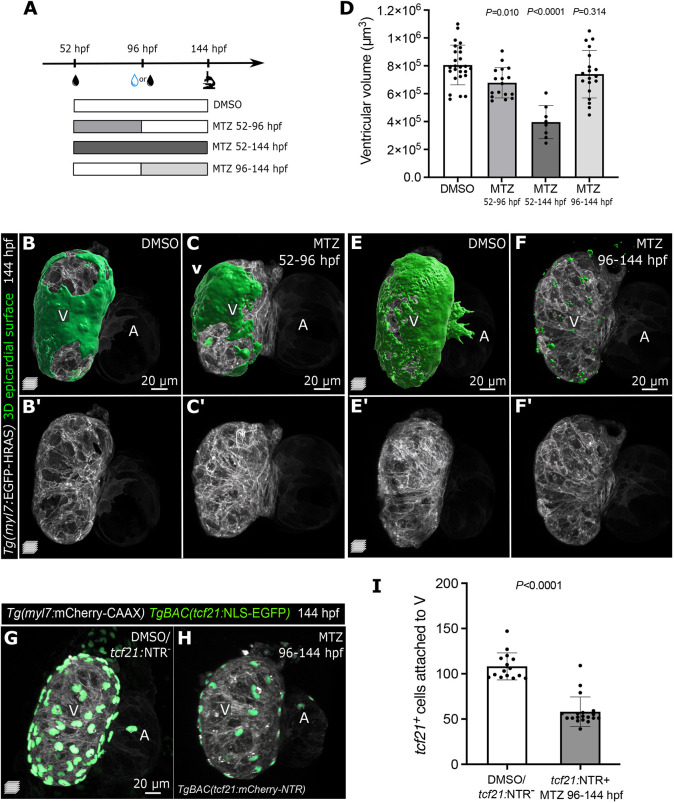
To further analyze the dependency of CM growth on the epicardium, we tested whether rescuing the epicardial coverage was sufficient to improve ventricular growth. Leveraging the temporal versatility of the NTR/MTZ system, we ablated the epicardium specifically from 52 to 96 hpf and then washed out the MTZ. We first confirmed that the EpiCs recovered by 144 hpf (Fig. 3A-C), as previously reported (Wang et al., 2015). Notably, epicardial restoration was sufficient to ameliorate the cardiac growth defects in MTZ-treated larvae (Fig. 3B′-D).
Fig. 3.
Epicardial cells are required for ventricular cardiomyocyte growth during early cardiac morphogenesis, but are dispensable at later time points. (A) Schematic of the MTZ treatment protocol. (B-C′,E-F′) Confocal images of 144 hpf Tg(myl7: EGFP-HRAS); TgBAC(tcf21:mCherry-NTR) DMSO and MTZ-treated larvae. Green, 3D reconstruction of epicardial coverage showing a partial recovery of EpiCs in 52-96 hpf MTZ-treated larvae, and a clear reduction in 96-144 hpf MTZ-treated larvae. (D) Quantification of ventricular volume at 144 hpf following epicardial regeneration or late epicardial ablation. (G-I) Confocal images and quantification of 144 hpf Tg(myl7:mCherry-CAAX); TgBAC(tcf21:NLS-EGFP); TgBAC(tcf21:mCherry-NTR) DMSO and MTZ-treated larvae, showing the reduction of tcf21+ EpiCs attached to the ventricular myocardium after their late-onset ablation. Controls were pooled, including tcf21:NTR+ DMSO-treated and tcf21:NTR− MTZ-treated larvae (see Materials and Methods). Data show the mean±s.d. (D,I) and P-values are from one-way ANOVA with Dunnett's post hoc test (D) or unpaired two-tailed t-test (I). A, atrium; V, ventricle.
Previous studies investigating the consequences of reduced epicardial coverage have used genetic models or physical ablation of the PEO, but the constitutive lack of the epicardium fails to pinpoint the time window in which EpiCs promote myocardial development. As mentioned above, we identified the 52-96 hpf window to be crucial for epicardial-myocardial interactions, which coincides with the period of epicardial attachment. We then tested the effects of epicardial ablation between 96 and 144 hpf (Fig. 3A). Surprisingly, although the reduction in epicardial coverage was significant (Fig. 3E-I), we did not observe any obvious morphological defects in ventricular morphology or size (Fig. 3D-F′).
Taken together, these results suggest that an epicardial-myocardial crosstalk is necessary to regulate ventricular volume at the early epicardial attachment stage (52-96 hpf), but dispensable once epicardial coverage is complete (>96 hpf). We propose that from 96 hpf onwards, CMs continue to grow due to epicardial-independent intrinsic or extrinsic cues. Therefore, at later stages, the epicardium might assume a different function, including preparing for the onset of EMT. Future investigations of later epicardial function during cardiac development (e.g. at the onset of EMT and EPDC formation) will greatly benefit from the temporal versatility of this tcf21:NTR/MTZ model.
Epicardial-derived secreted factors promote ventricular cardiomyocyte growth
Next, we aimed to understand how the epicardium promotes CM growth. The epicardium is an important signaling center during development and regeneration (Quijada et al., 2020). Nonetheless, the appearance of extruding CMs in epicardial-deficient larvae (Fig. S3), which was also observed following PEO ablation in chick (Manner et al., 2005), raised questions as to whether the epicardium primarily acts as a physical barrier to preserve myocardial integrity. To further investigate the role of the epicardium as a mechanical support and/or signaling source, we focused on the sizable subgroup of tcf21−/− hearts with substantial epicardial coverage on their ventricle (Fig. 1H; Fig. S4A-C). We first observed that these mutants display defects in ventricular size comparable with those in tcf21−/− hearts devoid of epicardial coverage (Fig. S4A-C). We found no significant correlation between the number of ventricular EpiCs and ventricular volume (Fig. S4D), or the number of extruding CMs (Fig. S4F). Notably, we also observed that, occasionally, extruding CMs were covered by EpiCs (Fig. S4E,E′,G-G″), suggesting that the physical presence of EpiCs alone does not prevent CM extrusion.
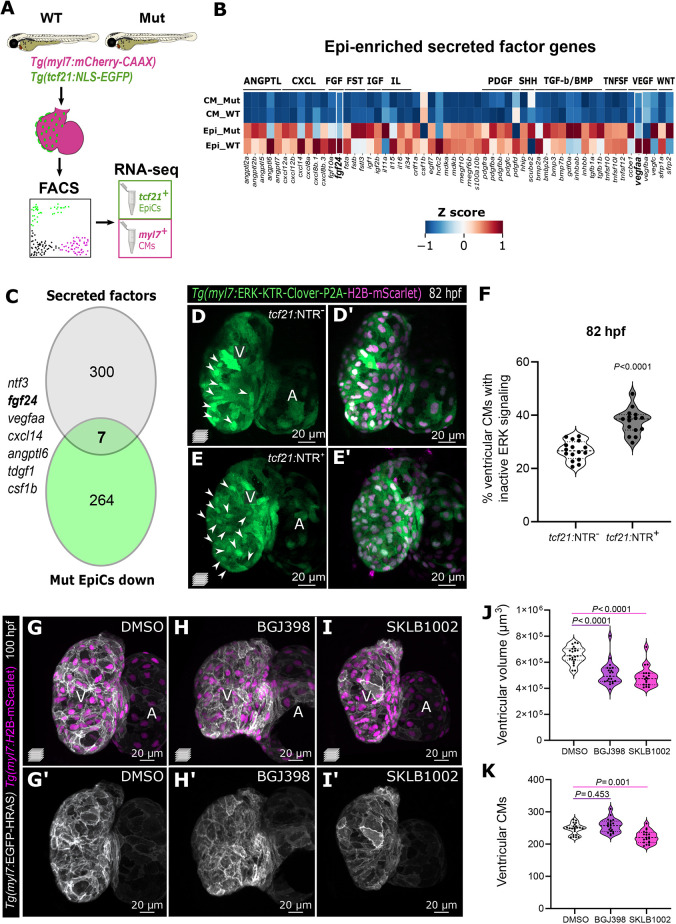
To identify epicardial factors necessary for CM growth, we compared the transcriptomes of sorted tcf21+/+ and tcf21−/− EpiCs and CMs at 96 hpf (Fig. 4A; Fig. S5A-C; Tables S2 and S3). To minimize bias in the analyses, we selected tcf21−/− larvae with similarly substantial ventricular epicardial coverage and collected the same number of EpiCs from the two genotypes (see Materials and Methods). We first determined the genes expressed in the two WT populations by RNA-sequencing (RNA-seq) and confirmed the expression of known cell type-specific markers, including postnb, wt1a, col1a2, cav1 and aldh1a2 in EpiCs and ttn.1, ttn.2, myh7 and myh6 in CMs. We also observed that the most enriched Gene Ontology (GO) terms reflected the biological processes prominent in the two cell types (e.g. EpiCs – extracellular matrix secretion, cell adhesion; CMs – mitochondrial processes and metabolism; Fig. S5C). Comparing the WT and tcf21−/− populations, we observed that genes upregulated in tcf21−/− EpiCs encode multiple extracellular matrix (ECM) components (including various collagens, as well as Postna and Has) and the related GO terms were amongst the most enriched (Fig. S5E). We envision that this dataset will be an important resource to further investigate epicardial development and regulation of epicardial function during embryogenesis.
Fig. 4.
Epicardial-enriched FGF and VEGF ligand genes in epicardial-myocardial crosstalk. (A) RNA-seq from tcf21+ and myl7+ cells from 96 hpf tcf21+/+ and tcf21−/− hearts. (B) Heatmap of zebrafish secreted factor genes in tcf21+/+ and tcf21−/− EpiCs and CMs, showing Z-scores normalized per row. The genes highlighted in bold are differentially expressed between tcf21+/+ and tcf21−/− EpiCs (log2FC<−0.7 or >0.7, Padj<0.05). (C) Venn diagram denoting the intersection between secreted factor genes in the zebrafish genome and genes that are downregulated (log2FC<−0.7, Padj<0.05) in tcf21−/− EpiCs. (D-F) Confocal images and quantification of 82 hpf Tg(myl7:ERK-KTR-Clover-P2A-H2B-mScarlet) ventricles in control (tcf21:NTR−) and TgBAC(tcf21:mCherry-NTR) (tcf21:NTR+) larvae treated with MTZ. White arrowheads point to CMs with nuclear Clover (inactive ERK), quantified in F. (G-K) Confocal images (G-I′), quantification of ventricular volume (J) and CM numbers (K) of 100 hpf larvae treated with FGFR (BGJ398) and VEGFR (SKLB1002) inhibitors starting at 65 hpf. The solid and dotted lines (F,J,K) indicate the median and quartiles, respectively, and P-values are from Kruskal-Wallis test with Dunn's post-hoc test. A, atrium; V, ventricle.
To identify potential molecular mechanisms disrupted in tcf21−/− hearts leading to the lack of CM growth, we explored GO terms that were enriched in the downregulated genes in tcf21−/− CMs (Fig. S5F). From this analysis, mitochondrial and ribosome-related terms were the most over-represented groups (Fig. S5F,G). In particular, genes that encode several mitochondrial ribosomal proteins (MRPs), which are necessary for mitochondrial protein synthesis and mitochondrial activity (Sylvester et al., 2004), were downregulated in tcf21−/− CMs. These data suggest that the defect in CM volume increase may be due to the failure of mitochondria to maintain normal metabolic function. Mutations in some MRP genes are correlated with cardiomyopathies in the human population (Huang et al., 2020; Friederich et al., 2021); however, further analysis is necessary to investigate how defects in MRP function lead to cardiac phenotypes.
For the purpose of this study, we decided to focus on epicardial-derived secreted factors that potentially mediate epicardial-myocardial crosstalk. Among the factors enriched in the EpiCs compared with CMs are members of the FGF, IGF, transforming growth factor (TGF)-β, and platelet-derived growth factor (PDGF) pathways (Fig. 4B; Table S3). These pathways are important for epicardial-myocardial crosstalk in mice (Olivey and Svensson, 2010; Li et al., 2017), suggesting that the molecular regulators of this crosstalk might be similar in zebrafish and humans.
Among the downregulated secreted factors in tcf21−/− EpiCs are FGF and VEGF ligand genes, including fgf10a, fgf24, vegfaa and vegfba (Fig. 4B,C; Table S3). fgf24 and vegfaa (Fig. 4C; Fig. S6A), in particular, are the FGF and VEGF ligand genes with the highest epicardial expression in our dataset and the only ones significantly downregulated (Padj<0.05; Table S3). The FGF pathway mediates epicardial-myocardial crosstalk in mouse and chicken embryos, where it is primarily known for its role in promoting CM proliferation (Pennisi et al., 2003; Lavine et al., 2005). By in situ hybridization, we observed fgf24 expression in 76 hpf hearts (Fig. S6B,B′), and it appeared to be enriched in the epicardium (Fig. S6C-C‴). Vegfaa promotes angiogenesis and coronary vessel formation (Liang et al., 2001; Wu et al., 2012; Marin-Juez et al., 2016; Rossi et al., 2016), but its role in the developing epicardium has not been investigated until recently (Bruton et al., 2022). We observed that vegfaa expression in the developing heart appeared to be initially limited to the epicardium until 100 hpf (Fig. S6D-E″; Bruton et al., 2022), at which point it was also expressed in the endocardium (Karra et al., 2018). The epicardial enrichment of fgf24 and vegfaa expression {log2[fold change (FC)]: +4.77 and +3.38, respectively} as well as their downregulation in tcf21−/− EpiCs led us to hypothesize that epicardial-derived Fgf24 and Vegfaa mediate signaling to the myocardium. To test this hypothesis, we first assessed the activation of the mitogen-activated protein kinase (MAPK)/extracellular regulated kinase (ERK) pathway, a downstream effector of both FGF and VEGF signaling, in CMs. We used the recently generated transgenic line Tg(myl7:ERK-KTR-Clover-P2A-H2B-mScarlet) (Qi et al., 2022) to monitor ERK activation through a kinase translocation reporter (de la Cova et al., 2017; Mayr et al., 2018; Okuda et al., 2021) (Fig. 4D). Following MTZ treatment between 52 and 80 hpf, Tg(myl7:ERK-KTR-Clover-P2A-H2B-mScarlet); TgBAC(tcf21:mCherry-NTR) hearts devoid of the epicardium exhibited a significantly increased number of CMs with inactive ERK signaling compared with control hearts (Fig. 4D-F). In addition, we used BGJ398 (De Simone et al., 2021) and SKLB1002 (Zhang et al., 2011) to broadly inhibit the FGF and VEGF signaling pathways, respectively, in a defined time window (65 to 100 hpf). We chose 65 hpf to avoid phenotypes caused by inhibiting the earlier roles of FGF and VEGF (Haigh, 2008; Khosravi et al., 2021). Notably, larvae treated with these compounds recapitulated the small ventricle phenotype observed in the epicardial-deficient hearts. FGF inhibition affected ventricular volume without causing any changes in CM numbers, whereas the VEGF inhibitor led to a mild decrease in ventricular CMs (∼10%; Fig. 4G-K). Thus, global inhibition of the VEGF pathway by SKLB1002 appeared to cause a stronger cardiac phenotype compared with the epicardial-specific downregulation of vegfaa in tcf21−/− hearts, and possibly affected additional signaling pathways.
Interestingly, the mammalian ortholog of Fgf24 binds Fgfr4 (Mok et al., 2014), which is highly expressed in zebrafish CMs and downregulated in tcf21−/− CMs, as per our transcriptomic datasets (Table S2). We speculate that the downregulation of Fgfr4 in tcf21−/− CMs might in part be due to the reduction in Fgf24 ligand in these mutants, and additional studies will be needed to investigate the involvement of different FGF ligands and receptors in regulating CM growth. However, Vegfaa is not known to bind receptors prominently expressed in CMs but binds Vegfr2, which is enriched in EpiCs. Therefore, Vegfaa might signal to EpiCs in an autocrine manner, similar to retinoic acid (Stuckmann et al., 2003; Brade et al., 2011), and regulate the production of other signaling molecules. It was also recently proposed that the epicardial expression of vegfaa (in response to macrophage activation) regulates Notch activity in the endocardium, which in turn signals to CMs (Bruton et al., 2022). A few studies have linked human FGF21 to mitochondrial function and disease (Tezze et al., 2019), but a clear mechanism is lacking.
Determining and comparing the molecular pathways impacted in each of the three different epicardial impairment models we present, through transcriptomic analysis for example, should give important insights into the epicardial-regulated mechanisms underlying CM growth. Also, we cannot exclude the possibility that the secreted factors that are upregulated in tcf21−/− hearts, such as il1a, scube2, ccbe1 and sfrp2, are involved in driving the cardiac phenotype. Further studies are needed to address the molecular mechanisms by which these signaling pathways mediate epicardial-myocardial crosstalk and how, in turn, this interaction promotes ventricular morphogenesis.
It is also likely that ECM-related components secreted by the epicardium play a role in CM growth and homeostasis. In particular, our transcriptomic analysis points to an altered ECM composition with the downregulation of some ECM genes, including col6a1, col6a2, col4a1, col4a2, lama5, hapln1a and hapln1b, and the upregulation of several other collagen isoforms, as well as postna, has and several metalloproteinases. Thus, it will also be interesting to investigate the potential role of the epicardial-derived ECM in promoting myocardial growth.
Taken together, our data uncover a previously unappreciated requirement for the epicardium in promoting CM growth at the cellular and tissue levels, which takes place prior to its previously reported role in stimulating CM proliferation. Moreover, we provide evidence that this inter-tissue crosstalk is mediated by the FGF and VEGF signaling pathways. The three epicardial-impairment models generated for and used in this study provide genetically tractable and, in one case, temporally manipulable systems that complement existing models to deepen our understanding of the cellular and molecular processes involved in epicardial-myocardial crosstalk during cardiac development.
MATERIALS AND METHODS
Zebrafish husbandry and lines
All zebrafish husbandry was performed under standard conditions, and all experiments were conducted in accordance with institutional (Max-Planck Gesellschaft) and national ethical and animal welfare guidelines. The following lines were used in the study: Tg(-0.8myl7:nlsDsRedExpress)hsc4, abbreviated Tg(myl7:nlsDsRed) (Takeuchi et al., 2011); Tg(myl7:mVenus-gmnn)ncv43 (Jimenez-Amilburu et al., 2016); Tg(myl7:EGFP-Hsa.HRAS)s883, abbreviated Tg(myl7:EGFP-HRAS) (D'Amico et al., 2007); TgBAC(tcf21:mCherry-NTR)pd108 (Wang et al., 2015); TgBAC(tcf21:NLS-EGFP)pd41 (Wang et al., 2015); Tg(myl7:BFP-CAAX)bns193 (Guerra et al., 2018); TgBAC(vegfaa:EGFP)pd260 (Karra et al., 2018); Tg(myl7:mCherry-CAAX)bns7 (Uribe et al., 2018); Tg(-0.8myl7:ERK-KTR-Clover-P2A-H2B-mScarlet)bns565, abbreviated Tg(myl7:ERK-KTR-Clover-P2A-H2B-mScarlet) (Qi et al., 2022); Tg(-0.8myl7:H2B-mScarlet)bns534, abbreviated Tg(myl7:H2B-mScarlet) (this study); tcf21bns427 (this study); and wt1abns428 (this study). All eggs, embryos and larvae were kept in egg water (3 g Instant Ocean, 0.75 g calcium sulfate, 10 l distilled water) unless otherwise stated.
Generation of transgenic lines
To generate Tg(-0.8myl7:H2B-mScarlet), the H2B and mScarlet sequences were cloned into a Tol2 backbone downstream of 800 bp of the myl7 promoter (-0.8myl7). Cloning was performed using InFusion Cloning (Takara Bio). The construct was injected into AB embryos at the one-cell stage (30 pg/embryo) together with Tol2 mRNA (25 pg/embryo) to establish the lines.
Generation of mutant lines using CRISPR/Cas9
To generate the tcf21bns427 and wt1abns428 mutant lines, the CRISPR design tool CHOPCHOP (https://chopchop.cbu.uib.no/) was used to design short guide RNAs (sgRNAs). The sgRNAs were assembled as described previously (Gagnon et al., 2014; Varshney et al., 2015) and transcribed using a MegaShortScript T7 Transcription Kit (Thermo Fisher Scientific). cas9 mRNA was transcribed using an mMESSAGE mMACHINE T3 Transcription Kit (Thermo Fisher Scientific) using pT3TS-nCas9n (Addgene plasmid #46757) as a template. sgRNAs and cas9 RNAs were purified with an RNA Clean and Concentrator Kit (Zymo Research). gRNAs (75 pg/embryo tcf21 gRNA; 25 pg/embryo wt1a gRNA) and cas9 mRNA (∼200 pg/embryo) were co-injected at the one-cell stage.
For tcf21bns427, an sgRNA targeting exon 1 (targeting sequence, 5′-CGCAGCTAACGCGCGC GAGA-3′) was used, resulting in a 10 bp deletion. To generate the wt1abns428 promoter-less allele, three sgRNAs targeting the promoter region of wt1a were designed and co-injected: two sgRNAs targeting the proximal promoter (sgRNA1, 5′-CAACTGGACAGCTTGGCCTA-3′; sgRNA2, 5′-AAAGGCGTCTAATAGACAAC-3′) and one sgRNA targeting intron 1 (sgRNA3, 5′-GGCAGTGCCACTCTTGCCAG-3′), resulting in a deletion of 8 kb.
tcf21 mutants were genotyped using high resolution melt analysis (Eco Illumina) with the following primers: tcf21 HRM fw, 5′-GCAAGCAGGTCCAGAGGA-3′, and tcf21 HRM rv, 5′-ACGGTAACGTCGTCTTCAGC-3′. tcf21 mutants carrying TgBAC(tcf21:NLS-EGFP) were additionally genotyped by PCR, to distinguish heterozygous and homozygous mutant embryos, using the following primers: tcf21 PCR fw, 5′-TGTCTCCAGCCAACATGTCCA-3′, and tcf21 PCR rev, 5′-GCGCATCCTCGCCCTCTCG-3′.
wt1a mutants were genotyped by PCR combining a common reverse primer (wt1a 3′ rv common, 5′-GCTGATCATCTCTGCGTTTG-3′) with one forward primer in the promoter region upstream of the deletion (wt1a 5′ fw1, 5′-TGTGAAATGAATGACACATCAAG-3′) or one forward primer in intron 1 inside the deleted region to detect the WT allele (wt1a 3′ fw2, 5′-CAATTGAAAAACTTTAAAAATCAGCA-3′).
Chemical treatments
For cell ablation experiments, metronidazole (MTZ, Sigma-Aldrich) treatment was performed as described previously (Curado et al., 2007, 2008; Pisharath et al., 2007; Wang et al., 2015) with some modifications. MTZ powder was freshly dissolved in DMSO at 1 M concentration and subsequently diluted in egg water to 5 mM (52-80 hpf, 52-100 hpf) or 7 mM (96-144 hpf) concentration. Embryos and larvae were treated in different time windows (52-80 hpf, 52-100 hpf, 96-144 hpf). The larvae were then immediately imaged or rinsed with egg water before growing in the incubator.
The broad FGFR inhibitor (BGJ398, Selleck Chemicals) (De Simone et al., 2021) and the VEGFR inhibitor (SKLB1002, Selleck Chemicals) (Zhang et al., 2011) were dissolved in DMSO at a concentration of 10 and 5 mM, respectively, and frozen in single-use aliquots at −80°C. Larvae were treated with a final concentration of 7.5 μM BGJ398 or 2.5μM SKLB1002 (Matsuoka et al., 2016). Different concentrations were first tested to minimize off-target developmental defects and the lowest effective doses were chosen. Control embryos were treated with the same concentrations of DMSO.
Whole-mount immunostaining
Whole-mount immunostaining was performed as described (Boezio et al., 2020). Larvae were fixed in 4% paraformaldehyde for 2 h at room temperature after stopping the heart with 0.4% tricaine. The fixative was substituted with PBS containing 0.1% Tween-20 and the yolks were manually removed using forceps. The blocking step preceding primary antibody incubation was performed in PBS with 1% bovine serum albumin, 1% DMSO and 0.5% Triton X-100 supplemented with 5% goat serum. Primary antibody incubations were performed overnight at 4°C at the following dilutions: anti-GFP (1:400, chicken; cat. no GFP-1020, AvesLabs), anti-Cav1 (1:50, mouse; cat. no 610406, BD Biosciences) and DsRed polyclonal antibody (1:200, rabbit; cat. no. 632496, Takara). All secondary antibodies tagged with Alexa Fluor 568, Alexa Fluor 488 and Alexa Fluor 647 (Thermo Fisher Scientific) were incubated overnight at 4°C at a 1:500 dilution. Larvae were incubated with 1 µg/ml DAPI with the secondary antibody.
Whole-mount in situ hybridization and fluorescence in situ hybridization
The following primers were used to generate the DNA template for the in situ RNA probes: tcf21 ISH fw, 5′-CGCATGACACGTTTCCACAT-3′; tcf21 ISH T7 rv, 5′-GGTAATACGACTCACTATAGGTGACATGACACTCGGCGT-3′; wt1a ISH fw, 5′-AAATGGCGTCACAGTTGGAG-3′; wt1a ISH T7 rv, 5′-GGTAATACGACTCACTATAGGTGTAATCAATCGACCTGCAGTG-3′; fgf24 ISH fw, 5′-ATGTCTGTTCTGCCGTCAAGG-3′; and fgf24 ISH T7 rv, 5′-TAATACGACTCACTATAGGAGTTTGTATTGGGGTTGGGT-3′. Digoxigenin (DIG)-labeled probes were transcribed in vitro from PCR products, using T7 polymerase (Promega) and a DIG RNA labeling kit (Roche).
In situ hybridization (ISH) was performed as described previously (Thisse and Thisse, 2008). Anti-MF20 primary antibody [mouse, 1:100, Developmental Studies Hybridoma Bank (DSHB); MF 20 was deposited to the DSHB by D.A. Fischman (DSHB Hybridoma Product MF 20)] to label the heart was added together with the anti-DIG antibody (1:5000, cat. no. 11093274910, Roche). The secondary antibody goat anti-mouse Alexa Fluor 488 (1:500, cat. no. A-11001, Thermo Fisher Scientific) was added after treatment with BM Purple (Roche) for 2 h at room temperature and washed in PBS with 0.1% Tween-20 before imaging.
Fluorescence in situ hybridization (FISH) was performed as described previously (Gunawan et al., 2019) and following the same protocol as for ISH, with some modifications. Briefly, after the hybridization with the probe and the blocking step, larvae were incubated with anti-DIG-POD (1:500, cat. no. 11207733910, Roche), anti-dsRed (rabbit, 1:200; cat. no. 632496, Takara), and anti-MF20 (mouse, 1:500; cat. no. 14-6503-82, Invitrogen) primary antibodies at 4°C overnight. After several washes with PBS containing 0.1% Tween-20, larvae were incubated in amplification buffer with TSA fluorescein reagent (1:100; Roche) at 37°C for 1-2 h. Samples were protected from light during the staining reaction. The reaction was stopped by a wash with PBS with 0.1% Tween-20 (15 min) followed by a 6% H2O2 wash for 30 min.
Larvae were incubated with goat anti-rabbit Alexa Fluor 647 and goat-anti mouse Alexa Fluor 568 (1:500, cat. no. A-21244, A-11004, Thermo Fisher Scientific) and DAPI (10 µg/ml) for 2 h at room temperature and washed in PBS with 0.1% Tween-20 before imaging with a Nikon SMZ25 stereomicroscope with a 2×/0.3 objective (ISH) or a Zeiss LSM880 confocal microscope with a 20× objective (FISH).
Confocal microscopy imaging
After immunostaining, fixed larvae were mounted in 1% low-melting agarose/egg water without tricaine, imaged with a Zeiss LSM700 confocal microscope with a W Plan-Apochromat 40×/1.0 dipping lens, and genotyped after imaging. For confocal imaging of live animals, larvae were embedded in 1% low-melting agarose with 0.2% or 0.01% tricaine to image stopped or beating hearts, respectively.
Videos of beating hearts were acquired with an exposure time of 5 ms for 20-40 s with a Zeiss spinning-disk confocal microscope. No tricaine was added to the agarose to avoid alteration of the heart rate. Confocal imaging of live hearts was performed with a Zeiss LSM700 or LSM800 Observer confocal microscope. Ventricular fractional shortening was quantified with ImageJ, using the following formula: [(width at diastole−width at systole)/width at diastole]×100.
Mosaic myl7:mScarlet analyses
The myl7:mScarlet plasmid (Uribe et al., 2018) was injected at the one-cell stage (25 pg/embryo) together with Tol2 mRNA (25 pg/embryo) into Tg(myl7:BFP-CAAX) embryos derived from tcf21+/− intercrosses. Injected embryos were selected for fluorescence at approximately 48 hpf. The same larvae were consecutively imaged at 76 and 100 hpf. Considering the variability in size and shape of the CMs depending on their location (Auman et al., 2007; Priya et al., 2020), only myl7:mScarlet+ compact-layer CMs in the ventricular outer curvature were analyzed. The larvae were genotyped after imaging and analysis.
Image analyses
The CM apical areas were manually quantified on a 3D reconstruction of the entire heart in ImageJ. CMs with inactive ERK signaling were defined as CMs with nuclear enrichment of the Clover signal and manually quantified in 2D sections in ImageJ. The numbers of total CMs, trabecular CMs, Venus-Gmnn+ CMs and EpiCs, and average internuclear distance were analyzed using the Spots function of the Imaris (Bitplane) software. 3D cardiac surface rendering, ventricular volume and individual CM volume quantifications were obtained with the Surfaces function of the Imaris (Bitplane) software. The numbers of extruding CM were quantified on the 3D myocardial surface rendering. Illustrations were done in Inkscape (XQuartz X11).
Statistical analyses
All statistical analyses were performed in GraphPad Prism (Version 8.4). Samples were tested for normal distribution using the D'Agostino–Pearson normality test. The following parametric tests were performed: unpaired two-tailed Student's t-test or one-way ANOVA, followed by Dunnett’s multiple comparison test, unless specified otherwise, for comparison of 2 or ≥3 samples, respectively. Non-parametric tests used were the Mann–Whitney test or Kruskal–Wallis test, followed by Dunn's multiple comparison test, for the comparison of 2 or ≥3 samples, respectively.
Randomization and blinding procedures
After selecting embryos or larvae for the relevant fluorescence signal, they were allocated randomly to different experimental groups. Animals were collected, grown or processed in the same dish or tube and genotyped after the imaging and analysis. For all experiments using the NTR-MTZ system, embryos and larvae from outcrosses of TgBAC(tcf21:mCherry-NTR); Tg(myl7:EGFP-HRAS) and AB were used. For the experiments performed to assess the epicardial coverage of MTZ-treated fish (Fig. 1M,N, Fig. S1, Fig. 3), DMSO-treated larvae were used as control. For most other experiments, all larvae were treated with MTZ to exclude contribution of the MTZ treatment to the observed phenotypes. TgBAC(tcf21:mCherry-NTR)− (tcf21:NTR−) larvae were used as controls and compared with TgBAC(tcf21:mCherry-NTR)+ (tcf21:NTR+) larvae. For the experiments shown in Fig. 1L and Fig. 3G-I designed to count surviving EpiCs after ablation, both tcf21:NTR+ DMSO-treated larvae and tcf21:NTR− MTZ-treated larvae were used as controls, and no significant difference was observed between the two groups. Therefore, the samples were pooled into the control group. Whenever possible, the investigators were blinded to allocation during experiments, data collection and analyses.
Heart isolation and fluorescence-activated cell sorting
Hearts from 96 hpf TgBAC(tcf21:NLS-EGFP); Tg(myl7:mCherry-CAAX) larvae were manually dissected in Dulbecco's modified Eagle's medium (DMEM, cat. no. 21969-035, Gibco) with 10% fetal bovine serum (FBS, S0615, Sigma/Merck). tcf21−/− larvae, derived from tcf21+/− intercrosses, were selected based on the phenotype (i.e. lack of head muscles), whereas tcf21+/+ larvae were obtained from tcf21+/+ sibling intercrosses. To minimize bias in our analyses and to collect enough ventricular EpiCs to investigate the EpiC-CM crosstalk, we selected only tcf21−/− larvae that exhibited a substantial ventricular epicardial coverage. Although this choice might have led to the selection of larvae with milder transcriptomic differences, we observed that these hearts displayed a morphologically comparable phenotype with the tcf21 mutants with less ventricular epicardial coverage (Fig. S4D,F). Hearts were centrifuged for 60 s at 2300 g and collected, washed with 1 ml Hanks' Balanced Salt Solution (HBSS) and dissociated into single cells by incubating in 100 μl Enzyme 1 and 5 μl Enzyme 2 (Pierce Cardiomyocytes Dissociation Kit, Thermo Fisher Scientific) for 20 min at 300-350 rpm in a 30°C shaker. The digestion was stopped by adding 1 ml DMEM with FBS. After centrifuging for 3 min at 800 g and discarding the supernatant, fresh DMEM with FBS was added to the dissociated cells and passed through 40 μl-filtered fluorescence-activated cell sorting (FACS) tubes. Cell viability was assessed with DAPI. Negative controls consisting of non-fluorescent hearts or single-color fluorescent hearts were prepared to adjust the gating. Cell filtering was performed by forward scatter amplitude (FSC-A) versus side scatter amplitude (SSC-A). Notably, from the FACS analyses, we observed that a population of tcf21+ cells was also mCherry+ (Fig. S5A). We confirmed, through confocal imaging (Fig. S5B,B′), that a subset of ventricular tcf21+ EpiCs displayed low levels of Tg(myl7:mCherry-CAAX) expression. According to this confocal imaging analysis, the double-positive population was composed of ventricular EpiCs. The single TgBAC(tcf21:NLS-EGFP)+ population was likely composed of OFT EpiCs and some ventricular EpiCs not expressing the myl7 transgene. In order to sort a sufficient number of ventricular EpiCs necessary to investigate the ventricular intercellular crosstalk between EpiCs and CMs, we decided to include the double-positive cells in the sorted epicardial population.
TgBAC(tcf21:NLS-EGFP)+ cells (EpiCs) and Tg(myl7:EGFP-HRAS)+/ TgBAC(tcf21:NLS-EGFP)− cells (CMs) were sorted on a FACSAria III machine (BD Biosciences), directly collected in TRIzol, and frozen at −80°C before RNA sequencing. Approximately 7000-10,000 EpiCs and CMs were collected per biological replicate. The same number of cells was collected per genotype, over five sorting sessions.
Transcriptomic analysis
Total RNA from sorted cells was isolated using the miRNeasy micro kit (QIAGEN), combined with on-column DNase digestion (DNase-Free DNase Set, QIAGEN). Total RNA and library integrity were verified with LabChip Gx Touch 24 (Perkin Elmer). Approximately 4 ng of total RNA was used as input for SMART-Seq v4 Ultra Low Input RNA Kit (Takara Clontech) for cDNA pre-amplification. Obtained full-length cDNA was checked on LabChip and fragmented by Ultrasonication using an E220 machine (Covaris). Final library preparation was performed using a Low Input Library Prep Kit v2 (Takara Clontech). Sequencing was performed on a NextSeq500 instrument (Illumina) using v2 chemistry, resulting in an average of 37 million reads per library with 1×75 bp single end setup. The resulting raw reads were assessed for quality, adapter content and duplication rates with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). Trimmomatic version 0.39 was used to trim reads with a quality drop below a mean of Q20 in a window of ten nucleotides (Davis et al., 2013). Only reads between 30 and 150 nucleotides were used in subsequent analyses. Trimmed and filtered reads were aligned versus the Ensembl Zebrafish genome version DanRer11 (ensemble release 99) using STAR 2.7.3a with the parameter ‘outFilterMismatchNoverLmax 0.1’ to set the maximum ratio of mismatches to mapped length at 10% (Dobin et al., 2013). The number of reads aligning to genes was counted with featureCounts 1.6.5 tool from the Subread package (Liao et al., 2014). Only reads mapping at least partially inside exons were admitted and aggregated per gene, whereas reads overlapping multiple genes or aligning to multiple regions were excluded from further analyses. Differentially expressed genes were identified using DESeq2 version 1.26.0 (Love et al., 2014). Genes were classified to be differentially expressed genes (DEGs) with the Benjamini–Hochberg-corrected P-value<0.05 and −0.59≤ Log2FC≥+0.59. The Ensembl annotation was enriched with UniProt data (release 06.06.2014) based on Ensembl gene identifiers. The top DEGs among the different samples are listed in Tables S2 and S3.
Differentially expressed genes encoding secreted factors were manually curated and annotated based on the Zebrafish matrisome genes (Nauroy et al., 2018). Expression data were imported in Python, Z-score transformed and plotted as heatmaps using Seaborn (https://joss.theoj.org/papers/10.21105/joss.03021).
Gene Ontology analyses
GO enrichment of differentially expressed genes (log2FC>|0.585|; P<0.05) of individual sub-ontologies (Biological Processes, Molecular Functions and Cellular Components) was calculated using Cluster Profiler (v 4.4.0) (Yu et al., 2012) and with annotations derived from org.Dr.eg.db (v 3.14.0). For all Gene Set Enrichment analyses, the R package fgsea (v.1.20.0) was used.
Scripts and data are available at https://github.com/giulia-boezio/epicardium_analyses. The data used for the plots, including individual gene names for each GO term, are available in Table S4.
RT-qPCR
Expression of tcf21 and wt1a was analyzed in single 96 hpf larvae deriving from tcf21bns427 or wt1abns428 heterozygous intercrosses, respectively. DNA and RNA were extracted from single embryos using TRIzol reagent, followed by TRIzol-chloroform extraction, as described previously (El-Brolosy et al., 2019).
To analyze fgf24 and vegfaa expression, hearts were manually dissected from 96 hpf tcf21+/+ and tcf21−/− larvae. tcf21−/− larvae were selected based on the lack of head muscles. RNA was extracted with the standard TRIzol-chloroform extraction and purified with RNA Clean and Concentrator Kit (Zymo Research).
Approximately 500 ng mRNA was used to synthesize cDNA using the Maxima First Strand cDNA kit (Thermo Fisher Scientific). For all experiments, DyNAmo ColorFlash SYBR Green qPCR Mix (Thermo Fisher Scientific) was used on a CFX connect Real-time System (Bio-Rad). All reactions were performed in technical triplicates and from three or more biological replicates. Every biological replicate consisted of cDNA from five to ten larvae or from 10-12 hearts per genotype. Gene expression values were normalized to the the zebrafish rpl13a housekeeping gene. The primers used are listed in Table S1.
Supplementary Material
Acknowledgments
This article is part of a collection ‘Moving Heart Failure to Heart Success: Mechanisms, Regeneration & Therapy’, which was launched in a dedicated Special Issue guest edited by Jeroen Bakkers, Milena Bellin and Ravi Karra. See related articles in this collection at https://journals.biologists.com/collection/8169/Moving-Heart-Failure-to-Heart-Success.
Acknowledgements
We would like to thank Matteo Perino for help with the RNA-seq analyses and critical comments on the manuscript; Ann Atzberger and Khrievono Kikhi for help with the FACS experiments; Michelle Collins, Alessandra Gentile, Srinivas Allanki and Hadil El-Sammak for valuable discussions and comments on the manuscript; Dr Radhan Ramadass for expert help with microscopy; Helen Allmendinger for experimental assistance; and all the fish facility staff for technical support.
Footnotes
Author contributions
Conceptualization: G.L.M.B., F.G., D.Y.R.S.; Methodology: G.L.M.B., R.P., S.M., S.G., N.F., F.G.; Validation: G.L.M.B.; Formal analysis: G.L.M.B., S.Z., J.G., S.G.; Investigation: G.L.M.B., S.Z., J.G., S.G.; Writing - original draft: G.L.M.B., F.G., D.Y.R.S.; Writing - review & editing: G.L.M.B., S.Z., J.G., R.P., S.M., S.G., F.G., D.Y.R.S.; Visualization: G.L.M.B.; Supervision: F.G., D.Y.R.S.; Project administration: D.Y.R.S.; Funding acquisition: D.Y.R.S.
Funding
This work was supported by funds from the Max-Planck-Gesellschaft to D.Y.R.S., a European Molecular Biology Organization (EMBO) Advanced Fellowship (ALTF 642-2018) and a Canadian Institutes of Health Research Fellowship (293898) to F.G., and an EMBO fellowship (LTF 1569-2016), a Humboldt fellowship from the Alexander von Humboldt-Stiftung and a Cardio-Pulmonary Institute grant (EXC 2026, project ID 390649896) to R.P. Open Access funding provided by Max Planck Society. Deposited in PMC for immediate release.
Data availability
The RNA-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database with the accession GSE174505. The scripts for all Gene Ontology analyses are available at https://github.com/giulia-boezio/epicardium_analyses.
References
- Acharya, A., Baek, S. T., Huang, G., Eskiocak, B., Goetsch, S., Sung, C. Y., Banfi, S., Sauer, M. F., Olsen, G. S., Duffield, J. S.et al. (2012). The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139, 2139-2149. 10.1242/dev.079970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman, H. J., Coleman, H., Riley, H. E., Olale, F., Tsai, H. J. and Yelon, D. (2007). Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 5, e53. 10.1371/journal.pbio.0050053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boezio, G. L., Bensimon-Brito, A., Piesker, J., Guenther, S., Helker, C. S. and Stainier, D. Y. (2020). Endothelial TGF-beta signaling instructs smooth muscle cell development in the cardiac outflow tract. Elife 9, e57603. 10.7554/eLife.57603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade, T., Kumar, S., Cunningham, T. J., Chatzi, C., Zhao, X., Cavallero, S., Li, P., Sucov, H. M., Ruiz-Lozano, P. and Duester, G. (2011). Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development 138, 139-148. 10.1242/dev.054239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton, F. A., Kaveh, A., Ross-Stewart, K. M., Matrone, G., Oremek, M. E. M., Solomonidis, E. G., Tucker, C. S., Mullins, J. J., Lucas, C. D., Brittan, M.et al. (2022). Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration. Dev. Cell 57, 1512-1528. 10.1016/j.devcel.2022.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg, L., Zhang, K., Bonawitz, T., Grajevskaja, V., Bellipanni, G., Waring, R. and Balciunas, D. (2016). Internal epitope tagging informed by relative lack of sequence conservation. Sci. Rep. 6, 36986. 10.1038/srep36986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. and Poss, K. D. (2018). The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 15, 631-647. 10.1038/s41569-018-0046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J., Navis, A., Cox, B. D., Dickson, A. L., Gemberling, M., Karra, R., Bagnat, M. and Poss, K. D. (2016). Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development 143, 232-243. 10.1242/dev.130534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W. Y., Gemberling, M., Wang, J., Holdway, J. E., Shen, M. C., Karlstrom, R. O. and Poss, K. D. (2013). In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 140, 660-666. 10.1242/dev.088526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado, S., Anderson, R. M., Jungblut, B., Mumm, J., Schroeter, E. and Stainier, D. Y. (2007). Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 236, 1025-1035. 10.1002/dvdy.21100 [DOI] [PubMed] [Google Scholar]
- Curado, S., Stainier, D. Y. and Anderson, R. M. (2008). Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 3, 948-954. 10.1038/nprot.2008.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico, L., Scott, I. C., Jungblut, B. and Stainier, D. Y. (2007). A mutation in zebrafish hmgcr1b reveals a role for isoprenoids in vertebrate heart-tube formation. Curr. Biol. 17, 252-259. 10.1016/j.cub.2006.12.023 [DOI] [PubMed] [Google Scholar]
- Davis, M. P., van Dongen, S., Abreu-Goodger, C., Bartonicek, N. and Enright, A. J. (2013). Kraken: a set of tools for quality control and analysis of high-throughput sequence data. Methods 63, 41-49. 10.1016/j.ymeth.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova, C., Townley, R., Regot, S. and Greenwald, I. (2017). A real-time biosensor for ERK activity reveals signaling dynamics during C. elegans cell fate specification. Dev. Cell 42, 542-553. 10.1016/j.devcel.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone, A., Evanitsky, M. N., Hayden, L., Cox, B. D., Wang, J., Tornini, V. A., Ou, J., Chao, A., Poss, K. D. and Di Talia, S. (2021). Control of osteoblast regeneration by a train of Erk activity waves. Nature 590, 129-133. 10.1038/s41586-020-03085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M. and Gingeras, T. R. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15-21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid, H., Larson, D. M., Springhorn, J. P., Attawia, M. A., Nayak, R. C., Smith, T. W. and Kelly, R. A. (1992). Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ. Res. 71, 40-50. 10.1161/01.RES.71.1.40 [DOI] [PubMed] [Google Scholar]
- El-Brolosy, M. A., Kontarakis, Z., Rossi, A., Kuenne, C., Gunther, S., Fukuda, N., Kikhi, K., Boezio, G. L. M., Takacs, C. M., Lai, S. L.et al. (2019). Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193-197. 10.1038/s41586-019-1064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, M. W., Geddes, G. C., Wortmann, S. B., Punnoose, A., Wartchow, E., Knight, K. M., Prokisch, H., Creadon-Swindell, G., Mayr, J. A. and Van Hove, J. L. K. (2021). Pathogenic variants in MRPL44 cause infantile cardiomyopathy due to a mitochondrial translation defect. Mol. Genet. Metab. 133, 362-371. 10.1016/j.ymgme.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, J. A., Valen, E., Thyme, S. B., Huang, P., Akhmetova, L., Pauli, A., Montague, T. G., Zimmerman, S., Richter, C. and Schier, A. F. (2014). Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One 9, e98186. 10.1371/journal.pone.0098186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile, A., Bensimon-Brito, A., Priya, R., Maischein, H. M., Piesker, J., Guenther, S., Gunawan, F. and Stainier, D. Y. (2021). The EMT transcription factor Snai1 maintains myocardial wall integrity by repressing intermediate filament gene expression. Elife 10, e66143. 10.7554/eLife.66143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, A., Germano, R. F., Stone, O., Arnaout, R., Guenther, S., Ahuja, S., Uribe, V., Vanhollebeke, B., Stainier, D. Y. and Reischauer, S. (2018). Distinct myocardial lineages break atrial symmetry during cardiogenesis in zebrafish. Elife 7, e32833. 10.7554/eLife.32833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan, F., Gentile, A., Fukuda, R., Tsedeke, A. T., Jimenez-Amilburu, V., Ramadass, R., Iida, A., Sehara-Fujisawa, A. and Stainier, D. Y. R. (2019). Focal adhesions are essential to drive zebrafish heart valve morphogenesis. J. Cell Biol. 218, 1039-1054. 10.1083/jcb.201807175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh, J. J. (2008). Role of VEGF in organogenesis. Organogenesis 4, 247-256. 10.4161/org.4.4.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, G., Li, H. and Zhang, H. (2020). Abnormal expression of mitochondrial ribosomal proteins and their encoding genes with cell apoptosis and diseases. Int. J. Mol. Sci. 21, 8879. 10.3390/ijms21228879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Amilburu, V., Rasouli, S. J., Staudt, D. W., Nakajima, H., Chiba, A., Mochizuki, N. and Stainier, D. Y. R. (2016). In Vivo visualization of cardiomyocyte apicobasal polarity reveals epithelial to mesenchymal-like transition during cardiac trabeculation. Cell Rep 17, 2687-2699. 10.1016/j.celrep.2016.11.023 [DOI] [PubMed] [Google Scholar]
- Jorgensen, P. and Tyers, M. (2004). How cells coordinate growth and division. Curr. Biol. 14, R1014-R1027. 10.1016/j.cub.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Karra, R., Foglia, M. J., Choi, W. Y., Belliveau, C., DeBenedittis, P. and Poss, K. D. (2018). Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proc. Natl. Acad. Sci. USA 115, 8805-8810. 10.1073/pnas.1722594115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner, P., Grondona, J. M., Mark, M., Gansmuller, A., LeMeur, M., Decimo, D., Vonesch, J. L., Dolle, P. and Chambon, P. (1994). Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell 78, 987-1003. 10.1016/0092-8674(94)90274-7 [DOI] [PubMed] [Google Scholar]
- Khosravi, F., Ahmadvand, N., Bellusci, S. and Sauer, H. (2021). The multifunctional contribution of FGF signaling to cardiac development, homeostasis, disease and repair. Front. Cell Dev. Biol. 9, 672935. 10.3389/fcell.2021.672935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine, K. J., Yu, K., White, A. C., Zhang, X., Smith, C., Partanen, J. and Ornitz, D. M. (2005). Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell 8, 85-95. 10.1016/j.devcel.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Li, P., Cavallero, S., Gu, Y., Chen, T. H., Hughes, J., Hassan, A. B., Bruning, J. C., Pashmforoush, M. and Sucov, H. M. (2011). IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 138, 1795-1805. 10.1242/dev.054338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Urban, A., Midura, D., Simon, H. G. and Wang, Q. T. (2017). Proteomic characterization of epicardial-myocardial signaling reveals novel regulatory networks including a role for NF-kappaB in epicardial EMT. PLoS One 12, e0174563. 10.1371/journal.pone.0174563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, D., Chang, J. R., Chin, A. J., Smith, A., Kelly, C., Weinberg, E. S. and Ge, R. (2001). The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech. Dev. 108, 29-43. 10.1016/S0925-4773(01)00468-3 [DOI] [PubMed] [Google Scholar]
- Liao, Y., Smyth, G. K. and Shi, W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923-930. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- Liu, J. and Stainier, D. Y. (2010). Tbx5 and Bmp signaling are essential for proepicardium specification in zebrafish. Circ. Res. 106, 1818-1828. 10.1161/CIRCRESAHA.110.217950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, M. I., Huber, W. and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtab, E. A., Vicente-Steijn, R., Hahurij, N. D., Jongbloed, M. R., Wisse, L. J., DeRuiter, M. C., Uhrin, P., Zaujec, J., Binder, B. R., Schalij, M. J.et al. (2009). Podoplanin deficient mice show a RhoA-related hypoplasia of the sinus venosus myocardium including the sinoatrial node. Dev. Dyn. 238, 183-193. 10.1002/dvdy.21819 [DOI] [PubMed] [Google Scholar]
- Manner, J. (1993). Experimental study on the formation of the epicardium in chick embryos. Anat. Embryol. (Berl) 187, 281-289. 10.1007/BF00195766 [DOI] [PubMed] [Google Scholar]
- Manner, J., Schlueter, J. and Brand, T. (2005). Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev. Dyn. 233, 1454-1463. 10.1002/dvdy.20487 [DOI] [PubMed] [Google Scholar]
- Marin-Juez, R., Marass, M., Gauvrit, S., Rossi, A., Lai, S. L., Materna, S. C., Black, B. L. and Stainier, D. Y. (2016). Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 113, 11237-11242. 10.1073/pnas.1605431113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, I. J., Ernst, A., Arora, P., Vianin, A., Hetke, T., Sanz-Morejon, A., Naumann, U., Odriozola, A., Langa, X., Andres-Delgado, L.et al. (2022). Wt1 transcription factor impairs cardiomyocyte specification and drives a phenotypic switch from myocardium to epicardium. Development 149, dev200375. 10.1242/dev.200375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, M. and Riley, P. R. (2014). The epicardium signals the way towards heart regeneration. Stem Cell Res. 13, 683-692. 10.1016/j.scr.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, R. L., Marass, M., Avdesh, A., Helker, C. S., Maischein, H. M., Grosse, A. S., Kaur, H., Lawson, N. D., Herzog, W. and Stainier, D. Y. (2016). Radial glia regulate vascular patterning around the developing spinal cord. Elife 5, e20253. 10.7554/eLife.20253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, V., Sturtzel, C., Stadler, M., Grissenberger, S. and Distel, M. (2018). Fast dynamic in vivo monitoring of Erk activity at single cell resolution in DREKA Zebrafish. Front. Cell Dev. Biol. 6, 111. 10.3389/fcell.2018.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok, G. F., Cardenas, R., Anderton, H., Campbell, K. H. and Sweetman, D. (2014). Interactions between FGF18 and retinoic acid regulate differentiation of chick embryo limb myoblasts. Dev. Biol. 396, 214-223. 10.1016/j.ydbio.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Moore, A. W., McInnes, L., Kreidberg, J., Hastie, N. D. and Schedl, A. (1999). YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126, 1845-1857. 10.1242/dev.126.9.1845 [DOI] [PubMed] [Google Scholar]
- Nagelberg, D., Wang, J., Su, R., Torres-Vazquez, J., Targoff, K. L., Poss, K. D. and Knaut, H. (2015). Origin, specification, and plasticity of the great vessels of the heart. Curr. Biol. 25, 2099-2110. 10.1016/j.cub.2015.06.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauroy, P., Hughes, S., Naba, A. and Ruggiero, F. (2018). The in-silico zebrafish matrisome: a new tool to study extracellular matrix gene and protein functions. Matrix Biol. 65, 5-13. 10.1016/j.matbio.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Okuda, K. S., Keyser, M., Gurevich, D. B., Sturtzel, C., Patterson, S., Chen, H., Scott, M., Condon, N. D., Martin, P., Distel, M.et al. (2021). Live-imaging of endothelial Erk activity reveals dynamic and sequential signalling events during regenerative angiogenesis. Elife, 10.7554/eLife.62196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivey, H. E. and Svensson, E. C. (2010). Epicardial-myocardial signaling directing coronary vasculogenesis. Circ. Res. 106, 818-832. 10.1161/CIRCRESAHA.109.209197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi, D. J., Ballard, V. L. and Mikawa, T. (2003). Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev. Dyn. 228, 161-172. 10.1002/dvdy.10360 [DOI] [PubMed] [Google Scholar]
- Peralta, M., Steed, E., Harlepp, S., Gonzalez-Rosa, J. M., Monduc, F., Ariza-Cosano, A., Cortes, A., Rayon, T., Gomez-Skarmeta, J. L., Zapata, A.et al. (2013). Heartbeat-driven pericardiac fluid forces contribute to epicardium morphogenesis. Curr. Biol. 23, 1726-1735. 10.1016/j.cub.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Peralta, M., Gonzalez-Rosa, J. M., Marques, I. J. and Mercader, N. (2014). The epicardium in the embryonic and adult Zebrafish. J Dev Biol 2, 101-116. 10.3390/jdb2020101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pomares, J. M., Phelps, A., Sedmerova, M. and Wessels, A. (2003). Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev. Dyn. 227, 56-68. 10.1002/dvdy.10284 [DOI] [PubMed] [Google Scholar]
- Pisharath, H., Rhee, J. M., Swanson, M. A., Leach, S. D. and Parsons, M. J. (2007). Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 124, 218-229. 10.1016/j.mod.2006.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya, R., Allanki, S., Gentile, A., Mansingh, S., Uribe, V., Maischein, H. M. and Stainier, D. Y. R. (2020). Tension heterogeneity directs form and fate to pattern the myocardial wall. Nature 588, 130-134. 10.1038/s41586-020-2946-9 [DOI] [PubMed] [Google Scholar]
- Qi, J., Rittershaus, A., Priya, R., Mansingh, S., Stainier, D. Y. R. and Helker, C. S. M. (2022). Apelin signaling dependent endocardial protrusions promote cardiac trabeculation in zebrafish. Elife 11, e73231. 10.7554/eLife.73231.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada, P., Trembley, M. A. and Small, E. M. (2020). The role of the epicardium during heart development and repair. Circ. Res. 126, 377-394. 10.1161/CIRCRESAHA.119.315857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli, S. J., El-Brolosy, M., Tsedeke, A. T., Bensimon-Brito, A., Ghanbari, P., Maischein, H. M., Kuenne, C. and Stainier, D. Y. (2018). The flow responsive transcription factor Klf2 is required for myocardial wall integrity by modulating Fgf signaling. Elife 7, e38889. 10.7554/eLife.38889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, L. S., Lalani, S., Runyan, R. B. and Camenisch, T. D. (2008). Differential growth and multicellular villi direct proepicardial translocation to the developing mouse heart. Dev. Dyn. 237, 145-152. 10.1002/dvdy.21378 [DOI] [PubMed] [Google Scholar]
- Rossi, A., Gauvrit, S., Marass, M., Pan, L., Moens, C. B. and Stainier, D. Y. R. (2016). Regulation of Vegf signaling by natural and synthetic ligands. Blood 128, 2359-2366. 10.1182/blood-2016-04-711192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serluca, F. C. (2008). Development of the proepicardial organ in the zebrafish. Dev. Biol. 315, 18-27. 10.1016/j.ydbio.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Smith, C. L., Baek, S. T., Sung, C. Y. and Tallquist, M. D. (2011). Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 108, e15-e26. 10.1161/CIRCRESAHA.110.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, A. M., Dronkers, E. and Goumans, M. J. (2018). The epicardium as a source of multipotent adult cardiac progenitor cells: their origin, role and fate. Pharmacol. Res. 127, 129-140. 10.1016/j.phrs.2017.07.020 [DOI] [PubMed] [Google Scholar]
- Son, S., Kang, J. H., Oh, S., Kirschner, M. W., Mitchison, T. J. and Manalis, S. (2015). Resonant microchannel volume and mass measurements show that suspended cells swell during mitosis. J. Cell Biol. 211, 757-763. 10.1083/jcb.201505058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckmann, I., Evans, S. and Lassar, A. B. (2003). Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev. Biol. 255, 334-349. 10.1016/S0012-1606(02)00078-7 [DOI] [PubMed] [Google Scholar]
- Sugiyama, M., Sakaue-Sawano, A., Iimura, T., Fukami, K., Kitaguchi, T., Kawakami, K., Okamoto, H., Higashijima, S. and Miyawaki, A. (2009). Illuminating cell-cycle progression in the developing zebrafish embryo. Proc. Natl. Acad. Sci. USA 106, 20812-20817. 10.1073/pnas.0906464106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester, J. E., Fischel-Ghodsian, N., Mougey, E. B. and O'Brien, T. W. (2004). Mitochondrial ribosomal proteins: candidate genes for mitochondrial disease. Genet. Med. 6, 73-80. 10.1097/01.GIM.0000117333.21213.17 [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Yamagishi, T., Narematsu, M., Kamimura, T., Kai, M. and Nakajima, Y. (2014). Epicardium is required for sarcomeric maturation and cardiomyocyte growth in the ventricular compact layer mediated by transforming growth factor beta and fibroblast growth factor before the onset of coronary circulation. Congenit Anom (Kyoto 54, 162-171. 10.1111/cga.12048 [DOI] [PubMed] [Google Scholar]
- Takeuchi, J. K., Lou, X., Alexander, J. M., Sugizaki, H., Delgado-Olguin, P., Holloway, A. K., Mori, A. D., Wylie, J. N., Munson, C., Zhu, Y.et al. (2011). Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat. Commun. 2, 187. 10.1038/ncomms1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezze, C., Romanello, V. and Sandri, M. (2019). FGF21 as modulator of metabolism in health and disease. Front Physiol 10, 419. 10.3389/fphys.2019.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse, C. and Thisse, B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59-69. 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- Uribe, V., Ramadass, R., Dogra, D., Rasouli, S. J., Gunawan, F., Nakajima, H., Chiba, A., Reischauer, S., Mochizuki, N. and Stainier, D. Y. R. (2018). In vivo analysis of cardiomyocyte proliferation during trabeculation. Development 145, dev164194. 10.1242/dev.164194 [DOI] [PubMed] [Google Scholar]
- van Wijk, B., Gunst, Q. D., Moorman, A. F. and van den Hoff, M. J. (2012). Cardiac regeneration from activated epicardium. PLoS One 7, e44692. 10.1371/journal.pone.0044692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, G. K., Pei, W., LaFave, M. C., Idol, J., Xu, L., Gallardo, V., Carrington, B., Bishop, K., Jones, M., Li, M.et al. (2015). High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 25, 1030-1042. 10.1101/gr.186379.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Hernandez, M., Kovacs, A., De Langhe, S. and Ornitz, D. M. (2011). FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development 138, 3331-3340. 10.1242/dev.064410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Cao, J., Dickson, A. L. and Poss, K. D. (2015). Epicardial regeneration is guided by cardiac outflow tract and Hedgehog signalling. Nature 522, 226-230. 10.1038/nature14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, M., Simoes, F. C., Patient, R., Sauka-Spengler, T. and Riley, P. R. (2020). Functional Heterogeneity within the Developing Zebrafish Epicardium. Dev. Cell 52, 574-590. 10.1016/j.devcel.2020.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, M., Simões, F. C., Sauka-Spengler, T. and Riley, P. R. (2021). Distinct epicardial gene regulatory programmes drive development and regeneration of the zebrafish heart. bioRxiv. 10.1101/2021.06.29.450229 [DOI] [PubMed] [Google Scholar]
- Wu, B., Zhang, Z., Lui, W., Chen, X., Wang, Y., Chamberlain, A. A., Moreno-Rodriguez, R. A., Markwald, R. R., O'Rourke, B. P., Sharp, D. J.et al. (2012). Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151, 1083-1096. 10.1016/j.cell.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, G., Wang, L.-G., Han, Y. and He, Q.-Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 5, 284-287. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Cao, Z., Tian, H., Shen, G., Ma, Y., Xie, H., Liu, Y., Zhao, C., Deng, S., Yang, Y.et al. (2011). SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 17, 4439-4450. 10.1158/1078-0432.CCR-10-3109 [DOI] [PubMed] [Google Scholar]
- Zlotek-Zlotkiewicz, E., Monnier, S., Cappello, G., Le Berre, M. and Piel, M. (2015). Optical volume and mass measurements show that mammalian cells swell during mitosis. J. Cell Biol. 211, 765-774. 10.1083/jcb.201505056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.